Physical Properties of Gelatin-Based Hydrogels Incorporated with Soybean Straw Nanocellulose Obtained by Enzymatic Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Soybean Straw Nanofibrils
2.3. Production of Gelatin Hydrogels Containing Cellulose Nanofibrils
2.4. Characterization of the Soybean Straw and the Cellulose Nanofibrils
2.4.1. Chemical Composition of Soybean Straw
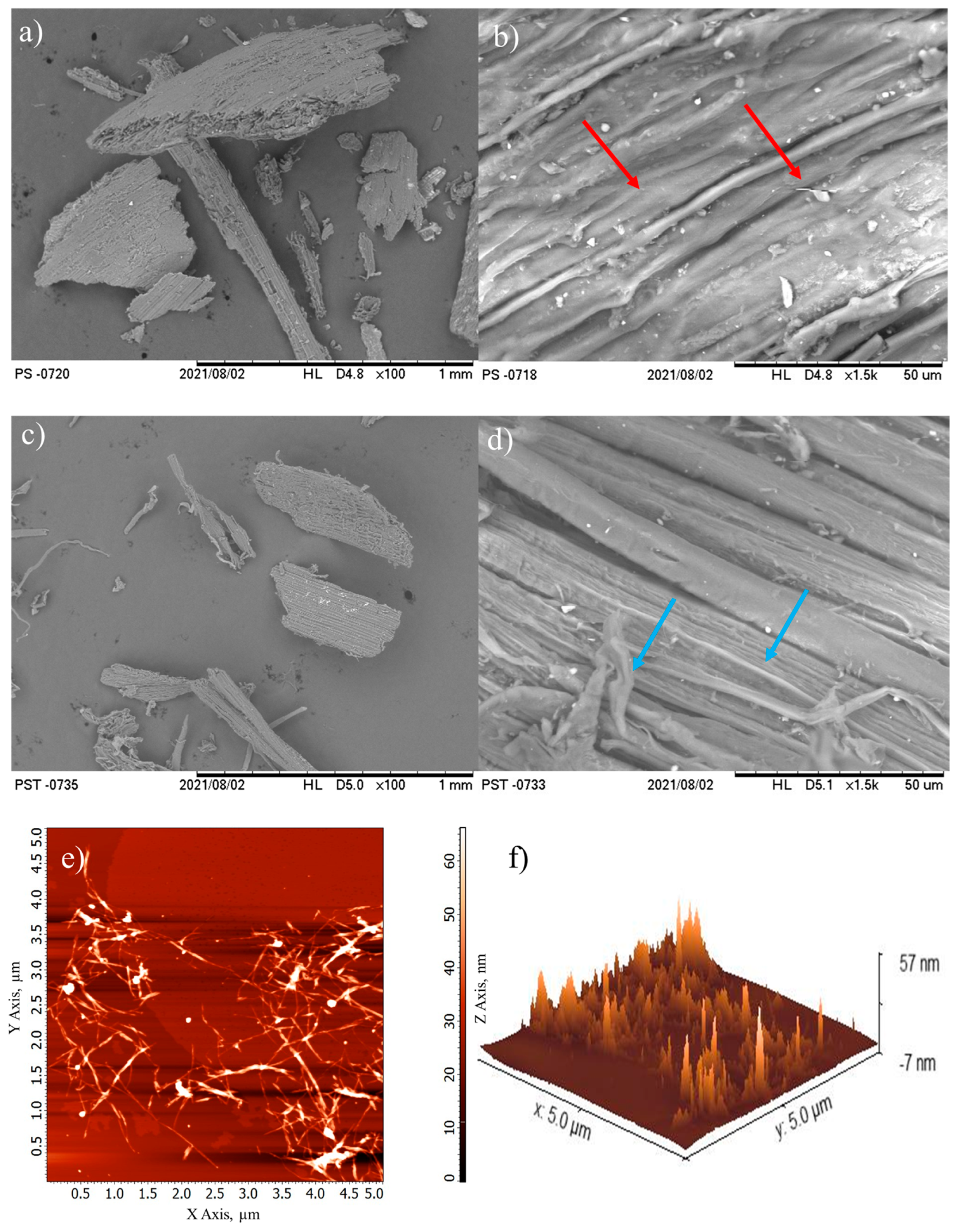
2.4.2. Morphology
2.4.3. X-Ray Diffraction
2.4.4. Zeta Potential
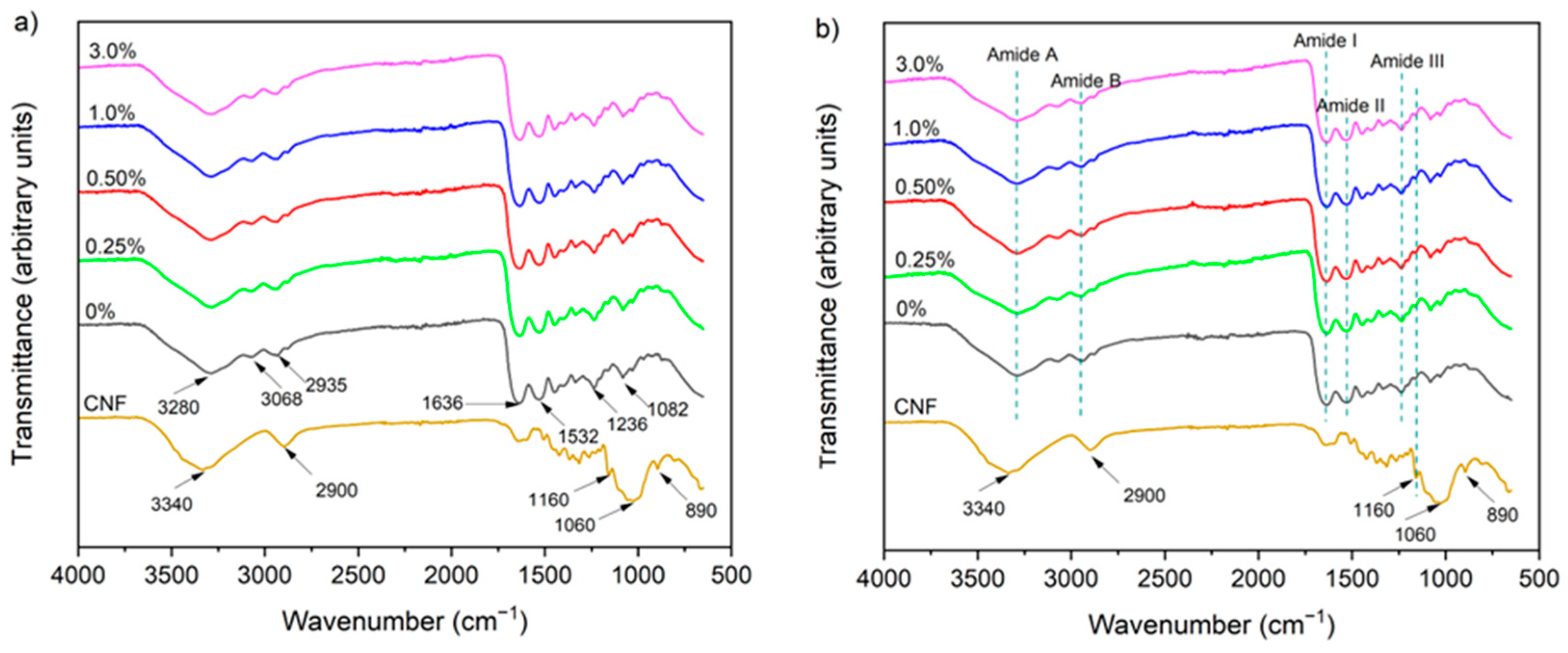
2.4.5. Chemical Structure
2.5. Characterization of Gelatin Hydrogels Containing Cellulose Nanofibrils
2.5.1. Visual Aspects
2.5.2. Color and Opacity
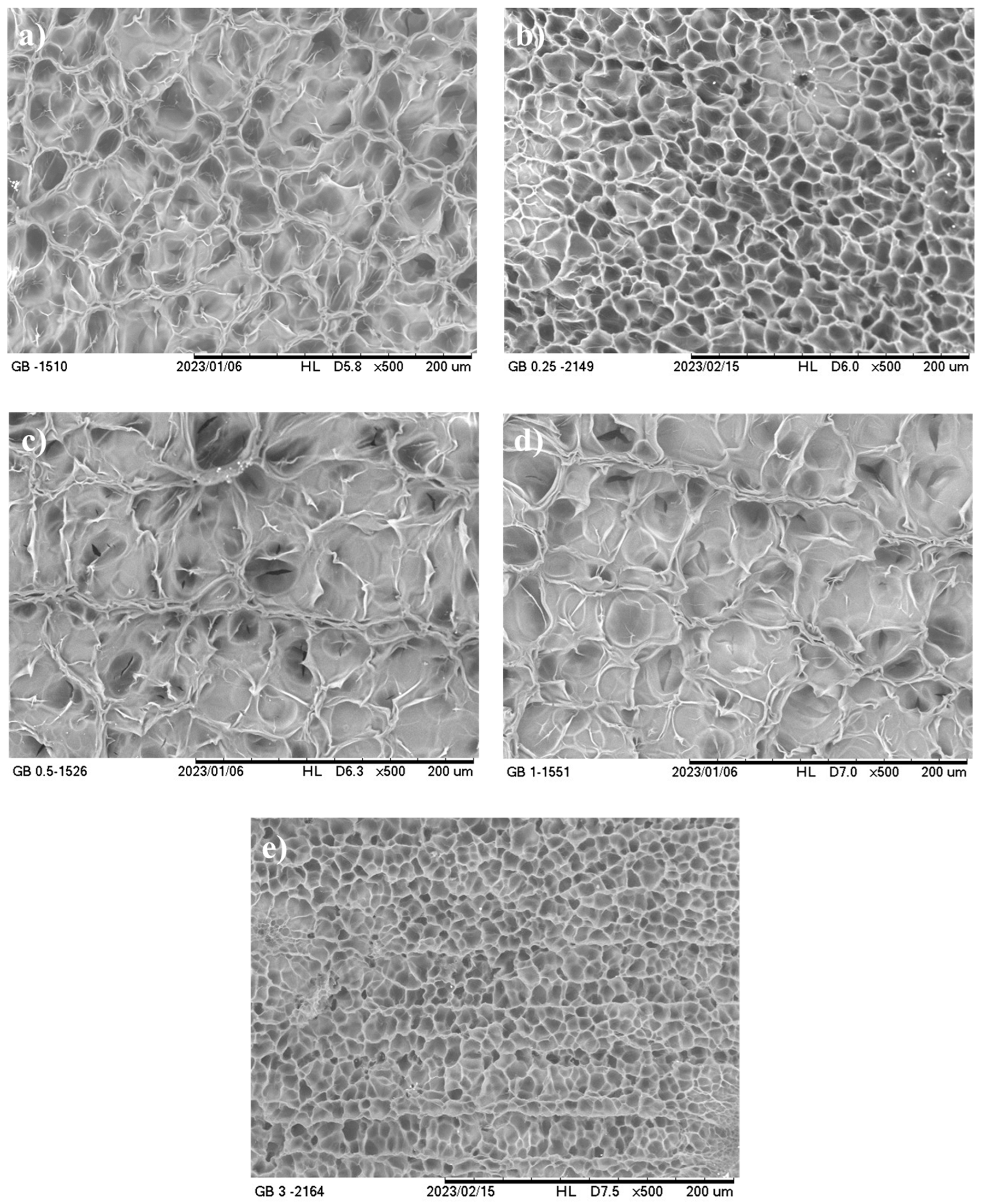
2.5.3. Microstructure
2.5.4. X-Ray Diffraction Pattern
2.5.5. Mechanical and Viscoelastic Properties
2.5.6. Swelling and Water Retention Capacity
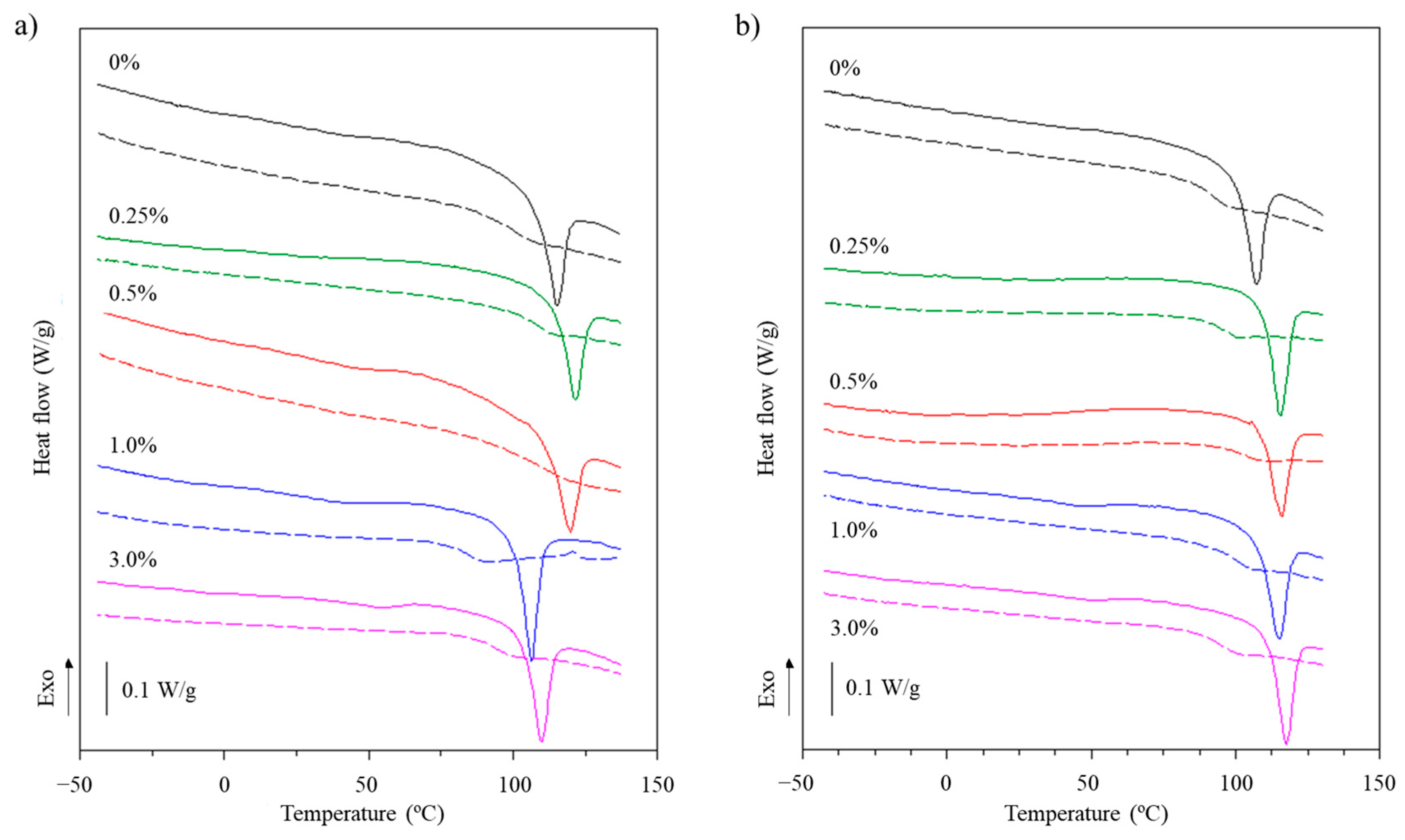
2.5.7. Thermal Properties
2.5.8. Chemical Structure
