Ultrasound-Assisted Extraction and Microencapsulation of Durvillaea incurvata Polyphenols: Toward a Stable Anti-Inflammatory Ingredient for Functional Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasound-Assisted Extract (UAE) Obtention
2.3. Cytotoxicity Assessment of UAE
2.4. Hyaluronidase Inhibitory Activity of UAE
2.5. Microencapsulation of UAE
2.6. Microparticle Analysis
2.6.1. Encapsulation Efficiency and Recovery
Total Polyphenols
Surface Polyphenols
2.6.2. Moisture Content
2.6.3. Hygroscopicity
2.6.4. Microparticle Size and Shape
2.6.5. Differential Scanning Calorimetry
2.6.6. X-Ray Diffraction
2.7. Statistical Analysis
3. Results and Discussion
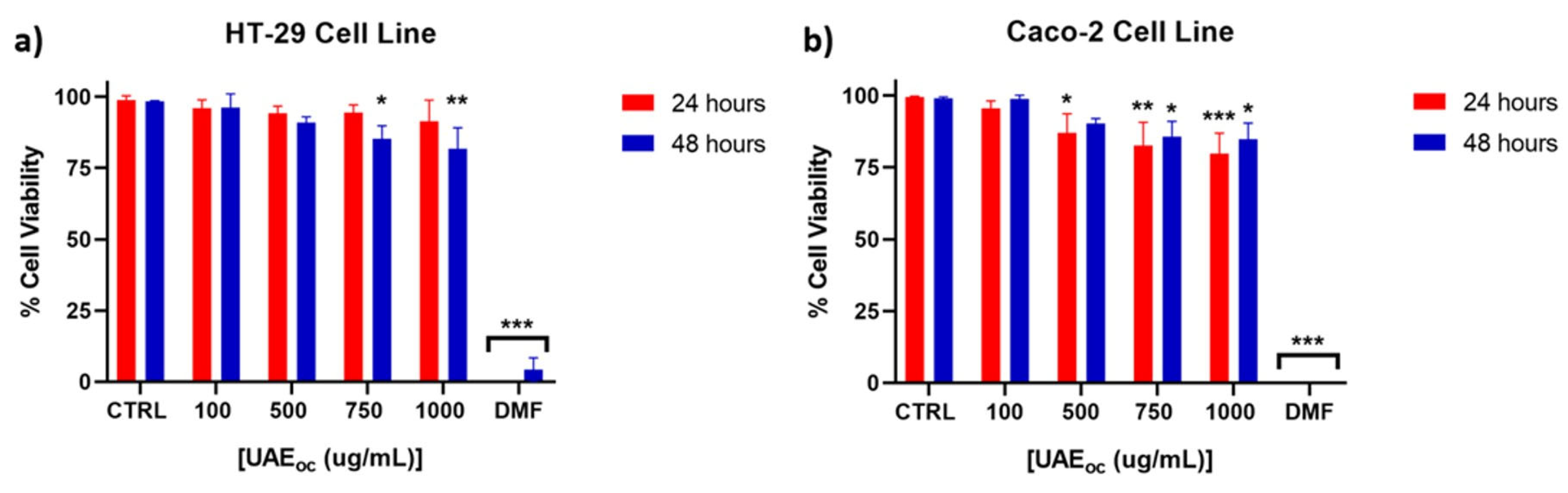
3.1. Cytotoxicity of UAE
3.2. Inhibition of Hyaluronidase by UAE
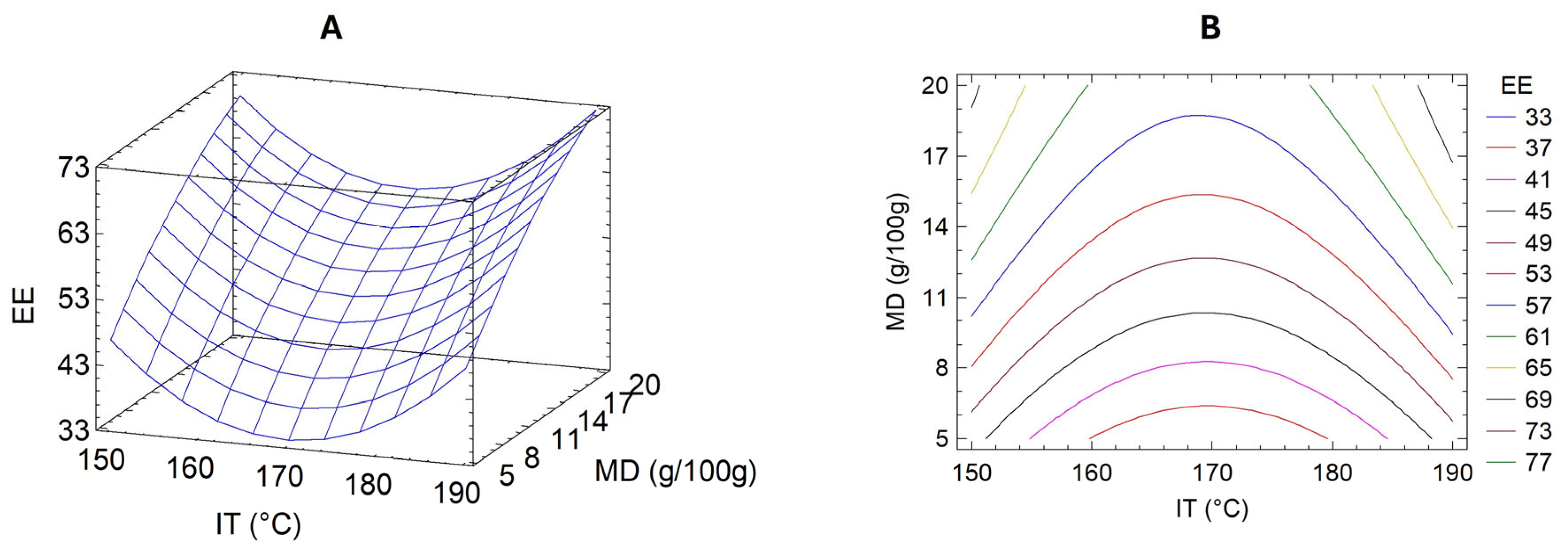
3.3. Optimization of UAE Microencapsulation Conditions
3.4. Microparticles Analysis
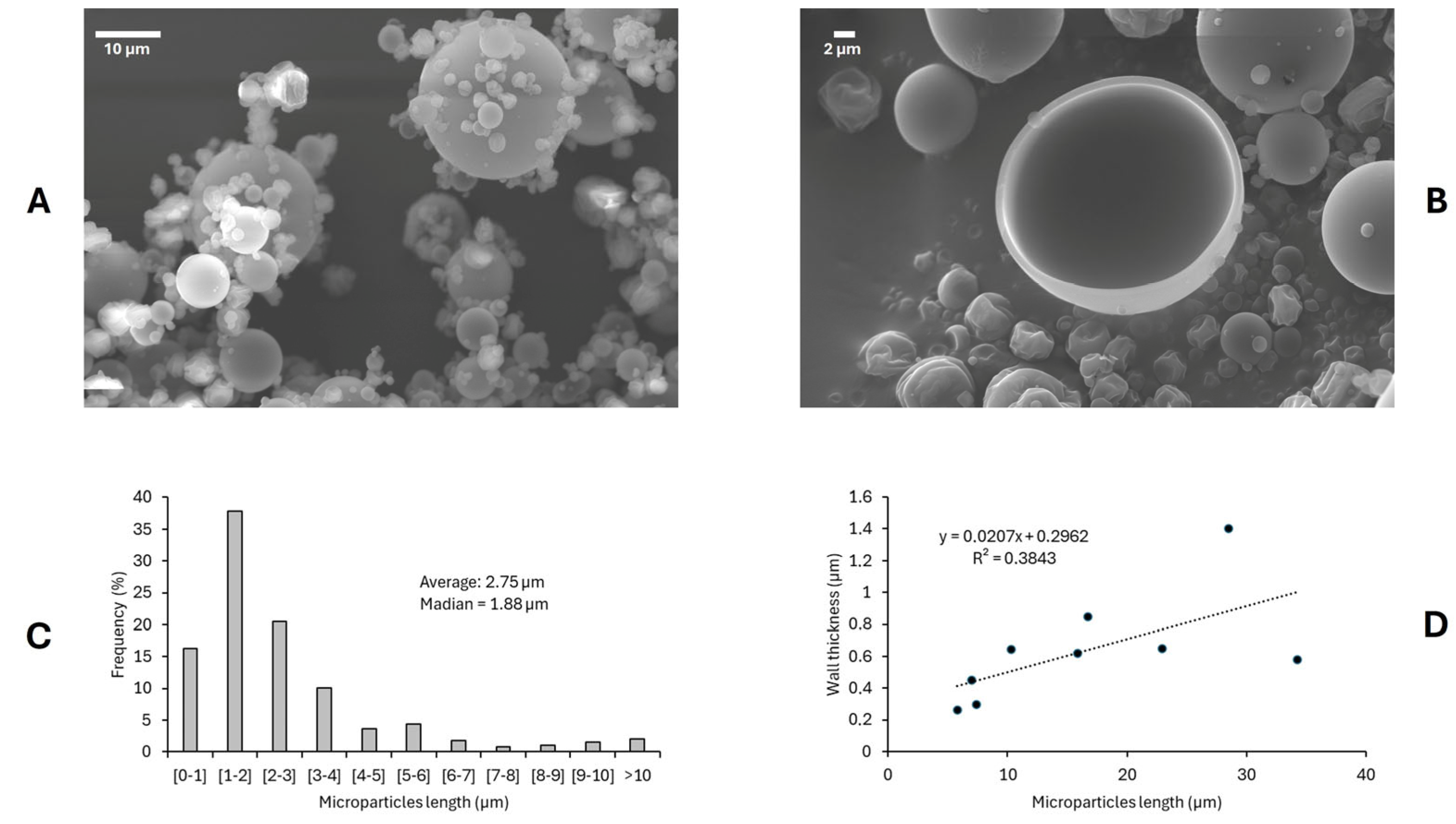
3.4.1. Microparticle Morphology by Scanning Electron Microscope
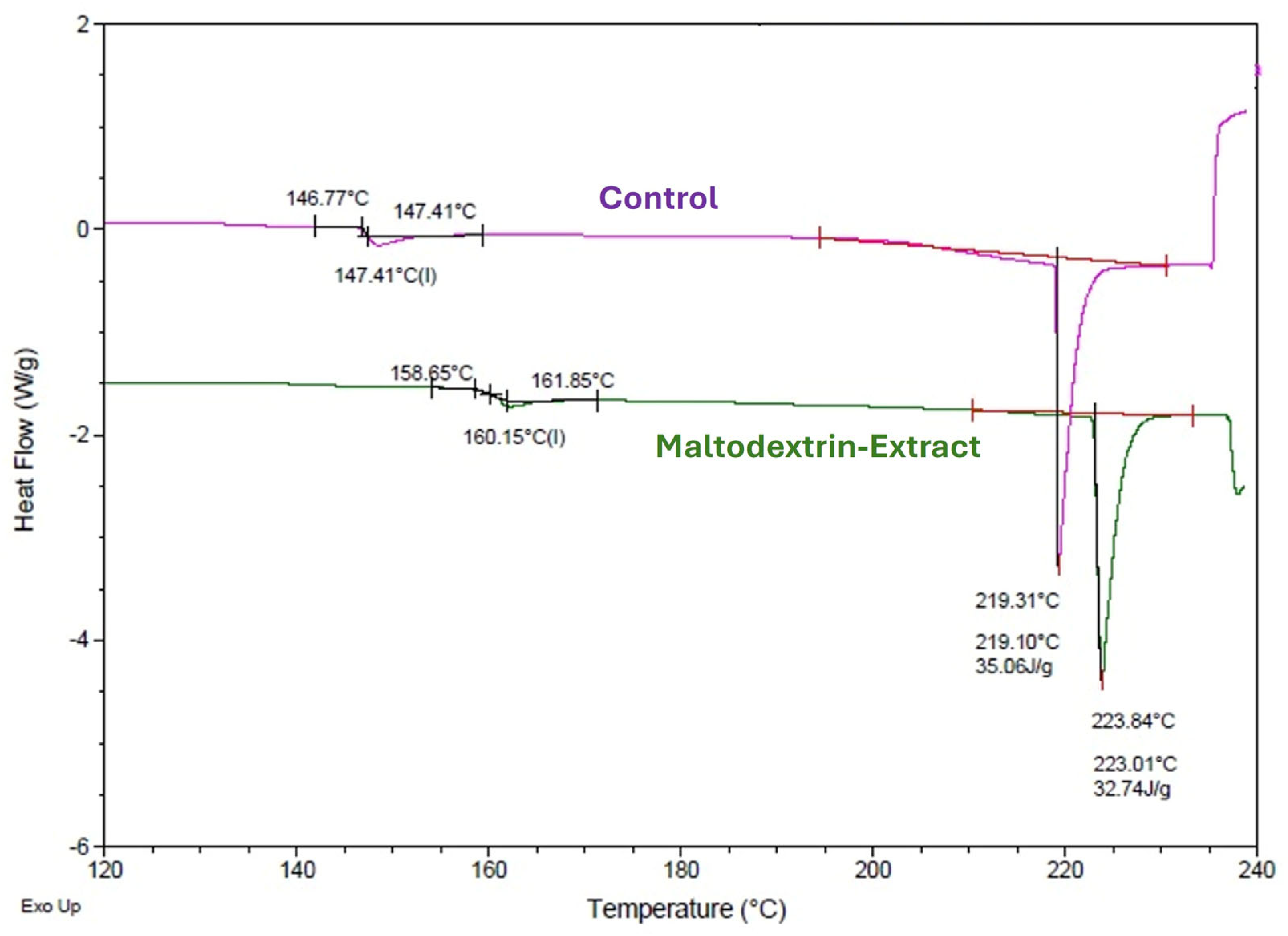
3.4.2. Differential Scanning Calorimetry of the Microparticles
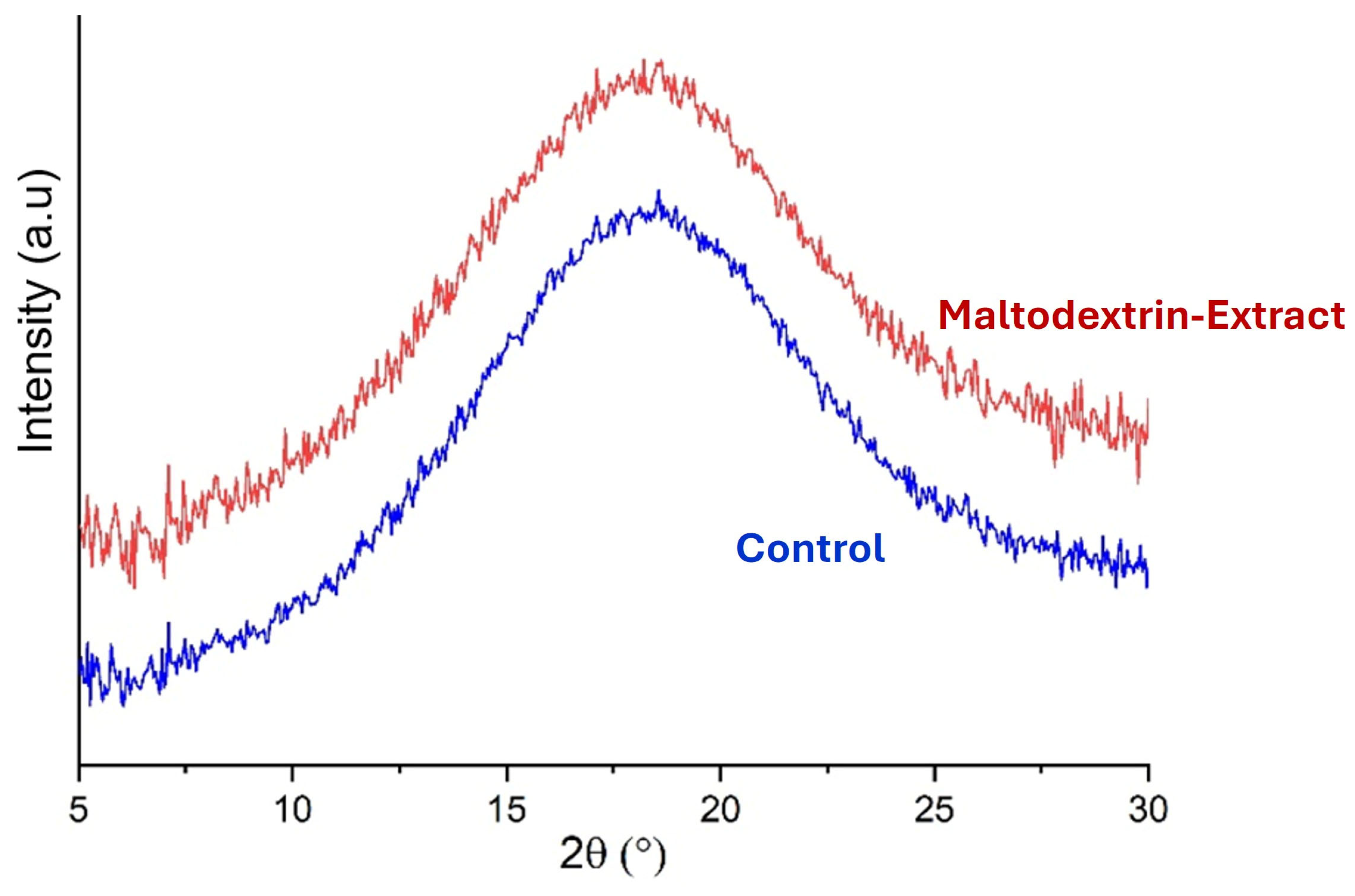
3.4.3. X-Ray Diffraction of the Microparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, T.; Zhang, Z.; Li, Z.; Guo, Y.; Zhao, G.; Wu, L. Recent advances in the effects of dietary polyphenols on inflammation in vivo: Potential molecular mechanisms, receptor targets, safety issues, and uses of nanodelivery system and polyphenol polymers. Curr. Opin. Food Sci. 2022, 48, 100921. [Google Scholar] [CrossRef]
