Extraction and Valorization of Oilseed Cakes for Value-Added Food Components—A Review for a Sustainable Foodstuff Production in a Case Process Approach
Abstract
1. Introduction
2. Oilseeds and Oilseed Cakes: Composition and Global Significance
2.1. Nutritional and Functional Profiles of Major Oilseed Crops
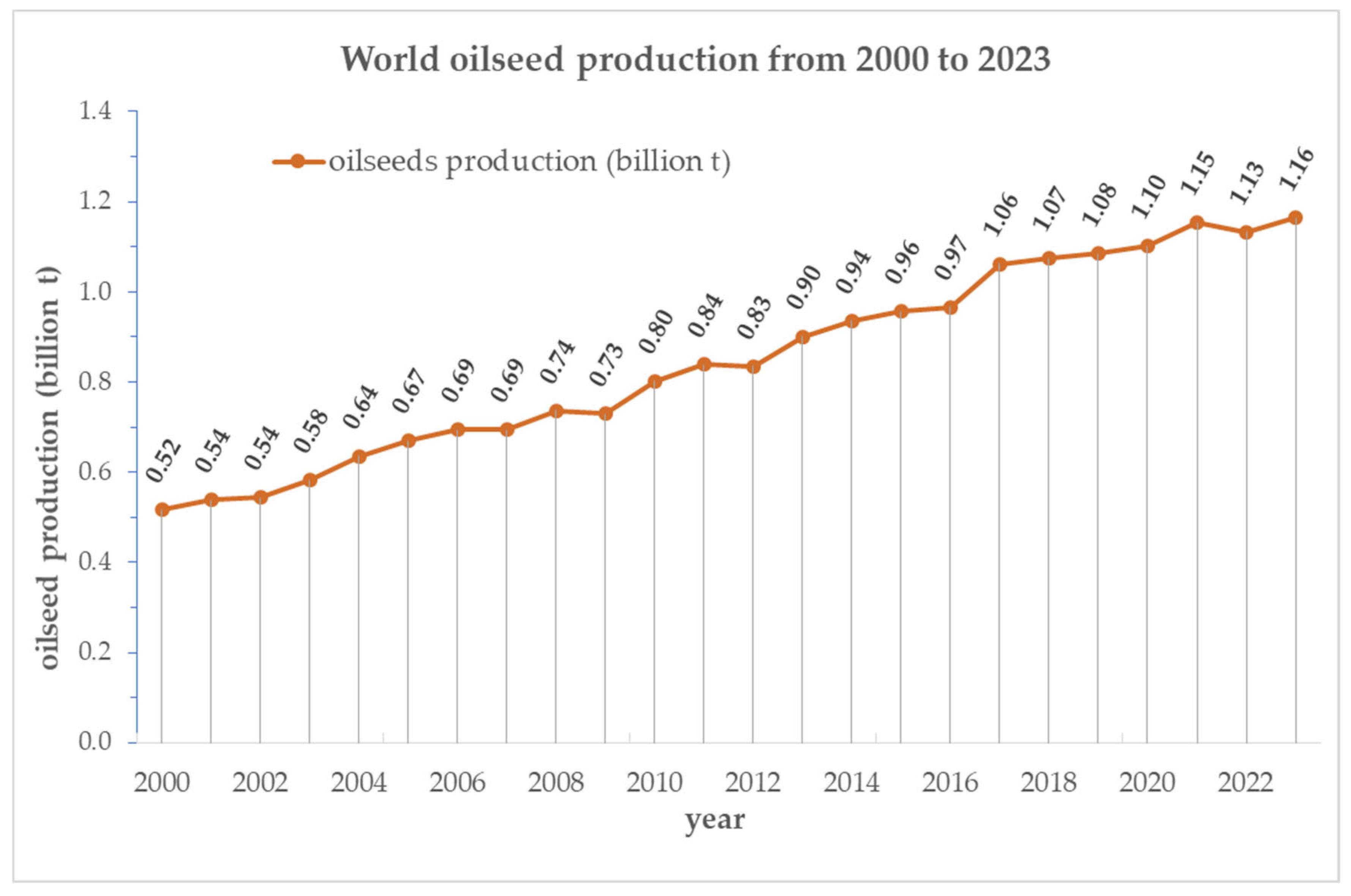
Global Production Trends and Market Importance
3. Current and Emerging Applications of Oilseed Cakes
4. Extraction Techniques for Value-Added Compounds from Oilseed Cakes
4.1. Protein Recovery from Oilseed Cakes: Challenges and Opportunities
4.1.1. Conventional Protein Extraction Methods
4.1.2. Emerging Technologies for Enhanced Protein Extraction
Ultrasonic-Assisted Extraction: Efficiency and Optimization
Enzymatic Hydrolysis for Improved Protein Yield
Microwave-Assisted Extraction: Mechanisms and Applications
Pulsed Electric Field (PEF) Extraction: A Non-Thermal Alternative
High-Pressure and Hydrostatic Extraction Techniques
4.2. Recovery of Bioactive Polyphenols from Oilseed By-Products
4.2.1. Conventional Methods for Polyphenol Extraction
4.2.2. Advanced Techniques for Polyphenol Isolation
Ultrasonic-Assisted Polyphenol Extraction
Supercritical Fluid and Pressurized Liquid Extraction of Phenolic Compounds
Pulsed Electric Field (PEF) for Polyphenol Recovery
4.3. Extraction and Processing of Dietary Fiber from Oilseed By-Products
4.3.1. Traditional Fiber Extraction Methods
Dry Processing Techniques
Wet Processing: Acidic and Alkaline Approaches
Gravimetric and Enzymatic Methods
- Non-enzymatic methods (acid-detergent and neutral-detergent extractions), which suffer from fiber loss or incompatibility with soluble fiber-rich materials;
- Enzymatic methods that use α-amylase, protease, and amyloglucosidase to break down starch and proteins before ethanol precipitation.