2.5.9. Statistical Analysis
3. Results
3.1. Characterization of Soybean Straw and Cellulose Nanofibrils
3.1.1. Chemical Composition of Soybean Straw
3.1.2. Yield and Zeta Potential of CNFs
3.1.3. Morphology
3.1.4. X-Ray Diffraction Patterns and Relative Crystallinity
3.1.5. Chemical Structure
3.2. Characteristics of Gelatin Hydrogels Containing Soybean Straw Cellulose Nanofibrils
3.2.1. Visual Aspect
3.2.2. Color and Opacity
3.2.3. Microstructure
3.2.4. Chemical Structure
3.2.5. X-Ray Diffraction
3.2.6. Thermal Properties
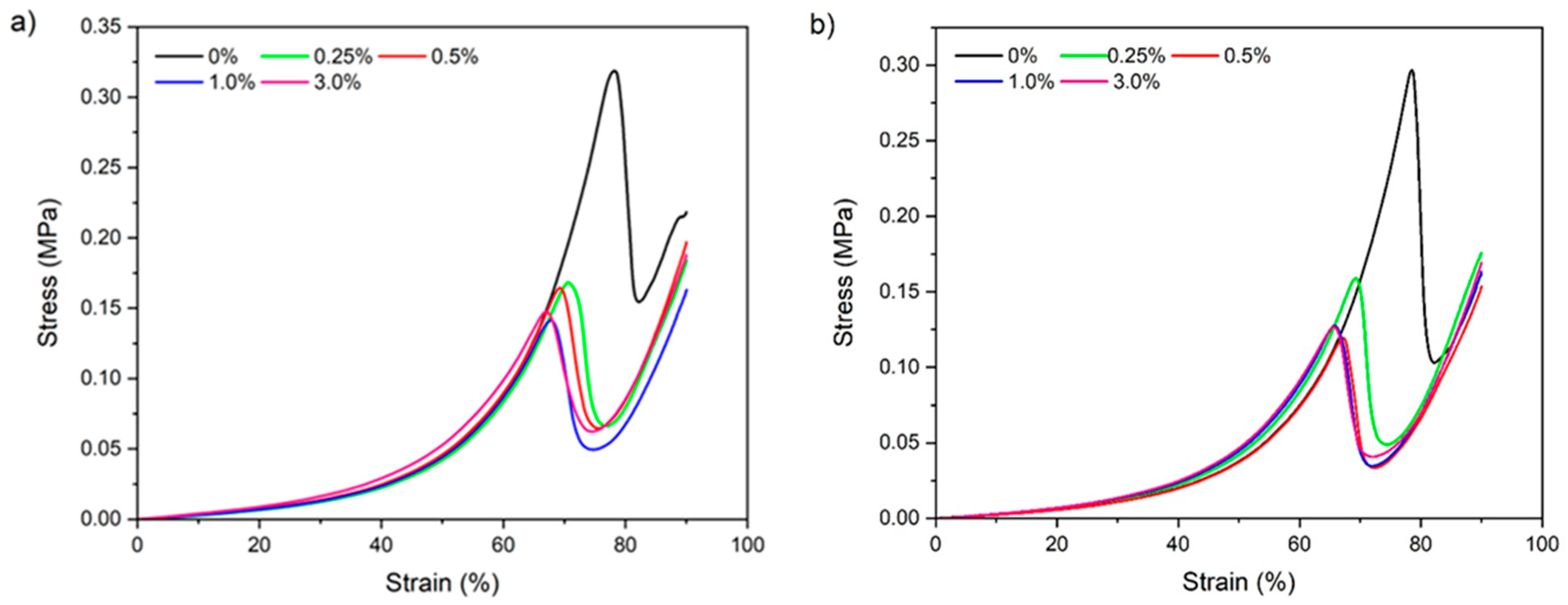
3.2.7. Mechanical Properties
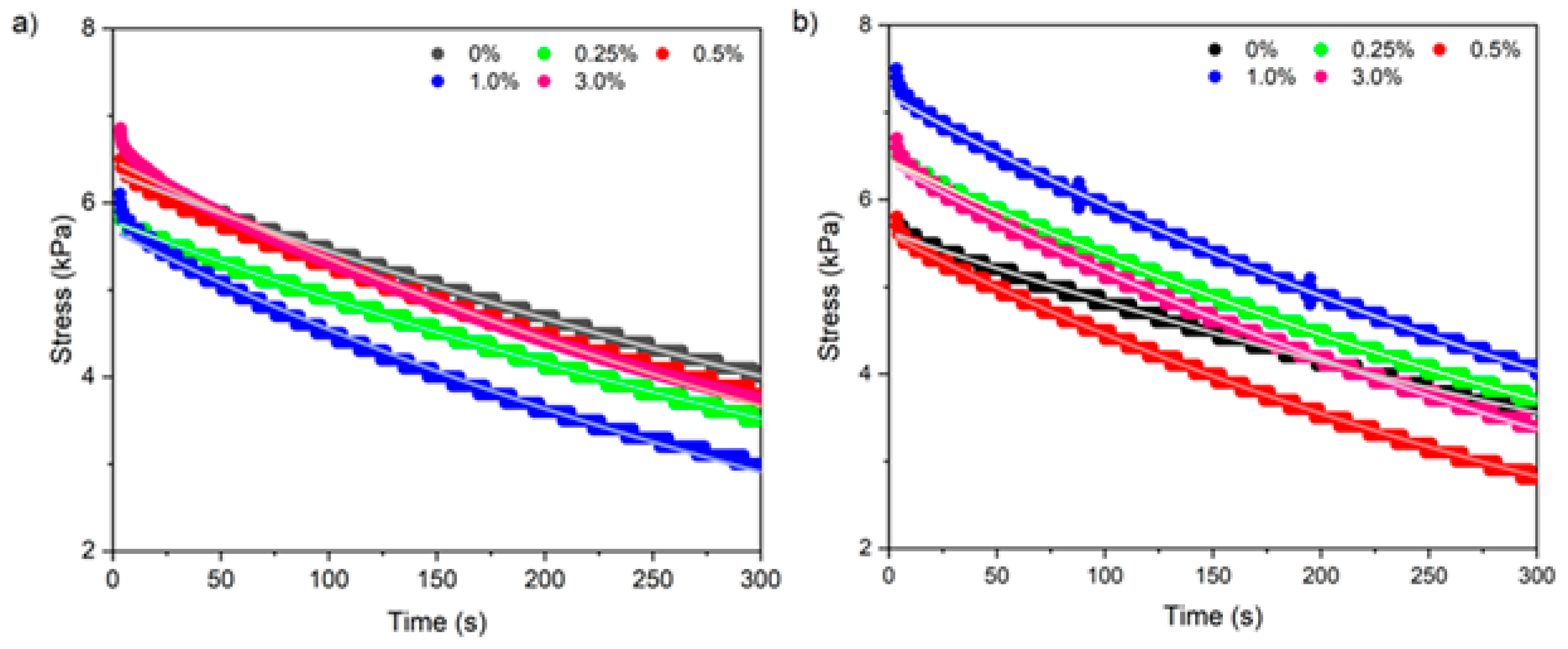
3.2.8. Viscoelastic Properties by Stress Relaxation Test
3.2.9. Swelling and Water Retention Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaczmarek, B.K.; Nadolna, K.; Owczarek, A. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymer, 1st ed.; Chen, Y., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 151–172. [Google