Exploring the Bioavailability of Red Grape Skin Extract Polyphenols: A Caco-2 Cell Model Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Grape Samples
2.3. Preparation of Polyphenolic Grape Skin Extract
2.4. HPLC Analysis of Polyphenols
2.5. Cell Culture
2.5.1. Caco-2 Cell Growth Conditions
2.5.2. Cytotoxicity Evaluation
2.5.3. TEER Assay and Bioavailability Study
2.6. Statistical Analysis
3. Results and Discussion
3.1. Polyphenolic Composition of Grape Skin Extract
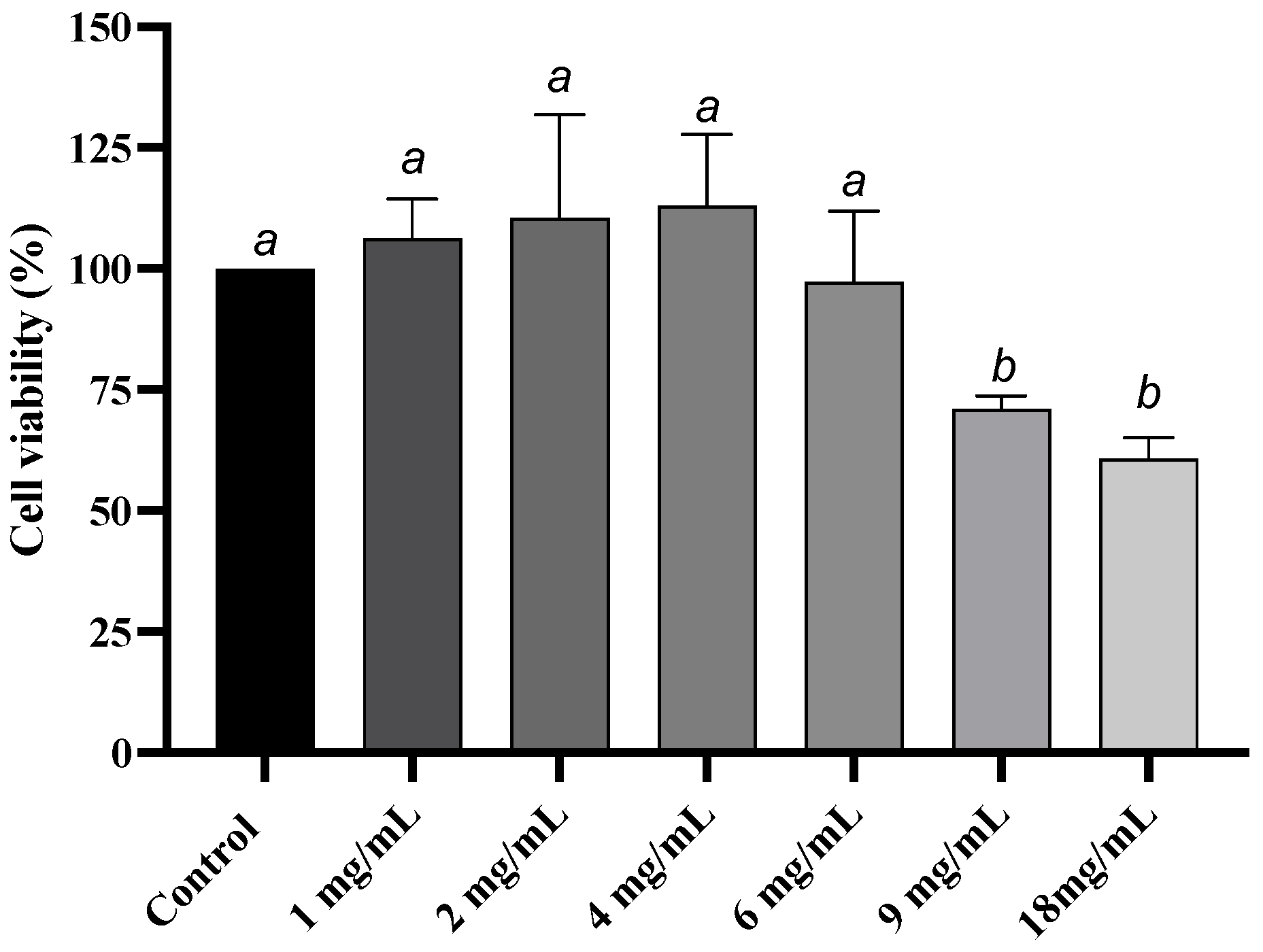
3.2. Cytotoxicity of Grape Skin Extract
3.3. Transport of Grape Skin Extract Polyphenols in the Human Colorectal Adenocarcinoma Cells Caco-2 Monolayer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Surface area of the Caco-2 cell monolayer |
| AB | Apical to basolateral side |
| AP | Apical side |
| BA | Basolateral to apical side |
| BL | Basolateral side |
| C | Polyphenol concentration at the acceptor side |
| C0 | Polyphenol initial concentration at the donor side |
| CA | Caftaric acid |
| Caco-2 | Caucasian colon adenocarcinoma cell line |
| D3G | Delphinidin-3-O-glucoside |
| DAD | Diode array detector |
| DMEM | Dulbecco’s modified eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| dQ | Change of polyphenol concentration at the acceptor side |
| dt | Variation in time |
| ER | Efflux ratio |
| FBS | Fetal bovine serum |
| GA | Gallic acid |
| GSE | Grape skin polyphenolic extract |
| HBSS | Hank’s balanced salt solution |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| HPLC | High performance liquid chromatography |
| K3G | Kaempferol-3-glucoside |
| K3Gal | Kaempferol-3-galactoside |
| LSD | Fisher‘s Least Significant Difference |
| M3G | Malvidin-3-O-glucoside |
| MIN | Minute |
| MTT | 3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| NEAA | Non-essential amino acid |
| Papp | Apparent permeability coefficient |
| PBS | Phosphate-buffered saline solution |
| Pet3G | Petunidin-3-O-glucoside |
| Q3G | Quercetin-3-glucoside |
| S1 | Solution 1 (15 mg grape skin polyphenolic extract/mL) |
| S2 | Solution 2 (22 mg grape skin polyphenolic extract/mL) |
| SA | Syringic acid |
| SD | Standard deviation |
| TE | Transport efficiency |
| TEER | Transepithelial electrical resistance |
| VR | Volume of the solution of the acceptor side |
References
- Ghani, U. Polyphenols. In Alpha-Glucosidase Inhibitors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–100. ISBN 978-0-08-102779-0. [Google Scholar]