- Guerrero-Wyss, M.; Yans, C.; Boscán-González, A.; Duran, P.; Parra-Soto, S.; Angarita, L. Durvillaea antarctica: A seaweed for enhancing immune and cardiometabolic health and gut microbiota composition modulation. Int. J. Mol. Sci. 2023, 24, 10779. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Contreras-Contreras, G.; Rivas-Reyes, P.I.; Pérez-Jiménez, J.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S. Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization. Antioxidants 2025, 14, 250. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Muñoz-Molina, N.; Parada, J.; Simirgiotis, M.; Montecinos-González, R. The Potential of Using Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound-Assisted Extraction to Fight against Aging-Related Diseases. Foods 2024, 13, 269. [Google Scholar] [CrossRef]
- Norcino, L.B.; Mendes, J.F.; Figueiredo, J.d.A.; Oliveira, N.L.; Botrel, D.A.; Mattoso, L.H.C. Development of alginate/pectin microcapsules by a dual process combining emulsification and ultrasonic gelation for encapsulation and controlled release of anthocyanins from grapes (Vitis labrusca L.). Food Chem. 2022, 391, 133256. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Singh, S.M.; Tripathy, S.; Ghodki, B.M.; Srivastav, P.P. Optimization of Wall Material Composition for Encapsulating Bioactive Compounds From Giloy (Tinospora cordifolia) Stems. J. Food Process. Preserv. 2025, 2025, 6459848. [Google Scholar] [CrossRef]
