Millet Quinic Acid Relieves Colitis by Regulating Gut Microbiota and Inhibiting MyD88/NF-κB Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Fermentation
2.3. Animals
2.4. Experimental Design
2.5. Evaluation of the Disease Activity Index (DAI)
2.6. Histopathological Analysis of the Colon
2.7. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Western Blot Analysis
2.9. 16S rRNA Sequencing
2.10. Statistical Analysis
3. Results
3.1. Quinic Acid Improved the Microbial Composition of IBD Patients by In Vitro Fermentation
3.2. Quinic Acid Alleviated the Symptoms of DSS-Induced Colitis
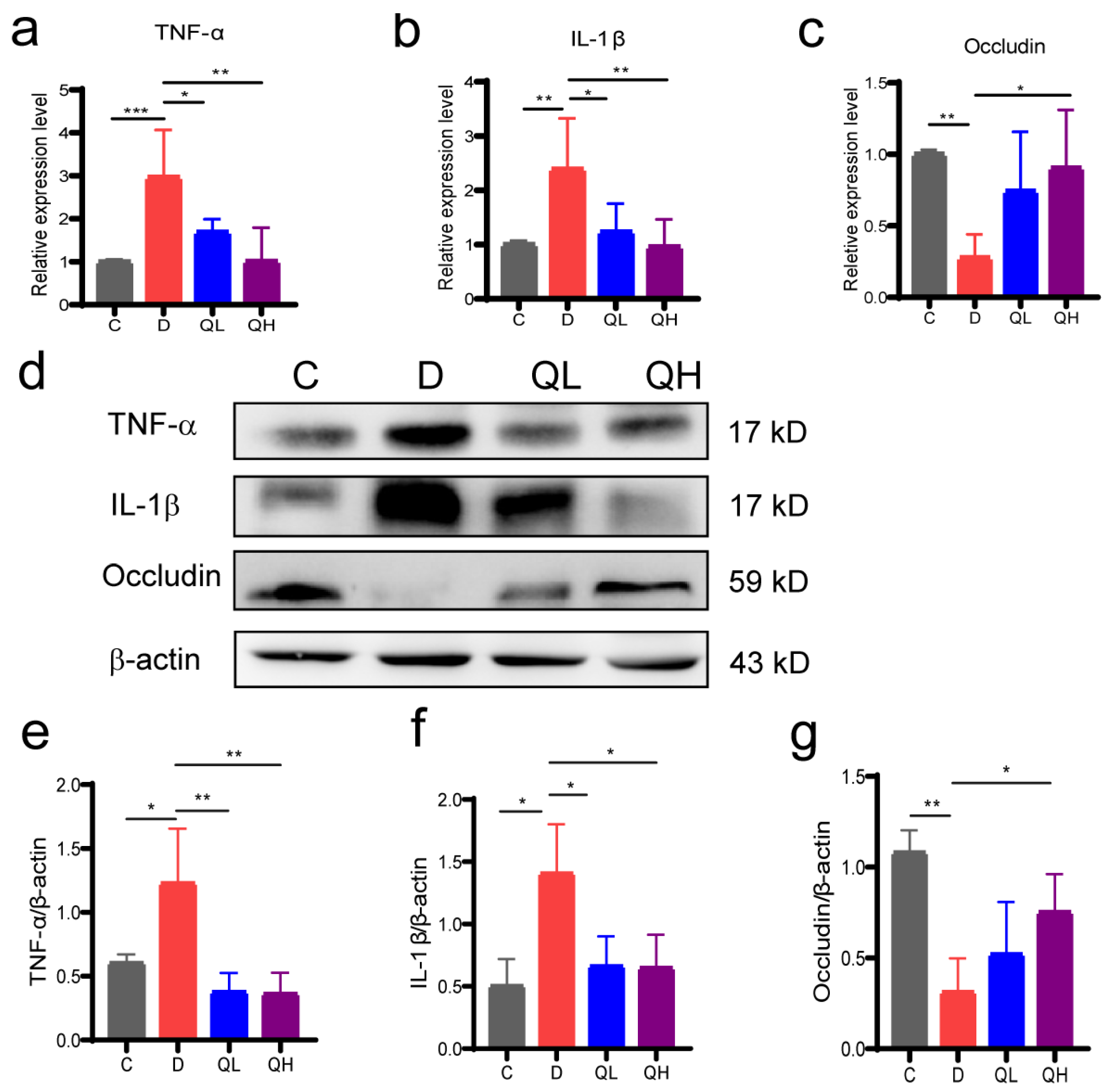
3.3. Quinic Acid Suppressed Intestinal Inflammation and Up-Regulated Barrier Protein Expression
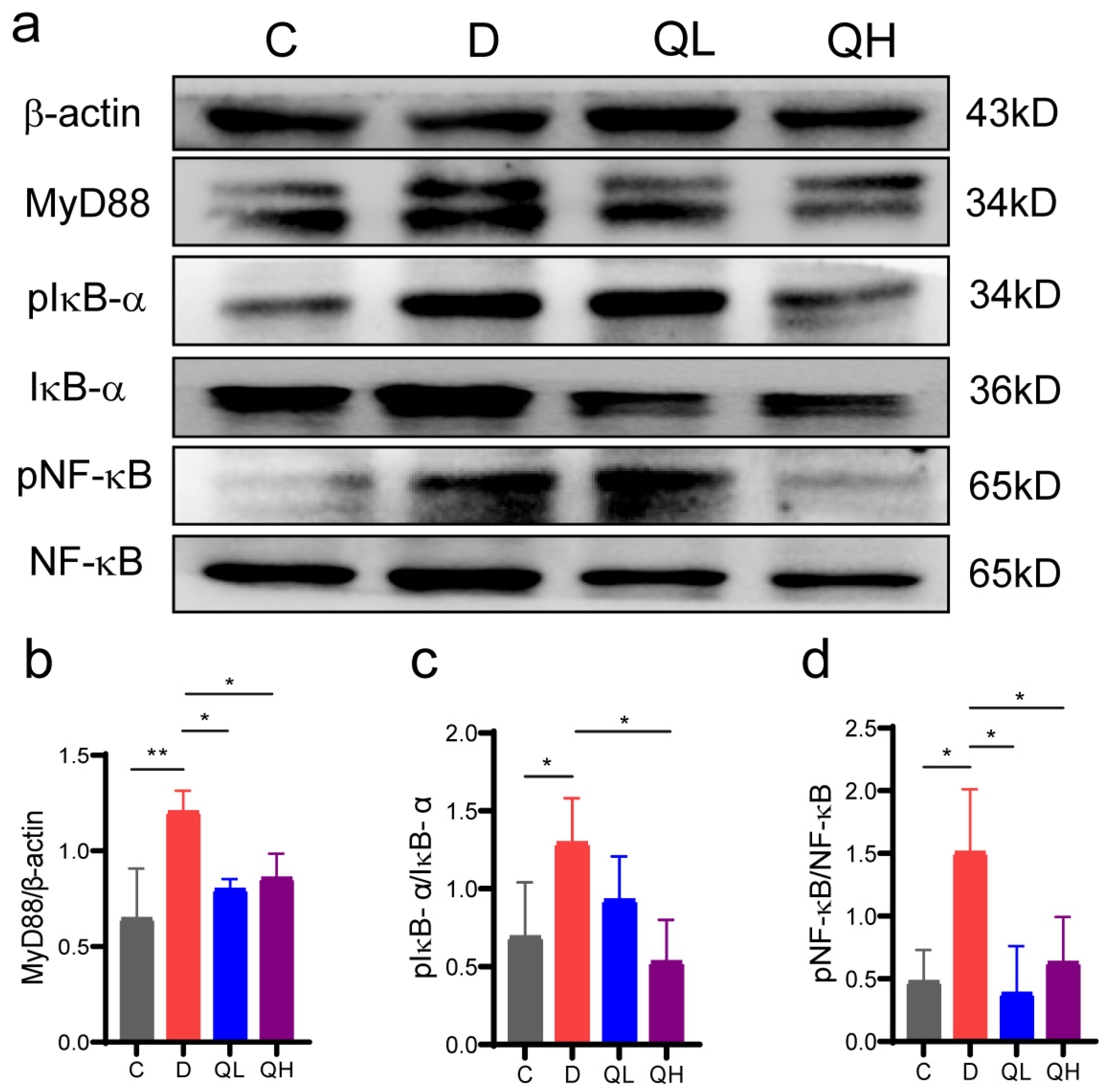
3.4. Quinic Acid Regulated the NF-κB Signaling Pathway in DSS-Induced Colitis Mice
3.5. Quinic Acid Improved Gut Microbiota Composition of DSS-Induced Colitis Mice
3.6. Effect of Quinic Acid on the Pathological Symptoms of Pseudo-Germ-Free Ccolitis Mice
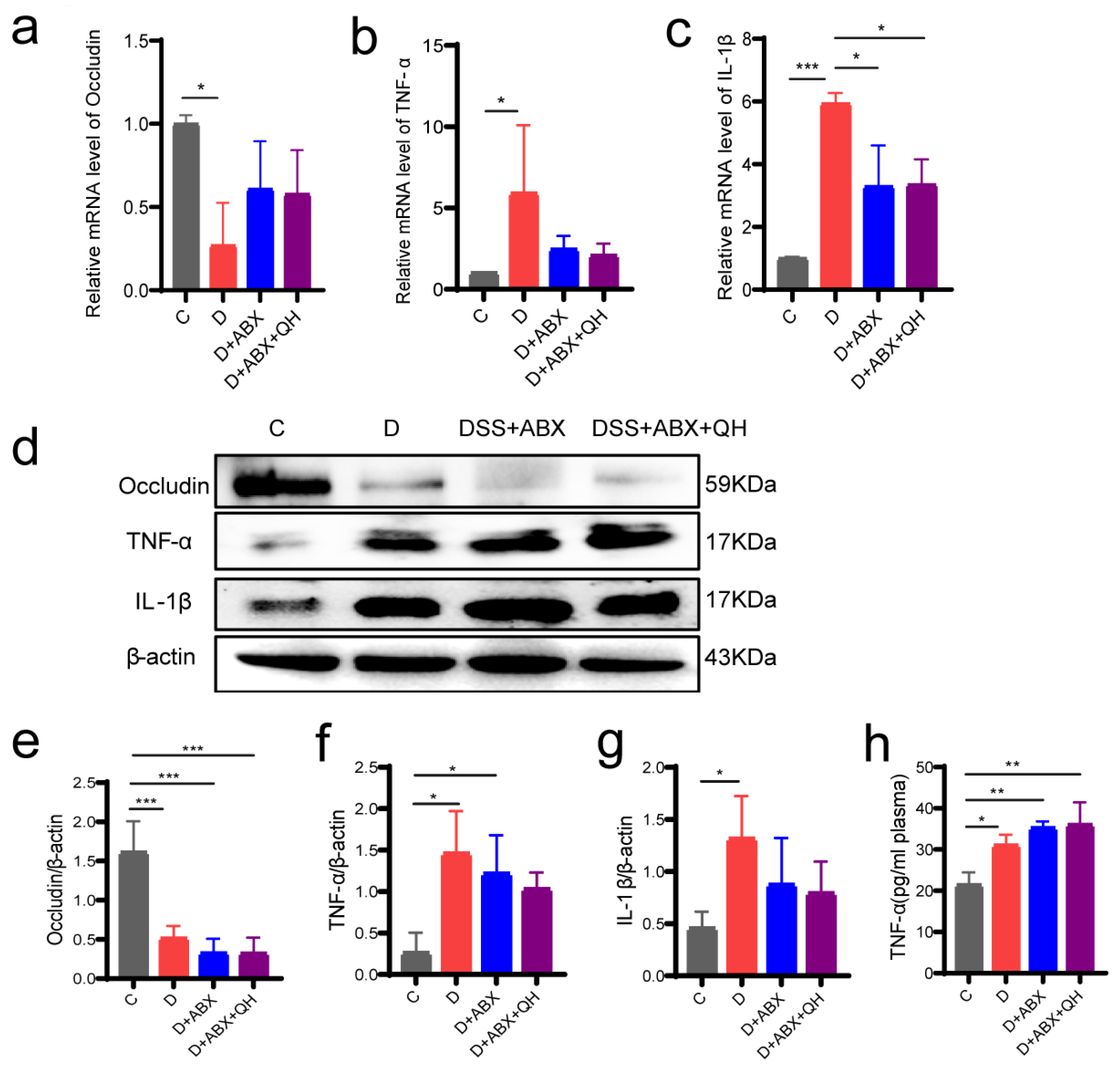
3.7. Effects of Quinic Acid on Barrier Proteins and Inflammatory Factors in Pseudo-Germ-Free Colitis Mice
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijnands, A.M.; de Jong, M.E.; Lutgens, M.W.M.D.; Hoentjen, F.; Elias, S.G.; Oldenburg, B. Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology 2021, 160, 1584–1598. [Google Scholar] [CrossRef]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.-F.; Vermeire, S.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions and diagnosis. J. Crohn’s Colitis 2012, 6, 965–990. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhu, Q.; Pan, L.-L.; Sun, J. Macrophage immunometabolism in inflammatory bowel diseases: From pathogenesis to therapy. Pharmacol. Ther. 2022, 238, 108176. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; Bergen, M.v.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Veltkamp, C.; Tonkonogy, S.L.; de Jong, Y.P.; Albright, C.; Grenther, W.B.; Balish, E.; Terhorst, C.; Sartor, R.B. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tgϵ26 mice. Gastroenterology 2001, 120, 900–913. [Google Scholar] [CrossRef]