- González de Mejía, E.; Rebollo-Hernanz, M.; Aguilera, Y.; Martín-Cabrejas, M.A. Chapter 45—Role of anthocyanins in oxidative stress and the prevention of cancer in the digestive system. In Cancer: Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 265–280. ISBN 9780128195475. [Google Scholar]
- Philips, N.; Richardson, R.; Siomyk, H.; Bynum, D.; Gonzalez, S. Chapter 39—“Skin cancer, polyphenols, and oxidative stress” or Counteraction of oxidative stress, inflammation, signal transduction pathways, and extracellular matrix remodeling that mediate skin carcinogenesis by polyphenols. In Cancer: Oxidative Stress and Dietary Antioxidants; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 439–450. ISBN 9780128195475. [Google Scholar]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Daniela, M.V.; Llano-ramirez, M.A.; Ram, C.; Bedoya, C.; Arango-varela, S.; Santa-gonz, G.A. Antioxidant capacity and cytotoxic effect of an optimized extract of isabella grape (Vitis labrusca) on breast cancer cells. Heliyon 2023, 9, e16540. [Google Scholar] [CrossRef]
- Hasan, M.; Tripathi, K.; Harun, M.; Krishnan, V.; Kaushik, R.; Chawla, G.; Shakil, N.A.; Verma, M.K.; Dahuja, A.; Sachdev, A.; et al. Unravelling the effect of extraction on anthocyanin functionality and prebiotic potential. Heliyon 2024, 10, e31780. [Google Scholar] [CrossRef]
- Gollucke, A.P.; Peres, R.C.; Odair, A.; Ribeiro, D.A. Polyphenols: A nutraceutical approach against diseases. Recent Pat. Food Nutr. Agric. 2013, 5, 214–219. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Dzitkowska-Zabielska, M. Polyphenol supplementation and antioxidant status in athletes: A narrative review. Nutrients 2023, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Elejalde, E.; Villarán, M.C.; Alonso, R.M. Grape polyphenols supplementation for exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2021, 18, 3. [Google Scholar] [CrossRef]
- Guaita, M.; Bosso, A. Polyphenolic characterization of grape skins and seeds of four Italian red cultivars at harvest and after fermentative maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Sasireka, A.; Chandrasekaran, M.; Chung, I.M. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2011, 44, 3134–3148. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, H.; Kaur, R.; Garg, R.; Prasad, R.; Assouguem, A.; Kara, M.; Bahhou, J. A review on the nutritional value and health benefits of different parts of grape (Vitis vinifera L.). Trop. J. Nat. Prod. Res. 2023, 7, 3874–3880. [Google Scholar] [CrossRef]
- World Statistics. Available online: https://www.oiv.int/what-we-do/global-report?oiv= (accessed on 19 April 2024).
