Recent Insights into Eco-Friendly Extraction Techniques for Obtaining Bioactive Compounds from Fruit Seed Oils
Abstract
1. Introduction
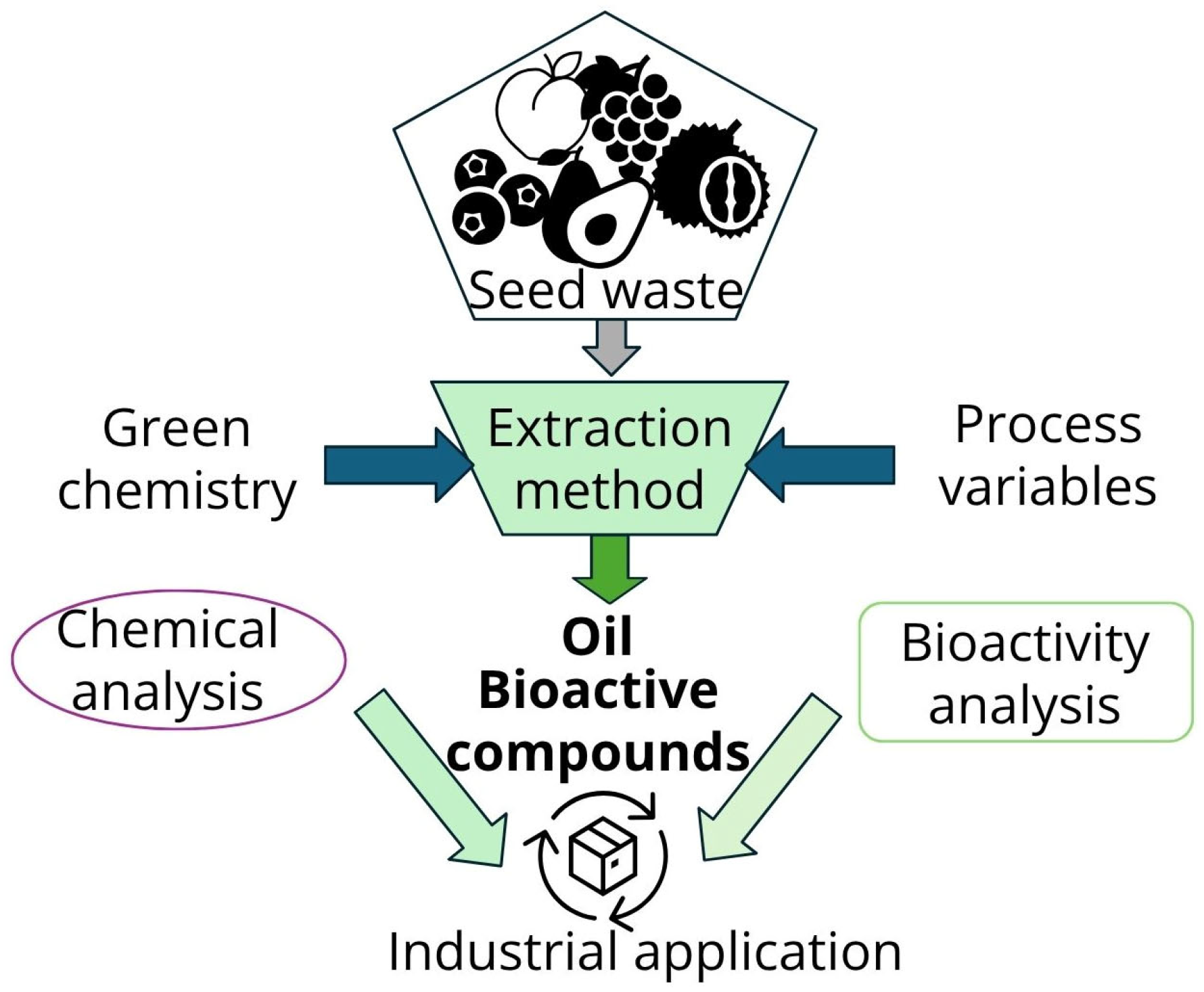
2. Valorization of Fruit Seeds as Agri-Food Residues
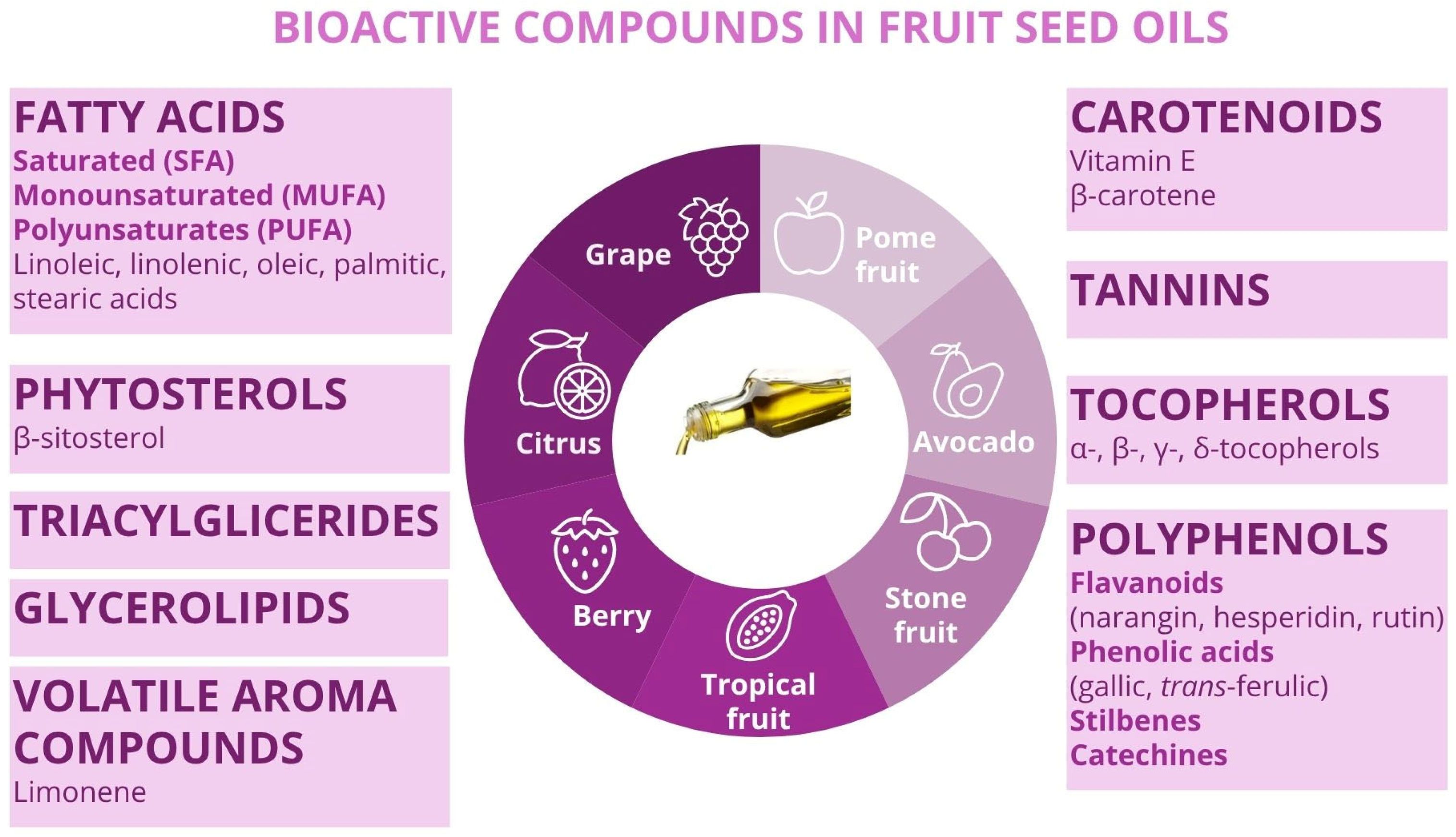
3. Bioactive Compounds in Fruit Seed Oils: Nutritional Value, Application Potential, and Determination Methods
4. Conventional Extraction Methods: Limitations and Challenges
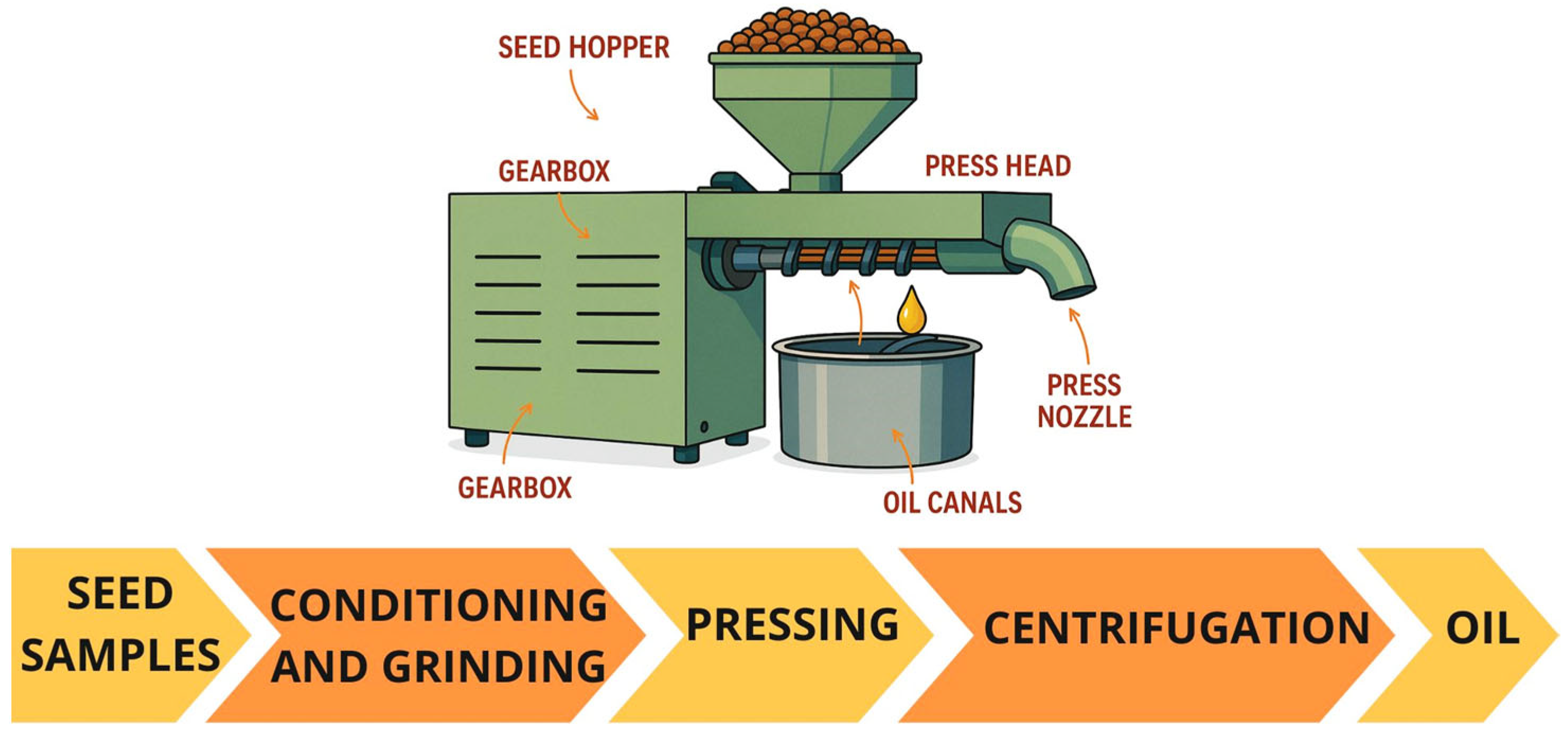
4.1. Mechanical Pressing
4.2. Solvent Extraction
4.3. Supercritical CO2 Extraction
5. Recent Advances in Eco-Friendly Extraction Methods and Factors Affecting Extraction Efficiency and Oil Quality
5.1. Ultrasound-Assisted Extraction (UAE)
5.2. Microwave-Assisted Extraction (MAE)
| Food Waste | Solvent | Processing conditions | Oil Recovery (wt%) | Bioactive Compounds | References |
|---|---|---|---|---|---|
| Pomegranate seeds | n-hexane or petroleum ether | Power: 500 W Frequency: 40 kHz Time: 9 min; Sample-to-solvent ratio: 1:6 (w/v) | 11.72–12.47 | Palmitic acid: 29.3% Oleic acid: 49.5% Stearic acid: 19.9% Linoleic acid: 53.6% Punicic acid: 776.5% α-Eleoestearic acid: 5.3% β-Eleoestearic acid: 33.0% Catalpic acid: 10.2% Arachidic acid: 6.2% Arachidonic acid: 7.6% ꞵ-Sitosterol: 3.47 mg·g−1 Squalene: 1.59 mg·g−1 α-tocopherol: 17.24 mg·100 g−1 γ-tocopherol: 322.7 mg·100 g−1 δ-tocopherol: 8.23 mg·100 g−1 | [38] |
| n-hexane | Power: 220 W, Time: 5 min; Sample-to-solvent ratio: 1:10 (w/v) | 35.19 | Palmitic acid: 2.04% Oleic acid: 4.10% Stearic acid: 1.71% Linoleic acid: 3.84% α-Linolenic acid: 86.53% Margaric acid: 0.04% Arachidic acid: 0.37% Gadoleic acid: 0.69.0% TPC: 7.42 mg·g−1 | [86] | |
| Dragon fruit seeds | - | Power: 450 W; Time: 10 min | 34.3 | Palmitic acid: 14.69% Stearic acid: 7.30% Oleic acid: 18.66% Linolenic acid: 52.14% Arachidonic acid:0.04% TPC: 96.71 mg GAE·g−1 α-tocopherol: 52.19 mg·kg−1 γ-tocopherol: 921.58 mg·kg−1 δ-tocopherol: 76.6 mg·kg−1 | [8] |
5.3. Enzyme-Assisted Extraction (EAE)
5.4. Pressurized Liquid Extraction (PLE)
5.5. Pulsed Electric Fields (PEF) Extraction
5.6. Subcritical Extraction
5.7. Hybrid Extraction Technologies
6. Green Assessment of Extraction Techniques
7. Integrating Artificial Intelligence into Waste Valorization Processes
8. Industrial Applications and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGREE | Analytical Greenness Metric Approach |
| AGREEprep | Analytical Greenness Metric for Sample Preparation |
| AI | Artificial intelligence |
| ANN | Artificial neural network |
| BAGI | Blue Applicability Grade Index |
| CLCAs | Life Cycle Assessments |
| ACLCAs | Attributional Life Cycle Assessments |
| DAD | Diode-array detector |
| EAE | Enzyme-assisted extraction |
| EFSA | European Food Safety Authority |
| FDA | Food and Drug Administration |
| FAMEs | Fatty acid methyl esters |
| FID | Flame ionization detection |
| GAC | Green Analytical Chemistry |
| GC | Gas chromatography |
| GAMs | Green Analytical Metrics |
| GAPI | Green Analytical Procedure Index |
| HCUEPM | Homogenization-circulating ultrasound with enzymatic pretreatment microwave-assisted extraction |
| HPLC | High-performance liquid chromatography |
| MAE | Microwave-assisted extraction |
| MAEB | Microwave-assisted extraction based on emulsion breaking |
| MS | Mass spectrometry |
| MUFAs | Monounsaturated fatty acids |
| NEMI | National Index of Environmental Methods |
| PEFs | Pulsed electric fields |
| PEF-SFE | Pulsed electric field–supercritical fluid extraction |
| PLE | Pressurized liquid extraction |
| PUFAs | Polyunsaturated fatty acids |
| RSM | Response surface methodology |
| ScCO2 | Supercritical carbon dioxide extraction |
| SDGs | Sustainable Development Goals |
| SFAs | Saturated fatty acids |
| S/F | Solvent-to-sample feed mass ratio |
| SFE | Supercritical fluid extraction |
| SFE-UAE | Supercritical fluid extraction–ultrasound-assisted extraction |
| TEA | Technical–economic analysis |
| TPC | Total polyphenol content |
| TFC | Total flavonoid content |
| UAE | Ultrasound-assisted extraction |
| UFAs | Unsaturated fatty acids |
| UMAE | Ultrasound–microwave assisted extraction |
| UMAEE | Ultrasound–microwave-assisted aqueous enzymatic extraction |
References
- Rani, H.; Sharma, S.; Bala, M. Technologies for Extraction of Oil from Oilseeds and Other Plant Sources in Retrospect and Prospects: A Review. J. Food Process Eng. 2021, 44, e13851. [Google Scholar] [CrossRef]
- Rodríguez-Blázquez, S.; Pedrera-Cajas, L.; Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Rodríguez-Bencomo, J.J.; Miranda, R. The Potential of Plum Seed Residue: Unraveling the Effect of Processing on Phytochemical Composition and Bioactive Properties. Int. J. Mol. Sci. 2024, 25, 1236. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E. Unlocking the in Vitro Neuroprotection of Sloe Residues Phenolic Extracts by Bioanalytical and Chemometric Strategies. Food Chem. 2025, 463, 141208. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, L.P.; Silva, E.K. Pulsed Electric Field-Assisted Extraction Techniques for Obtaining Vegetable Oils and Essential Oils: Recent Progress and Opportunities for the Food Industry. Sep. Purif. Technol. 2025, 354, 128833. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. [Google Scholar] [CrossRef]
- Dhiman, S.; Thakur, B.; Kaur, S.; Ahuja, M.; Gantayat, S.; Sarkar, S.; Singh, R.; Tripathi, M. Closing the Loop: Technological Innovations in Food Waste Valorisation for Global Sustainability. Discov. Sustain. 2025, 6, 258. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Boyapati, T.; Rana, S.S.; Ghosh, P. Microwave-Assisted Extraction of Dragon Fruit Seed Oil: Fatty Acid Profile and Functional Properties. J. Saudi Soc. Agric. Sci. 2023, 22, 149–157. [Google Scholar] [CrossRef]
- Ćurko, N.; Lukić, K.; Tušek, A.J.; Balbino, S.; Vukušić Pavičić, T.; Tomašević, M.; Redovniković, I.R.; Ganić, K.K. Effect of Cold Pressing and Supercritical CO2 Extraction Assisted with Pulsed Electric Fields Pretreatment on Grape Seed Oil Yield, Composition and Antioxidant Characteristics. LWT 2023, 184, 114974. [Google Scholar] [CrossRef]
- Wang, W.Y.; Yan, Y.Y.; Liu, H.M.; Qi, K.; Zhu, X.L.; Wang, X.D.; Qin, G.Y. Subcritical Low Temperature Extraction Technology and Its Application in Extracting Seed Oils. J. Food Process Eng. 2021, 44, e13805. [Google Scholar] [CrossRef]
- Massaa, T.B.; Iwassaa, I.J.; Stevanatoa, N.; Garciab, V.A.S.; Silva, C. Passion Fruit Seed Oil: Extraction and Subsequent Transesterification Reaction. Grasas Y Aceites 2021, 72, 2. [Google Scholar] [CrossRef]