- Atli, O.; Can Karaca, A.; Ozcelik, B. Encapsulation of cumin (Cuminum cyminum L.) seed essential oil in the chickpea protein–maltodextrin matrix. ACS Omega 2023, 8, 4156–4164. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Román-Aguirre, M.; Toxqui-Terán, A.; Espinosa-Solís, V.; Franco-Vega, A.; Leyva-Porras, C. Blends of Carbohydrate Polymers for the Co-Microencapsulation of Bacillus clausii and Quercetin as Active Ingredients of a Functional Food. Polymers 2022, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-Shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. 2024, 24, 101953. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Sampaio, F.C.; de Faria, J.T.; Pitangui, C.G.; Lovaglio, F.; Casazza, A.A.; Converti, A.; Perego, P. Optimization of spray drying microencapsulation of olive pomace polyphenols using response surface methodology and artificial neural network. Lwt 2018, 93, 220–228. [Google Scholar] [CrossRef]
- Pacheco, L.V.; Parada, J.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Erpel, F.; Zambrano, A.; Palacios, M. Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Mar. Drugs 2020, 18, 353. [Google Scholar] [CrossRef]
- Ling, S.K.; Tanaka, T.; Kouno, I. Effects of iridoids on lipoxygenase and hyaluronidase activities and their activation by β-glucosidase in the presence of amino acids. Biol. Pharm. Bull. 2003, 26, 352–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Bernal-Millán, M.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-Dried microencapsulation of oregano (Lippia graveolens) polyphenols with maltodextrin enhances their stability during in vitro digestion. J. Chem. 2022, 2022, 8740141. [Google Scholar] [CrossRef]
- Porras-Saavedra, J.; Palacios-González, E.; Lartundo-Rojas, L.; Garibay-Febles, V.; Yáñez-Fernández, J.; Hernandez-Sanchez, H.; Gutiérrez-López, G.; Alamilla-Beltran, L. Microstructural properties and distribution of components in microparticles obtained by spray-drying. J. Food Eng. 2015, 152, 105–112. [Google Scholar] [CrossRef]
- Shi, Q.; Lin, W.; Zhao, Y.; Zhang, P. Thermal characteristics and state diagram of Penaeus vannamei meat with and without maltodextrin addition. Thermochim. Acta 2015, 616, 92–99. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Paul Ross, R. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Erpel, F.; Mariotti-Celis, M.S.; Parada, J.; Pedreschi, F.; Pérez-Correa, J.R. Pressurized hot liquid extraction with 15% v/v glycerol-water as an effective environment-friendly process to obtain durvillaea incurvata and lessonia spicata phlorotannin extracts with antioxidant and antihyperglycemic potential. Antioxidants 2021, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MS n: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Li, Z.; Mou, Q. The anti-allergic activity of polyphenol extracted from five marine algae. J. Ocean Univ. China 2015, 14, 681–684. [Google Scholar] [CrossRef]