- Pezzuto, J.M. Grapes and human health: A perspective. J. Agric. Food Chem. 2008, 56, 6777–6784. [Google Scholar] [CrossRef]
- Rastija, V.; Drenjančević, M.; Kujundžić, T.; Zmaić, L.; Karnaš, M. The antidiabetic effect of grape skin extracts of selected indigenous Croatian white grapevine varieties. Foods 2024, 13, 4143. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Xu, T.; Du, Q.; Yang, R.; Ai, M.; Han, X.; Wang, W. Ultrasound-assisted polysaccharide extraction from grape skin and assessment of in vitro hypoglycemic activity of polysaccharides. Foods 2025, 14, 1801. [Google Scholar] [CrossRef] [PubMed]
- Mateus Martins, I.; Alves Macedo, G.; Alves Macedo, J. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: Effects in Caco-2 cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Koch, W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Atnip, A.; Bomser, J.; Giusti, M.M. Aglycone structures and glycosylations affect anthocyanin transport and uptake in human gastric epithelial (NCI-N87) cells. J. Food Compos. Anal. 2018, 65, 33–39. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Cuadrado, C.; Redondo, I.B.; Giampieri, F.; González-Paramás, A.M.; Santos-Buelga, C. Novel approaches in anthocyanin research—Plant fortification and bioavailability. Trends Food Sci. Technol. 2021, 117, 92–105. [Google Scholar] [CrossRef]
- Cahyana, Y.; Gordon, M.H.; Gibson, T.M. Urinary excretion of anthocyanins following consumption of strawberry and red grape juice. Vitam. Nutr. Res. 2019, 89, 29–36. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P.; Sharma, J.; Dixit, A. Flavonoids modulate tight junction barrier functions in hyperglycemic human intestinal Caco-2 cells. Nutrition 2020, 78, 110792. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Bornhorst, G.M.; Oteiza, P.I.; Mitchell, A.E. Assessing the fate and bioavailability of glucosinolates in kale (Brassica oleracea) using simulated human digestion and Caco-2 cell uptake models. J. Agric. Food Chem. 2019, 67, 9492–9500. [Google Scholar] [CrossRef]
- Ke, X.; Hu, X.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Xue, C. A novel zinc-binding peptide identified from tilapia (Oreochromis niloticus) skin collagen and transport pathway across Caco-2 monolayers. Food Biosci. 2021, 42, 101127. [Google Scholar] [CrossRef]
- Cui, N.; Yang, C.; Chen, X.; Zhang, J.; Zhang, L. Anti-oxidative and anti-inflammatory effects of different lutein isomers in Caco-2 cell model. Food Biosci. 2023, 53, 102646. [Google Scholar] [CrossRef]
- Soler, A.; Romero, M.P.; Macià, A.; Saha, S.; Furniss, C.S.M.; Kroon, P.A.; Motilva, M.J. Digestion stability and evaluation of the metabolism and transport of olive oil phenols in the human small-intestinal epithelial Caco-2/TC7 cell line. Food Chem. 2010, 119, 703–714. [Google Scholar] [CrossRef]
- Food and Drug Administration. M9 Biopharmaceutics Classification System-Based Biowaivers Guidance for Industry M9 Biopharmaceutics Classification System-Based Biowaivers Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2021.
- European Medicines Agency. ICH M9 Guideline on Biopharmaceutics Classification System-Based Biowaivers; European Medicines Agency: Amsterdan, The Netherlands, 2020; Volume 31.
- Kus, M.; Ibragimow, I.; Piotrowska-kempisty, H. Caco-2 cell line standardization with pharmaceutical requirements and in vitro model suitability for permeability assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.L.; Jourdes, M.; Teissedre, P.L. Valorization of grape pomace: A review of phenolic composition, bioactivity, and therapeutic potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape pomace extract attenuates inflammatory response in intestinal epithelial and endothelial cells: Potential health-promoting properties in bowel inflammation. Nutrients 2022, 14, 1175. [Google Scholar] [CrossRef]
- Taladrid, D.; González de Llano, D.; Zorraquín-Peña, I.; Tamargo, A.; Silva, M.; Molinero, N.; Moreno-Arribas, M.V.; Bartolomé, B. Gastrointestinal digestion of a grape pomace extract: Impact on intestinal barrier permeability and interaction with gut microbiome. Nutrients 2021, 13, 2467. [Google Scholar] [CrossRef] [PubMed]
- Milella, R.A.; Gasparro, M.; Debiase, G.; Notarangelo, L.; Notarnicola, M.; Tutino, V.; Antonacci, D. Antitumor effects of table grape extracts. BIO Web Conf. 2023, 68, 04013. [Google Scholar] [CrossRef]
- El 96% de la Superficie de Viñedo en España Está en Territorio de Alguna Denominación de Calidad. Available online: https://www.mapa.gob.es/es/prensa/ultimas-noticias/el-96--de-la-superficie-de-viñedo-en-españa-está-en-territorio-de-alguna-denominación-de-calidad-/tcm:30-561255 (accessed on 1 September 2024).