- Barrales, F.M.; Rezende, C.A.; Martínez, J. Supercritical CO2 Extraction of Passion Fruit (Passiflora Edulis sp.) Seed Oil Assisted by Ultrasound. J. Supercrit. Fluids 2015, 104, 183–192. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K. Ultrasound and Microwave-Assisted Solvent Extraction of Mango Kernel Oil and Evaluation of Physicochemical Properties and Fatty Acid Profile. J. Food Process Preserv. 2021, 45, e16090. [Google Scholar] [CrossRef]
- Lin, Z.; Grasso, S. Exploring Seed-Based Upcycled Oils: Types, Extraction Processes, and Emerging Applications. Crit. Rev. Food Sci. Nutr. 2025, 1–20. [Google Scholar] [CrossRef]
- Choe, U.; Childs, H.; Zeng, M.; Zheng, W.; Zhu, H.; Zhu, L.; Xie, Z.; Gao, B.; Yu, L. Value-Added Utilization of Fruit Seed Oils for Improving Human Health: A Progress Review. ACS Food Sci. Technol. 2023, 3, 528–538. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Puranik, S.; Radha; Natta, S.; Senapathy, M.; Dhumal, S.; Singh, S.; Kumar, S.; Deshmukh, V.P.; et al. Combination of Green Extraction Techniques and Smart Solvents for Bioactives Recovery. TrAC Trends Anal. Chem. 2023, 169, 117286. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent Advances in Scaling-up of Non-Conventional Extraction Techniques: Learning from Successes and Failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction Methods of Oils and Phytochemicals from Seeds and Their Environmental and Economic Impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Araújo, A.S.; de Lima, G.S.; Nunes, I.d.S.; Aguiar, J.C.R.d.O.F.d.; Navarro, D.M.D.A.F.; Melo, N.F.C.B.; Magalhães, N.S.S.; França, R.; Carvalho, R.d.S.F.; Stamford, T.C.M. Chitosan Hydrochloride-Gum Arabic-Passion Fruit Seed Oil Nanoparticle Edible Coating to Control Fungal Infection and Maintain Quality Parameters of Strawberries. Food Control 2024, 161, 110360. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, S.; Yang, Y.; Zheng, X.; Xiao, D.; Ai, B.; Sheng, Z. Volatile Aroma Compounds of Passion Fruit Seed Oils: HS-GC-IMS Analysis and Interpretation. Food Chem. X 2024, 21, 101212. [Google Scholar] [CrossRef]
- Le, X.-T.; Ly, T.-T.; Tong, T.-D.; Luu, X.-C.; Thuy Nguyen Pham, D.; Hien Tran, T. Oil Extraction of Purple Passion Fruit Seeds Grown in Vietnam. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Yang, Q.; Li, L.; Cao, Y.; Zhong, R.; Miao, J. Extraction and Characterization of Passion Fruit Seed Oil and Investigation of Its Hypolipidemic Activity. J. Agric. Food Res. 2025, 19, 101672. [Google Scholar] [CrossRef]
- Antoniassi, R.; Wilhelm, A.E.; Rosa Reis, S.L.; Regis, S.A.; Faria-Machado, A.F.; Bizzo, H.R.; Cenci, S.A. Expeller Pressing of Passion Fruit Seed Oil: Pressing Efficiency and Quality of Oil. Braz. J. Food Technol. 2022, 25, e2021168. [Google Scholar] [CrossRef]
- Pereira, M.G.; Maciel, G.M.; Haminiuk, C.W.I.; Bach, F.; Hamerski, F.; de Paula Scheer, A.; Corazza, M.L. Effect of Extraction Process on Composition, Antioxidant and Antibacterial Activity of Oil from Yellow Passion Fruit (Passiflora Edulis Var. Flavicarpa) Seeds. Waste Biomass Valorization 2019, 10, 2611–2625. [Google Scholar] [CrossRef]
- Surlehan, H.F.; Azman, N.; Zakaria, R.; Amin, M. Extraction of Oil from Passion Fruit Seeds Using Surfactant-Assisted Aqueous Extraction. Food Res. 2019, 3, 348–356. [Google Scholar] [CrossRef]
- Liu, N.; Ren, G.; Faiza, M.; Li, D.; Cui, J.; Zhang, K.; Yao, X.; Zhao, M. Comparison of Conventional and Green Extraction Methods on Oil Yield, Physicochemical Properties, and Lipid Compositions of Pomegranate Seed Oil. J. Food Compos. Anal. 2022, 114, 104747. [Google Scholar] [CrossRef]
- Goula, A.M.; Papatheodorou, A.; Karasavva, S.; Kaderides, K. Ultrasound-Assisted Aqueous Enzymatic Extraction of Oil from Pomegranate Seeds. Waste Biomass Valorization 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Lolli, V.; Viscusi, P.; Bonzanini, F.; Conte, A.; Fuso, A.; Larocca, S.; Leni, G.; Caligiani, A. Oil and Protein Extraction from Fruit Seed and Kernel By-Products Using a One Pot Enzymatic-Assisted Mild Extraction. Food Chem. X 2023, 19, 100819. [Google Scholar] [CrossRef]
- Rosa, A.; Era, B.; Masala, C.; Nieddu, M.; Scano, P.; Fais, A.; Porcedda, S.; Piras, A. Supercritical CO2 Extraction of Waste Citrus Seeds: Chemical Composition, Nutritional and Biological Properties of Edible Fixed Oils. Eur. J. Lipid Sci. Technol. 2019, 121, 1800502. [Google Scholar] [CrossRef]
- Garrido, G.; Chou, W.-H.; Vega, C.; Goïty, L.; Valdés, M. Influence of Extraction Methods on Fatty Acid Composition, Total Phenolic Content and Antioxidant Capacity of Citrus Seed Oils from the Atacama Desert, Chile. J. Pharm. Pharmacogn. Res. 2019, 7, 389–407. [Google Scholar] [CrossRef]
- Hasanov, J.H.; Mirzaxmedov, S.D.; Sultonova, E.M.; Salikhov, S.I. Effect of Moisture Content on the Quality and Quantity of Screw-Pressed Flax Seed Oil. Food Process. Tech. Technol. 2023, 53, 309–315. [Google Scholar] [CrossRef]
- Arturo-Perdomo, D.; Mora, J.P.J.; Ibáñez, E.; Cifuentes, A.; Hurtado-Benavides, A.; Montero, L. Extraction and Characterization of the Polar Lipid Fraction of Blackberry and Passion Fruit Seeds Oils Using Supercritical Fluid Extraction. Food Anal. Methods 2021, 14, 2026–2037. [Google Scholar] [CrossRef]
- Bose, P.; Pradhan, D.; Sonawane, A.; Pathak, S.S.; Anupriya, S.; Pradhan, R.C. Optimization of Hot Air and Microwave Pretreatment for Enhancing the Mechanical Expression Yield of Simarouba glauca Oil. J. Agric. Food Res. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Dimić, I.; Pezo, L.; Rakić, D.; Teslić, N.; Zeković, Z.; Pavlić, B. Supercritical Fluid Extraction Kinetics of Cherry Seed Oil: Kinetics Modeling and Ann Optimization. Foods 2021, 10, 1513. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ghazani, S.M.; Corazza, M.L.; Marangoni, A.G.; Ribani, R.H. Assessment of Subcritical Propane, Supercritical CO2 and Soxhlet Extraction of Oil from Sapucaia (Lecythis pisonis) Nuts. J. Supercrit. Fluids 2018, 133, 122–132. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Maciel, L.G.; Mazzutti, S.; Gonçalves, C.B.; Ferreira, S.R.S.; Block, J.M. Composition, Thermal Behavior and Antioxidant Activity of Pracaxi (Pentaclethra macroloba) Seed Oil Obtained by Supercritical CO2. Biocatal. Agric. Biotechnol. 2020, 24, 101521. [Google Scholar] [CrossRef]
- Correa, M.S.; Fetzer, D.L.; Hamerski, F.; Corazza, M.L.; Scheer, A.P.; Ribani, R.H. Pressurized Extraction of High-Quality Blackberry (Rubus spp. Xavante Cultivar) Seed Oils. J. Supercrit. Fluids 2021, 169, 105101. [Google Scholar] [CrossRef]
