Physicochemical and Sensory Evaluation of Spreads Derived from Fruit Processing By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Pomace Materials
2.2. Pomace Spread Production
2.3. pH, aw, Total Titratable Acidity and Moisture Content Determination
2.4. Protein and Total Dietary Fiber Content of Pomaces
2.5. Color Measurements
2.6. Rheometry
2.7. Texture Analysis
2.8. Antioxidant Activity and Phenolic Compound Profile
2.8.1. Extraction Procedure
2.8.2. Determination of Total Phenolic Content
2.8.3. Determination of the Antioxidant Activity
2.8.4. Chromatographic Analysis of Phenolic Compounds
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Fruit Pomace Spreads
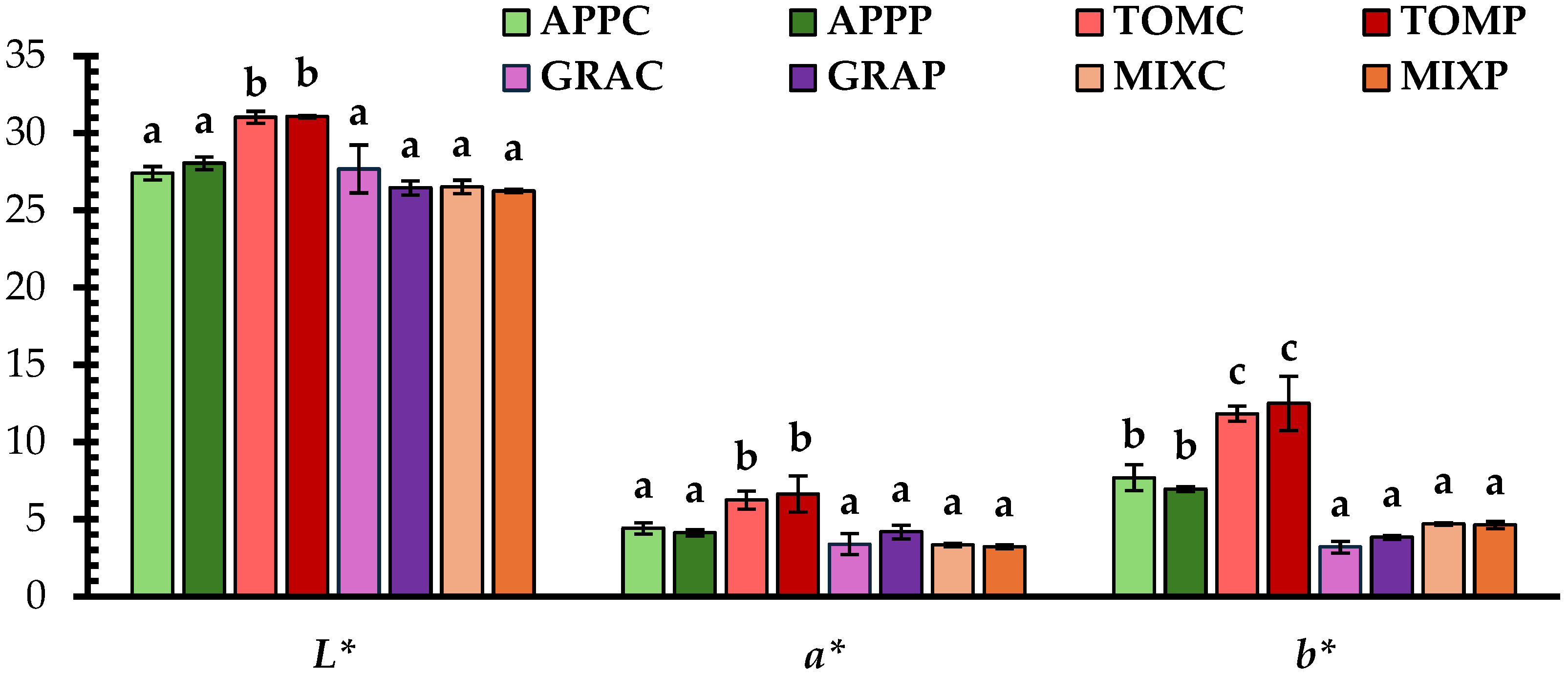
3.2. Color Parameters
3.3. Rheological Properties of Fruit Pomace Spreads
3.4. Textural Properties of Fruit Pomace Spreads
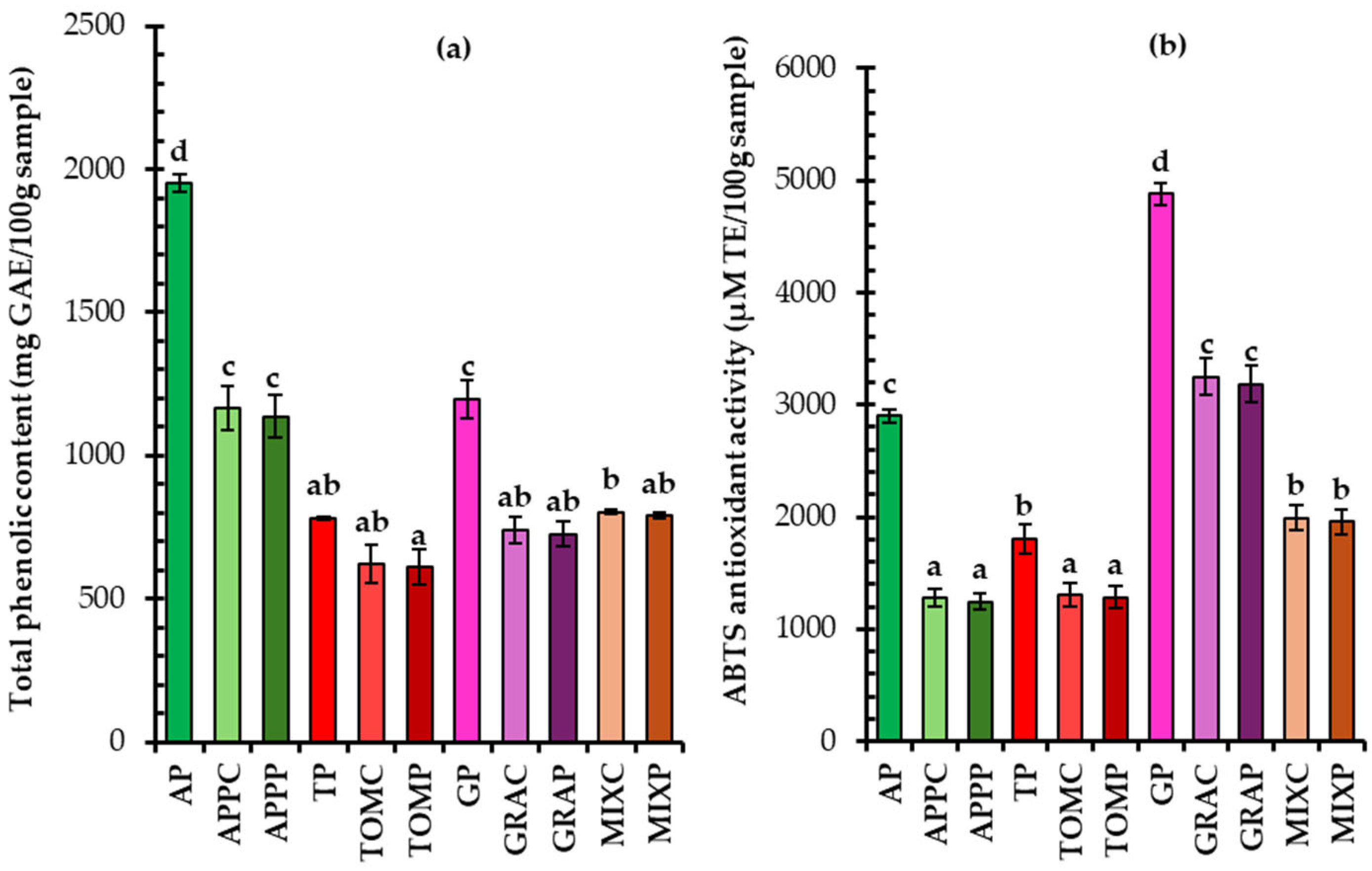
3.5. Total Phenolic Content, Antioxidant Capacity, and Phenolic Compounds of Dried Fruit Pomaces and Pomace Spreads
3.5.1. Total Phenolic Content and Antioxidant Capacity
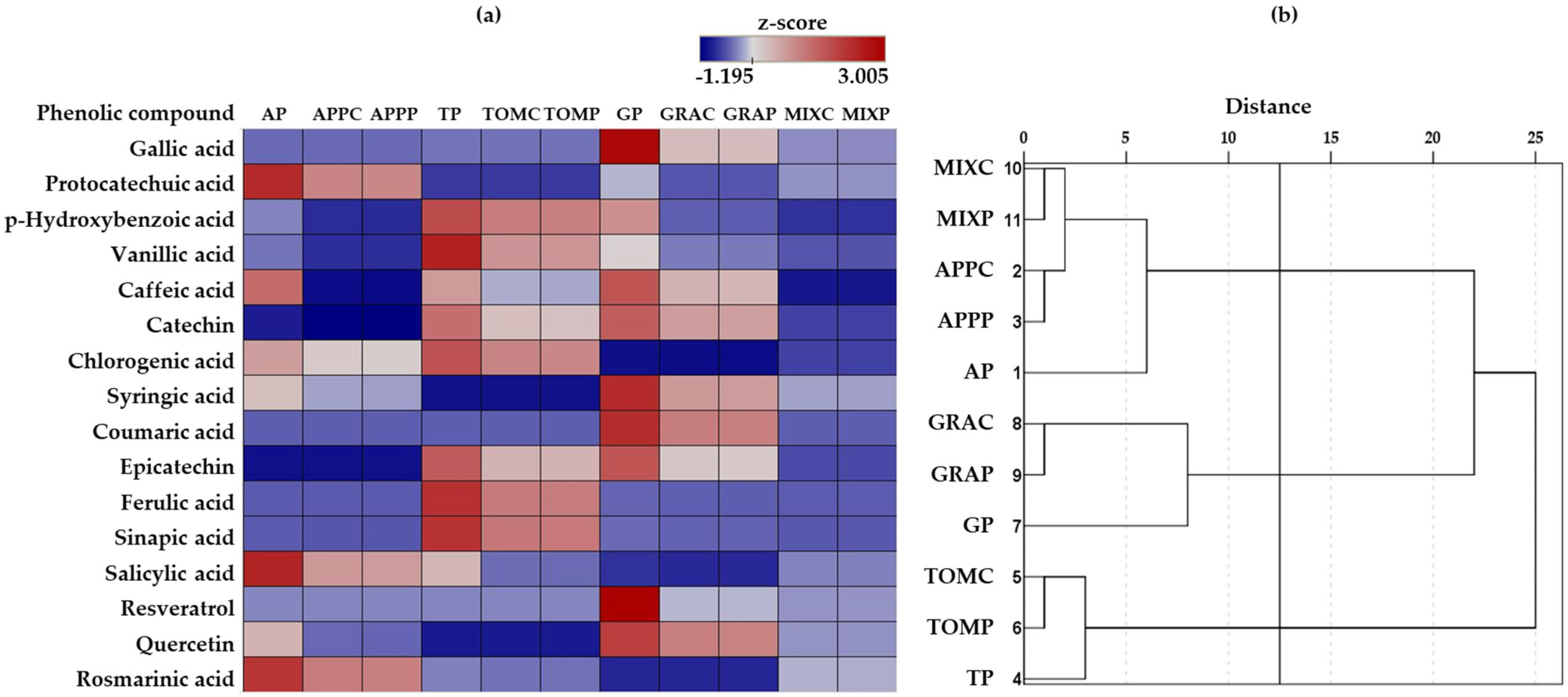
3.5.2. Phenolic Compounds
3.6. Sensory Attributes
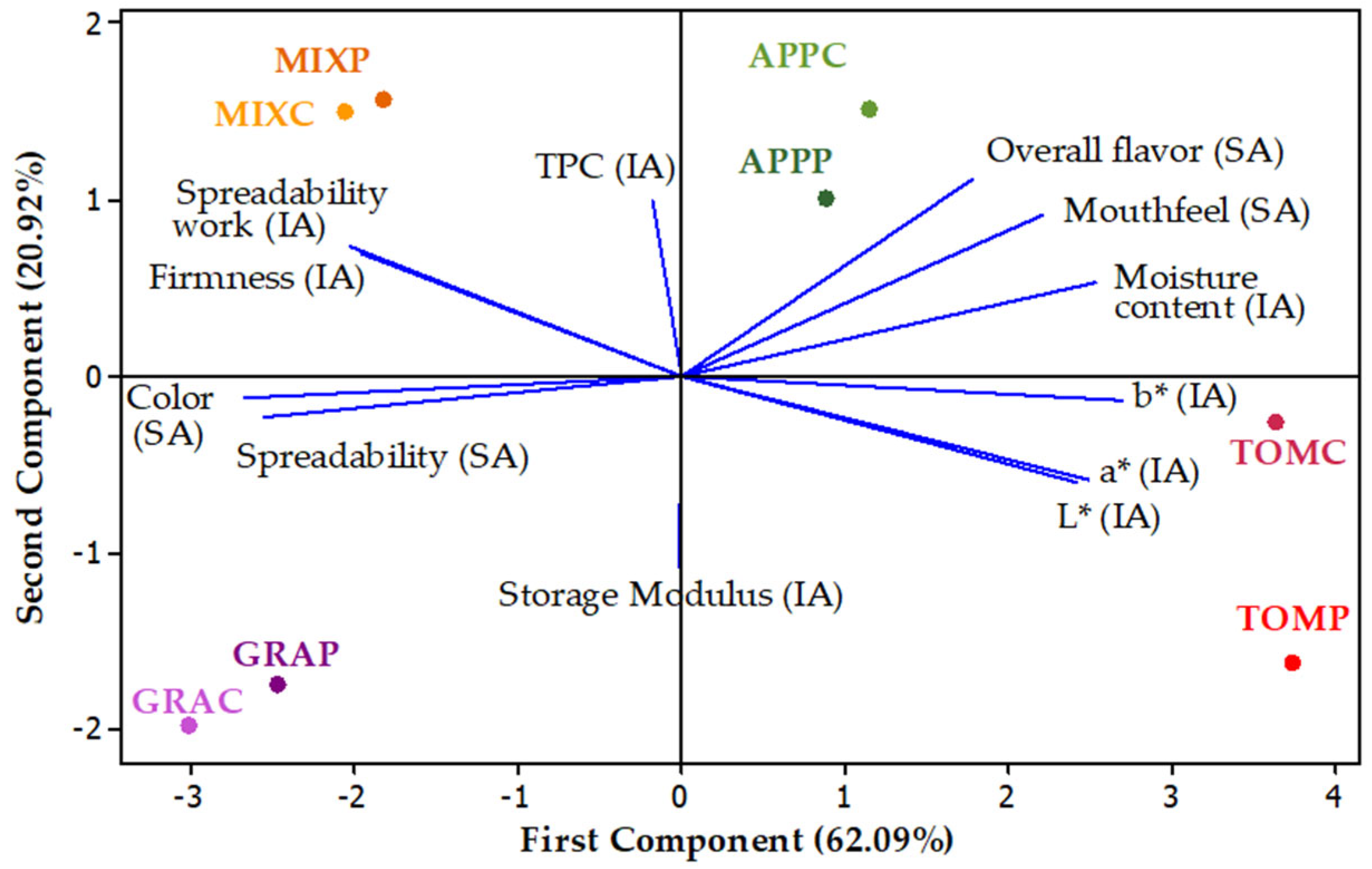
3.7. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amaya-Cruz, D.M.; Rodríguez-González, S.; Pérez-Ramírez, I.F.; Loarca-Piña, G.; Amaya-Llano, S.; Gallegos-Corona, M.A.; Reynoso-Camacho, R. Juice By-Products as a Source of Dietary Fibre and Antioxidants and Their Effect on Hepatic Steatosis. J. Funct. Foods 2015, 17, 93–102. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Pintado, M.; Lopes da Silva, J.A. Potential Nutritional and Functional Improvement of Extruded Breakfast Cereals Based on Incorporation of Fruit and Vegetable By-Products—A Review. Trends Food Sci. Technol. 2022, 125, 136–153. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Kapoor, A.; Kapoor, S.; Aggarwal, P. Rheological, Microstructural and Biochemical Characterisation of Fruit Pomace Jams. J. Food Meas. Charact. 2023, 17, 4267–4279. [Google Scholar] [CrossRef]
- Sereti, V.; Kotsiou, K.; Ciurlă, L.; Patras, A.; Irakli, M.; Lazaridou, A. Valorizing Apple Pomace as Stabilizer of Olive Oil-Water Emulsion Used for Reduction of Saturated Fat in Biscuits. Food Hydrocoll. 2024, 151, 109746. [Google Scholar] [CrossRef]