- OIV. Distribution of the World’s Grapevine Varieties; OIV: Paris, France, 2017. [Google Scholar]
- Elejalde, E.; Villarán, M.C.; Esquivel, A.; Alonso, R.M. Bioaccessibility and antioxidant capacity of grape seed and grape skin phenolic compounds after simulated In vitro gastrointestinal digestion. Plant Foods Hum. Nutr. 2024, 79, 432–439. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem. 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Natolino, A.; Da Porto, C. Kinetic models for conventional and ultrasound assistant extraction of polyphenols from defatted fresh and distilled grape marc and its main components skins and seeds. Chem. Eng. Res. Des. 2020, 156, 1–12. [Google Scholar] [CrossRef]
- Elejalde, E.; Villarán, M.C.; Lopez-de-Armentia, I.; Ramón, D.; Murillo, R.; Alonso, R.M. Study of unpicked grapes valorization: A natural source of polyphenolic compounds and evaluation of their antioxidant capacity. Resources 2022, 11, 33. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Przybylla, R.; Mullins, C.S.; Krohn, M.; Oswald, S.; Linnebacher, M. Establishment and characterization of novel human intestinal in vitro models for absorption and first-pass metabolism studies. Int. J. Mol. Sci. 2022, 23, 9861. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Amorim, M.; Vilas-Boas, A.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M. Impact of: In vitro gastrointestinal digestion on the chemical composition, bioactive properties, and cytotoxicity of Vitis vinifera L. cv. Syrah grape pomace extract. Food Funct. 2019, 10, 1856–1869. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.W. Caco-2: Predicting Drug Permeability and Relationship with COVID-19 Infected Caco-2 Cells; HAL Science Ouverte: Adelaide, South Australia, 2021. [Google Scholar]
- Kotze, S.; Ebert, A.; Goss, K.U. Effects of aqueous boundary layers and paracellular transport on the efflux ratio as a measure of active transport across cell layers. Pharmaceutics 2024, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC-DAD-ESI-MSn: Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H. Bin Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Chengolova, Z.; Ivanov, Y.; Godjevargova, T. Comparison of identification and quantification of polyphenolic compounds in skins and seeds of four grape varieties. Molecules 2023, 28, 4061. [Google Scholar] [CrossRef]
- Otun, S.; Achilonu, I.; Odero-Marah, V. Unveiling the potential of Muscadine grape skin extract as an innovative therapeutic intervention in cancer treatment. J. Funct. Foods 2024, 116, 106146. [Google Scholar] [CrossRef]
- Polat, İ.; Balkıç, R.; Uysal Seçkin, G. Quality characteristics, phenolic composition and potential antioxidant activities of some table grape varieties in highland viticulture. Appl. Fruit Sci. 2024, 66, 1819–1829. [Google Scholar] [CrossRef]
- Phajon, Y.; Tan, H.; Liu, B.; Zhang, Y.; Ju, Y.; Shen, T.; Xu, M.; Fang, Y. Effect of terroir on phenolic content and aroma properties of grapes and wines. Foods 2025, 14, 1409. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A.; Roberto, B.S.; Chen, Q.; Blumberg, J.B.; Chen, C.-Y.O. Tannase enhances the anti-inflammatory effect of grape pomace in Caco-2 cells treated with IL-1β. J. Funct. Foods 2017, 29, 69–76. [Google Scholar] [CrossRef]