- Arunkumar, K.; Nalluri, M.; Anjana, K.; Mohan, G.; Raja, R. Fucoxanthin as antioxidant, anti-hyaluronidase and cytotoxic agent: Potential of brown seaweeds decoction for tea supplement. J. Food Meas. Charact. 2023, 17, 3980–3989. [Google Scholar] [CrossRef]
- Arunkumar, K.; Raj, R.; Raja, R.; Carvalho, I.S. Brown seaweeds as a source of anti-hyaluronidase compounds. S. Afr. J. Bot. 2021, 139, 470–477. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Sivagnanam, S.P.; Park, J.S.; Cho, Y.J.; Chun, B.S. Effect of wall materials on the spray drying encapsulation of brown seaweed bioactive compounds obtained by subcritical water extraction. Algal Res. 2021, 58, 102381. [Google Scholar] [CrossRef]
- Neves, M.A.; Hashemi, J.; Prentice, C. Development of novel bioactives delivery systems by micro/nanotechnology. Curr. Opin. Food Sci. 2015, 1, 7–12. [Google Scholar] [CrossRef]
- Beirão-da-Costa, S.; Duarte, C.; Bourbon, A.; Pinheiro, A.; Januário, M.; Vicente, A.A.; Beirão-da-Costa, M.L.; Delgadillo, I. Inulin potential for encapsulation and controlled delivery of Oregano essential oil. Food Hydrocoll. 2013, 33, 199–206. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Deroanne, C.; Paquot, M.; Fougnies, C.; Lambrechts, J.C.; Blecker, C.S. Characterization of the physical state of spray-dried inulin. Food Biophys. 2007, 2, 83–92. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Spray drying of nopal mucilage (Opuntia ficus-indica): Effects on powder properties and characterization. Carbohydr. Polym. 2010, 81, 864–870. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Palma-Rodríguez, H.M.; Alvarez-Ramírez, J.; Vargas-Torres, A. Using Modified Starch/Maltodextrin Microparticles for Enhancing the Shelf Life of Ascorbic Acid by the Spray-Drying Method. Starch—Stärke 2018, 70, 1700323. [Google Scholar] [CrossRef]
- Pai, D.A.; Vangala, V.R.; Ng, J.W.; Ng, W.K.; Tan, R.B. Resistant maltodextrin as a shell material for encapsulation of naringin: Production and physicochemical characterization. J. Food Eng. 2015, 161, 68–74. [Google Scholar] [CrossRef]

| Independent Variable | Unit | Symbol | Coded and Actual Levels of Factors | ||||
|---|---|---|---|---|---|---|---|
| −1.41 | −1 | 0 | +1 | 1.41 | |||
| Inlet temperature (IT) | °C | x1 | 141.7 | 150.0 | 170.0 | 190.0 | 198.3 |
| Maltodextrin concentration (MD) | g/100 g | x2 | 1.9 | 5.0 | 12.5 | 20.0 | 23.1 |
| Run | Inlet Temperature (°C) | Maltodextrin Concentration (g/100 g) | EE (%) |
|---|---|---|---|
| 1 | 150.00 | 5.00 | 43.3 ± 4.9 |
| 2 | 190.00 | 5.00 | 43.2 ± 4.0 |
| 3 | 150.00 | 20.00 | 69.0 ± 1.5 |
| 4 | 190.00 | 20.00 | 71.0 ± 1.9 |
| 5 | 141.72 | 12.50 | 75.3 ± 0.8 |
| 6 | 198.28 | 12.50 | 79.1 ± 1.4 |
| 7 | 170.00 | 1.89 | 30.1 ± 2.5 |
| 8 | 170.00 | 23.11 | 61.4 ± 0.4 |
| 9 | 170.00 | 12.50 | 52.7 ± 1.7 |