- Britton, G.J.; Contijoch, E.J.; Mogno, I.; Vennaro, O.H.; Llewellyn, S.R.; Ng, R.; Li, Z.; Mortha, A.; Merad, M.; Das, A.; et al. Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORγt+ Regulatory T Cells and Exacerbate Colitis in Mice. Immunity 2019, 50, 212–224.e214. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Liu, S.; Lv, H.; Hu, Y.; Wang, Y.; Li, Z.; Wang, J.; Ji, X.; Ma, H.; et al. Dietary Supplementation of Foxtail Millet Ameliorates Colitis-Associated Colorectal Cancer in Mice via Activation of Gut Receptors and Suppression of the STAT3 Pathway. Nutrients 2020, 12, 2367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Zhang, D.; Yang, Y.; Wang, X.; Li, L. Fermented and Germinated Processing Improved the Protective Effects of Foxtail Millet Whole Grain Against Dextran Sulfate Sodium-Induced Acute Ulcerative Colitis and Gut Microbiota Dysbiosis in C57BL/6 Mice. Front. Nutr. 2021, 8, 694936. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Guan, T.; Zhang, Y.; Huang, K.; Zhang, Z.; Cao, W.; Guan, X. Quinic acid alleviates high-fat diet-induced neuroinflammation by inhibiting DR3/IKK/NF-κB signaling via gut microbial tryptophan metabolites. Gut Microbes 2024, 16, 2374608. [Google Scholar] [CrossRef]
- Åkesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100®. Int. Immunopharmacol. 2005, 5, 219–229. [Google Scholar] [CrossRef]
- Pero, R.W.; Lund, H.; Leanderson, T. Antioxidant metabolism induced by quinic acid. increased urinary excretion of tryptophan and nicotinamide. Phytother. Res. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Jang, S.-A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.-H.; Koo, H.J.; Kang, S.C. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Dehnoo, M.; Lorigooini, Z.; Amini-Khoei, H.; Sabzevary-Ghahfarokhi, M.; Rafieian-Kopaei, M. Quinic acid ameliorates ulcerative colitis in rats, through the inhibition of two TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Immun. Inflamm. Dis. 2023, 11, e926. [Google Scholar] [CrossRef] [PubMed]
- Mortelé, O.; Jörissen, J.; Spacova, I.; Lebeer, S.; van Nuijs, A.L.N.; Hermans, N. Demonstrating the involvement of an active efflux mechanism in the intestinal absorption of chlorogenic acid and quinic acid using a Caco-2 bidirectional permeability assay. Food Funct. 2021, 12, 417–425. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Verny, M.-A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic Acid Bioavailability Largely Depends on Its Metabolism by the Gut Microflora in Rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, C.; Chen, R.; Jiang, L.-p.; Li, H.; Wu, P.; Li, L. Quinic acid regulated TMA/TMAO-related lipid metabolism and vascular endothelial function through gut microbiota to inhibit atherosclerotic. J. Transl. Med. 2024, 22, 352. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Guan, X.; Cao, H.; Li, L.; Yu, J.; Liu, H. Characterization of Saponins from Differently Colored Quinoa Cultivars and Their In Vitro Gastrointestinal Digestion and Fermentation Properties. J. Agric. Food Chem. 2022, 70, 1810–1818. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Cueva, C.; Ángeles Pozo-Bayón, M.; Victoria Moreno-Arribas, M. Ability of human oral microbiota to produce wine odorant aglycones from odourless grape glycosidic aroma precursors. Food Chem. 2015, 187, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N.S.; Cooper, H.S.; Shim, H.; Shah, R.S.; Ibrahim, S.A.; Sedergran, D.J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig. Dis. Sci. 1993, 38, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Aitken, J.; Carvalho, F.; Cullender, T.