- Kuntz, S.; Asseburg, H.; Dold, S.; Römpp, A.; Fröhling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. Biofactors 2013, 39, 448–456. [Google Scholar] [CrossRef]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Iametti, S.; Bonomi, F.; Di Nunzio, M. Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review. Int. J. Mol. Sci. 2022, 23, 4355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, J.; Shi, X.; Miao, S.; Li, Y.; Wen, A. Absorption and metabolism of rutin in Caco-2 cells. Sci. World J. 2013, 2013, 382350. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Herranz-López, M.; Barrajón-Catalán, E.; Arráez-Román, D.; González-Álvarez, I.; Bermejo, M.; Gutiérrez, A.F.; Micol, V.; Segura-Carretero, A. Permeability study of polyphenols derived from a phenolic-enriched hibiscus sabdariffa extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int. J. Mol. Sci. 2015, 16, 18396–18411. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Wu, Y.; Wang, D.; Wei, Y.; Wu, J.; Ji, B. Stability and absorption of anthocyanins from blueberries subjected to a simulated digestion process. Int. J. Food Sci. Nutr. 2014, 65, 440–448. [Google Scholar] [CrossRef]
- Cahyana, Y.; Mills, C.E.; Huda, S.; Gordon, M.H. Factors affecting cellular uptake of anthocyanins: The role of pH, glucose and anthocyanin structure. Nutrients 2022, 14, 4807. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Wang, J.; Chai, Z.; Zhang, X.; Wang, J.; Wang, N.; Huang, W. Impact of in vitro gastrointestinal digestion on rabbiteye blueberry anthocyanins and their absorption efficiency in Caco-2 cells. Food Biosci. 2023, 52, 102424. [Google Scholar] [CrossRef]
- Pintać Šarac, D.; Tremmel, M.; Vujetić, J.; Torović, L.; Orčić, D.; Popović, L.; Mimica-Dukić, N.; Lesjak, M. How do in vitro digestion and cell metabolism affect the biological activity and phenolic profile of grape juice and wine. Food Chem. 2024, 449, 139228. [Google Scholar] [CrossRef]
- Chen, M.; Yu, S. Lipophilized grape seed proanthocyanidin derivatives as novel antioxidants. J. Agric. Food Chem. 2017, 65, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Oliveira, H.; Brás, N.F.; Fernandes, I.; Cruz, L.; De Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins—Impact of structural complexity and phase II metabolization. Food Chem. 2020, 317, 126398. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Molonia, M.S.; Muscarà, C.; Cristani, M.; Salamone, F.L.; Saija, A.; Cimino, F. An overview on the cellular mechanisms of anthocyanins in maintaining intestinal integrity and function. Fitoterapia 2024, 175, 105953. [Google Scholar] [CrossRef]
- Achour, M.; Saguem, S.; Sarriá, B.; Bravo, L.; Mateos, R. Bioavailability and metabolism of rosemary infusion polyphenols using Caco-2 and HepG2 cell model systems. J. Sci. Food Agric. 2018, 98, 3741–3751. [Google Scholar] [CrossRef]
- Rastogi, H.; Jana, S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 33–43. [Google Scholar] [CrossRef]