Chemical Extraction: Challenges and Limitations
4.3.2. Innovative Fiber Extraction Technologies
Microwave Extraction Assisted Fiber Extraction
Ultrasonic Extraction for Enhanced Fiber Recovery
Pulsed Electric Field (PEF) Extraction for Fiber Isolation
Subcritical Water Extraction (SWE): A Green Alternative
5. Functional and Biological Properties of Oilseed Components
5.1. Functional Characteristics of Extracted Proteins
5.2. Bioactive Potential of Oilseed Proteins
5.3. Health Benefits and Applications of Dietary Fibers
5.4. Health Benefits and Applications of Polyphenols
5.4.1. Bioactive Properties of Extracted Polyphenols
5.4.2. Biological Potential of Oilseed-Derived Polyphenols
5.4.3. Applications in Functional Foods and Nutraceuticals
6. Application and Valorization Strategies of Oilseed Cakes and Their Protein Isolates
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil Cakes and Their Biotechnological Applications—A Review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Lužaić, T.; Romanić, R.; Grahovac, N.; Jocić, S.; Cvejić, S.; Hladni, N.; Pezo, L. Prediction of Mechanical Extraction Oil Yield of New Sunflower Hybrids: Artificial Neural Network Model. J. Sci. Food. Agric. 2021, 101, 5827–5833. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.S.; Bekhit, A.E.D.A. Utilization of Oilseed Cakes for Human Nutrition and Health Benefits. In Agricultural Biomass Based Potential Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 191–229. [Google Scholar] [CrossRef]
- Sunil, L.; Prakruthi, A.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Development of Health Foods from Oilseed Cakes. J. Food Process Technol. 2016, 7, 631–640. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, R.; Savita, S.; Singh, B. Oilseed as Potential Functional Food Ingredient. In Trends and Prospects in Food Technology, Processing and Preservation; Today and Tomorrow’s Printers and Publishers: New Delhi, India, 2019; pp. 191–215. [Google Scholar]
- Ancuţa, P.; Sonia, A. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Food and Agriculture Data. Available online: https://www.fao.org/faostat/ (accessed on 19 May 2025).
- Vichare, S.A.; Morya, S. Exploring Waste Utilization Potential: Nutritional, Functional and Medicinal Properties of Oilseed Cakes. Front. Food Sci. Technol. 2024, 4, 1441029. [Google Scholar] [CrossRef]
- Tileuberdi, N.; Turgumbayeva, A.; Yeskaliyeva, B.; Sarsenova, L.; Issayeva, R. Extraction, Isolation of Bioactive Compounds and Therapeutic Potential of Rapeseed (Brassica napus L.). Molecules 2022, 27, 8824. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Cozea, A.; Ionescu (Bordei), N.; Popescu, M.; Neagu, M.; Gruia, R. Comparative Study Concerning the Composition of Certain Oil Cakes with Phytotherapeutical Potential. Rev. Chim 2016, 67, 422–425. [Google Scholar]
- Grahovac, N.; Lužaić, T.; Živančev, D.; Stojanović, Z.; Đurović, A.; Romanić, R.; Kravić, S.; Miklič, V. Assessing Nutritional Characteristics and Bioactive Compound Distribution in Seeds, Oil, and Cake from Confectionary Sunflowers Cultivated in Serbia. Foods 2024, 13, 1882. [Google Scholar] [CrossRef]
- Vidal, N.P.; Roman, L.; Swaraj, V.J.S.; Ragavan, K.V.; Simsek, S.; Rahimi, J.; Kroetsch, B.; Martinez, M.M. Enhancing the Nutritional Value of Cold-Pressed Oilseed Cakes through Extrusion Cooking. Innov. Food Sci. Emerg. Technol. 2022, 77, 102956. [Google Scholar] [CrossRef]
- McKevith, B. Nutritional Aspects of Oilseeds. Nutr. Bull. 2005, 30, 13–26. [Google Scholar] [CrossRef]
- Jithender, B.; Upendar, K.; Nickhil, C.; Rathod, P. Nutritional and Anti-Nutritional Factors Present in Oil Seeds: An Overview. Int. J. Chem. Stud. 2019, 7, 1159–1165. [Google Scholar]
- Nagaraj, G. Oilseeds: Properties, Processing, Products and Procedures; New India Publishing Agency: New Delhi, India, 2021; ISBN 9788190723756. [Google Scholar]
- Strahinja, V. The Examination of the Potential of Various Dry Fractionation Process for Obtaining High Protein Sunflower Meal Fractions. Ph.D. Thesis, Faculty of Technology, University of Novi Sad, Novi Sad, Serbia, 25 September 2024. [Google Scholar]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower Meal/Cake as a Sustainable Protein Source for Global Food Demand: Towards a Zero-Hunger World. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Nehmeh, M.; Rodriguez-Donis, I.; Cavaco-Soares, A.; Evon, P.; Gerbaud, V.; Thiebaud-Roux, S. Bio-Refinery of Oilseeds: Oil Extraction, Secondary Metabolites Separation towards Protein Meal Valorisation—A Review. Processes 2022, 10, 841. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, Detoxification, Utilization, and Opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Imran, S.; Munir, S.; Altemimi, A.B.; Fatima, I.; Rabail, R.; Batool, I.; Khalid, N.; Abdi, G.; Shabbir, M.A.; Aadil, R.M. Therapeutic Implications of Flaxseed Peptides and Bioactive Components against Various Diseases. J. Funct. Foods 2024, 119, 106324. [Google Scholar] [CrossRef]
- Leming, R.; Lember, A. Chemical Composition of Expeller-Extracted and Cold-Pressed Rapeseed Cake. Agric. Food Sci. Chem. 2005, 16, 96–109. [Google Scholar]