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.; Knight, R.; Ley, R.; Gewirtz, A. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Graef, F.A.; Healey, G.R.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B.A. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018, 155, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, C.; Chen, D.; Zeng, X.; Cao, Y. Prebiotic effects in vitro of anthocyanins from the fruits of Lycium ruthenicum Murray on gut microbiota compositions of feces from healthy human and patients with inflammatory bowel disease. LWT 2021, 149, 111829. [Google Scholar] [CrossRef]
- Zhang, J.; Hoedt, E.C.; Liu, Q.; Berendsen, E.; Teh, J.J.; Hamilton, A.; O’ Brien, A.W.; Ching, J.Y.L.; Wei, H.; Yang, K.; et al. Elucidation of Proteus mirabilis as a Key Bacterium in Crohn’s Disease Inflammation. Gastroenterology 2021, 160, 317–330.e311. [Google Scholar] [CrossRef]
- Aktories, K. Semaphorins or Frizzled –it is the receptor that direct the action of clostridial glucosylating toxins. Signal Transduct. Target. Ther. 2020, 5, 206. [Google Scholar] [CrossRef]
- Heidarian, F.; Alebouyeh, M.; Shahrokh, S.; Balaii, H.; Zali, M.R. Altered fecal bacterial composition correlates with disease activity in inflammatory bowel disease and the extent of IL8 induction. Curr. Res. Transl. Med. 2019, 67, 41–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, Y.; Gan, H.; Zhao, X.; Zhi, F. Streptococcus gallolyticus conspires myeloid cells to promote tumorigenesis of inflammatory bowel disease. Biochem. Biophys. Res. Commun. 2018, 506, 907–911. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef] [PubMed]