- Mao, X.; Wu, L.F.; Zhao, H.J.; Liang, W.Y.; Chen, W.J.; Han, S.X.; Qi, Q.; Cui, Y.P.; Li, S.; Yang, G.H.; et al. Transport of corilagin, gallic acid, and ellagic acid from Fructus phyllanthi tannin fraction in Caco-2 cell monolayers. Evid.-Based Complement. Altern. Med. 2016, 2016, 9205379. [Google Scholar] [CrossRef]
| Compound | Polyphenol Family | Concentration (µg/g) |
|---|---|---|
| Delphinidin-3-O-glucoside (D3G) | Anthocyanin | 212 ± 15 |
| Petunidin-3-O-glucoside (Pet3G) | Anthocyanin | 196 ± 15 |
| Malvidin-3-O-glucoside (M3G) | Anthocyanin | 548 ± 35 |
| Quercetin-3-glucoside (Q3G) | Flavonol | 28 ± 3 |
| Kaempferol-3-galactoside (K3Gal) | Flavonol | 58 ± 5 |
| Kaempferol-3-glucoside (K3G) | Flavonol | 254 ± 26 |
| Gallic acid (GA) | Hydroxybenzoic acid | 38 ± 4 |
| Syringic acid (SA) | Hydroxybenzoic acid | 59 ± 6 |
| Caftaric acid (CA) | Hydroxycinnamic acid | 88 ± 8 |
| Experiment | Solution | GSE (mg/mL) | TEER (Ω cm2) | |
|---|---|---|---|---|
| Before | After | |||
| AB | S1 | 15 | 602 ± 8 | 536 ± 31 |
| AB | S2 | 22 | 350 ± 25 | 300 ± 15 |
| BA | S1 | 15 | 357 ± 32 | 340 ± 17 |
| BA | S2 | 22 | 320 ± 9 | 300 ± 9 |
| AB Experiments | ||||||
|---|---|---|---|---|---|---|
| GSE Concentration | Polyphenol | Apical Side (mg/L) | ||||
| 0 min | 75 min | 90 min | 115 min | 180 min | ||
| S1 (15 mg/mL) | D3G | 2.1 ± 0.2 a | 1.9 ± 0.2 a,b | 1.8 ± 0.1 b,c | 1.6 ± 0.1 c | 1.5 ± 0.1 c |
| Pet3G | 1.8 ± 0.1 a | 1.4 ± 0.1 b | 1.2 ± 0.1 c | 1.02 ± 0.10 c,d | 0.9 ± 0.1 d | |
| Q3G | 0.23 ± 0.01 a | 0.18 ± 0.01 b | 0.14 ± 0.01 c | 0.10 ± 0.00 d | 0.08 ± 0.00 c | |
| K3Gal | 0.63 ± 0.02 a | 0.52 ± 0.02 b | 0.45 ± 0.02 c | 0.39 ± 0.01 d | 0.35 ± 0.01 c | |
| SA | 0.70 ± 0.03 a | 0.54 ± 0.03 b | 0.42 ± 0.03 c | 0.32 ± 0.03 d | 0.23 ± 0.03 c | |
| S2 (22 mg/mL) | D3G | 4.7 ± 0.1 a | 4.4 ± 0.1 b | 4.3 ± 0.1 c | 4.05 ± 0.09 c | 3.94 ± 0.08 c |
| Pet3G | 4.4 ± 0.2 a | 4.0 ± 0.1 b | 3.7 ± 0.1 c | 3.33 ± 0.09 c | 3.16 ± 0.08 c | |
| Q3G | 0.61 ± 0.01 a | 0.52 ± 0.01 b | 0.47 ± 0.01 c | 0.41 ± 0.01 d | 0.37 ± 0.01 c | |
| K3Gal | 1.23 ± 0.02 a | 1.09 ± 0.02 b | 1.01 ± 0.02 c | 0.88 ± 0.02 d | 0.79 ± 0.02 c | |
| SA | 1.48 ± 0.06 a | 1.21 ± 0.04 b | 1.03 ± 0.03 c | 0.82 ± 0.02 d | 0.67 ± 0.03 c | |
| GSE Concentration | Polyphenol | Basolateral Side (mg/L) | ||||
| 0 min | 75 min | 90 min | 115 min | 180 min | ||
| S1 (15 mg/mL) | D3G | nd | nd | nd | nd | nd |
| Pet3G | nd | nd | nd | nd | nd | |
| Q3G | nd | nd | nd | nd | nd | |
| K3Gal | nd | nd | nd | nd | nd | |
| SA | nd | nd | nd | nd | nd | |
| S2 (22 mg/mL) | D3G | nd | nd | nd | nd | nd |
| Pet3G | nd | nd | nd | nd | nd | |
| Q3G | nd | nd | nd | nd | nd | |
| K3Gal | nd | nd | nd | nd | nd | |
| SA | nd | nd | nd | nd | nd | |
| BA Experiments | ||||||
|---|---|---|---|---|---|---|
| GSE Concentration | Polyphenol | Basolateral Side (mg/L) | ||||
| 0 min | 75 min | 90 min | 115 min | 180 min | ||
| S1 (15 mg/mL) | D3G | 2.7 ± 0.2 a | 2.6 ± 0.2 a,b | 2.5 ± 0.2 b,c | 2.4 ± 0.2 b,c | 2.3 ± 0.2 c |
| Pet3G | 4.3 ± 0.1 a | 4.0 ± 0.1 b | 3.8 ± 0.1 b,c | 3.65 ± 0.09 c,d | 3.49 ± 0.08 d | |
| K3Gal | 0.26 ± 0.01 a | 0.19 ± 0.01 b | 0.13 ± 0.01 c | 0.08 ± 0.01 d | 0.05 ± 0.01 c | |