- Gallon, R.; Draszewski, C.P.; Santos, J.A.A.; Wagner, R.; Brondani, M.; Zabot, G.L.; Tres, M.V.; Hoffmann, R.; Castilhos, F.; Abaide, E.R.; et al. Obtaining Oil, Fermentable Sugars, and Platform Chemicals from Butia odorata Seed Using Supercritical Fluid Extraction and Subcritical Water Hydrolysis. J. Supercrit. Fluids 2023, 203, 106062. [Google Scholar] [CrossRef]
- Gasparini, A.; Ferrentino, G.; Angeli, L.; Morozova, K.; Zatelli, D.; Scampicchio, M. Ultrasound Assisted Extraction of Oils from Apple Seeds: A Comparative Study with Supercritical Fluid and Conventional Solvent Extraction. Innov. Food Sci. Emerg. Technol. 2023, 86, 103370. [Google Scholar] [CrossRef]
- Dimić, I.; Pavlić, B.; Rakita, S.; Cvetanović Kljakić, A.; Zeković, Z.; Teslić, N. Isolation of Cherry Seed Oil Using Conventional Techniques and Supercritical Fluid Extraction. Foods 2023, 12, 11. [Google Scholar] [CrossRef]
- Pavlić, B.; Pezo, L.; Marić, B.; Tukuljac, L.P.; Zeković, Z.; Solarov, M.B.; Teslić, N. Supercritical Fluid Extraction of Raspberry Seed Oil: Experiments and Modelling. J. Supercrit. Fluids 2020, 157, 104687. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Fatty Acid Composition, Bioactive Phytochemicals, Antioxidant Properties and Oxidative Stability of Edible Fruit Seed Oil: Effect of Preharvest and Processing Factors. Heliyon 2020, 6, e04962. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Aydeniz Guneser, B.; Ok, S. Valorization of Grapefruit Seeds: Cold Press Oil Production. Waste Biomass Valorization 2019, 10, 2713–2724. [Google Scholar] [CrossRef]
- Cui, J.; Li, T.; Zhou, Y.; Wang, L.; Li, T.; Zhang, W. Comparative Lipidomics Analysis of Seed Oils from Nine Tropical Fruits: Emphasizing the Fatty Acid and Lipid Molecule Profiles. Food Res. Int. 2024, 198, 115334. [Google Scholar] [CrossRef]
- Li, X.; Qi, B.; Zhang, S.; Li, Y. Foodomics Revealed the Effects of Ultrasonic Extraction on the Composition and Nutrition of Cactus Fruit (Opuntia ficus-indica) Seed Oil. Ultrason. Sonochem 2023, 97, 106459. [Google Scholar] [CrossRef]
- Rodríguez-Blázquez, S.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; García-Sánchez, B.; Miranda, R. Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability. Molecules 2023, 28, 7045. [Google Scholar] [CrossRef]
- Umeagukwu, O.E.; Onukwuli, D.O.; Ude, C.N.; Chizoo, E.; Nnamdi Ekwueme, B.; Asadu, C.O.; Okey-Onyesolu, F.C.; Ikenna, M.U.; Innocent Chukwudi, E.; Ugwele, F.O. Techno-Economic Analysis of the Statistically Optimized Biodiesel Production Process Using African Pear Seed Oil and Activated Empty Palm Fruit Bunch Biocatalyst. Waste Manag. Bull. 2024, 2, 95–112. [Google Scholar] [CrossRef]
- García-Ramón, D.F.; Valdiviezo-Marcelo, J.; Rodrigues, C.E.d.C.; Viganó, J.; de Oliveira, A.L. Advances and Trends in Compressed Fluid Extraction to Recovering Bioactive Compounds from Avocado: Effect of Extraction Parameters, Biological Properties, and Applications. J. Supercrit. Fluids 2025, 218, 106505. [Google Scholar] [CrossRef]
- Chifomboti, L.P.; Chimphango, A.F.A. Development and Optimization of a Two-Step Co-Extraction Process for the Recovery of Pumpkin Seed Oil and in-Situ Enrichment with β-Carotene Compounds from Pumpkin Peel. Food Bioprod. Process. 2025, 149, 211–223. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Zhang, K.; Zhao, R.; Tian, H.; Yang, L.; Zhao, X. Homogenization-Circulating Ultrasound in Combination with Aqueous Enzymatic Pretreatment for Microwave-Assisted Extraction of Kernel Oil and Essential Oil from the Fruit of Litsea cubeba. Ultrason. Sonochem 2024, 111, 107093. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioproc. Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Niu, Z.; Zhu, Z.; Zhou, J.; Xu, C.; Wei, C.; Liu, W.; Liu, Z.; Wang, T.; Xiao, H. Effect of Roasting on the Chemical Composition and Oxidative Stability of Tomato (Solanum lycopersicum L.) Seed Oil. Foods 2024, 13, 1682. [Google Scholar] [CrossRef]
- Hou, N.C.; Gao, H.H.; Qiu, Z.J.; Deng, Y.H.; Zhang, Y.T.; Yang, Z.C.; Gu, L.B.; Liu, H.M.; Zhu, X.L.; Qin, Z.; et al. Quality and Active Constituents of Safflower Seed Oil: A Comparison of Cold Pressing, Hot Pressing, Soxhlet Extraction and Subcritical Fluid Extraction. LWT 2024, 200, 116184. [Google Scholar] [CrossRef]
- Morya, S.; Menaa, F.; Jiménez-López, C.; Lourenço-Lopes, C.; Binmowyna, M.N.; Alqahtani, A. Nutraceutical and Pharmaceutical Behavior of Bioactive Compounds of Miracle Oilseeds: An Overview. Foods 2022, 11, 1824. [Google Scholar] [CrossRef]
- Zeng, J.; Xiao, T.; Ni, X.; Wei, T.; Liu, X.; Deng, Z.Y.; Li, J. The Comparative Analysis of Different Oil Extraction Methods Based on the Quality of Flaxseed Oil. J. Food Compos. Anal. 2022, 107, 104373. [Google Scholar] [CrossRef]
- Siol, M.; Piasecka, I.; Mańko-Jurkowska, D.; Górska, A.; Bryś, J. Optimization and Impact of Ultrasound-Assisted Extraction on Pomegranate Seed Oil Quality: A Comparative Study of Bioactive Potential and Oxidation Parameters. Molecules 2025, 30, 1837. [Google Scholar] [CrossRef]
- Andasuryani; Ifmalinda; Derosya, V.; Syukri, D. The Effect of Moisture on the Yield of Sweet Passion Fruit (Passiflora lingularis Juss Cv. Gumanti) Seed Oil from Indonesia by Mechanical Extraction. IOP Conf. Ser. Earth Environ. Sci. 2020, 515, 012021. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Zhu, X.; Xu, R.; Shen, H.; Zhang, Q.; Ge, X. The Effect of Different Extraction Methods on Extraction Yield, Physicochemical Properties, and Volatile Compounds from Field Muskmelon Seed Oil. Foods 2022, 11, 721. [Google Scholar] [CrossRef]
- Yilmaz, E.; Güneşer, B.A. Cold Pressed versus Solvent Extracted Lemon (Citrus limon L.) Seed Oils: Yield and Properties. J. Food Sci. Technol. 2017, 54, 1891–1900. [Google Scholar] [CrossRef]
- De Filette, M.; Schatteman, K.; Geuens, J. Characterization of Six Cold-Pressed Berry Seed Oils and Their Seed Meals. Appl. Sci. 2024, 14, 439. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green Solvents and Technologies for Oil Extraction from Oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Gonfa Keneni, Y.; Bahiru, A.; Marchetti, J.M.; Marchetti, J.M. Effects of Different Extraction Solvents on Oil Extracted from Jatropha Seeds and the Potential of Seed Residues as a Heat Provider. Bioenergy Res. 2021, 14, 1207–1222. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Zappia, C.; Capocasale, M. Tomato Seed Oil: A Comparison of Extraction Systems and Solvents on Its Biodiesel and Edible Properties. Riv. Ital. Delle Sostanze Grasse 2017, 94, 140–160. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Method 960.39, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Ndayishimiye, J.; Getachew, A.T.; Chun, B.S. Comparison of Characteristics of Oils Extracted from a Mixture of Citrus Seeds and Peels Using Hexane and Supercritical Carbon Dioxide. Waste Biomass Valorization 2017, 8, 1205–1217. [Google Scholar] [CrossRef]