- Sandes, S.; Figueiredo, N.; Pedroso, S.; Sant’Anna, F.; Acurcio, L.; Abatemarco Junior, M.; Barros, P.; Oliveira, F.; Cardoso, V.; Generoso, S.; et al. Weissella paramesenteroides WpK4 plays an immunobiotic role in gut-brain axis, reducing gut permeability, anxiety-like and depressive-like behaviors in murine models of colitis and chronic stress. Food Res. Int. 2020, 137, 109741. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guo, K.; Chen, Q.; Wang, Y.; Jirimutu. Camel milk modulates the gut microbiota and has anti-inflammatory effects in a mouse model of colitis. J. Dairy Sci. 2022, 105, 3782–3793. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Zhang, Y.; Lv, H.; Luo, L.; Wang, S.; Guan, X. Polyphenolic Extracts of Coffee Cherry Husks Alleviated Colitis-Induced Neural Inflammation via NF-κB Signaling Regulation and Gut Microbiota Modification. J. Agric. Food Chem. 2022, 70, 6467–6477. [Google Scholar] [CrossRef]
- Huang, J.; Wu, T.; Zhong, Y.; Huang, J.; Kang, Z.; Zhou, B.; Zhao, H.; Liu, D. Effect of curcumin on regulatory B cells in chronic colitis mice involving TLR/MyD88 signaling pathway. Phytother. Res. 2023, 37, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, H.; Huang, H.; Xiao, Y.; Wu, X.; Li, M.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; et al. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. 2021, 12, 3142–3158. [Google Scholar] [CrossRef]
- Chen, L.; Tai, W.C.S.; Hsiao, W.L.W. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J. Funct. Foods 2015, 17, 892–902. [Google Scholar] [CrossRef]
- Miao, S.T.; Lu, Q.S.; Zhou, Y.J.; Chang, Y.N.; Xu, T.; Zhu, M.Y. Oral administration of octacosanol modulates the gut bacteria and protects the intestinal barrier in ulcerative colitis mice. J. Food Biochem. 2022, 46, e14284. [Google Scholar] [CrossRef]
- Mao, N.; Yu, Y.; He, J.; Yang, Y.; Liu, Z.; Lu, Y.; Wang, D. Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 6613. [Google Scholar] [CrossRef]