- Rakita, S.; Kokić, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L. Cold-Pressed Oilseed Cakes as Alternative and Sustainable Feed Ingredients: A Review. Foods 2023, 12, 432. [Google Scholar] [CrossRef]
- de Morais Oliveira, V.R.; de Arruda, A.M.V.; Silva, L.N.S.; de Souza, J.B.F.; de Queiroz, J.P.A.F.; da Silva Melo, A.; Holanda, J.S. Sunflower Meal as a Nutritional and Economically Viable Substitute for Soybean Meal in Diets for Free-Range Laying Hens. Anim. Feed. Sci. Technol. 2016, 220, 103–108. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Sánchez-Vega, R.; Pérez-Leal, R.; Soto-Caballero, M.C.; Salas-Salazar, N.A.; Flores-Córdova, M.A. By-Products from Oilseed Processing and Their Potential Applications. In Oil and Oilseed Processing: Opportunities and Challenges; Lafarga, T., Bobo, G., Aguilo-Aguayo, I., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 183–201. [Google Scholar]
- González-Pérez, S.; Merck, K.B.; Vereijken, J.M.; Van Koningsveld, G.A.; Gruppen, H.; Voragen, A.G.J. Isolation and Characterization of Undenatured Chlorogenic Acid Free Sunflower (Helianthus annuus) Proteins. J. Agric. Food Chem. 2002, 50, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Usman, I.; Saif, H.; Imran, A.; Afzaal, M.; Saeed, F.; Azam, I.; Afzal, A.; Ateeq, H.; Islam, F.; Shah, Y.A.; et al. Innovative Applications and Therapeutic Potential of Oilseeds and Their By-Products: An Eco-Friendly and Sustainable Approach. Food Sci. Nutr. 2023, 11, 2599–2609. [Google Scholar] [CrossRef]
- Rani, R.; Badwaik, L.S. Functional Properties of Oilseed Cakes and Defatted Meals of Mustard, Soybean and Flaxseed. Waste Biomass Valorization 2021, 12, 5639–5647. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Oseyko, M.; Romanovska, T.; Shevchyk, V. Justification of the Amino Acid Composition of Sunflower Proteins for Dietary and Functional Products. Ukr. Food J. 2020, 9, 394–403. [Google Scholar] [CrossRef]
- Mohanty, A.; Rout, P.R.; Dubey, B.; Meena, S.S.; Pal, P.; Goel, M. A Critical Review on Biogas Production from Edible and Non-Edible Oil Cakes. Biomass Convers. Biorefin. 2022, 12, 949–966. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Sangwan, S.; Rohtagi, B.; Khandelwal, A.; Shrivastava, M. Protein for Human Consumption From Oilseed Cakes: A Review. Front. Sustain. Food Syst. 2022, 6, 856401. [Google Scholar] [CrossRef]
- Mashanova, N.; Satayeva, Z.; Smagulova, M.; Kundyzbayeva, N.; Karimova, G. Nutritional and Structural Evaluation of Gluten-Free Flour Mixtures Incorporating Various Oilseed Cakes. Processes 2024, 12, 1616. [Google Scholar] [CrossRef]
- Nega, T. Review on Nutritional Limitations and Opportunities of Using Rapeseed Meal and Other Rape Seed by-products in Animal Feeding. J. Nutr. Health Food Eng. 2018, 8, 43–48. [Google Scholar] [CrossRef]
- Eklund, M.; Sauer, N.; Schöne, F.; Messerschmidt, U.; Rosenfelder, P.; Htoo, J.K.; Mosenthin, R. Effect of Processing of Rapeseed under Defined Conditions in a Pilot Plant on Chemical Composition and Standardized Ileal Amino Acid Digestibility in Rapeseed Meal for Pigs. J. Anim. Sci. 2015, 93, 2813–2825. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B.; Kaur, A.; Singh, N. Proximate, Mineral, Amino Acid Composition, Phenolic Profile, Antioxidant and Functional Properties of Oilseed Cakes. Int. J. Food Sci. Technol. 2021, 56, 6732–6741. [Google Scholar] [CrossRef]
- Girotto, F.; Merlino, M.; Giovanelli, G.; Condurso, C.; Piazza, L. Unveiling the Potential of Micronized Dehulled Sunflower Press-Cake: A Breakthrough in Sustainable Plant-Based Protein-Rich Sport Beverages. Int. J. Food Sci. Technol. 2024, 59, 4784–4796. [Google Scholar] [CrossRef]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef]
- Kolláthová, R.; Varga, B.; Ivanišová, E.; Bíro, D.; Rolinec, M.; Juráček, M.; Šimko, M.; Gálik, B. Mineral Profile Analysis of Oilseeds and Their By-Products as Feeding Sources for Animal Nutrition. Slovak. J. Anim. Sci. 2019, 52, 9–15. [Google Scholar]
- Lužaić, T.; Kravić, S.; Stojanović, Z.; Grahovac, N.; Jocić, S.; Cvejić, S.; Pezo, L.; Romanić, R. Investigation of Oxidative Characteristics, Fatty Acid Composition and Bioactive Compounds Content in Cold Pressed Oils of Sunflower Grown in Serbia and Argentina. Heliyon 2023, 9, e18201. [Google Scholar] [CrossRef]
- Deng, Z.; Kim, S.W. Opportunities and Challenges of Soy Proteins with Different Processing Applications. Antioxidants 2024, 13, 569. [Google Scholar] [CrossRef]
- Kao, T.H.; Chen, B.H. Functional Components in Soybean Cake and Their Effects on Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef]
- Manikandan, A.; Muthusamy, S.; Wang, E.S.; Ivarson, E.; Manickam, S.; Sivakami, R.; Narayanan, M.B.; Zhu, L.H.; Rajasekaran, R.; Kanagarajan, S. Breeding and Biotechnology Approaches to Enhance the Nutritional Quality of Rapeseed Byproducts for Sustainable Alternative Protein Sources—A Critical Review. Front. Plant Sci. 2024, 15, 1468675. [Google Scholar] [CrossRef]