- Onyedikachi, U.B.; Nkwocha, C.C.; Ejiofor, E.; Nnanna, C.C. Investigation of Chemical Constituents, Antioxidant, Anti-Inflammatory and Nutritional Properties of Oil of Persea americana (Avocado) Seeds. Food Chem. Adv. 2024, 5, 100770. [Google Scholar] [CrossRef]
- Ferrentino, G.; Giampiccolo, S.; Morozova, K.; Haman, N.; Spilimbergo, S.; Scampicchio, M. Supercritical Fluid Extraction of Oils from Apple Seeds: Process Optimization, Chemical Characterization and Comparison with a Conventional Solvent Extraction. Innov. Food Sci. Emerg. Technol. 2020, 64, 102428. [Google Scholar] [CrossRef]
- Versteeg, F.A.; Picchioni, F.; Versteeg, G.F. On the Mass Transfer of Supercritical Fluids, Specifically Super Critical CO2: An Overview. Chem. Eng. J. 2024, 493, 152521. [Google Scholar] [CrossRef]
- Dhara, O.; Rani, K.N.P.; Chakrabarti, P.P. Supercritical Carbon Dioxide Extraction of Vegetable Oils: Retrospective and Prospects. Eur. J. Lipid Sci. Technol. 2022, 124, 2200006. [Google Scholar] [CrossRef]
- de Souza Correa, M.; Boschen, N.L.; Rodrigues, P.R.P.; Corazza, M.L.; de Paula Scheer, A.; Ribani, R.H. Supercritical CO2 with Co-Solvent Extraction of Blackberry (Rubus spp. Xavante Cultivar) Seeds. J. Supercrit. Fluids 2022, 189, 105702. [Google Scholar] [CrossRef]
- Macawile, M.C.; Auresenia, J. Utilization of Supercritical Carbon Dioxide and Co-Solvent n-Hexane to Optimize Oil Extraction from Gliricidia Sepium Seeds for Biodiesel Production. Appl. Sci. Eng. Prog. 2022, 15, 1. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak-Błaszczyk, K.; Sójka, M.; Kosmala, M.; Dobrzyńska-Inger, A.; Rój, E. Changes of Bioactive Components in Berry Seed Oils during Supercritical CO2 Extraction. J. Food Process Preserv. 2018, 42, e13368. [Google Scholar] [CrossRef]
- Silva, L.d.O.; Ranquine, L.G.; Monteiro, M.; Torres, A.G. Pomegranate (Punica granatum L.) Seed Oil Enriched with Conjugated Linolenic Acid (CLnA), Phenolic Compounds and Tocopherols: Improved Extraction of a Specialty Oil by Supercritical CO2. J. Supercrit. Fluids 2019, 147, 126–137. [Google Scholar] [CrossRef]
- Borah, K.; Kumar, R.; Purohit, S.; Goud, V.V. Comparative Study on Phytochemical Extraction from Passion Fruit Wastes Using Ultrasound and Supercritical Fluid Extraction. J. Food Meas. Charact. 2025, 19, 2831–2843. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Mechlinska, A.; Namie, J. Green Analytical Chemistry—Theory and Practice. Chem. Soc. Rev. 2010, 39, 2869–2878. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.K.; Lee, Y.Y. A Review on Application of Ultrasound and Ultrasound Assisted Technology for Seed Oil Extraction. J. Food Sci. Technol. 2023, 60, 1222–1236. [Google Scholar] [CrossRef]
- Bao, J.; Li, L.; Ban, L.; Pi, X.; Li, C. A Novel Efficient Method for the Simultaneous Recycle of Amygdalin and Oil from Bitter Apricot Kernels: Ultrasonication Based on Natural Deep Eutectic Solvent. J. Mol. Liq. 2025, 429, 127592. [Google Scholar] [CrossRef]
- Marinaccio, L.; Gentile, G.; Zengin, G.; Pieretti, S.; Stefanucci, A.; Cichelli, A.; Mollica, A. Ultrasound Assisted Deep Eutectic Solvent-Based Extraction of Montepulciano d’ Abruzzo Grape Seeds for the Recovery of the Grape Seed Oil and Its Biological Evaluation. Food Chem. X 2025, 26, 102273. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of Ultrasound-Assisted Extraction (UAE) Process for the Recovery of Bioactive Compounds from Bitter Gourd Using Response Surface Methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Hu, T.; Subbiah, V.; Wu, H.; BK, A.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidant Properties. ACS Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Yu, M.H.; Hung, T.W.; Wang, C.C.; Wu, S.W.; Yang, T.W.; Yang, C.Y.; Tseng, T.H.; Wang, C.J. Neochlorogenic Acid Attenuates Hepatic Lipid Accumulation and Inflammation via Regulating Mir-34a in Vitro. Int. J. Mol. Sci. 2021, 22, 13163. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shang, K.; Lin, C.; Wang, C.; Shi, X.; Wang, H.; Li, H. Processing Technologies, Phytochemical Constituents, and Biological Activities of Grape Seed Oil (GSO): A Review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Satriana, S.; Supardan, M.D.; Arpi, N.; Wan Mustapha, W.A. Development of Methods Used in the Extraction of Avocado Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800210. [Google Scholar] [CrossRef]
- Keskin Çavdar, H.; Koçak Yanık, D.; Gök, U.; Göğüş, F. Optimisation of Microwave-Assisted Extraction of Pomegranate (Punica granatum L.) Seed Oil and Evaluation of Its Physicochemical and Bioactive Properties. Food Technol. Biotechnol. 2017, 55, 86–94. [Google Scholar] [CrossRef]
- da Silva, R.F.; Carneiro, C.N.; Cheila, C.B.; Gomez, J.V.F.; Espino, M.; Boiteux, J.; de los Á Fernández, M.; Silva, M.F.; Dias, F.d.S. Sustainable Extraction Bioactive Compounds Procedures in Medicinal Plants Based on the Principles of Green Analytical Chemistry: A Review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Amador-Luna, V.M.; Montero, L.; Herrero, M. Compressed Fluids for the Extraction of Bioactive Compounds from Plants, Food by-Products, Seaweeds and Microalgae—An Update from 2019 to 2023. TrAC Trends Anal. Chem. 2023, 169, 117410. [Google Scholar] [CrossRef]
- Cheriyan, B.V.; Karunakar, K.K.; Anandakumar, R.; Murugathirumal, A.; Kumar, A.S. Eco-Friendly Extraction Technologies: A Comprehensive Review of Modern Green Analytical Methods. Sustain. Chem. Clim. Action 2025, 6, 100054. [Google Scholar] [CrossRef]
- Višnjevec, A.M.; Barp, L.; Lucci, P.; Moret, S. Pressurized Liquid Extraction for the Determination of Bioactive Compounds in Plants with Emphasis on Phenolics. TrAC Trends Anal. Chem. 2024, 173, 117620. [Google Scholar] [CrossRef]
- Piasecka, I.; Górska, A.; Obranović, M.; Kalisz, S.; Dobrinčić, A.; Dobroslavić, E.; Ostrowska-Ligęza, E.; Brzezińska, R.; Dragović-Uzelac, V. The Quality Assessment of Oils Obtained from Berry Fruit Seeds Using Pressurized Liquid Extraction. Biol. Life Sci. Forum 2023, 26, 84. [Google Scholar] [CrossRef]