- Beresford-Jones, B.S.; Forster, S.C.; Stares, M.D.; Notley, G.; Viciani, E.; Browne, H.P.; Boehmler, D.J.; Soderholm, A.T.; Kumar, N.; Vervier, K.; et al. The Mouse Gastrointestinal Bacteria Catalogue enables translation between the mouse and human gut microbiotas via functional mapping. Cell Host Microbe 2022, 30, 124–138.e128. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Feng, Q.; Liang, S.; Sonne, S.B.; Xia, Z.; Qiu, X.; Li, X.; Long, H.; Zhang, J.; Zhang, D.; et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015, 33, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Cheng, R.; Zhang, Y.; Liang, H.; Jiang, F.; Shen, X.; Chen, G.; Zhang, Q.; He, F.; Li, M. Antibiotics can cause weight loss by impairing gut microbiota in mice and the potent benefits of lactobacilli. Biosci. Biotechnol. Biochem. 2020, 84, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Q.; Mi, X.; Qiu, L.; Tao, X.; Zhang, Z.; Xia, J.; Wu, Q.; Wei, H. Ripened Pu-erh Tea Extract Promotes Gut Microbiota Resilience against Dextran Sulfate Sodium Induced Colitis. J. Agric. Food Chem. 2021, 69, 2190–2203. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, Z.; Luo, L.; Zhang, Y.; Huang, K.; Guan, X. Millet Quinic Acid Relieves Colitis by Regulating Gut Microbiota and Inhibiting MyD88/NF-κB Signaling Pathway. Foods 2025, 14, 2267. https://doi.org/10.3390/foods14132267
Li S, Zhang Z, Luo L, Zhang Y, Huang K, Guan X. Millet Quinic Acid Relieves Colitis by Regulating Gut Microbiota and Inhibiting MyD88/NF-κB Signaling Pathway. Foods. 2025; 14(13):2267. https://doi.org/10.3390/foods14132267
Chicago/Turabian StyleLi, Sen, Ze Zhang, Lei Luo, Yu Zhang, Kai Huang, and Xiao Guan. 2025. "Millet Quinic Acid Relieves Colitis by Regulating Gut Microbiota and Inhibiting MyD88/NF-κB Signaling Pathway" Foods 14, no. 13: 2267. https://doi.org/10.3390/foods14132267
APA StyleLi, S., Zhang, Z., Luo, L., Zhang, Y., Huang, K., & Guan, X. (2025). Millet Quinic Acid Relieves Colitis by Regulating Gut Microbiota and Inhibiting MyD88/NF-κB Signaling Pathway. Foods, 14(13), 2267. https://doi.org/10.3390/foods14132267