- Montalvo-González, E.; Aguilar-Hernández, G.; Hernández-Cázares, A.S.; Ruiz-López, I.I.; Pérez-Silva, A.; Hernández-Torres, J.; Vivar-Vera, M.d.l.Á. Production, Chemical, Physical and Technological Properties of Antioxidant Dietary Fiber from Pineapple Pomace and Effect as Ingredient in Sausages. CyTA-J. Food 2015, 16, 831–839. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary Fibre Content and Antioxidant Activity of Manto Negro Red Grape (Vitis Vinifera): Pomace and Stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; Tommasi, N.D. Polyphenol Constituents and Antioxidant Activity of Grape Pomace Extracts from Five Sicilian Red Grape Cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Silveira, S.T.; Daroit, D.J.; Brandelli, A. Pigment Production by Monascus Purpureus in Grape Waste Using Factorial Design. LWT Food Sci. Technol. 2008, 41, 170–174. [Google Scholar] [CrossRef]
- Balbinoti, T.C.V.; Stafussa, A.P.; Haminiuk, C.W.I.; Maciel, G.M.; Sassaki, G.L.; Jorge, L.M.d.M.; Jorge, R.M.M. Addition of Grape Pomace in the Hydration Step of Parboiling Increases the Antioxidant Properties of Rice. Int. J. Food Sci. Technol. 2020, 55, 2370–2380. [Google Scholar] [CrossRef]
- Acun, S.; Gül, H. Effects of Grape Pomace and Grape Seed Flours on Cookie Quality. Qual. Assur. Saf. Crops Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Kaur, D.; Wani, A.A.; Oberoi, D.P.S.; Sogi, D.S. Effect of Extraction Conditions on Lycopene Extractions from Tomato Processing Waste Skin Using Response Surface Methodology. Food Chem. 2008, 108, 711–718. [Google Scholar] [CrossRef]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-Assisted Extraction of Lycopene from Tomato Processing Waste. Enzym. Microb. Technol. 2011, 49, 567–573. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Keum, Y.-S. An Updated Review on Use of Tomato Pomace and Crustacean Processing Waste to Recover Commercially Vital Carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable Valorisation of Tomato Pomace: A Comprehensive Review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Development of Cold Plasma Pretreatment for Improving Phenolics Extractability from Tomato Pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Chabi, I.B.; Zannou, O.; Dedehou, E.S.C.A.; Ayegnon, B.P.; Odouaro, O.B.O.; Maqsood, S.; Galanakis, C.M.; Kayodé, A.P.P. Tomato Pomace as a Source of Valuable Functional Ingredients for Improving Physicochemical and Sensory Properties and Extending the Shelf Life of Foods: A Review. Heliyon 2024, 10, e25261. [Google Scholar] [CrossRef]