- Bakhshabadi, H.; Mirzaei, H.O.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M. The Influence of Pulsed Electric Fields and Microwave Pretreatments on Some Selected Physicochemical Properties of Oil Extracted from Black Cumin Seed. Food Sci. Nutr. 2018, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, I.; Ostrowska-Ligęza, E.; Wiktor, A.; Górska, A. Ultrasound and Pulsed Electric Field Treatment Effect on the Thermal Properties, Oxidative Stability and Fatty Acid Profile of Oils Extracted from Berry Seeds. J. Therm. Anal. Calorim. 2024, 150, 1311–1325. [Google Scholar] [CrossRef]
- Ferreira, I.J.B.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Green Emerging Extraction Technologies to Obtain High-Quality Vegetable Oils from Nuts: A Review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Rivas, S.; Rivas, J.; Domínguez, H. Subcritical Water Extraction of Essential Oils and Plant Oils. Sustain. Chem. Pharm. 2023, 36, 101332. [Google Scholar] [CrossRef]
- Quaisie, J.; Ma, H.; Golly, M.K.; Tuly, J.A.; Amaglo, N.K.; Jiaqi, Z. Effect of Ultrasound-Microwave Irradiation Hybrid Technique on Extraction, Physicochemical, Antioxidative, and Structural Properties of Stearic Acid-Rich Allanblackia parviflora Seed Oil. Chem. Pap. 2021, 75, 4527–4541. [Google Scholar] [CrossRef]
- Vovk, H.; Karnpakdee, K.; Ludwig, R.; Nosenko, T. Enzymatic Pretreatment of Plant Cells for Oil Extraction. Food Technol. Biotechnol. 2023, 61, 160–178. [Google Scholar] [CrossRef]
- Candan, A.; Arslan, D. Enzymatic Pre-Treatment in Cold Pressing: Influence on Flaxseed, Apricot Kernel and Grape Seed Oils. Grasas Y Aceites 2021, 72, 4. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; He, L.; Li, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhou, K.; Zhang, Q.; Liu, A.; et al. A Method for Extracting Oil from Cherry Seed by Ultrasonic-Microwave Assisted Aqueous Enzymatic Process and Evaluation of Its Quality. J. Chromatogr. A 2019, 1587, 50–60. [Google Scholar] [CrossRef]
- Imam, M.S.; Abdelrahman, M.M. How Environmentally Friendly Is the Analytical Process? A Paradigm Overview of Ten Greenness Assessment Metric Approaches for Analytical Methods. Trends Environ. Anal. Chem. 2023, 38, e00202. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Green Analytical Chemistry Metrics: A Review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- Martínez, J.; Cortés, J.F.; Miranda, R. Green Chemistry Metrics, A Review. Processes 2022, 10, 1274. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Ferrara, D.; Beccaria, M.; Cordero, C.E.; Purcaro, G. Comprehensive Comparison of Fatty Acid Methyl Ester Profile in Different Food Matrices Using Microwave-Assisted Extraction and Derivatization Methods and Comprehensive Two-Dimensional Gas Chromatography Coupled with Flame Ionization Detection. Adv. Sample Prep. 2024, 11, 100124. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Szydłowska-Czerniak, A. Antioxidant Potential Evaluation at Various Stages of Black Cumin Oil Production. Foods 2024, 13, 3518. [Google Scholar] [CrossRef]
- Costa, F.S.; Moreira, L.S.; Ludovico, L.L.; Volpe, J.; de Oliveira, A.C.; dos Santos, M.P.; da Silva, E.G.P.; Souto, D.E.P.; Grassi, M.T.; Gonzalez, M.H.; et al. Microwave-Assisted Extraction Based on Emulsion Breaking with Natural Deep Eutectic Solvent for Vegetable Oil Sample Preparation Prior to Elemental Determination by ICP OES. Talanta 2024, 266, 125108. [Google Scholar] [CrossRef] [PubMed]
- El Sayed Mehanni, A.-H. Impact of Enzymatic Aqueous Extraction Mango Seed Oil on Physicochemical Properties and Oxidative Stability. J. Food Dairy Sci. 2021, 12, 195–201. [Google Scholar] [CrossRef]
- Ferreira de Mello, B.T.; Stevanato, N.; Filho, L.C.; da Silva, C. Pressurized Liquid Extraction of Radish Seed Oil Using Ethanol as Solvent: Effect of Pretreatment on Seeds and Process Variables. J. Supercrit. Fluids 2021, 176, 105307. [Google Scholar] [CrossRef]
- Onyeaka, H.; Tamasiga, P.; Nwauzoma, U.M.; Miri, T.; Juliet, U.C.; Nwaiwu, O.; Akinsemolu, A.A. Using Artificial Intelligence to Tackle Food Waste and Enhance the Circular Economy: Maximising Resource Efficiency and Minimising Environmental Impact: A Review. Sustainability 2023, 15, 10482. [Google Scholar] [CrossRef]
- Cheraghi, Y.; Kord, S.; Mashayekhizadeh, V. Application of Machine Learning Techniques for Selecting the Most Suitable Enhanced Oil Recovery Method; Challenges and Opportunities. J. Pet. Sci. Eng. 2021, 205, 108761. [Google Scholar] [CrossRef]
- Iweka, S.C.; Akpomedaye, O.; Emu, A.O.; Adepoju, T.F. Impact of Optimization Parameters on Green Sustainable Oil from Ripe Palm Kernel Seeds for Energy Utilities: A Machine Learning Approach. Sci. Afr. 2024, 23, e02097. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, Z.; Jia, Z.; Hou, M.; Yang, X.; Ying, X.; Ji, Z. Artificial Neural Network-Based Shelf Life Prediction Approach in the Food Storage Process: A Review. Crit. Rev. Food Sci. Nutr. 2023, 64, 12009–12024. [Google Scholar] [CrossRef] [PubMed]
- Agu, C.M.; Menkiti, M.C.; Ekwe, E.B.; Agulanna, A.C. Modeling and Optimization of Terminalia catappa L. Kernel Oil Extraction Using Response Surface Methodology and Artificial Neural Network. Artif. Intell. Agric. 2020, 4, 1–11. [Google Scholar] [CrossRef]
- Rozina; Ahmad, M.; Zafar, M. Conversion of Waste Seed Oil of Citrus aurantium into Methyl Ester via Green and Recyclable Nanoparticles of Zirconium Oxide in the Context of Circular Bioeconomy Approach. Waste Manag. 2021, 136, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Nicolalde, J.F.; Martinez-Gómez, J.; Guerrero, V.H.; Chico-Proano, A. Experimental Performance of Avocado Seed Oil as a Bio-Based Phase Change Material for Refrigeration Applications. Int. J. Refrig. 2024, 168, 632–647. [Google Scholar] [CrossRef]
- Chu, C.C.; Hasan, Z.A.B.A.; Chua, S.K.; Nyam, K.L. Formulation and Characterization of Novel Nanostructured Lipid Carriers with Photoprotective Properties Made from Carnauba Wax, Beeswax, Pumpkin Seed Oil, and UV Filters. JAOCS J. Am. Oil Chem. Soc. 2020, 97, 531–542. [Google Scholar] [CrossRef]
- Tsiapali, O.I.; Ayfantopoulou, E.; Tzourouni, A.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Unveiling the Utilization of Grape and Winery By-Products in Cosmetics with Health Promoting Properties. Appl. Sci. 2025, 15, 1007. [Google Scholar] [CrossRef]