- Đermanovć, B.; Vujetić, J.; Sedlar, T.; Dragojlović, D.; Popović, L.; Kojić, P.; Jovanov, P.; Šarić, B. Optimization of Protein Extraction from Rapeseed Oil Cake by Dephenolization Process for Scale-Up Application Using Artificial Neural Networks. Foods 2025, 14, 1762. [Google Scholar] [CrossRef]
- Mosenthin, R.; Messerschmidt, U.; Sauer, N.; Carré, P.; Quinsac, A.; Schöne, F. Effect of the Desolventizing/Toasting Process on Chemical Composition and Protein Quality of Rapeseed Meal. J. Anim. Sci. Biotechnol. 2016, 7, 36. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Antioxidant Capacity of Rapeseed Extracts Obtained by Conventional and Ultrasound-Assisted Extraction. J. Am. Oil Chem. Soc. 2014, 91, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Chen, D. Solid State Fermentation of Rapeseed Cake with Aspergillus niger for Degrading Glucosinolates and Upgrading Nutritional Value. J. Anim. Sci. Biotechnol. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and Flaxseed Cake as a Source of Compounds for Food Industry. J. Soil. Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef]
- Jarošová, M.; Roudnický, P.; Bárta, J.; Zdráhal, Z.; Bártová, V.; Stupková, A.; Lorenc, F.; Bjelková, M.; Kyselka, J.; Jarošová, E.; et al. Proteomic Profile of Flaxseed (Linum usitatissimum L.) Products as Influenced by Protein Concentration Method and Cultivar. Foods 2024, 13, 1288. [Google Scholar] [CrossRef] [PubMed]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef]
- Ogunronbi, O.; Jooste, P.J.; Abu, J.O.; Van Der Merwe, B. Chemical Composition, Storage Stability and Effect of Cold-Pressed Flaxseed Oil Cake Inclusion on Bread Quality. J. Food Process Preserv. 2011, 35, 64–79. [Google Scholar] [CrossRef]
- Talwar, B.; Chopra, R.; Taneja, N.K.; Chand, M.; Homroy, S.; Dhiman, A.; Singh, P.K.; Chaudhary, S. Use of Flaxseed Cake as a Source of Nutrients in the Food Industry and Possible Health Benefits—A Review. Food Prod. Process. Nutr. 2025, 7, 22. [Google Scholar] [CrossRef]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Berčíková, M.; Zdráhal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef]
- Raole, V.M.; Raole, V.V. Flaxseed and Seed Oil: Functional Food and Dietary Support for Health. EAS J. Nutr. Food Sci. 2022, 4, 68–77. [Google Scholar] [CrossRef]
- Ermosh, L.G.; Prisuhina, N.V.; Koch, D.A.; Eremina, E.V. The Use of Oilseed Cake for Supplementation of Bakery Products. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Krasnoyarsk, Russian Federation, 18–20 November 2020; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 677. [Google Scholar]
- Usman, I.; Imran, A.; Arshad, M.U.; Saeed, F.; Afzaal, M.; Sana, S.; Islam, F.; Siddiqua, A.; Naeem, U.; Islam, S. Valorization of Mustard and Sesame Oilseed Cakes for Food Application through Eco-Innovative Technologies. Food Sci. Nutr. 2023, 11, 1818–1825. [Google Scholar] [CrossRef]
- Sobczak, P.; Zawislak, K.; Starek, A.; Zukiewicz-Sobczak, W.; Sagan, A.; Zdybel, B.; Andrejko, D. Compaction Process as a Concept of Press-Cake Production from Organic Waste. Sustainability 2020, 12, 1567. [Google Scholar] [CrossRef]
- Panhwar, F. Anti-Nutritional Factors in Oil Seeds as Aflatoxin in Ground Nut Anti-Nutritional Factors in Oil Seeds as Aflatoxin in Ground Nut. ChemLin 2005, 1–7. [Google Scholar]
- Duodu, C.P.; Adjei-Boateng, D.; Edziyie, R.E.; Agbo, N.W.; Owusu-Boateng, G.; Larsen, B.K.; Skov, P.V. Processing Techniques of Selected Oilseed By-Products of Potential Use in Animal Feed: Effects on Proximate Nutrient Composition, Amino Acid Profile and Antinutrients. Anim. Nutr. 2018, 4, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Larroche, C.; Dussap, C.-G.; Gnansounou, E.; Ricke, S.C. Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Rodrigues, I.M.; Coelho, J.F.J.; Carvalho, M.G.V.S. Isolation and Valorisation of Vegetable Proteins from Oilseed Plants: Methods, Limitations and Potential. J. Food Eng. 2012, 109, 337–346. [Google Scholar] [CrossRef]
- Parodi, E.; La Nasa, J.; Ribechini, E.; Petri, A.; Piccolo, O. Extraction of Proteins and Residual Oil from Flax (Linum usitatissimum), Camelina (Camelina sativa), and Sunflower (Helianthus annuus) Oilseed Press Cakes. Biomass Convers. Biorefin 2023, 13, 1915–1926. [Google Scholar] [CrossRef]
- Nandasiri, R.; Michael Eskin, N.A.; Eck, P.; Thiyam-Höllander, U. Application of Green Technology on Extraction of Phenolic Compounds in Oilseeds (Canola). In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 81–96. ISBN 9780128181881. [Google Scholar]
- Piccolo, O.; Parodi, E.; Petri, A. Biocatalyzed Hydrolysis of Residual Oils and Proteins from Flax and Camelina Oilseed Press Cakes Using Lipase and Protease. Catal. Res. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Chandran, A.S.; Suri, S.; Choudhary, P. Sustainable Plant Protein: An up-to-Date Overview of Sources, Extraction Techniques and Utilization. Sustain. Food Technol. 2023, 1, 466–483. [Google Scholar] [CrossRef]
- Yang, J.; Kornet, R.; Ntone, E.; Meijers, M.G.J.; van den Hoek, I.A.F.; Sagis, L.M.C.; Venema, P.; Meinders, M.B.J.; Berton-Carabin, C.C.; Nikiforidis, C.V.; et al. Plant Protein Aggregates Induced by Extraction and Fractionation Processes: Impact on Techno-Functional Properties. Food Hydrocoll. 2024, 155, 110223. [Google Scholar] [CrossRef]