| SA | 0.84 ± 0.08 a | 0.72 ± 0.07 a,b | 0.63 ± 0.07 b,c | 0.53 ± 0.06 c,d | 0.44 ± 0.06 d | |
| S2 (22 mg/mL) | D3G | 3.6 ± 0.2 a | 3.5 ± 0.2 a | 3.4 ± 0.2 a | 3.4 ± 0.2 d | 3.4 ± 0.2 a |
| Pet3G | 3.1 ± 0.2 a | 3.0 ± 0.2 a | 2.9 ± 0.2 a | 2.7 ± 0.2 a | 2.7 ± 0.2 a | |
| K3Gal | 0.79 ± 0.02 a | 0.75 ± 0.02 b | 0.71 ± 0.02 c | 0.67 ± 0.02 d | 0.64 ± 0.01 c | |
| SA | 1.1 ± 0.1 a | 1.10 ± 0.09 a,b | 1.03 ± 0.09 a,b,c | 0.96 ± 0.08 b,c | 0.901 ± 0.08 c | |
| GSE Concentration | Polyphenol | Apical Side (mg/L) | ||||
| 0 min | 75 min | 90 min | 115 min | 180 min | ||
| S1 (15 mg/mL) | D3G | nd | nd | nd | nd | nd |
| Pet3G | nd | nd | nd | nd | nd | |
| K3Gal | nd | nd | nd | nd | nd | |
| SA | nd | nd | nd | nd | nd | |
| S2 (22 mg/mL) | D3G | nd | nd | nd | nd | nd |
| Pet3G | nd | nd | nd | nd | nd | |
| K3Gal | nd | nd | nd | nd | nd | |
| SA | nd | nd | nd | nd | nd | |
| AB Experiments | |||||||
|---|---|---|---|---|---|---|---|
| GSE Concentration | Polyphenol | Apical Side (mg/L) | |||||
| 0 min | 75 min | 90 min | 115 min | 180 min | |||
| S1 (15 mg/mL) | M3G | 6.1 ± 0.3 a | 4.5 ± 0.2 b | 3.2 ± 0.1 c | 2.26 ± 0.06 d | 1.53 ± 0.04 c | |
| K3G | 7.7 ± 0.3 a | 7.1 ± 0.2 b | 6.7 ± 0.2 b,c | 6.4 ± 0.2 c | 5.1 ± 0.2 d | ||
| GA | 6.1 ± 0.5 a | 5.0 ± 0.5 b | 4.1 ± 0.4 c | 3.4 ± 0.4 c,d | 2.9 ± 0.3 d | ||
| CA | 1.04 ± 0.08 a | 0.84 ± 0.07 b | 0.67 ± 0.07 c | 0.54 ± 0.07 d | 0.43 ± 0.07 d | ||
| S2 (22 mg/mL) | M3G | 14 ± 1 a | 11.6 ± 0.9 b | 9.2 ± 0.9 c | 7.2 ± 0.9 d | 6.0 ± 0.8 d | |
| K3G | 12.7 ± 0.3 a | 11.8 ± 0.3 b | 11.3 ± 0.3 b,c | 10.6 ± 0.23 c | 9.0 ± 0.2 d | ||
| GA | 5.9 ± 0.3 a | 5.0 ± 0.3 b | 4.4 ± 0.3 c | 3.7 ± 0.3 d | 3.2 ± 0.3 d | ||
| CA | 1.82 ± 0.03 a | 1.54 ± 0.03 b | 1.37 ± 0.02 c | 1.16 ± 0.02 d | 1.01 ± 0.01 c | ||
| GSE Concentration | Polyphenol | Basolateral Side (mg/L) | TE (%) | ||||
| 0 min | 75 min | 90 min | 115 min | 180 min | |||
| S1 (15 mg/mL) | M3G | nd | nd | nd | nd | nd | |
| K3G | nd | 6.2 ± 0.1 b | 6.7 ± 0.1 c | 7.1 ± 0.1 c | 7.4 ± 0.1 c | 97 ± 2 | |
| GA | nd | 8.1 ± 0.9 b | 8.6 ± 0.9 b | 9.1 ± 0.9 b | 9.4 ± 0.9 b | 156 ± 26 | |
| CA | nd | 0.06 ± 0.01 b | 0.06 ± 0.01 b,c | 0.06 ± 0.01 b,c | 0.07 ± 0.01 c | 7 ± 1 | |
| S2 (22 mg/mL) | M3G | nd | 0.12 ± 0.01 b | 0.13 ± 0.01 c,d | 0.14 ± 0.01 d,c | 0.15 ± 0.01 c | 1.08 ± 0.01 |
| K3G | nd | 6.4 ± 0.1 b | 6.8 ± 0.2 c | 7.2 ± 0.2 d,c | 7.6 ± 0.2 c | 60.2 ± 0.9 | |
| GA | nd | 8.1 ± 0.9 b | 8.6 ± 0.9 b | 9.1 ± 0.9 b | 9.6 ± 0.9 b | 164 ± 23 | |
| CA | nd | 0.07 ± 0.01 b | 0.07 ± 0.01 b,c | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 4.4 ± 0.4 | |
| BA Experiments | |||||||
|---|---|---|---|---|---|---|---|
| GSE Concentration | Polyphenol | Basolateral Side (mg/L) | |||||
| 0 min | 75 min | 90 min | 115 min | 180 min | |||
| S1 (15 mg/mL) | M3G | 10.4 ± 0.7 a | 9.7 ± 0.6 b | 9.1 ± 0.6 c | 8.5 ± 0.6 d | 8.0 ± 0.6 c | |
| K3G | 7.6 ± 0.2 a | 7.2 ± 0.2 b | 6.9 ± 0.2 c | 6.6 ± 0.1 d | 6.1 ± 0.1 c | ||
| Q3G | 0.28 ± 0.02 a | 0.24 ± 0.02 b | 0.21 ± 0.02 c | 0.18 ± 0.01 d | 0.15 ± 0.01 c | ||
| GA | 6.9 ± 0.3 a | 6.5 ± 0.3 b | 6.2 ± 0.3 c | 5.9 ± 0.3 d | 5.5 ± 0.2 c | ||