- Sandhu, D.K.; Joshi, V.K. Solid State Fermentation of Apple Pomace for Concomitant Production of Ethanol and Animal Feed. J. Sci. Ind. Res. 1997, 56, 86–90. [Google Scholar]
- Hwang, J.-K.; Kim, C.-J.; Kim, C.-T. Extrusion of Apple Pomace Facilitates Pectin Extraction. J. Food Sci. 1998, 63, 841–844. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of Apple Pomace Extracts as a Source of Bioactive Compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Rohou, Y.; Hubert, C.; Della Valle, G.; Thibault, J.-F.; Savina, J.-P. Bleaching of Apple Pomace by Hydrogen Peroxide in Alkaline Conditions: Optimisation and Characterisation of the Products. LWT Food Sci. Technol. 1997, 30, 398–405. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical Properties of Pectin from Malus Domestica ‘Fălticeni’ Apple Pomace as Affected by Non-Conventional Extraction Techniques. Food Hydrocoll. 2020, 100, 105383. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2012, 83, 1–295. [Google Scholar]
- Yang, H.; Chou, L.-Y.; Hua, C.-C. Effects of Calcium and pH on Rheological Thermal Resistance of Composite Xanthan Gum and High-Methoxyl Apple Pectin Matrices Featuring Dysphagia-Friendly Consistency. Foods 2024, 13, 90. [Google Scholar] [CrossRef]
- Razak, R.A.; Karim, R.; Sulaiman, R.; Hussain, N. Effects of Different Types and Concentration of Hydrocolloids on Mango Filling. Int. Food Res. J. 2018, 25, 1109–1119. [Google Scholar]
- Ishwarya, S.P.; Sandhya, R.; Nisha, P. Advances and Prospects in the Food Applications of Pectin Hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef]
- European Commission. Council Directive 2001/113/EC of 20 December 2001 relating to fruit jams, jellies, and marmalades and sweetened chestnut purée intended for human consumption. Off. J. Eur. Comm. 2002, L10, 67–72. [Google Scholar]
- AOAC Method 925.10; Moisture in Fruits and Fruit Products. AOAC International: Rockville, MD, USA, 2000.
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of Variety on the Quality of Tomato Stored under Ambient Conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef] [PubMed]