- Hadnadjev, M.; Dapcevic-Hadnadjev, T.; Pojic, M.; Saric, B.; Misan, A.; Jovanov, P.; Sakac, M. Progress in Vegetable Proteins Isolation Techniques: A Review. Food Feed. Res. 2017, 44, 11–21. [Google Scholar] [CrossRef]
- Salaria, A.; Kaur, N. A Detailed Review on Valorizing Oilseed Cakes: Extraction of Bioactive Compounds from Agro-Industrial Waste. Bioscene 2024, 21, 254–278. [Google Scholar]
- Helstad, A.; Forsén, E.; Ahlström, C.; Mayer Labba, I.C.; Sandberg, A.S.; Rayner, M.; Purhagen, J.K. Protein Extraction from Cold-Pressed Hempseed Press Cake: From Laboratory to Pilot Scale. J. Food Sci. 2022, 87, 312–325. [Google Scholar] [CrossRef]
- Li, Z.; Huang, D.; Tang, Z.; Deng, C.; Zhang, X. Fast Determination of Chlorogenic Acid in Tobacco Residues Using Microwave-Assisted Extraction and Capillary Zone Electrophoresis Technique. Talanta 2010, 82, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Elsorady, M.E. Characterization and Functional Properties of Proteins Isolated from Flaxseed Cake and Sesame Cake. Croat. J. Food Sci. Technol. 2020, 12, 77–83. [Google Scholar] [CrossRef]
- Salgado, P.R.; Molina Ortiz, S.E.; Petruccelli, S.; Mauri, A.N. Sunflower Protein Concentrates and Isolates Prepared from Oil Cakes Have High Water Solubility and Antioxidant Capacity. J. Am. Oil Chem. Soc. 2011, 88, 351–360. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of Ultrasound Assisted Extraction of Protein from Sunflower Meal and Its Physicochemical and Functional Properties. J. Food Process Eng. 2018, 41, e12799. [Google Scholar] [CrossRef]
- Zhou, J.; Li, D.; Zhang, X.; Liu, C.; Chen, Y. Valorization of Protein-Rich Waste and Its Application. Sci. Total Environ. 2023, 901, 166141. [Google Scholar] [CrossRef]
- Fatima, A.; Singh, P.; Pandey, V.K.; Singh, R.; Rustagi, S. Exploring the Significance of Protein Concentrate: A Review on Sources, Extraction Methods, and Applications. Food Chem. Adv. 2024, 5, 100771. [Google Scholar] [CrossRef]
- Niu, Y.X.; Li, W.; Zhu, J.; Huang, Q.; Jiang, M.; Huang, F. Aqueous Enzymatic Extraction of Rapeseed Oil and Protein from Dehulled Cold-Pressed Double-Low Rapeseed Cake. Int. J. Food Eng. 2012, 8, 1–14. [Google Scholar] [CrossRef]
- Kvist, U.S.; Carlsson, I.T.; Lawther, M.J.; De Castro, B.F. Process for the Fractionation of Oilseed Press Cakes and Meals. U.S. Patent 10/815,045, 23 June 2005. [Google Scholar]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-Innovative Technologies for Extraction of Proteins for Human Consumption from Renewable Protein Sources of Plant Origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Hadidi, M.; Tan, C.; Assadpour, E.; Jafari, S.M. Oilseed Meal Proteins: From Novel Extraction Methods to Nanocarriers of Bioactive Compounds. Food Chem. 2024, 438, 137971. [Google Scholar] [CrossRef]
- Choi, I.; Jun Choi, S.; Keun Chun, J.; Wha Moon, T. Extraction Yield of Soluble Protein and Microstructure of Soybean Affected by Microwave Heating. J. Food Process Preserv. 2006, 30, 407–419. [Google Scholar] [CrossRef]
- Varghese, T.; Pare, A. Effect of Microwave Assisted Extraction on Yield and Protein Characteristics of Soymilk. J. Food Eng. 2019, 262, 92–99. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Optimization of Microwave-Assisted Extraction of Rice Bran Protein and Its Hydrolysates Properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic Promises of Chlorogenic Acid with Special Emphasis on Its Anti-Obesity Property. Curr. Mol. Pharmacol. 2019, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bals, O.; Grimi, N.; Vorobiev, E. A New Way for the Oil Plant Biomass Valorization: Polyphenols and Proteins Extraction from Rapeseed Stems and Leaves Assisted by Pulsed Electric Fields. Ind. Crops Prod. 2015, 74, 309–318. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Blouet, C.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Effect of Pulsed Electric Fields and High Voltage Electrical Discharges on Polyphenol and Protein Extraction from Sesame Cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.J.; Fryer, P.J.; Zuidam, N.J. Intensification of Protein Extraction from Soybean Processing Materials Using Hydrodynamic Cavitation. Innov. Food Sci. Emerg. Technol. 2017, 41, 47–55. [Google Scholar] [CrossRef]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis Improved Protein Digestion of Flaxseed Protein Isolate and Generation of Peptides with Antioxidant Activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef]
- Tang, S.; Hettiarachchy, N.S.; Shellhammer, T.H. Protein Extraction from Heat-Stabilized Defatted Rice Bran. 1. Physical Processing and Enzyme Treatments. J. Agric. Food Chem. 2002, 50, 7444–7448. [Google Scholar] [CrossRef]
- Jung, S.; Mahfuz, A.A. Low Temperature Dry Extrusion and High-Pressure Processing Prior to Enzyme-Assisted Aqueous Extraction of Full Fat Soybean Flakes. Food Chem. 2009, 114, 947–954. [Google Scholar] [CrossRef]
- Petraru, A.; Amariei, S. Recovery of Bioactive Compounds from Oilcakes-A Review. Food Environ. Saf. J. 2022, 21, 364–381. [Google Scholar] [CrossRef]