Scholar]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A comprehensive review of food hydrogels: Principles, formation mechanisms, microstructure, and its applications. Gels 2022, 9, 1. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Xia, X.; Tan, M.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. High-performance carboxymethyl cellulose-based hydrogel film for food packaging and preservation system. Int. J. Biol. Macromol. 2022, 223, 1126–1137. [Google Scholar] [CrossRef]
- Sudheer, S.; Bandyopadhyay, S.; Bhat, R. Sustainable polysaccharide and protein hydrogel-based packaging materials for food products: A review. Int. J. Biol. Macromol. 2023, 248, 125845. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; Wiley-Vch Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 45–117. [Google Scholar]
- Shen, R.; Xue, S.; Xu, Y.; Liu, Q.; Feng, Z.; Ren, H.; Zhai, H.; Kong, F. Research progress and development demand of nanocellulose reinforced polymer composites. Polymers 2020, 12, 2113. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and characterization of cellulose nanofibers from Rose stems (Rosa spp.). Carbohydr. Polymers 2019, 220, 53–59. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lin, Y.; Qin, Y.; He, R.; Wang, M.; Sun, Q.; Peng, Y. Nanocellulose from agro-industrial wastes: A review on sources, production, applications, and current challenges. Food Res. Int. 2024, 192, 114741. [Google Scholar] [CrossRef]
- Longaresi, R.H.; De Menezes, A.J.; Pereira-da-Silva, M.A.; Baron, D.; Mathias, S.L. The maize stem as a potential source of cellulose nanocrystal: Cellulose characterization from its phenological growth stage dependence. Ind. Crops Prod. 2019, 133, 232–240. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Oilseeds: World Markets and Trade, Foreign Agricultural Service, Office of Global Analysis, Washington. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 19 November 2024).