- AOAC Method 950.48; Protein (Total Nitrogen) in Fruits and Fruit Products. AOAC International: Rockville, MD, USA, 2000.
- AACC Method 32–21.01; Insoluble and Soluble Dietary Fiber in Oat Products. American Association of Cereal Chemists International: St. Paul, MN, USA, 2010.
- AOAC Method 985.29; Total Dietary Fiber in Foods. AOAC International: Rockville, MD, USA, 2012.
- Kotsiou, K.; Sacharidis, D.-D.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Impact of Roasted Yellow Split Pea Flour on Dough Rheology and Quality of Fortified Wheat Breads. Foods 2021, 10, 1832. [Google Scholar] [CrossRef]
- Nouska, C.; Irakli, M.; Palakas, P.; Lytou, A.E.; Bouloumpasi, E.; Biliaderis, C.G.; Lazaridou, A. Influence of Sesame Cake on Physicochemical, Antioxidant and Sensorial Characteristics of Fortified Wheat Breads. Food Res. Int. 2024, 178, 113980. [Google Scholar] [CrossRef] [PubMed]
- Bajić, A.; Pezo, L.L.; Stupar, A.; Filipčev, B.; Cvetković, B.R.; Horecki, A.T.; Mastilović, J. Application of Lyophilized Plum Pomace as a Functional Ingredient in a Plum Spread: Optimizing Texture, Colour and Phenol Antioxidants by ANN Modelling. LWT 2020, 130, 109588. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Filimon, R.V.; Bunea, C.I.; Bora, F.D.; Filimon, R.M.; Dunca, S.I.; Rózsa, S.; Ciurlă, L.; Patraș, A. Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis Vinifera L.) Canes. Horticulturae 2023, 9, 1164. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Comm. 2006, L404, 9–25. [Google Scholar]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of Soluble Dietary Fibre on Postprandial Blood Glucose Response and Its Potential as a Functional Food Ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Xie, J.; Peng, G.; Hu, X.; Xie, J.; Chen, Y.; Dong, R.; Si, J.; Yang, C.; Yu, Q. Physicochemical Characteristics of Soluble Dietary Fiber Obtained from Grapefruit Peel Insoluble Dietary Fiber and Its Effects on Blueberry Jam. Foods 2022, 11, 3735. [Google Scholar] [CrossRef]
- Huang, S.; He, Y.; Zou, Y.; Liu, Z. Modification of Insoluble Dietary Fibres in Soya Bean Okara and Their Physicochemical Properties. Int. J. Food Sci. Technol. 2015, 50, 2606–2613. [Google Scholar] [CrossRef]
