Mechanistic Insights into Vegetable Color Stability: Discoloration Pathways and Emerging Protective Strategies
Abstract
1. Introduction
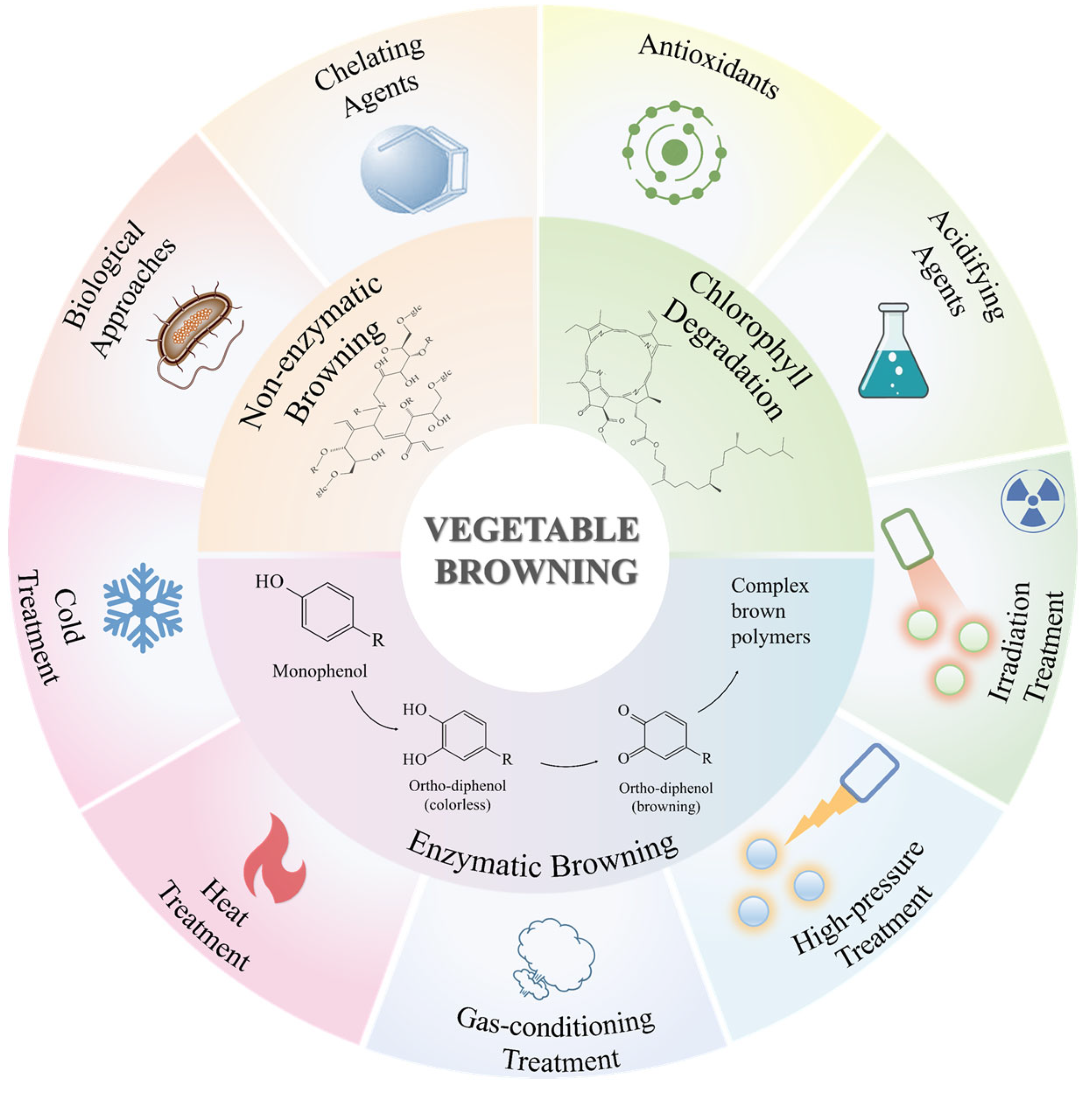
2. Mechanism of Vegetable Color Change
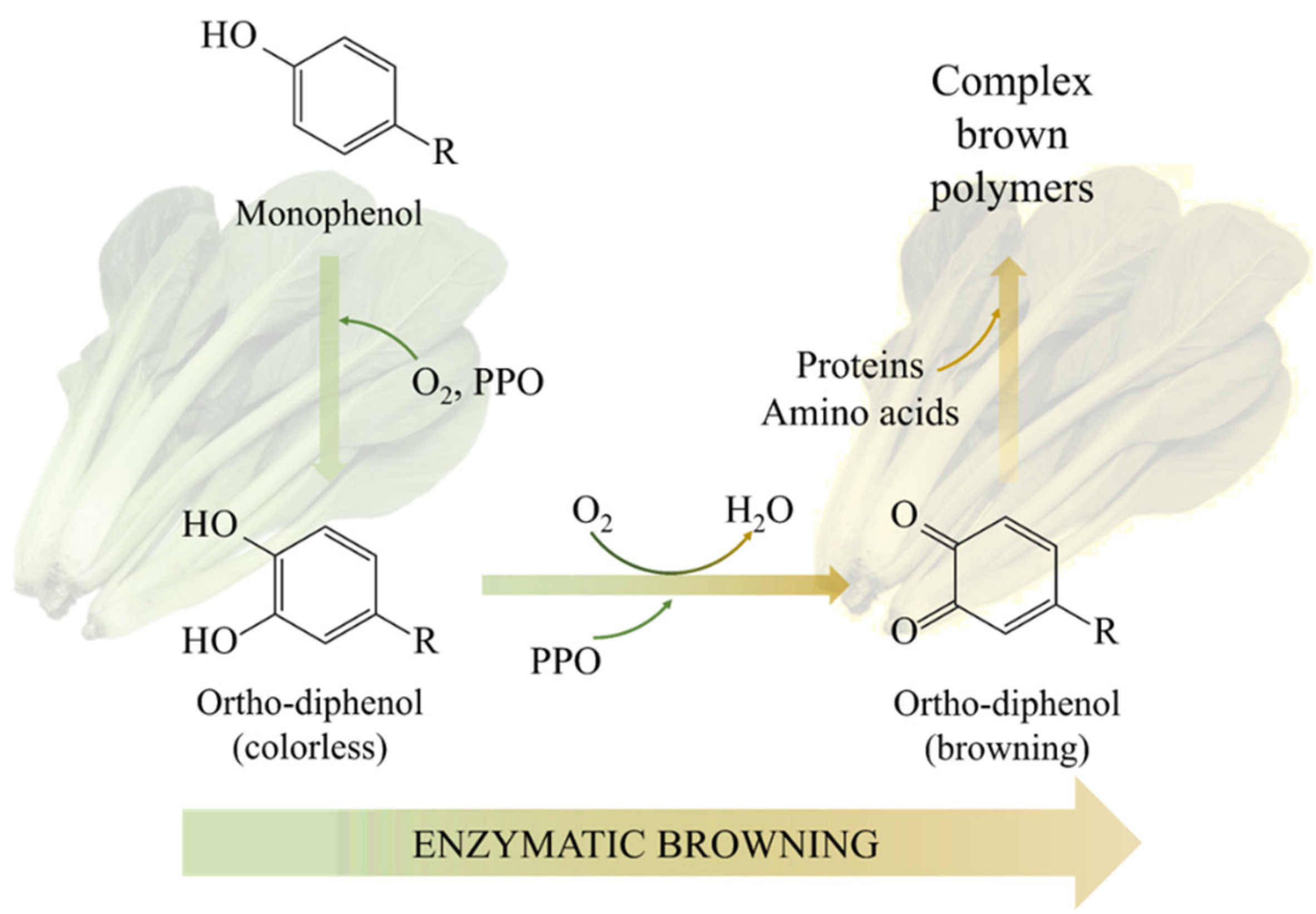
2.1. Enzymatic Browning
2.1.1. Mechanism of Enzymatic Browning
2.1.2. Conditions of Enzymatic Browning
2.2. Non-Enzymatic Browning
2.2.1. Mechanism of Non-Enzymatic Browning
2.2.2. Conditions of Non-Enzymatic Browning
2.3. Chlorophyll Degradation
2.3.1. Mechanism of Chlorophyll Degradation
2.3.2. Conditions of Chlorophyll Degradation
3. Methods to Inhibit Browning
3.1. Physical Methods
3.1.1. Cold Treatment
3.1.2. Heat Treatment
3.1.3. Gas-Conditioning Treatment
3.1.4. High-Pressure Treatment
3.1.5. Irradiation Treatment
3.2. Chemical Methods
3.2.1. Acidifying Agents
3.2.2. Antioxidants
3.2.3. Chelating Agents
3.3. Natural Anti-Browning Method
4. Conclusions and Prospects
4.1. Current Research and Gaps in Understanding Mechanisms
4.2. Outlook on Novel Anti-Browning Agents and Natural Preservatives
4.3. The Promise of Biotechnology: Gene Editing and Plant Breeding
4.4. Towards Integrated, Holistic Preservation Systems