| 10 | 170.00 | 12.50 | 42.8 ± 0.4 |
| 11 | 170.00 | 12.50 | 48.5 ± 3.6 |
| 12 | 170.00 | 12.50 | 51.0 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Molina, N.; Parada, J.; Zambrano, A.; Chipon, C.; Robert, P.; Mariotti-Celis, M.S. Ultrasound-Assisted Extraction and Microencapsulation of Durvillaea incurvata Polyphenols: Toward a Stable Anti-Inflammatory Ingredient for Functional Foods. Foods 2025, 14, 2240. https://doi.org/10.3390/foods14132240
Muñoz-Molina N, Parada J, Zambrano A, Chipon C, Robert P, Mariotti-Celis MS. Ultrasound-Assisted Extraction and Microencapsulation of Durvillaea incurvata Polyphenols: Toward a Stable Anti-Inflammatory Ingredient for Functional Foods. Foods. 2025; 14(13):2240. https://doi.org/10.3390/foods14132240
Chicago/Turabian StyleMuñoz-Molina, Nicolás, Javier Parada, Angara Zambrano, Carina Chipon, Paz Robert, and María Salomé Mariotti-Celis. 2025. "Ultrasound-Assisted Extraction and Microencapsulation of Durvillaea incurvata Polyphenols: Toward a Stable Anti-Inflammatory Ingredient for Functional Foods" Foods 14, no. 13: 2240. https://doi.org/10.3390/foods14132240
APA StyleMuñoz-Molina, N., Parada, J., Zambrano, A., Chipon, C., Robert, P., & Mariotti-Celis, M. S. (2025). Ultrasound-Assisted Extraction and Microencapsulation of Durvillaea incurvata Polyphenols: Toward a Stable Anti-Inflammatory Ingredient for Functional Foods. Foods, 14(13), 2240. https://doi.org/10.3390/foods14132240