- Grigelmo-Miguel, N.; Martín-Belloso, O. Influence of Fruit Dietary Fibre Addition on Physical and Sensorial Properties of Strawberry Jams. J. Food Eng. 1999, 41, 13–21. [Google Scholar] [CrossRef]
- Kong, K.-W.; Khoo, H.-E.; Prasad, K.N.; Ismail, A.; Tan, C.-P.; Rajab, N.F. Revealing the Power of the Natural Red Pigment Lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.; Hussein, D. Tomato Pomace Pigment: Extraction and Use as Food Colorant. Jordan J. Agric. Sci. 2013, 9, 72–85. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, Technological and in Vitro Antioxidant Properties of Mango, Guava, Pineapple and Passion Fruit Dietary Fibre Concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Garrido, J.I.; Lozano, J.E.; Genovese, D.B. Effect of Formulation Variables on Rheology, Texture, Colour, and Acceptability of Apple Jelly: Modelling and Optimization. LWT Food Sci. Technol. 2015, 62, 325–332. [Google Scholar] [CrossRef]
- Viegas, Â.; Alegria, M.J.; Raymundo, A. Sustainable Jam with Apple Pomace: Gelling, Rheology, and Composition Analysis. Gels 2024, 10, 580. [Google Scholar] [CrossRef]
- Mohammadi-Moghaddam, T.; Firoozzare, A.; Daryadar, S.; Rahmani, Z. Black Plum Peel Jam: Physicochemical Properties, Sensory Attributes, and Antioxidant Capacity. Int. J. Food Prop. 2020, 23, 1737–1747. [Google Scholar] [CrossRef]
- Acan, B.G.; Kilicli, M.; Bursa, K.; Toker, O.S.; Palabiyik, I.; Gulcu, M.; Yaman, M.; Gunes, R.; Konar, N. Effect of Grape Pomace Usage in Chocolate Spread Formulation on Textural, Rheological and Digestibility Properties. LWT 2021, 138, 110451. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of Polyphenols in Apple Pomace: A Comparative Study of Different Extraction and Hydrolysis Procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Hussein, A.M.S.; Kamil, M.M.; Hegazy, N.A.; Mahmoud, K.F.; Ibrahim, M.A. Utilization of Some Fruits and Vegetables By-Products to Produce High Dietary Fiber Jam. Food Sci. Qual. Manag. 2015, 37, 39–45. [Google Scholar]



| Sample | Abbreviation | Pomace (g) | Sugar (g) | Citric Acid (g) | Pectin (g) | CaCl2 (g) | Water (g) |
|---|---|---|---|---|---|---|---|
| Apple pomace spread | APPC | 100.00 | 100.00 | - | - | - | 400.00 |
| APPP | 100.00 | 100.00 | - | 5.00 | 5.00 | 400.00 | |