- Afzal, M.F.; Khalid, W.; Armghan Khalid, M.; Zubair, M.; Akram, S.; Kauser, S.; Noreen, S.; Jamal, A.; Kamran Khan, M.; Al-Farga, A. Recent Industrials Extraction of Plants Seeds Oil Used in the Development of Functional Food Products: A Review. Int. J. Food Prop. 2022, 25, 2530–2550. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Contieri, L.S.; e Silva, F.A.; Bagini, R.H.; Bragagnolo, F.S.; Strieder, M.M.; Sosa, F.H.B.; Schaeffer, N.; Freire, M.G.; Ventura, S.P.M.; et al. Path2Green: Introducing 12 Green Extraction Principles and a Novel Metric for Assessing Sustainability in Biomass Valorization. Green Chem. 2024, 26, 10087–10106. [Google Scholar] [CrossRef]
- Agarwal, P.; Kumar, D.; Katiyar, R. Antecedents of Continuous Purchase Behavior for Sustainable Products: An Integrated Conceptual Framework and Review. J. Consum. Behav. 2025. [Google Scholar] [CrossRef]
- Buffi, M.; Georgakaki, A.; Hurtig, O.; Joanny, G.; Letout, S.; Motola, V.; Mountraki, A.; Scarlat, N. Clean Energy Technology Observatory Advanced Biofuels-KJ0124063ENN; European Commission: Bruxelles, Belgium, 2022. [Google Scholar] [CrossRef]

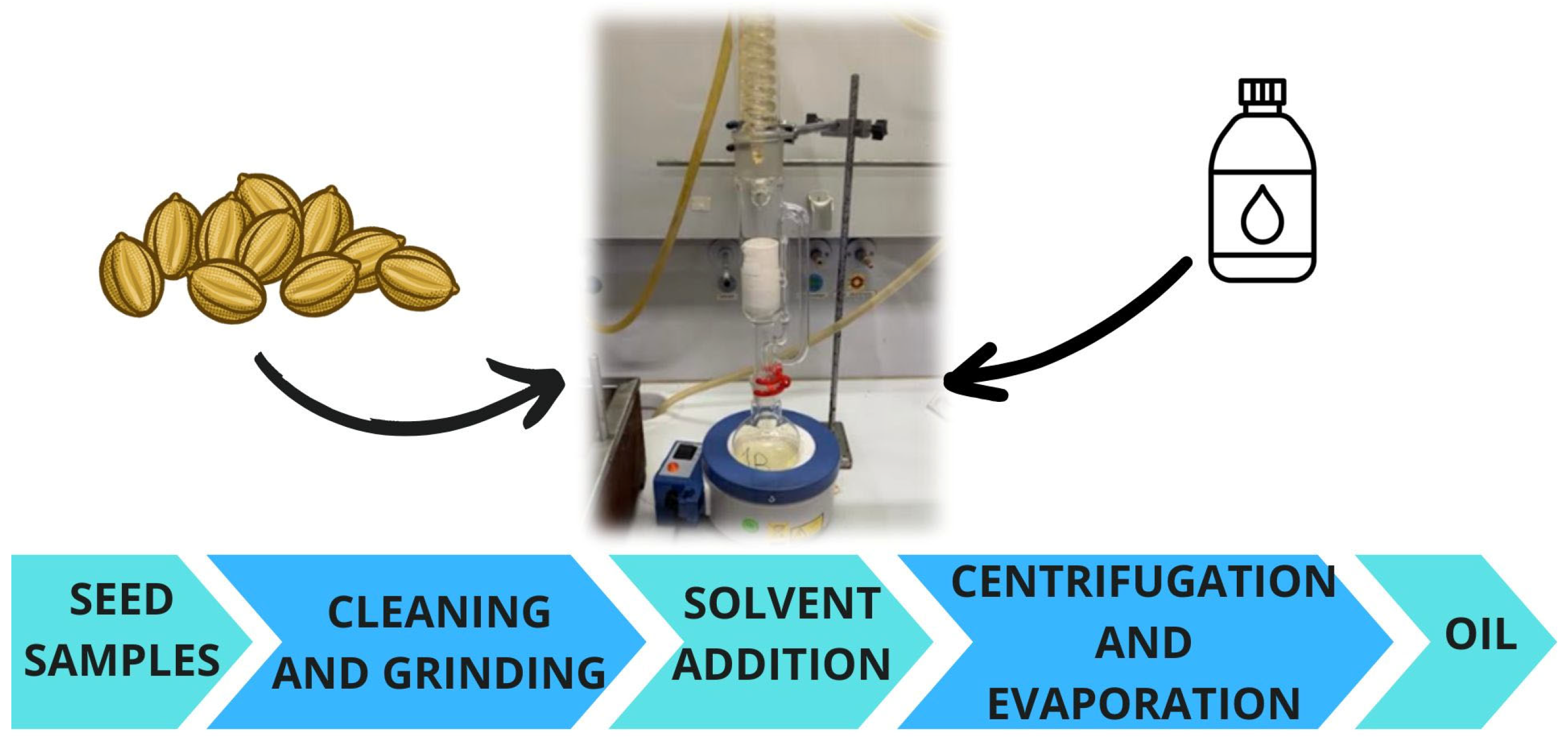


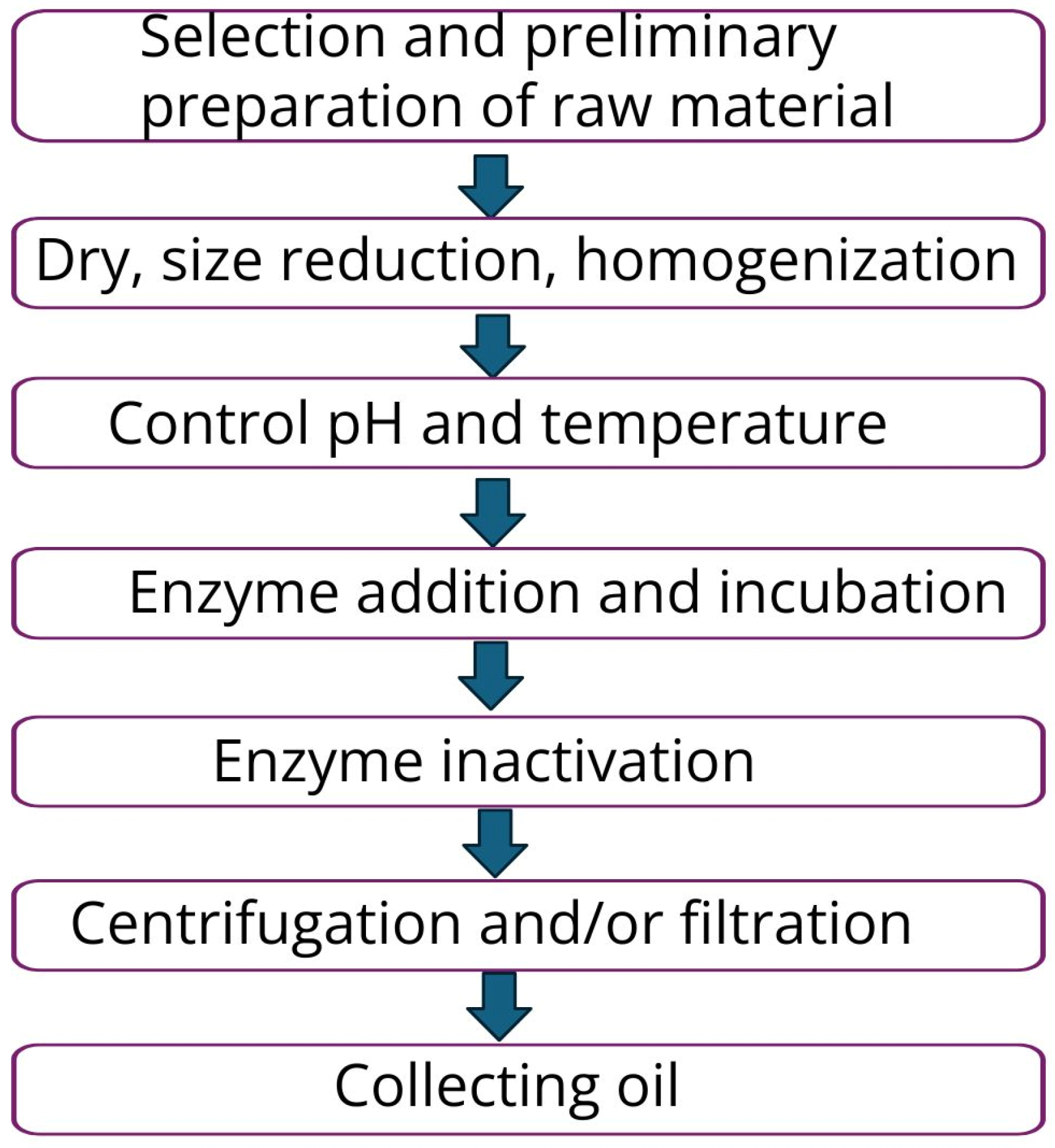
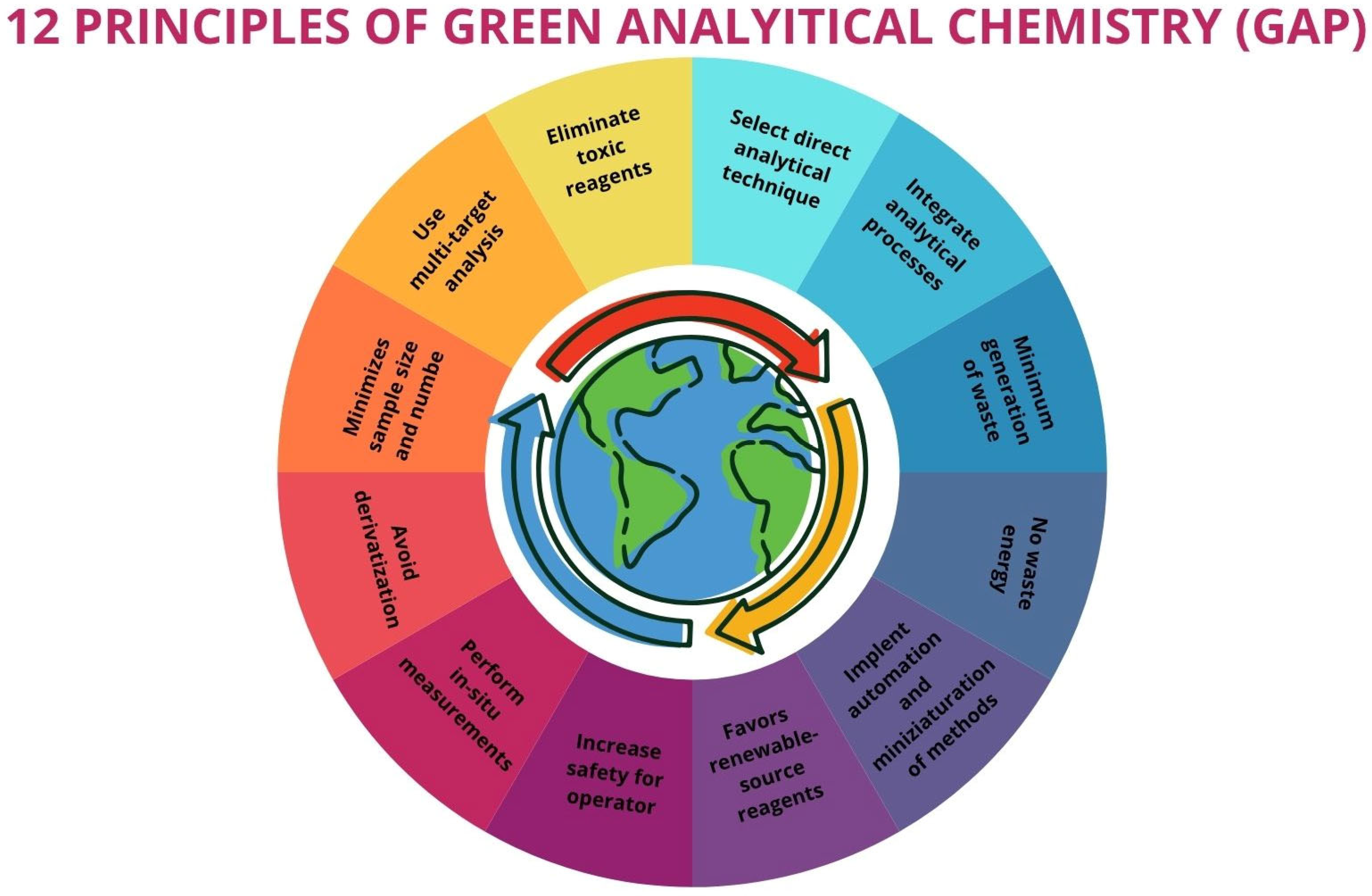
| Extraction Method | Solvent | Extraction Time | Oil Recovery (wt%) | References |
|---|---|---|---|---|
| Soxhlet | n-hexane | 8 h | 15.66 | [25] |
| Soxhlet | Petroleum ether | 8 h | 14.92 | [25] |
| Shaking extraction | n-hexane | 2 h | 12.10 | [25] |
| Shaking extraction | Petroleum ether | 2 h | 11.32 | [25] |
| Mechanical pressing | - | 8 min | 9.00 | [31] |
| MAE | n-hexane | 9 min | 12.47 | [25] |
| MAE | Petroleum ether | 9 min | 11.72 | [25] |
| MAE | n-hexane or petroleum ether | 9 min | 11.72–12.47 | [32] |
| MAE | n-hexane | 5 min | 35.19 | [33] |
| UAE | n-hexane | 18 min | 13.63 | [25] |
| UAE | Petroleum ether | 18 min | 12.82 | [25] |
| UAE | water | 2–8 h | 4.29–25.11 | [26] |
| SubE | n-butane | 40 min | 14.5 | [25] |
| Sc-CO2 | CO2 | 2 h | 16 | [34] |
| Food Waste Material | Seed Mass (g) | Mechanical Pressing Method | Temperature Pressing (°C) | Oil Recovery (wt%) | Content of Principal Bioactive Compounds | References |
|---|---|---|---|---|---|---|
| Pomegranate seeds (Punica granatum L.) | 200 | Cold pressing YODA YD-ZY-02A (Yoda, Warsaw, Poland) | 28–32 | 9 | Linolenic acid: 80% Oleic acid: 5% Linoleic acid: 5% | [57] |
| Cherry seeds | 6000 | Cold pressing (SZR “Mikron”, Temerin, Serbia) | 70 | 4 | Linoleic acid: 46.82% Oleic acid: 41.92% α-tocopherol: 6.39 mg·100 g−1 ꞵ-tocopherol: 1.07 mg·100 g−1 γ-tocopherol: 25.22 mg·100 g−1 δ-tocopherol: 5.41 mg·100 g−1 | [40] |
| Passion fruit seeds | 200 | Cold pressing (grain type MKS-J03) | 62 | 6–12 | Linoleic acid: 38–70% Oleic acid: 9–17% | [58] |
| Muskmelon (Cucumis bisexualis) seeds | 100 | Hot pressing (DH-50 screw oil press) | 90 | 23 | Oleic acid: 49.60% Linoleic acid: 26.60% | [59] |
| Lemon (Citrus limon L.) seeds | 3000 | Cold pressing (Koçmaksan, ESM 3710, İzmir, Turkey) | 40 | 17 | Palmitic acid: 20.88% Oleic acid: 30.86% Linoleic acid: 30.77% Beta-sitosterol: 76.55% α-tocopherol: 155 mg·kg−1 | [60] |
| Berry seeds | n.d. | Cold pressing (IBG Momforts Oekotec, DD85G, Mönchengladbach, Germany) | 50 | n.d. | TPC: 8.9–19.3 mg GAE·100 g−1 Brassicasterol: <0.1–465.7 mg·kg−1 Campesterol: <0.1–200 mg·kg−1 Stigmasterol: <0.1–34 mg·kg−1 | [61] |
| Food Waste Material | Mass Seed to Solvent Ratio (w/v) | Time of Extraction (h) | Oil Recovery (wt%) | Content of Principal Bioactive Compounds | References |
|---|---|---|---|---|---|
| Pomegranate seeds (Punica granatum L.) | 1:6 | 8 | 15.66 | Punicic acid: 762.2 mg·g−1 Oleic acid: 55.4 mg·g−1 Linoleic acid: 57.5 mg·g−1 Squalene: 1.20 mg·g−1 ꞵ-Sitosterol: 3.23 mg·g−1 α-tocopherol: 17.66 mg·100 g−1 γ-tocopherol: 325.3 mg·100 g−1 δ-tocopherol: 8.33 mg·100 g−1 | [26] |
| Passion seeds | 1:20 | 6 | 26.12 | Palmitic acid: 10.22% Oleic acid: 17.11% Linoleic acid: 68.97% α-tocopherol: 1.68 mg·100 g−1 γ-tocopherol: 1.62 mg·100 g−1 δ-tocopherol: 4.93 mg·100 g−1 | [24] |
| Citrus seeds | 1:20 | 20 | 24.94 | Palmitic acid: 21.72% Oleic acid: 26.00% Linoleic acid: 34.32% ꞵ-Sitosterol: 32.25% Limonene: 5.1% ∆-Avenasterol: 5.69% Campesterol: 7.55% 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester: 18.66% | [66] |
| Avocado seeds (Persea Americana L.) | 1:10 | 8 | 41.3 | TPC: 25.78 mg GAE·g−1 TFC: 72.69 mg QE·100 mg−1 Myristic acid: 33.33% Linoleic acid: 20.12% Oleic acid: 11.60% Palmitic acid: 7.07% | [67] |
| Prunus seeds (peach, apricot, plum, and cherry) | 1:10 | 6 | Peach: 30% Apricot: 38% Plum: 37.4% Cherry: 36.0% | Palmitic acid: 5.71–8.1% Oleic acid: 48.6–72.7% Linoleic acid: 16.4–39.3% | [46] |
| Apple seeds | 1:15 | 10 | 22.5 | Quercetin: 0.01 μg·g−1 p-coumaric: 0.098 μg·g−1 Trans-ferulic acid: 0.104 μg·g−1 Phloridzin dihydrate: 0.01 μg·g−1 Naringenin: 0.31 μg·g−1 Palmitic acid: 6.24% Stearic acid: 2.97% Oleic acid: 24.15% Linoleic acid: 49.03% Linolenic acid: 1.87% Arachidic acid: 1.22% | [68] |
| Cherry seeds | 1:4 | 6 | 3.19 | Linoleic acid: 41.44% Oleic acid: 47.17% α-tocopherol: 7.13 mg·100 g−1 ꞵ-tocopherol: 1.112 mg·100 g−1 γ-tocopherol: 31.47 mg·100 g−1 δ-tocopherol: 6.64 mg·100 g−1 | [40] |
| Food Waste Material | Pressure (bar) | Temperature (°C) | CO2 Flow Rate (kg·h−1) | Particle Size (μm) | Oil Recovery (wt%) | Content of Principal Bioactive Compounds | References |
|---|---|---|---|---|---|---|---|
| Cherry seeds | 200–350 bar | 40–70 | 0.2–0.4 | 800 | 3.62–13.02 | Oleic acid: 40.89–41.65% Linoleic acid: 46.32–47.37% α-tocopherol: 1.75–10.54 mg·100 g−1 ꞵ-tocopherol: 0.78–1.11 mg·100 g−1 γ-tocopherol: 6.23–40.60 mg·100 g−1 δ-tocopherol: 2.08–8.42 mg·100 g−1 | [40] |
| Citrus seeds | 200–250 | 45–60 | 1.62 | n.d. | 15.2–19.6 | Palmitic acid: 17.68–20.01% Oleic acid: 29.51–30.67% Linoleic acid: 36–39.44% Limonene: 5.73–6.39% ꞵ-sitosterol: 26.63–28.37% 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester: 26.55–30.34% | [66] |
| Berry seeds | 1000 | 100 | 240 | n.d. | 12–18 | α-tocopherol: 21.7–176.1 mg·kg−1 ꞵ + γ-tocopherol: 50.6–1027.7 mg·kg−1 δ-tocopherol: 2.1–1104.8 mg·kg−1 α-linolenic acid: 3.2–38.0% Linoleic acid: 47.9–72.9% Palmitic acid: 2.1–4.3% Oleic acid: 10.5–18.0% | [73] |
| Apple seeds | 75–350 | 25–90 | 0.12–7.6 | n.d. | 20.5 | Quercetin: 0.03 μg·g−1 p-coumaric: 0.17 μg·g−1 Trans-ferulic acid: 0.12 μg·g−1 Phloridzin dihydrate: 2.97 μg·g−1 Naringenin: 0.63 μg·g−1 Palimitic acid: 13.39% Stearic acid: 7.69% Oleic acid: 34.84% Linoleic acid: 63.76% Linolenic acid: 1.61% Arachidic acid: 2.04% | [68] |