- Souza, A.G.; Santos, D.F.; Ferreira, R.R.; Pinto, V.Z.; Rosa, D.S. Innovative process for obtaining modified nanocellulose from soybean straw. Int. J. Biol. Macromol. 2020, 165, 1803–1812. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.; Tapia-Blácido, D.R. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef]
- Khorairi, A.N.S.A.; Sofian-Seng, N.S.; Othaman, R.; Rahman, H.A.; Razali, N.S.M.; Lim, S.J.; Mustapha, W.A.W. A review on agro-industrial waste as cellulose and nanocellulose source and their potentials in food applications. Food Rev. Int. 2023, 39, 663–688. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Torricillas, M.D.; Martins, M.A.; Assis, O.B.; Tapia-Blácido, D.R. Using commercial enzymes to produce cellulose nanofibers from soybean straw. J. Nanomater. 2016, 2016, 8106814. [Google Scholar] [CrossRef]
- Li, P.; Zhou, M.; Jian, B.; Lei, H.; Liu, R.; Zhou, X.; Li, X.; Wang, Y.; Zhou, B. Paper material coated with soybean residue nanocellulose waterproof agent and its application in food packaging. Ind. Crops Prod. 2023, 199, 116749. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Q.; Pan, S.; Li, Y.; Jin, Y.; Yang, H.; Li, T.; Zhang, Q. Facile isolation of cellulose nanofibers from soybean residue. Carbohydr. Polym. Technol. Appl. 2021, 2, 100172. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Zhao, R.; Shi, Q.; Nian, Y.; Hu, B. Hydrogels as promising carriers for the delivery of food bioactive ingredients. Front. Nutr. 2022, 9, 1006520. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Liu, Q.; Li, M.; Liu, J.; Lu, H.; Li, F.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers. Food Hydrocoll. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Teng, A.; Liu, A. Mechanical reinforcement of gelatin hydrogel with nanofiber cellulose as a function of percolation concentration. Int. J. Biol. Macromol. 2017, 103, 226–233. [Google Scholar] [CrossRef]
- Shang, X.; Xiaojin, G.; Huiping, L.; Jing, T.; Chunyan, X. Preparation and properties of hydrogels with different forms of nanocellulose and low methoxyl pectin. Food Sci. Biotechnol. 2025, 34, 629–636. [Google Scholar] [CrossRef]
- Ooi, S.Y.; Ahmad, I.; Amin, M.C. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crops Prod. 2016, 93, 227–234. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.h.o.u.w.e.i.; Lu, W.; Chen, N.; Zhu, D.; Li, Y. Preparation and characterization of enzymatically cross-linked gelatin/cellulose nanocrystal composite hydrogels. RSC Adv. 2021, 11, 10794–10803. [Google Scholar] [CrossRef]
- Marciano, J.S. Biodegradable gelatin composite hydrogels filled with cellulose for chromium (VI) adsorption from contaminated water. Int. J. Biol. Macromol. 2021, 181, 112–124. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Assis, O.B.; Silva, N.C.; Esposto, B.S.; Martins, M.A.; Tapia-Blácido, D.R. Chemical treatment and characterization of soybean straw and soybean protein isolate/straw composite films. Carbohydr. Polym. 2017, 157, 512–520. [Google Scholar] [CrossRef]
- Silva, D.J.; Queiroz, A.D. Análise de Alimentos: Métodos Químicos e Biológicos, 3rd ed.; UFV: Viçosa, Brazil, 2005; pp. 97–127. [Google Scholar]
- Soest, P.V. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. AOAC Int. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crops Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Xu, Y.; Atrens, A.D.; Stokes, J.R. Rheology and microstructure of aqueous suspensions of nanocrystalline cellulose rods. J. Colloid Interface Sci. 2017, 496, 130–140. [Google Scholar] [CrossRef]
- Bertsch, P.; Andrée, L.; Besheli, N.H.; Leeuwenburgh, S.C. Colloidal hydrogels made of gelatin nanoparticles exhibit fast stress relaxation at strains relevant for cell activity. Acta Biomater. 2022, 138, 124–132. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: New York, NY, USA, 1996; pp. 1–418. [Google Scholar]