| Tomato pomace spread | TOMC | 100.00 | 100.00 | 2.85 | - | - | 400.00 |
| TOMP | 100.00 | 100.00 | 2.85 | 5.00 | 5.00 | 400.00 | |
| Grape pomace spread | GRAC | 100.00 | 100.00 | 1.60 | - | - | 300.00 |
| GRAP | 100.00 | 100.00 | 1.60 | 5.00 | 5.00 | 300.00 | |
| Apple–grape (1:1) pomace spreads | MIXC | 100.00 | 100.00 | - | - | - | 400.00 |
| MIXP | 100.00 | 100.00 | - | 5.00 | 5.00 | 400.00 |
| Sample 1 | pH | Titratable Acidity (Expressed as g of Citric Acid/100 g Spread) | aw | Moisture (%) |
|---|---|---|---|---|
| APPC | 3.22 ± 0.06ab 2 | 1.36 ± 0.03c | 0.94 ± 0.01c | 58.90 ± 3.65d |
| APPP | 3.30 ± 0.00b | 1.19 ± 0.08bc | 0.95 ± 0.00c | 62.43 ± 0.55d |
| TOMC | 3.36 ± 0.12b | 1.15 ± 0.58bc | 0.96 ± 0.02c | 65.86 ± 0.86d |
| TOMP | 3.34 ± 0.06b | 1.06 ± 0.16abc | 0.97 ± 0.00c | 62.99 ± 1.99d |
| GRAC | 3.34 ± 0.11b | 1.18 ± 0.03bc | 0.88 ± 0.03a | 36.14 ± 3.14a |
| GRAP | 3.29 ± 0.01b | 0.51 ± 0.13a | 0.90 ± 0.00ab | 38.97 ± 6.46ab |
| MIXC | 3.42 ± 0.02b | 0.66 ± 0.03ab | 0.91 ± 0.01b | 47.77 ± 1.47bc |
| MIXP | 3.05 ± 0.08a | 0.60 ± 0.06ab | 0.90 ± 0.0ab | 49.23 ± 0.25c |
| Sample 1 | Protein (%) | SDF (%) 2 | IDF (%) 2 | TDF (%) 2 | IDF/SDF 2 |
|---|---|---|---|---|---|
| APPC | 1.52 ± 0.12a 3 | 4.28 ± 0.42c | 9.85 ± 0.87a | 14.13 ± 0.44e | 2.36 ± 0.44a |
| APPP | 1.55 ± 0.14a | 3.98 ± 0.48c | 9.16 ± 0.99a | 13.14 ± 0.51de | 2.36 ± 0.53a |
| TOMC | 3.21 ± 0.24bc | 2.01 ± 0.03a | 8.10 ± 0.17a | 10.11 ± 0.13a | 4.03 ± 0.15bc |
| TOMP | 3.13 ± 0.28b | 1.96 ± 0.04a | 7.89 ± 0.20a | 9.85 ± 0.16a | 4.03 ± 0.18bc |
| GRAC | 3.96 ± 0.23d | 1.99 ± 0.07a | 9.00 ± 0.87a | 10.99 ± 0.80abc | 4.55 ± 0.60c |
| GRAP | 3.76 ± 0.27cd | 1.89 ± 0.08a | 8.54 ± 1.01a | 10.42 ± 0.93ab | 4.55 ± 0.73c |
| MIXC | 2.81 ± 0.05b | 3.14 ± 0.18b | 9.42 ± 0.00a | 12.56 ± 0.18cde | 3.02 ± 0.17ab |
| MIXP | 2.65 ± 0.06b | 2.94 ± 0.20b | 8.85 ± 0.01a | 11.78 ± 0.21bcd | 3.03 ± 0.20ab |
| Sample 1 | Storage Modulus, G′ (Pa) | Loss Modulus, G″ (Pa) | Complex Viscosity, η* (Pa∙s) | Damping Factor, tanδ | Firmness, Fmax (N) | Spreadability Work, SW (N∙mm) |
|---|---|---|---|---|---|---|
| APPC | 50,000 ± 6124ab 2 | 16,015 ± 2870ab | 1385.9 ± 306.9abc | 0.32 ± 0.02b | 59.22 ± 2.05b | 59.23 ± 2.66b |
| APPP | 73,900 ± 6777abc | 22,300 ± 1633ab | 3270.0 ± 253.1bc | 0.30 ± 0.01b | 58.85 ± 2.00b | 49.07 ± 10.29b |