| Pomegranate seeds (Punica granatum L.) | 200 | 40 | 0.108 | n.d. | 16 | Punicic acid: 80.7% Oleic acid: 1.58% Linoleic acid: 3.43% 2,4-Dihydroxyphenylacetic acid: 0.14 mg·100 g−1 Ferulic acid: 0.29 mg·100 g−1 Trans-Cinnamic acid: 0.14 mg·100 g−1 p-hydroxibenzoic acid acid: 0.11 mg·100 g−1 p-Coumaric acid: 0.07 mg·100 g−1 Naringenin: 0.31 mg·100 g−1 Vanillic acid: 0.07 mg·100 g−1 ꞵ-Carotene: 0.02 mg·100 g−1 ꞵ-Sitosterol≈ 1000 mg·100 g−1 Stigmasterol≈ 1000–1500 mg·100 g−1 Campesterol≈ 50 mg·100 g−1 α-tocopherol≈ 5 mg·100 g−1 γ-tocopherol≈ 2000 mg·100 g−1 δ-tocopherol≈ 5 mg·100 g−1 | [74] |
| Passion seeds | 284 | 56 | 1.02 | n.d. | 2.67 | Isovitexin: 90.78 mg·g−1 Ferulic acid: 31.08 mg·g−1 p-coumaric: 24.74 mg·g−1 Gallic acid: 13.99 mg·g−1 Isorhmnetin: 7.40 mg·g−1 Quercetin: 3.05 mg·g−1 | [75] |
| Food Waste | Solvent | Processing Conditions | Oil Recovery (wt%) | Bioactive Compounds | References |
|---|---|---|---|---|---|
| Cactus fruit seeds | n-hexane | Ultrasound intensity: 150–600 W Time: 60 min; Temperature: 40 °C Solid–solvent ratio 1:10 (w/v) | 20.28 ± 0.68 | α-tocopherol: 20.01–36.94 mg·100 g−1 γ-tocopherol: 12.73–19.15 mg·100 g−1 δ-tocopherol: 0.88–2.95 mg·100 g−1 Sinapic acid: 0.87–1.31 μg·g−1 Syringic acid: 0.14–0.15 μg·g−1 Salicylic acid: 0.21–0.22 μg·g−1 Palmitic acid: 10.01–12.71% Stearic acid: 5.14–6.33% Oleic acid: 10.04–11.46% Linoleic acid: 57.46–61.93% Linolenic acid: 5.41–7.20% | [45] |
| Passion seeds | ethanol | Power: 165 W; Frequency: 25 kHz Time: 10–50 min; Temperature: 30–60 °C Solid–solvent ratio 2–4 mL·g−1 | 12.32–21.76 | Palmitic acid: 12.31% Palmitoleic acid: 0.19% Oleic acid: 17.03 Stearic acid: 2.44 Linoleic acid: 61.07 Linolenic acid: 0.12 Stigmasterol: 75.67 mg·100 g−1 Campesterol: 21.55 mg·100 g−1 ꞵ-Sitosterol: 69.11 mg·100 g−1 | [11] |
| Apple seeds | n-hexane | Ultrasound power: 18–70 W Amplitude: 20–80%, Temperature: 30 °C Solid–solvent ratio 1:15 (w/v) | 17.20 ± 2.3 | Phloretin and phloridzin | [39] |
| Passion seeds | supercritical CO2 | Ultrasound power: 160 W Pressure: 16 MPa, Temperature: 40 (w/v) | 29 | γ -tocopherol: 6.7–10.6 mg·100 g−1 γ-tocotrienol: 24–36 mg·100 g−1 δ-tocotrienol: 33–45 mg·100 g−1 Palmitic acid: 10.9–11.8% Oleic acid: 15.89–19% Linoleic acid: 63–67.91% | [12] |
| Mango seeds | n-hexane | Power: 70 W; Frequency: 42 kHz Time: 20–60 min; Temperature: 35–55 (w/v) Solid–solvent ratio 1:5 (w/v) | 96.6 ± 1.3 | Palmitic acid: 4.95% Stearic acid: 39.59% Oleic acid: 32.38% Linoleic acid: 3.31% Eicosadienoic acid: 2.32% Docosahexaenoic acid: 15.74% | [13] |
| Pomegranate seeds | water | Power: 130 W; Frequency: 20 kHz Temperature: 35–55 °C Time: 2–8 h | 4.29–25.11 | n.d. | [27] |
| Citrus seeds | n-hexane | Temperature: 25 °C Time: 90 min | Lime seeds: 22.07–22.08 Sweet orange seeds: 22.84–23.11 | Lime seed TPC: ≈ 0.6–0.8 mg GAE·g−1 Palmitic acid: 19.83–20.79% Oleic acid: 17.27–18.07% Linoleic acid: 34.07–34.25% α-Linolenic acid: 10.96–11.45% Orange sweet seed TPC: ≈0.6–1 mg GAE·g−1 Palmitic acid: 23.56–23.73% Oleic acid: 20.95–21.58% Linoleic acid: 35.74–36.32% α-Linolenic acid: 3.67–3.98% | [30] |
| Bitter apricot kernels | natural deep eutectic solvent (choline chloride–lactic acid) | Liquid-to-solid ratio: 18 mL·g−1; Ultrasonic power: 420 W; Temperature: 42 °C; Extraction time: 32 min | 45.64 ± 0.24 | Palmitic acid:16.63 ±0.52 mg·g−1 Palmitoleic acid: 3.15 ± 0.16 mg·g−1 Ginkgolic acid: 0.62 ± 0.02 mg·g−1 Stearic acid: 3.86 ± 0.20 mg·g−1 Oleic acid: 309.18 ± 9.47 mg·g−1 Linoleic acid: 109.40 ± 8.35 mg·g−1 Arachidic acid: 0.36 ± 0.01 mg·g−1 cis-11-eicosenoic acid: 0.42 ± 0.02 mg·g−1 Octadecatrienoic acid: 0.60 ± 0.01 mg·g−1 | [78] |
| Grape seeds | natural deep eutectic solvent (menthol–thymol) | Sample-to-solvent ratio: 1:4 Ultrasound sonicator (20 kHz, 400 W, and 70% amplitude) Temperature: 70 °C Time extraction: 20 min | Palmitic acid: 14.47% Stearic acid: 5.48% Oleic acid: 15.49% Linoleic acid: 56.17% α-linolenic acid: 5.18% TPC: 18.65 mg GAE·g−1 TFC: 0.73 mg RE·g−1 | [79] |
| Extraction Method | Food Waste Material | Processing Conditions | Oil Recovery (wt%) | Content of Principal Bioactive Compounds | References |
|---|---|---|---|---|---|
| PLE | Avocado seeds | 200 °C 11 MPa ethanol 20 min 5 g | 39 | Total polyphenols: 88 mg GAE·100 g−1 | [48] |
| PLE | Avocado seeds | 200 °C 11 MPa Ethanol–water 50:50 (v/v) | n.d | Organic acids, phenolic acids, flavonoids, catechins, and condensed tannins | [81] |
| Hot PLE | Grape pomace seeds | 160 °C 10 atm 60% ethanol | n.d | Gallic acid: 21.66 μg·g−1 Ellagic acid: 4.55 μg·g−1 Protocatechuic acid: 5.31 μg·g−1 Caffeic acid: 4.92 μg·g−1 Chlorogenic acid: 6.66 μg·g−1 p-oumaric acid: 2.24 μg·g−1 Catechin: 85.91 μg·g−1 Epicatechin: 28.53 μg·g−1 Epigallocatechin: 13.21 μg·g−1 Gallocatechin: 3.27 μg·g−1 | [87] |
| PLE | Berry fruit seeds | 150 °C 10.34 MPa hexane 4 cycles × 5 min | 16.00 (cranberry) 13.53 (strawberry) 5.08 (chokeberry) 7.60 (black currant) 1.70 (red currant) | Palmitic acid: 5.54–9.14% Stearic acid: 1.23–2.62% Palmitoleic acid: 0.11–0.36% Elaidic acid: 13.73–23.52% Linoleic acid: 36.35–67.23% γ-Linoleic acid: 0.12–8.72% Linolenic acid: 1.99–31.80% | [88] |
| Extraction Method | Food Waste Material | Processing Conditions | Oil Recovery | Content of Principal Bioactive Compounds | References |
|---|---|---|---|---|---|
| SFE-UAE | Passion seeds | Temperature: 40–50 °C Pressure: 16–29 MPa CO2 flow rate: 0.63 kg·h−1 Solvent-seed mass ratio: 210 kg CO2·g−1 Ultrasound power: 160–800 W Time: 20 min | 13.5–20.9% | γ-tocopherol: 6.7–9.4 mg·100 g−1 γ-tocotrienol: 24–34 mg·100 g−1 δ-tocotrienol: 33–44 mg·100 g−1 Palmitic acid: 10.9–11.6% Stearic acid: 3.01–5% Oleic acid: 15.89–19% Linoleic acid: 63–68.2% | [12] |
| PEF-SFE | Grape seeds | Solvent-seed mass ratio: 1/2 Temperature: 35–45 °C Pressure: 35–50 MPa CO2 flow rate: 0.9–2.7 kg·h−1 | 70–80 g·kg−1 | Campesterol: 370–395.6 mg·kg−1 Stigmasterol: 508.8–527.2 mg·kg−1 β-Sitosterol: 3784.4–3973.9 mg·kg−1 α-tocopherol: 63.1–73.6 mg·kg−1 α-tocotrienol: 60.9–77.8 mg·kg−1 γ-tocopherol: 16.1–20.5 mg·kg−1 γ-tocotrienol: 88.2–108.1 mg·kg−1 Oleic acid: 210.8–211.1 g·kg−1 Linoleic acid: 645.2–646.1 g·kg−1 Procyanidin dimer B1: 912.1–1338.0 µg·kg−1 (+)-Catequin: 293.8–450.3 µg·kg−1 Procyanidin dimer B1: 912.1–1338.0 µg·kg−1 p-coumaric acid: 130.6–282.0 µg·kg−1 Gallic acid: 130.0–582.5 µg·kg−1 Dihydroxibenzoic acid: 191.5–267.4 µg·kg−1 | [9] |
| Enzyme-assisted pressing | Apricot seeds, flaxseeds, and grape seeds | Types of enzymes: pectolytic, cellulotic, and hemicellulotic; time and temperature during incubation: 60 °C and 3 h; pH of enzyme solution: 6 | 11.05–30.85% | Total carotenoids: 0.037–0.731 mg·kg−1 Total phenolic compounds: 31.08–127.92 mg·kg−1 α-tocopherol: 111.60–156.59 mg·L−1 β-tocopherol: 0.147–154.29 mg·L−1 γ-tocopherol: 413.00–619.04 mg·L−1 δ-tocopherol: 0.147–150.97 mg·L−1 Oleic acid: 18.10–69.28% Linoleic acid: 14.32–68.33% α-linoleic acid: 0.08–57.49% | [98] |