- Şahin, S.; Elhussein, E.A.A. Valorization of a Biomass: Phytochemicals in Oilseed by-Products. Phytochem. Rev. 2018, 17, 657–668. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Alli, I. Distribution, Antioxidant and Characterisation of Phenolic Compounds in Soybeans, Flaxseed and Olives. Food Chem. 2013, 139, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mei, H.; Xu, L.; Zhan, L.; Yang, Y.; Zhao, D.; Bao, G.; Li, X.; Cao, Z. Impact of Fermented Feed of Soybean Hulls and Rapeseed Cake on Immunity, Antioxidant Capacity and Gut Microbiota in Chahua Chicken. Poult. Sci. 2024, 103, 103451. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.; Rawat, H.; Verma, N.; Mishra, S.; Nautiyal, A.; Bhatt, S.; Bisht, N.; Aggarwal, K.; Bora, A.; Kumar, H.; et al. Analytical Approach to Assess Anti-Nutritional Factors of Grains and Oilseeds: A Comprehensive Review. J. Agric. Food Res. 2023, 14, 100877. [Google Scholar] [CrossRef]
- Teh, S.S.; El-Din Bekhit, A.; Birch, J. Antioxidative Polyphenols from Defatted Oilseed Cakes: Effect of Solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Ultrasound-Assisted Extraction of Antioxidant Bioactive Compounds from Wastes of Rapeseed Industry and Their Application in Delaying Rapeseed Oil Oxidation. Environ. Technol. Innov. 2023, 30, 103081. [Google Scholar] [CrossRef]
- Grahovac, N.; Aleksić, M.; Đurović, A.; Stojanović, Z.; Cvejić, S.; Jocić, S.; Lužaić, T.; Romanić, R. Optimizacija ekstrakcije hlorogene kiseline iz uzoraka suncokreta za određivanje visokopritsnom tečnom hromatografijom. Uljarstvo 2023, 54, 29–38. [Google Scholar]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Z. Concurrent Extraction and Transformation of Bioactive Phenolic Compounds from Rapeseed Meal Using Pressurized Solvent Extraction System. Ind. Crops Prod. 2016, 94, 152–159. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Rodrigues, F.; Moreira, M.M.; Delerue-Matos, C.; Morais, S.; Dorosh, O.; Silva, A.M.; Bassani, A.; Dzedik, V.; Spigno, G. Chemical Composition and Bioactivity of Oilseed Cake Extracts Obtained by Subcritical and Modified Subcritical Water. Bioresour. Bioprocess. 2022, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioproc Tech. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. Dietary Fiber Extraction for Human Nutrition—A Review. Food Rev. Int. 2016, 32, 98–115. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Study on Functional Properties of Physically Modified Dietary Fibres Derived from Defatted Rice Bran. J. Agric. Sci. 2012, 4, 85–97. [Google Scholar] [CrossRef]
- Buljeta, I.; Šubarić, D.; Babić, J.; Pichler, A.; Šimunović, J.; Kopjar, M. Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review. Appl. Sci. 2023, 13, 9309. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Liu, J.; Zheng, T.; Li, Y.; He, S.; Jiang, M.; Wu, L.; Wang, S. Extraction Methods of Dietary Fiber and Effect on Bioactivity: A Review. Process Biochem. 2024, 146, 451–461. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Cai, W.D.; Xiao, G.S.; Duan, Y.; Zhang, H. Subcritical Water Extraction-Based Methods Affect the Physicochemical and Functional Properties of Soluble Dietary Fibers from Wheat Bran. Food Chem. 2019, 298, 124987. [Google Scholar] [CrossRef]
- Dungani, R.; Karina, M.; Subyakto; Sulaeman, A.; Hermawan, D.; Hadiyane, A. Agricultural Waste Fibers towards Sustainability and Advanced Utilization: A Review. Asian J. Plant Sci. 2016, 15, 42–55. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.H.; Wen, Q.H.; He, F.; Xu, F.Y.; Chen, B.R.; Zeng, X.A. Combination of Pulsed Electric Field and PH Shifting Improves the Solubility, Emulsifying, Foaming of Commercial Soy Protein Isolate. Food Hydrocoll. 2023, 134, 108049. [Google Scholar] [CrossRef]
- Kaur, R.P.; Ghoshal, G. Sunflower Protein Isolates-Composition, Extraction and Functional Properties. Adv. Colloid. Interface Sci. 2022, 306, 102725. [Google Scholar] [CrossRef]
- Cheung, L.; Wanasundara, J.; Nickerson, M.T. Effect of PH and NaCl on the Emulsifying Properties of a Napin Protein Isolate. Food Biophys. 2015, 10, 30–38. [Google Scholar] [CrossRef]
- Xu, W.; Nikolov, A.; Wasan, D.T.; Gonsalves, A.; Borwankar, R.P. Foam Film Rheology and Thickness Stability of Foam-Based Food Products. Colloids Surf. A. Physicochem. Eng. Aspects 2003, 214, 13–21. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive Overview of the Quality of Plant- And Animal-Sourced Proteins Based on the Digestible Indispensable Amino Acid Score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- Tessier, R.; Khodorova, N.; Calvez, J.; Kapel, R.; Quinsac, A.; Piedcoq, J.; Tomé, D.; Gaudichon, C. 15N and 2h Intrinsic Labeling Demonstrate That Real Digestibility in Rats of Proteins and Amino Acids from Sunflower Protein Isolate Is Almost as High as That of Goat Whey. J. Nutr. 2020, 150, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, O.; Cai, S.; Zhao, L.; Zhao, L. Composition, Functional Properties, Health Benefits and Applications of Oilseed Proteins: A Systematic Review. Food Res. Int. 2023, 171, 113061. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, Y.B.; Chi, M.H. Soy Protein Supplementation Reduces Clinical Indices in Type 2 Diabetes and Metabolic Syndrome. Yonsei Med. J. 2016, 57, 681–689. [Google Scholar] [CrossRef]