- Do Nascimento, D.M.; Nunes, Y.L.; Feitosa, J.P.; Dufresne, A.; Rosa, M.D. Cellulose nanocrystals-reinforced core-shell hydrogels for sustained release of fertilizer and water retention. Int. J. Biol. Macromol. 2022, 216, 24–31. [Google Scholar] [CrossRef]
- Choudhury, A.R. pH mediated rheological modulation of chitosan hydrogels. Int. J. Biol. Macromol. 2020, 156, 591–597. [Google Scholar] [CrossRef]
- Sobral, P.J.; Habitante, A.M. Phase transitions of pigskin gelatin. Food Hydrocoll. 2001, 15, 377–382. [Google Scholar] [CrossRef]
- Neto, W.P.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Barros, P.J.; Ascheri, D.P.; Santos, M.L.; Morais, C.C.; Ascheri, J.L.; Signini, R.D.; Santos, M.; Campos, A.J.; Devilla, I.A. Soybean hulls: Optimization of the pulping and bleaching processes and carboxymethyl cellulose synthesis. Int. J. Biol. Macromol. 2020, 144, 208–218. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Aslaniyan, A.; Ghanbari, F.; Kouhsar, J.B.; Shahraki, B.K. Comparing the effects of gamma ray and alkaline treatments of sodium hydroxide and calcium oxide on chemical composition, ruminal degradation kinetics and crystallinity degree of soybean straw. Appl. Radiat. Isot. 2023, 191, 110524. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT Food Sci. Technol. 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Rodrigues, M.I.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by enzymatic treatment: Study of process conditions. Ind. Crops Prod. 2017, 95, 664–674. [Google Scholar] [CrossRef]
- Mondragon, G.; Peña-Rodriguez, C.; González, A.; Eceiza, A.; Arbelaiz, A. Bionanocomposites based on gelatin matrix and nanocellulose. Eur. Polym. J. 2015, 62, 1–9. [Google Scholar] [CrossRef]
- Onyeaka, P.O.; Dai, H.; Feng, X.; Wang, H.; Fu, Y.; Yu, Y.; Zhu, H.; Chen, H.; Ma, L.; Zhang, Y. Effect of different types of nanocellulose on the structure and properties of gelatin films. Food Hydrocoll. 2023, 144, 108972. [Google Scholar] [CrossRef]
- Smyth, M.; García, A.; Rader, C.; Foster, E.J.; Bras, J. Extraction and process analysis of high aspect ratio cellulose nanocrystals from corn (Zea mays) agricultural residue. Ind. Crops Prod. 2017, 108, 257–266. [Google Scholar] [CrossRef]
- Althues, H.; Henle, J.; Kaskel, S. Functional inorganic nanofillers for transparent polymers. Chem. Soc. Rev. 2007, 36, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Dong, Y.; Liu, J.; Zheng, X.; Pei, Y.; Tang, K. Genipin-crosslinked gelatin-based composite hydrogels reinforced with amino-functionalized microfibrillated cellulose. Int. J. Biol. Macromol. 2022, 222, 3155–3167. [Google Scholar] [CrossRef]
- Boughriba, S.; Souissi, N.; Nasri, R.; Nasri, M.; Li, S. pH sensitive composite hydrogels based on gelatin and reinforced with cellulose microcrystals: In depth physicochemical and microstructural analyses for controlled release of vitamin B2. Mater. Today Commun. 2021, 27, 102334. [Google Scholar] [CrossRef]
- Yan, S.; Wu, S.; Zhang, J.; Zhang, S.; Huang, Y.; Zhu, H.; Li, Y.; Qi, B. Controlled release of curcumin from gelatin hydrogels by the molecular-weight modulation of an oxidized dextran cross-linker. Food Chem. 2023, 418, 135966. [Google Scholar] [CrossRef]
- Chiani, E.; Beaucamp, A.; Hamzeh, Y.; Azadfallah, M.; Thanusha, A.V.; Collins, M.N. Synthesis and characterization of gelatin/lignin hydrogels as quick release drug carriers for Ribavirin. Int. J. Biol. Macromol. 2023, 224, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Emami, Z.; Ehsani, M.; Zandi, M.; Daemi, H.; Ghanian, M.H.; Foudazi, R. Modified hydroxyapatite nanoparticles reinforced nanocomposite hydrogels based on gelatin/oxidized alginate via Schiff base reaction. Carbohydr. Polym. Technol. Appl. 2021, 2, 100056. [Google Scholar] [CrossRef]