| UMAE | Mango seeds | Bath of ultrasonicator (42 kHz, 70 WUltrasound); Ultrasound extraction time: 20–60 min Ultrasound extraction temperature: 35–55 °C Type of solvent: n-hexane Mass–solvent ratio: 1:3–1:8 Power MAE: 250–350 W MAE time: 5–15 min | 11.60% | Stearic acid: 39.59% Oleic acid: 32.33% Docosahexaenoic acid: 15.74% | [13] |
| HCUEPM | Litsea cubeba seeds | Types of enzymes: hemicellulose, pectinase, and neutral protease Incubation temperature: 30–50 °C | 204.23 mL·kg−1 | D-Limonene: ND-0.48% Lauric acid: 48.72–57.47% Decanoic acid: 10.89–11.67% Oleic acid: 8.93–14.37% Linoleic acid: 4.28–7.69% | [50] |
| UMAEE | Cherry seeds | Ultrasonic power: 560 W, Microwave power: 323 W for 38 min at 40 °C Enzyme: 2.7% (cellulase, hemicellulase, and pectinase) | 83.85% | Palmitic acid: 5.68% Oleic acid: 53.82% Linoleic acid: 38.29% | [99] |
| Extraction Techniques | Food Waste Material | Analytes | Processing Conditions | Analytical Techniques | Criterion | Metrics | References |
|---|---|---|---|---|---|---|---|
| UAE | Passion seeds | Sterols and fatty acids | Power 165 W, frequency 25 kHz, time 10–50 min, temperature 30–60 °C, and sample–solvent ratio 2–4 mL·g−1 | GC-MS | Criterion 1: ex situ Criterion 2: non-hazardous materials Criterion 3: only sustainable materials Criterion 4: no waste Criterion 5: 1 g of sample Criterion 6: 4 sample throughput Criterion 7: 2 steps or fewer and semi-automated Criterion 8: 82.5 W·h−1 Criterion 9: liquid chromatography; gas chromatography with quadrupole detection Criterion 10: non-hazardous or no exposure |  | [11] |
| MAE | Pomegranate seeds | Fatty acids, sterols, and tocopherols | Sample–solvent ratio: 1:6 (w/v) 500 W, and frequency of 40 kHz for 9 min | GC-FID HPLC-FLD | Criterion 1: ex situ Criterion 2: non-hazardous materials Criterion 3: only sustainable materials Criterion 4: no waste Criterion 5: 1 g of sample Criterion 6: 10 sample throughput Criterion 7: 3 steps or fewer and semi-automated Criterion 8: 41.65 W·h−1 Criterion 9: liquid chromatography; gas chromatography with quadrupole detection Criterion 10: 1 hazard |  | [26] |
| EAE | Mango seeds | Fatty acid | 15 g of seeds Sample–water ratio: 1:10 (w/v) 5 min 2% of enzymes | GC-FID | Criterion 1: ex situ Criterion 2: non-hazardous materials Criterion 3: only sustainable materials Criterion 4: no waste Criterion 5: 15 g of sample Criterion 6: 12 sample throughput Criterion 7: 2 steps or fewer and manual systems Criterion 8: 69.4 W·h−1 Criterion 9: gas chromatography with non-MS detection Criterion 10: non-hazardous or no exposure | 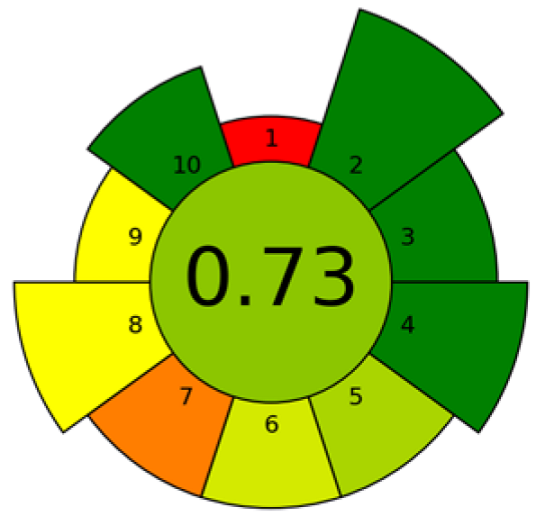 | [107] |
| PLE | Radish seeds | Phytosterols and tocopherols | 5.2 g of seeds 120, 135, or 150 °C 5, 10, or 15 MPa 10 or 30 min | GC-MS | Criterion 1: ex situ Criterion 2: non-hazardous materials Criterion 3: only sustainable materials Criterion 4: no waste Criterion 5: 15 g of sample Criterion 6: 12 samples throughout Criterion 7: 2 steps or fewer and manual systems Criterion 8: 250 W·h−1 Criterion 9: liquid chromatography; gas chromatography with quadrupole detection Criterion 10: non-hazardous or no exposure |  | [108] |
| PEF | Berry seeds | Fatty acids | 2 g of seeds 8 or 10 kV electrode voltage, 7 µs pulse width, 20 Hz frequency, and an adequate number of pulses to achieve energy intake of 50 kV kg−1 30 min | GC-FID | Criterion 1: ex situ Criterion 2: non-hazardous materials Criterion 3: only sustainable materials Criterion 4: no waste Criterion 5: 2 g of sample Criterion 6: 2 sample throughput Criterion 7: 2 steps or fewer and semi-automated Criterion 8: 13.89 W·h−1 Criterion 9: gas chromatography with non-MS detection Criterion 10: non-hazardous or no exposure |  | [93] |
| Cold pressing | Cherry seeds | Fatty acids and tocopherols | 6000 g of seed 70 °C | GC-MS and HPLC-FLD | Criterion 1: ex situ Criterion 2: non-hazardous materials Criterion 3: only sustainable materials Criterion 4: no waste Criterion 5: 2000 g of sample Criterion 6: 6 sample throughput Criterion 7: 2 steps or fewer and semi-automated Criterion 8: 200 W·h−1 Criterion 9: gas chromatography with non-MS detection Criterion 10: non-hazardous or no exposure |  | [40] |
| Soxhlet extraction | Passion seeds | Fatty acids and tocopherols | 10 g of seed Sample–solvent ratio: 1:20 (w/v) 4 h 60 °C | GC-MS and HPLC-DAD | Criterion 1: ex situ Criterion 2: 200 mL of hazardous materials Criterion 3: >75% sustainable materials Criterion 4: 200 mL of waste Criterion 5: 10 g of sample Criterion 6: 0.25 sample throughput Criterion 7: 3 steps or fewer and semi-automated Criterion 8: 2000 W·h−1 Criterion 9: liquid chromatography; gas chromatography with quadrupole detection Criterion 10: 1 hazard |  | [24] |
| Supercritical CO2 extraction | Berry seeds | Fatty acids and tocopherols | 14 kg of seed sample 1000 bar 100 °C 240 kg·h−1 | RP-HPLC-FLD | Criterion 1: ex situ Criterion 2: non-hazardous materials Criterion 3: only sustainable materials Criterion 4: no waste Criterion 5: 14,000 g of sample Criterion 6: 1 sample throughput Criterion 7: 3 steps and semi-automated Criterion 8: 3000 W·h−1 Criterion 9: liquid chromatography; gas chromatography with quadrupole detection Criterion 10: no hazards | 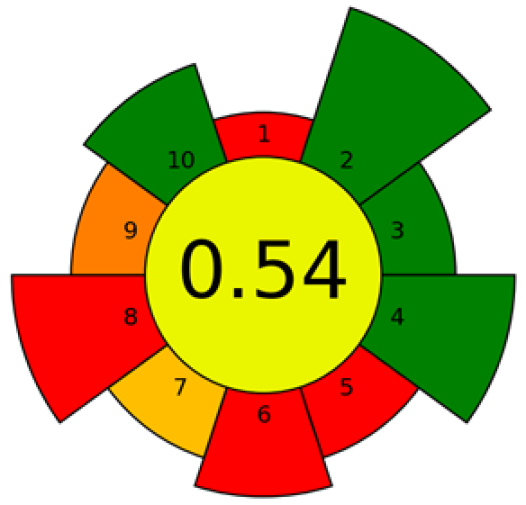 | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Blázquez, S.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E. Recent Insights into Eco-Friendly Extraction Techniques for Obtaining Bioactive Compounds from Fruit Seed Oils. Foods 2025, 14, 2271. https://doi.org/10.3390/foods14132271
Rodríguez-Blázquez S, Gómez-Mejía E, Rosales-Conrado N, León-González ME. Recent Insights into Eco-Friendly Extraction Techniques for Obtaining Bioactive Compounds from Fruit Seed Oils. Foods. 2025; 14(13):2271. https://doi.org/10.3390/foods14132271
Chicago/Turabian StyleRodríguez-Blázquez, Sandra, Esther Gómez-Mejía, Noelia Rosales-Conrado, and María Eugenia León-González. 2025. "Recent Insights into Eco-Friendly Extraction Techniques for Obtaining Bioactive Compounds from Fruit Seed Oils" Foods 14, no. 13: 2271. https://doi.org/10.3390/foods14132271
APA StyleRodríguez-Blázquez, S., Gómez-Mejía, E., Rosales-Conrado, N., & León-González, M. E. (2025). Recent Insights into Eco-Friendly Extraction Techniques for Obtaining Bioactive Compounds from Fruit Seed Oils. Foods, 14(13), 2271. https://doi.org/10.3390/foods14132271