- Doyen, A.; Udenigwe, C.C.; Mitchell, P.L.; Marette, A.; Aluko, R.E.; Bazinet, L. Anti-Diabetic and Antihypertensive Activities of Two Flaxseed Protein Hydrolysate Fractions Revealed Following Their Simultaneous Separation by Electrodialysis with Ultrafiltration Membranes. Food Chem. 2014, 145, 66–76. [Google Scholar] [CrossRef]
- Han, R.; Hernández Álvarez, A.J.; Maycock, J.; Murray, B.S.; Boesch, C. Comparison of Alcalase- And Pepsin-Treated Oilseed Protein Hydrolysates—Experimental Validation of Predicted Antioxidant, Antihypertensive and Antidiabetic Properties. Curr. Res. Food Sci. 2021, 4, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources-A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S.; Dimitrić Marković, J.M.; Mojović, M.; Milenković, D. Antioxidative Mechanisms in Chlorogenic Acid. Food Chem. 2017, 237, 390–398. [Google Scholar] [CrossRef]
- Morya, S.; Menaa, F.; Jiménez-lópez, C.; Lourenço-lopes, C.; Binmowyna, M.N.; Alqahtani, A. Nutraceutical and Pharmaceutical Behavior of Bioactive Compounds of Miracle Oilseeds: An Overview. Foods 2022, 11, 1824. [Google Scholar] [CrossRef]
- Pag, A.I.; Radu, D.G.; Drăgănescu, D.; Popa, M.I.; Sîrghie, C. Flaxseed Cake—A Sustainable Source Od Antioxidant and Antibacterial Extracts. Cellul. Chem. Technol. 2014, 48, 265–273. [Google Scholar]
- Majed, M.; Galala, A.A.; Amer, M.M.; Selmar, D.; Abouzeid, S. Oilseed Cakes: A Promising Source of Antioxidant, and Anti-Inflammatory Agents—Insights from Lactuca sativa. Int. J. Mol. Sci. 2024, 25, 11077. [Google Scholar] [CrossRef]
- Mazanko, M.; Prazdnova, E.; Statsenko, V.; Bren, A.; Rudoy, D.; Maltseva, T.; Chistyakov, V.; Chikindas, M. Oil Cakes of Essential Oil Plants as a Source of Prebiotics for Poultry Production. Agriculture 2023, 13, 591. [Google Scholar] [CrossRef]
- Nevara, G.A.; Giwa Ibrahim, S.; Syed Muhammad, S.K.; Zawawi, N.; Mustapha, N.A.; Karim, R. Oilseed Meals into Foods: An Approach for the Valorization of Oilseed by-Products. Crit. Rev. Food Sci. Nutr. 2023, 63, 6330–6343. [Google Scholar] [CrossRef]
- Hoek, A.C.; Luning, P.A.; Weijzen, P.; Engels, W.; Kok, F.J.; de Graaf, C. Replacement of Meat by Meat Substitutes. A Survey on Person- And Product-Related Factors in Consumer Acceptance. Appetite 2011, 56, 662–673. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Wada, Y.; Schott, M.; Wäsche, A. Functional and Bioactive Properties of Rapeseed Protein Concentrates and Sensory Analysis of Food Application with Rapeseed Protein Concentrates. LWT 2006, 39, 503–512. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, M.; Sun, Y.; Fang, L.; Sun, Y.; Qi, B.; Li, Y. Effects of PH on Ultrasonic-Modified Soybean Lipophilic Protein Nanoemulsions with Encapsulated Vitamin E. LWT 2021, 144, 111240. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef]
- Chmielewska, A.; Kozłowska, M.; Rachwał, D.; Wnukowski, P.; Amarowicz, R.; Nebesny, E.; Rosicka-Kaczmarek, J. Canola/Rapeseed Protein–Nutritional Value, Functionality and Food Application: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3836–3856. [Google Scholar] [CrossRef] [PubMed]
- Salah, K.; Olkhovatov, E.A.; Aïder, M. Effect of Canola Proteins on Rice Flour Bread and Mathematical Modelling of the Baking Process. J. Food Sci. Technol. 2019, 56, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Wanasundara, J.P.D.; McIntosh, T.C.; Perera, S.P.; Withana-Gamage, T.S.; Mitra, P. Canola/Rapeseed Protein-Functionality and Nutrition. OCL 2016, 23, D407. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Banerjee, R.; Biswas, S.; McClements, D.J. Application of Nanoemulsion-Based Approaches for Improving the Quality and Safety of Muscle Foods: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2677–2700. [Google Scholar] [CrossRef] [PubMed]
- Gaber Ahmed, G.H.; Fernández-González, A.; Díaz García, M.E. Nano-Encapsulation of Grape and Apple Pomace Phenolic Extract in Chitosan and Soy Protein via Nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Mohamad Ramlan, B.M.S.; Maruyama, N.; Adachi, M.; Hontani, N.; Saka, S.; Kato, N.; Ohkawa, Y.; Utsumi, S. Comparison of Protein Chemical and Physicochemical Properties of Rapeseed Cruciferin with Those of Soybean Glycinin. J. Agric. Food Chem. 2002, 50, 7380–7385. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, G.; Liu, C.; Fu, J.; Hu, D.; Rong, J.; Yang, X. Recent Progress in the Preparation, Chemical Interactions and Applications of Biocompatible Polysaccharide-Protein Nanogel Carriers. Food Res. Int. 2021, 147, 110564. [Google Scholar] [CrossRef]
| Oilseed and By-Product | Content (%) | References | ||
|---|---|---|---|---|
| Oil | Protein | Fiber | ||
| sunflower | 28.0–60.0 | 10.0–25.5 | 16.0–55.0 | [15,16,17,18,19] |
| soybean | 7.3–24.7 | 14.0–47.0 | 5.6–31.9 | [15,17,20] |
| rapeseed | 31.2–47.5 | 18.6–20.1 | 12.8 | [17] |
| flaxseed | 30.0–42.2 | 18.3–30.0 | 20.0–35.0 | [21,22] |
| rapeseed cake | 1.1–31.3 | 14.0–45.0 | 5.5–19.5 | [6,17,23,24] |