| TOMC | 57,225 ± 9941abc | 12,560 ± 2400a | 1343.0 ± 234.3abc | 0.23 ± 0.08ab | 5.40 ± 0.69a | 4.53 ± 0.80a |
| TOMP | 279,600 ± 61,564d | 51,178 ± 12,021c | 6502.5 ± 1435.0d | 0.18 ± 0.00a | 8.89 ± 0.13a | 7.51 ± 0.69a |
| GRAC | 4150 ± 776a | 3153 ± 549a | 134.6 ± 22.8a | 0.76 ± 0.01c | 151.69 ± 2.85c | 106.82 ± 2.42c |
| GRAP | 168,375 ± 41,335cd | 46,225 ± 8798c | 3933.3 ± 936.3cd | 0.28 ± 0.02ab | 163.19 ± 49.71c | 91.43 ± 7.45c |
| MIXC | 47,233 ± 6002ab | 13,027 ± 2957a | 1122.7 ± 154.2ab | 0.27 ± 0.03ab | 384.57 ± 15.33d | 214.71 ± 11.96d |
| MIXP | 160,333 ± 55,769bc | 36,700 ± 10,044bc | 3760.0 ± 1288.2bc | 0.23 ± 0.02ab | 440.86 ± 14.97e | 255.60 ± 13.72e |
| Phenolic Compound | AP 1 | APPC | APPP | TP | TOMC | TOMP | GP | GRAC | GRAP | MIXC | MIXP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | n.d. 2 | n.d. | n.d. | 1.95 ± 1.09a | 1.63 ± 0.06a | 1.61 ± 0.04a | 123.59 ± 2.92d | 31.05 ± 1.60c | 30.44 ± 1.28c | 8.31 ± 0.74b | 8.18 ± 0.73b |
| Protocatechuic acid | 337.60 ± 12.58f 3 | 196.41 ± 0.34e | 191.62 ± 0.27e | n.d. | n.d. | n.d. | 68.16 ± 3.32d | 18.47 ± 0.30b | 18.11 ± 0.25b | 52.19 ± 4.30c | 51.34 ± 3.46c |
| p-Hydroxybenzoic acid | 41.10 ± 0.98b | 20.93 ± 1.75a | 20.42 ± 1.40a | 129.95 ± 6.90e | 102.65 ± 2.12d | 100.98 ± 1.70d | 92.47 ± 4.61c | 33.78 ± 0.74b | 33.12 ± 0.60b | 23.05 ± 0.71a | 22.68 ± 0.57a |
| Vanillic acid | 7.23 ± 0.58b | 0.48 ± 0.22a | 0.46 ± 0.18a | 56.07 ± 4.43e | 28.92 ± 1.34d | 28.44 ± 1.08d | 16.54 ± 0.79c | 8.04 ± 0.39b | 7.88 ± 0.31b | 4.44 ± 0.22ab | 4.37 ± 0.18ab |
| Caffeic acid | 1.91 ± 0.26de | 0.49 ± 0.07a | 0.48 ± 0.07a | 1.56 ± 0.16cd | 1.02 ± 0.02abc | 1.00 ± 0.02ab | 2.08 ± 0.20e | 1.40 ± 0.30bcd | 1.37 ± 0.24bcd | 0.54 ± 0.17a | 0.54 ± 0.14a |
| Catechin | 8.60 ± 1.53ab | 1.05 ± 0.09a | 1.02 ± 0.12a | 83.99 ± 1.49e | 51.04 ± 3.93c | 50.20 ± 3.16c | 91.54 ± 2.66e | 64.60 ± 4.28d | 63.34 ± 3.42d | 17.08 ± 6.12b | 16.49 ± 4.59b |
| Chlorogenic acid | 27.51 ± 1.92d | 19.93 ± 0.64c | 19.45 ± 0.51c | 41.86 ± 0.59f | 32.08 ± 0.60e | 31.56 ± 0.18e | 1.85 ± 0.50a | 1.32 ± 0.13a | 1.35 ± 0.04a | 6.89 ± 0.95b | 6.78 ± 0.76b |
| Syringic acid | 28.09 ± 1.33b | 16.84 ± 0.95a | 16.42 ± 0.76a | n.d. | n.d. | n.d. | 67.80 ± 3.68d | 37.10 ± 3.90c | 36.38 ± 3.13c | 16.85 ± 0.85a | 16.57 ± 0.68a |