- Manish, V.; Arockiarajan, A.; Tamadapu, G. Influence of water content on the mechanical behavior of gelatin based hydrogels: Synthesis, characterization, and modeling. Int. J. Solids Struct. 2021, 233, 111219. [Google Scholar] [CrossRef]
- Zhan, Y.; Fu, W.; Xing, Y.; Ma, X.; Ma, C. Advances in versatile anti-swelling polymer hydrogels. Mater. Sci. Eng. C 2021, 127, 112208. [Google Scholar] [CrossRef]



| Parameter | CCNFs (%) | AGH | BGH |
|---|---|---|---|
| L* | 0 | 86.5 ± 0.2 a.A | 85.7 ± 0.3 a.B |
| 0.25 | 86.0 ± 0.1 a.A | 85.8 ± 0.2 a.A | |
| 0.5 | 85.9 ± 0.2 a.A | 85.7 ± 0.6 a.A | |
| 1 | 86.6 ± 0.5 a.A | 85.94 ± 0.8 a.A | |
| 3 | 85.5 ± 0.7 a.A | 83.63 ± 0.4 b.B | |
| a* | 0 | −0.8 ± 0.0 a.A | −1.3 ± 0.0 a.B |
| 0.25 | −0.9 ± 0.0 b.a.A | −1.3 ± 0.0 a.B | |
| 0.5 | −0.9 ± 0.0 b.A | −1.4 ± 0.0 a.B | |
| 1 | −1.1 ± 0.0 c.A | −1.5 ± 0.1 b.B | |
| 3 | −1.5 ± 0.1 d.A | −1.8 ± 0.0 c.B | |
| b* | 0 | 4.2 ± 0.2 d.B | 8.8 ± 0.2 c.b.A |
| 0.25 | 4.8 ± 0.1 d.c.B | 8.3 ± 0.1 c.A | |
| 0.5 | 5.1 ± 0.2 c.B | 8.8 ± 0.2 c.b.A | |
| 1 | 6.0 ± 0.2 b.B | 9.9 ± 0.8 b.A | |
| 3 | 7.4 ± 0.4 a.B | 12.9 ± 0.3 a.A | |
| ∆E* | 0 | 8.4 ± 0.3 b.B | 10.8 ± 0.3 c.b.A |
| 0.25 | 9.0 ± 0.2 b.A | 9.2 ± 0.8 c.A | |
| 0.5 | 9.1 ± 0.3 b.a.B | 11.1± 0.4 c.b.A | |
| 1 | 9.3 ± 0.5 b.a.B | 11.6 ± 1.0 b.A | |
| 3 | 10.4 ± 0.8 a.B | 15.3 ± 0.5 a.A | |
| Opacity | 0 | 2.4 ± 0.2 c.B | 3.3 ± 0.3 d.A |
| 0.25 | 3.2 ± 0.2 c.b.B | 3.9 ± 0.2 c.d.A | |
| 0.5 | 3.2 ± 0.3 c.b.B | 4.4 ± 0.1 c.b.A | |
| 1 | 3.5 ± 0.3 b.B | 4.8 ± 0.1 b.A | |
| 3 | 6.6 ± 0.3 a.A | 7.1 ± 0.3 a.A |
| Parameters | CCNFs (%) | AGH | BGH |
|---|---|---|---|
| Tm (°C) (I) | 0 | 115.1 ± 0.3 a.A | 106.1 ± 6.0 a.A |
| 0.25 | 115.7 ± 5.1 a.A | 111.3 ± 3.2 a.A | |
| 0.50 | 116.9 ± 4.2 a.A | 121.9 ± 7.7 a.A | |
| 1.00 | 108.7 ± 1.6 a.A | 117.7 ± 4.9 a.A | |
| 3.00 | 110.9 ± 5.2 a.A | 115.5 ± 1.4 a.A | |
| ΔHm (J/g) (I) | 0 | 17.6 ± 0.5 b.A | 17.7 ± 1.5 a.A |
| 0.25 | 16.7 ± 0.5 b.A | 16.9 ± 1.0 a.A | |
| 0.50 | 17.7 ± 0.9 b.A | 16.4 ± 2.4 a.A | |
| 1.00 | 21.2 ± 1.5 a.A | 17.2 ± 0.6 a.B | |
| 3.00 | 17.3 ± 0.8 b.A | 17.1 ± 0.4 a.A | |
| Tg (°C) (II) | 0 | 102.6 ± 8.3 a.A | 93.1 ± 4.8 a.A |
| 0.25 | 107.2 ± 6.6 a.A | 95.3 ± 2.9 a.A | |
| 0.50 | 99.8 ± 7.7 a.A | 110.6 ± 12.4 a.A | |
| 1.00 | 90.4 ± 5.0 a.A | 102.7 ± 13.5 a.A | |
| 3.00 | 92.7 ± 6.2 a.A | 94.0 ± 2.2 a.A |
| Properties | CCNFs (%) | AGH | BGH |
|---|---|---|---|
| Fracture stress (kPa) | 0 | 311.3 ± 10.5 a.A | 282.7 ± 12.9 a.A |
| 0.25 | 169.0 ± 3.5 b.A | 161.0 ± 2.1 b.B | |
| 0.5 | 163.5 ± 1.7 c.b.A | 119.2 ± 2.3 c.B | |
| 1 | 144.7 ± 6.3 c.A | 129.8 ± 7.6 c.A | |
| 3 | 140.5 ± 9.6 c.A | 126.5 ± 0.3 c.A | |
| Fracture strain (%) | 0 | 78.3 ± 0.4 a.A | 77.3 ± 0.6 a.A |
| 0.25 | 70.8 ± 0.4 b.A | 69.5 ± 0.3 b.B | |
| 0.5 | 70.2 ± 0.5 b.A | 66.7 ± 0.4 c.B | |
| 1 | 68.6 ± 0.4 c.A | 66.4 ± 0.8 c.B | |
| 3 | 67.4 ± 0.5 c.A | 65.95 ± 0.5 c.A | |
| Elastic modulus (kPa/%) | 0 | 0.30 ± 0.0 b.A | 0.28 ± 0.0 b.A |
| 0.25 | 0.29 ± 0.0 b.A | 0.29 ± 0.0 b.a.A | |
| 0.5 | 0.30 ± 0.0 b.A | 0.28± 0.0 b.A | |
| 1 | 0.30 ± 0.0 b.a.A | 0.31 ± 0.0 b.a.A | |
| 3 | 0.36 ± 0.0 a.A | 0.34 ± 0.0 a.A |
| Properties | CCNFs (%) | AGH | BGH |
|---|---|---|---|
| Hardness (g force) | 0 | 473.3 ± 8.8 b.A | 425.1 ± 5.4 c.B |
| 0.25 | 469.7 ± 7.0 b.B | 488.8 ± 0.9 b.a.A | |
| 0.5 | 507.8 ± 14.6 a.A | 432.3 ± 17.3 b.c.B | |
| 1 | 463.4 ± 4.8 b.A | 508.2 ± 26.1 a.A | |
| 3 | 522.1 ± 8.8 a.A | 533.3 ± 23.1 a.A | |
| Springiness | 0 | 1.00 ± 0.01 a.A | 0.99 ± 0.01 a.A |
| 0.25 | 0.99 ± 0.00 b.a.A | 0.98 ± 0.01 a.A | |
| 0.5 | 0.98 ± 0.0 b.B | 0.99 ± 0.00 a.A | |
| 1 | 0.99 ± 0.00 b.a.A | 0.99 ± 0.01 a.A | |
| 3 | 0.99 ± 0.00 b.a.A | 0.99 ± 0.00 a.A | |