| CA | 1.04 ± 0.03 a | 0.91 ± 0.02 b | 0.80 ± 0.02 c | 0.70 ± 0.02 c,d | 0.59 ± 0.02 c | ||
| S2 (22 mg/mL) | M3G | 17.5 ± 0.4 a | 16.4 ± 0.4 b | 15.4 ± 0.3 c | 14.5 ± 0.3 c,d | 13.7 ± 0.2 c | |
| K3G | 9.8 ± 0.2 a | 9.5 ± 0.2 b | 9.3 ± 0.2 c | 9.1 ± 0.2 d | 8.5 ± 0.2 c | ||
| Q3G | 0.41 ± 0.03 a | 0.38 ± 0.03 a,b | 0.36 ± 0.03 a,b,c | 0.34 ± 0.03 b,c | 0.32 ± 0.03 c | ||
| GA | 6.1 ± 0.4 a | 5.7 ± 0.4 b | 5.4 ± 0.4 c | 5.0 ± 0.4 d | 4.7 ± 0.3 d | ||
| CA | 1.4 ± 0.1 a | 1.3 ± 0.1 b | 1.23 ± 0.09 c | 1.15 ± 0.08 d | 1.08 ± 0.08 c | ||
| GSE Concentration | Polyphenol | Apical Side (mg/L) | TE (%) | ||||
| 0 min | 75 min | 90 min | 115 min | 180 min | |||
| S1 (15 mg/mL) | M3G | nd | 0.19 ± 0.02 a,b | 0.23 ± 0.02 b,c | 0.28 ± 0.02 c | 0.33 ± 0.02 c | 3.2 ± 0.3 |
| K3G | nd | 6.1 ± 0.3 b | 7.4 ± 0.3 c | 8.7 ± 0.4 d | 9.9 ± 0.4 c | 130 ± 3 | |
| Q3G | nd | 0.009 ± 0.001 b | 0.010 ± 0.001 b,c | 0.011 ± 0.001 c | 0.011 ± 0.001 c | 4.1 ± 0.1 | |
| GA | nd | 8.1 ± 0.3 a,b | 9.4 ± 0.2 b,c | 10.5 ± 0.3 c,d | 11.3 ± 0.3 d | 164 ± 6 | |
| CA | nd | 0.08 ± 0.01 b | 0.09 ± 0.01 c | 0.10 ± 0.01 d | 0.12 ± 0.01 c | 11.2 ± 0.8 | |
| S2 (22 mg/mL) | M3G | nd | 0.18 ± 0.01 b | 0.22 ± 0.01 c | 0.26 ± 0.01 d | 0.30 ± 0.01 c | 1.73 ± 0.04 |
| K3G | nd | 6.8 ± 0.2 a,b | 8.1 ± 0.2 b,c | 9.4 ± 0.3 c,d | 10.6 ± 0.3 c | 109.0 ± 0.9 | |
| Q3G | nd | 0.013 ± 0.001 b | 0.013 ± 0.001 b,c | 0.014 ± 0.001 c,d | 0.015 ± 0.001 d | 3.6 ± 0.1 | |
| GA | nd | 8.3 ± 0.5 a,b | 9.6 ± 0.5 b,c | 10.7 ± 0.6 c | 11.5 ± 0.7 c | 188 ± 3 | |
| CA | nd | 0.08 ± 0.01 a,b | 0.10 ± 0.01 a,b,c | 0.11 ± 0.01 b,c | 0.12 ± 0.01 c | 8.7 ± 0.9 | |
| Polyphenol | Papp AB (10−4 cm/s) | Papp BA (10−4 cm/s) | ER | |||
|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | |
| M3G | nd | 0.15 ± 0.01 | 0.33 ± 0.02 | 0.18 ± 0.01 | nd | 1.2 |
| K3G | 11.6 ± 0.2 | 7.3 ± 0.4 | 12.3 ± 0.3 | 9.7 ± 0.3 | 1.1 | 1.3 |
| GA | 16.9 ± 0.7 | 18 ± 2 | 11.5 ± 0.6 | 12.90 ± 0.05 | 0.7 | 0.7 |
| CA | 0.74 ± 0.03 | 0.51 ± 0.02 | 0.93 ± 0.07 | 0.73 ± 0.08 | 1.3 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elejalde, E.; Alonso, R.M.; Villarán, M.C.; Díez-Gutiérrez, L.; Chávarri, M.; López-de-Armentia, I. Exploring the Bioavailability of Red Grape Skin Extract Polyphenols: A Caco-2 Cell Model Study. Foods 2025, 14, 2253. https://doi.org/10.3390/foods14132253
Elejalde E, Alonso RM, Villarán MC, Díez-Gutiérrez L, Chávarri M, López-de-Armentia I. Exploring the Bioavailability of Red Grape Skin Extract Polyphenols: A Caco-2 Cell Model Study. Foods. 2025; 14(13):2253. https://doi.org/10.3390/foods14132253
Chicago/Turabian StyleElejalde, Edurne, Rosa María Alonso, María Carmen Villarán, Lucía Díez-Gutiérrez, María Chávarri, and Iratxe López-de-Armentia. 2025. "Exploring the Bioavailability of Red Grape Skin Extract Polyphenols: A Caco-2 Cell Model Study" Foods 14, no. 13: 2253. https://doi.org/10.3390/foods14132253
APA StyleElejalde, E., Alonso, R. M., Villarán, M. C., Díez-Gutiérrez, L., Chávarri, M., & López-de-Armentia, I. (2025). Exploring the Bioavailability of Red Grape Skin Extract Polyphenols: A Caco-2 Cell Model Study. Foods, 14(13), 2253. https://doi.org/10.3390/foods14132253