| Coumaric acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.68 ± 0.16a | 0.41 ± 0.22a | 0.40 ± 0.017a | n.d. | n.d. |
| Epicatechin | n.d. | n.d. | n.d. | 98.85 ± 3.69c | 57.01 ± 7.27b | 56.08 ± 5.84b | 102.54 ± 6.75c | 47.89 ± 1.09b | 46.95 ± 0.87b | 14.78 ± 3.60a | 14.53 ± 2.89a |
| Ferulic acid | n.d. | n.d. | n.d. | 110.68 ± 0.30d | 69.11 ± 0.47c | 67.98 ± 0.38c | 1.90 ± 0.45b | 0.74 ± 0.05a | 0.72 ± 0.04a | 0.22 ± 0.03a | 0.22 ± 0.02a |
| Sinapic acid | 3.36 ± 1.07a | 1.25 ± 0.08a | 1.22 ± 0.06a | 250.71 ± 4.97c | 164.07 ± 6.99b | 161.39 ± 5.61b | 10.75 ± 1.41a | 6.85 ± 0.84a | 7.00 ± 0.88a | 2.22 ± 0.35a | 2.18 ± 0.28a |
| Salicylic acid | 92.06 ± 1.47e | 47.37 ± 2.61d | 46.21 ± 2.08d | 37.05 ± 3.19c | 12.59 ± 1.40b | 12.38 ± 1.12b | 3.18 ± 0.32a | 1.54 ± 0.55a | 1.51 ± 0.44a | 15.71 ± 0.82b | 15.46 ± 0.66b |
| Resveratrol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6.15 ± 0.64b | 0.39 ± 0.05a | 0.38 ± 0.04a | 0.11 ± 0.02a | 0.11 ± 0.02a |
| Quercetin | 94.40 ± 0.76c | 31.21 ± 0.78a | 30.45 ± 0.63a | n.d. | n.d. | n.d. | 197.17 ± 12.07e | 138.08 ± 2.45d | 135.38 ± 1.97d | 45.77 ± 3.12b | 45.02 ± 2.50b |
| Rosmarinic acid | 59.86 ± 4.47d | 40.30 ± 0.16c | 39.32 ± 0.13c | 10.22 ± 1.13a | 8.89 ± 0.45a | 8.75 ± 0.37a | n.d. | n.d. | n.d. | 14.60 ± 0.42b | 14.36 ± 0.34b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouska, C.; Ciurla, L.; Patras, A.; Biliaderis, C.G.; Lazaridou, A. Physicochemical and Sensory Evaluation of Spreads Derived from Fruit Processing By-Products. Foods 2025, 14, 2224. https://doi.org/10.3390/foods14132224
Nouska C, Ciurla L, Patras A, Biliaderis CG, Lazaridou A. Physicochemical and Sensory Evaluation of Spreads Derived from Fruit Processing By-Products. Foods. 2025; 14(13):2224. https://doi.org/10.3390/foods14132224
Chicago/Turabian StyleNouska, Chrysanthi, Liliana Ciurla, Antoanela Patras, Costas G. Biliaderis, and Athina Lazaridou. 2025. "Physicochemical and Sensory Evaluation of Spreads Derived from Fruit Processing By-Products" Foods 14, no. 13: 2224. https://doi.org/10.3390/foods14132224
APA StyleNouska, C., Ciurla, L., Patras, A., Biliaderis, C. G., & Lazaridou, A. (2025). Physicochemical and Sensory Evaluation of Spreads Derived from Fruit Processing By-Products. Foods, 14(13), 2224. https://doi.org/10.3390/foods14132224