| Cohesiveness | 0 | 0.97 ± 0.00 a.A | 0.97 ± 0.00 a.A |
| 0.25 | 0.97 ± 0.00 a.A | 0.97 ± 0.00 a.A | |
| 0.5 | 0.96 ± 0.00 a.A | 0.97 ± 0.00 a.A | |
| 1 | 0.97 ± 0.00 a.A | 0.97 ± 0.00 a.A | |
| 3 | 0.97 ± 0.00 a.A | 0.97 ± 0.00 a.A | |
| Gumminess (g force) | 0 | 458.1 ± 7.8 b.A | 413.3 ± 5.8 b.B |
| 0.25 | 454.7 ± 6.1 b.B | 472.2 ± 3.0 b.a.A | |
| 0.5 | 490.6 ± 13.4 a.A | 419.8 ± 19.8 b.B | |
| 1 | 449.7 ± 5.2 b.A | 493.1 ± 25.8 a.A | |
| 3 | 505.0 ± 8.3 a.A | 518.9 ± 23.1 a.A | |
| Chewiness (g force) | 0 | 462.8 ± 12.3 b.c.A | 413.4 ± 10.5 b.B |
| 0.25 | 448.6 ± 4.9 c.A | 461.0± 7.8 b.a.A | |
| 0.5 | 478.4 ± 10.1 b.a.A | 423.1 ± 20.1 b.B | |
| 1 | 452.3 ± 1.6 b.c.B | 496.6 ± 15.0 a.A | |
| 3 | 497.9 ± 8.0 a.A | 513.50 ± 22.0 a.A | |
| Resilience | 0 | 0.94 ± 0.00 a.A | 0.94 ± 0.00 a.A |
| 0.25 | 0.93 ± 0.01 b.a.A | 0.91 ± 0.01 b.a.A | |
| 0.5 | 0.92 ± 0.01 b.c.A | 0.92 ± 0.00 b.a.A | |
| 1 | 0.93 ± 0.00 b.a.A | 0.91 ± 0.01 b.B | |
| 3 | 0.91 ± 0.00 c.B | 0.93 ± 0.00 b.a.A |
| Properties | CCNFs (%) | AGH | BGH |
|---|---|---|---|
| G (kPa) | 0 | 31.7 ± 0.6 a.A | 28.7 ± 1.4 a.A |
| 0.25 | 28.7 ± 0.4 b.a.B | 32.1 ± 1.1 a.A | |
| 0.5 | 31.3 ± 0.9 a.A | 27.9 ± 0.9 a.B | |
| 1 | 28.0 ± 1.2 b.B | 31.10 ± 0.6 a.A | |
| 3 | 29.4 ± 1.4 b.a.A | 31.55 ± 2.8 a.A | |
| μ (×106 Pa·s) | 0 | 15.8 ± 0.3 a.A | 14.3 ± 0.7 a.A |
| 0.25 | 14.3 ± 0.2 b.a.A | 16.0 ± 0.5 a.A | |
| 0.5 | 15.7 ± 0.4 a.A | 14.0 ± 0.5 a.B | |
| 1 | 14.0 ± 0.6 b.B | 15.6 ± 0.3 a.A | |
| 3 | 14.7 ± 0.7 b.a.A | 15.8 ± 1.4 a.A | |
| R2 | 0 | 0.998 ± 0.000 | 0.996 ± 0.001 |
| 0.25 | 0.997 ± 0.000 | 0.998 ± 0.000 | |
| 0.5 | 0.996 ± 0.001 | 0.997 ± 0.001 | |
| 1 | 0.997 ± 0.000 | 0.998 ± 0.000 | |
| 3 | 0.998 ± 0.001 | 0.998 ± 0.000 |
| Parameters | CCNFs (%) | AGH | BGH |
|---|---|---|---|
| Moisture content (%) | 0 | 6.0 ± 0.3 a.A | 5.3 ± 1.1 a.A |
| 0.25 | 5.5 ± 0.7 a.A | 5.2 ± 0.1 a.A | |
| 0.5 | 5.8 ± 0.2 a.A | 4.8 ± 0.2 a.B | |
| 1 | 4.9 ± 0.7 a.A | 5.8 ± 0.5 a.A | |
| 3 | 6.1 ± 0.7 a.A | 5.1 ± 0.3 a.A | |
| SW (%) | 0 | 2024 ± 33 a.A | 2023 ± 48 a.A |
| 0.25 | 1456 ± 9.4 c.B | 1578 ± 52 b.A | |
| 0.5 | 1841 ± 43 b.A | 1473 ± 34 c.b.B | |
| 1 | 1428 ± 8.3 c.A | 1309 ± 57 d.B | |
| 3 | 1257 ± 47 d.A | 1370 ± 16 c.d.A | |
| WR (%) | 0 | 89.4 ± 1.0 b.A | 91.2 ± 2.5 b.a.A |
| 0.25 | 92.6 ± 0.4 a.A | 93.1 ± 0.8 a.A | |
| 0.5 | 89.8 ± 0.7 b.A | 85.8 ± 0.9 b.B | |
| 1 | 92.7 ± 1.1 a.A | 89.6 ± 1.9 b.a.A | |
| 3 | 92.6 ± 0.3 a.A | 91.9 ± 0.2 a.A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Castillo, L.E.; de Freitas, G.I.; Moraes, I.C.F.; Tosi, M.M.; López Angulo, D.E.; do Amaral Sobral, P.J. Physical Properties of Gelatin-Based Hydrogels Incorporated with Soybean Straw Nanocellulose Obtained by Enzymatic Hydrolysis. Foods 2025, 14, 2269. https://doi.org/10.3390/foods14132269
Velásquez-Castillo LE, de Freitas GI, Moraes ICF, Tosi MM, López Angulo DE, do Amaral Sobral PJ. Physical Properties of Gelatin-Based Hydrogels Incorporated with Soybean Straw Nanocellulose Obtained by Enzymatic Hydrolysis. Foods. 2025; 14(13):2269. https://doi.org/10.3390/foods14132269
Chicago/Turabian StyleVelásquez-Castillo, Lía Ethel, Gisele Imoto de Freitas, Izabel Cristina Freitas Moraes, Milena Martelli Tosi, Daniel Enrique López Angulo, and Paulo José do Amaral Sobral. 2025. "Physical Properties of Gelatin-Based Hydrogels Incorporated with Soybean Straw Nanocellulose Obtained by Enzymatic Hydrolysis" Foods 14, no. 13: 2269. https://doi.org/10.3390/foods14132269
APA StyleVelásquez-Castillo, L. E., de Freitas, G. I., Moraes, I. C. F., Tosi, M. M., López Angulo, D. E., & do Amaral Sobral, P. J. (2025). Physical Properties of Gelatin-Based Hydrogels Incorporated with Soybean Straw Nanocellulose Obtained by Enzymatic Hydrolysis. Foods, 14(13), 2269. https://doi.org/10.3390/foods14132269