| soybean cake | 0.5–8.9 | 25.7–52.4 | 3.0–6.0 | [6,12,17,20,25,26] |
| sunflower cake | 1.0–23.6 | 29.0–43.4 | 13.1–36.0 | [18,19,27,28] |
| flaxseed cake | 7.0–21.4 | 36.0–56.0 | 66.0 | [24,29] |
| Amino Acid (%) | Type of Oilseed Cake | |||
|---|---|---|---|---|
| Sunflower | Soybean | Rapeseed | Flaxseed | |
| Isoleucine (Ile) | 1.20–4.20 | 2.10–5.20 | 1.19–3.80 | 2.14–2.17 * |
| Leucine (Leu) | 2.00–6.90 | 3.35–7.80 | 2.09–6.30 | |
| Lysine (Lys) | 0.86–3.5 | 2.02–2.66 | 1.62–5.40 | 0.25–1.40 |
| Methionine (Met) | 0.51–2.20 | 0.25–1.40 | 0.59–1.70 | 0.38–0.51 |
| Cysteine (Cys) | 0.78–1.80 | 0–0.57 | 0.72 | 0.36 |
| Phenylalanine (Phe) | 0.70–5.10 | 1.73–5.50 | 1.17–3.80 | 0.94–1.79 |
| Tyrosine (Tyr) | 0.61–1.40 | 1.15–1.42 | n.d ** | 0.57–0.83 |
| Threonine (Thr) | 1.20–3.40 | 1.01–3.80 | 1.34–3.80 | 0.65–1.46 |
| Tryptophan (Trp) | 0.80–1.40 | 0.57–1.15 | 0.80–1.30 | 0.46 |
| Valine (Val) | 1.50–5.80 | 1.44–5.20 | 1.56–5.20 | 0.96–2.41 |
| Arginine (Arg) | 1.83–9.10 | 1.41–1.79 | 1.77–6.40 | 2.16–4.76 |
| Histidine (His) | 0.70–2.80 | 0.78–2.40 | 0.78–2.60 | 0.48–0.53 |
| Alanine (Ala) | 0.60–2.04 | 1.21–2.15 | 1.32–1.50 | 0.77 |
| Aspartic acid(Asp) | 1.83–9.10 | 4.71 | 2.18 | 2.23 |
| Glutamic acid (Glu) | 0.13–4.22 | 7.85 | 4.70 | 2.96 |
| Glycine (Gly) | 1.48–5.60 | 1.15–4.50 | 1.56–4.90 | 1.46–2.23 |
| Proline (Pro) | 1.45–2.21 | 2.13–2.43 | 1.75 | 0.66–1.82 |
| Serine (Ser) | 1.11–1.92 | 1.27–2.60 | 1.25–1.5 | 0.84–1.82 |
| References | [30,31,32] | [32,33,34] | [32,35,36] | [34,37] |
| Type of Oilseed Cake | Protein Fractions | Minerals | Vitamins | Bioactive Compounds | Antinutritional Factors |
|---|---|---|---|---|---|
| Sunflower | 40.0–90.0% globulins (helianthinin), 10–30% albumin (2S albumin) [19,38] | 5.0–6.0% minerals: K(1.5%), P (1.2%), Ca (0.7%), Mg (0.4%), Na (0.03%) Tl, Cu, Zn, Cr, Mn [39,40] | nicotinic acid, thiamine, pantothenic acid, riboflavin [16,17,24,25,39] | 1.0–4.0% phenolic compounds (chlorogenic (70.0%), caffeic, and quinic acids) [19,27,41] | chlorogenic acid [27] |
| Soybean | 90.0% globulins (36 to 53% glycine, 30 to 46% β-conglycinin) 10.0% albumins) [17,42] | 5.9–6.0% minerals: K (2.1%), P (0.7%), Ca (0.3%), Mg (0.2%), Na (0.02%), Fe, Zn, Mn, Cu [12,15,40] | thiamine, riboflavin, niacin, folic acid, [15,26] | flavonoids, isoflavones (genistein, daidzein), lignans, and saponins [20,26,43] | trypsin inhibitors, lectins, goitrogens [42] |
| Rapeseed | 55.0–85.0% globulin (cruciferins) 15.0–45.0% albumin (napins) [44,45] | 6.5–6.8% minerals: K(0.8%), P (0.6%) (65% p as phytate), Ca(0.4%), Mg (0.259, Na (0.02%), Se, Fe, Mn [40,46] | choline (0.7%), niacin (0.02%), biotin, folic acid, niacin, [17,25] | polyphenols (sinapic acid), [45,47] | glucosinolates (progoitrin, gluconapin, glucobrassicanapin) [17,46,48] |
| Flaxseed | 45.0–80.0% globulins (linins), 6–17,7% albumins (colinis) [17,49,50,51] | 3.9–5.4% minerals: Na (1.0%), P (0.9%), Ca (0.3%), Mg (0.003%), K, Zn, Mn, Cu [37,40,48,52] | thiamine, riboflavin, pyridoxine, niacin folic acid [49,50,51,53] | phenolic acid (ellagic, ferulic, guercetic acids), flavonoids, and lignans (SDG) (0.2–2.4%) [54,55] | cyanogenic glycosides (linustatin, neolinustatin, linumarin), phytic acid (~1.5%), and tannins [53] |
| Oilseed | Bioactive Compounds | References |
|---|---|---|
| sunflower | p-coumaric acid, chlorogenic acid, caffeic acid, syringic, vanillic, gallic acid, vanillic acids, catechin, epicatechin | [93,94] |
| soybean | p-coumaric acid, ferulic acid, hesperidin, rutin, sinapic acid, syringic acid, gallic acid, hydroxybenzoic acid, vanillic acid | [91,95,96] |
| rapeseed (canola) | gallic acid, p-coumaric, caffeic acid, ferulic acid, epicatehin, sinapic, esters, and glycosides of phenolic acid | [3,91,95] |
| flaxseed | tannic acid, p-coumaric, ferulic acid, p-hydroxybenzoic acid, lignans, sinapic, lignin | [91,95,96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grahovac, N.; Aleksić, M.; Trajkovska, B.; Marjanović Jeromela, A.; Nakov, G. Extraction and Valorization of Oilseed Cakes for Value-Added Food Components—A Review for a Sustainable Foodstuff Production in a Case Process Approach. Foods 2025, 14, 2244. https://doi.org/10.3390/foods14132244
Grahovac N, Aleksić M, Trajkovska B, Marjanović Jeromela A, Nakov G. Extraction and Valorization of Oilseed Cakes for Value-Added Food Components—A Review for a Sustainable Foodstuff Production in a Case Process Approach. Foods. 2025; 14(13):2244. https://doi.org/10.3390/foods14132244
Chicago/Turabian StyleGrahovac, Nada, Milica Aleksić, Biljana Trajkovska, Ana Marjanović Jeromela, and Gjore Nakov. 2025. "Extraction and Valorization of Oilseed Cakes for Value-Added Food Components—A Review for a Sustainable Foodstuff Production in a Case Process Approach" Foods 14, no. 13: 2244. https://doi.org/10.3390/foods14132244
APA StyleGrahovac, N., Aleksić, M., Trajkovska, B., Marjanović Jeromela, A., & Nakov, G. (2025). Extraction and Valorization of Oilseed Cakes for Value-Added Food Components—A Review for a Sustainable Foodstuff Production in a Case Process Approach. Foods, 14(13), 2244. https://doi.org/10.3390/foods14132244