4.5. The Importance of Cross-Disciplinary Collaboration and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPO | polyphenol oxidase |
| MAP | modified atmosphere packaging |
| HHAIB | high-humidity hot air impingement blanching |
| MAB | microwave-assisted blanching |
| PEF | pulsed electric fields |
| HPP | High-pressure processing |
| 4-HR | 4-hexylresorcinol |
References
- Zhang, Y.; Zareef, M.; Rong, Y.; Lin, H.; Chen, Q.; Ouyang, Q. Application of colorimetric sensor array coupled with chemometric methods for monitoring the freshness of snakehead fillets. Food Chem. 2024, 439, 138172. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.M.R.; Ma, H.; Xu, B.; Devi, S.; Siddique, M.A.B.; Stanley, S.L.; Bhandari, B.; Zhu, J. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432. [Google Scholar] [CrossRef]
- Gao, Q.; Li, Y.; Li, Y.; Zhang, Z.; Liang, Y. Antioxidant and prooxidant activities of phenolic acids commonly existed in vegetables and their relationship with structures. Food Sci. Technol. 2022, 42, e07622. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Xu, B.; Sun, J.; Mujumdar, A.S. Artificial intelligence assisted technologies for controlling the drying of fruits and vegetables using physical fields: A review. Trends Food Sci. Technol. 2020, 105, 251–260. [Google Scholar] [CrossRef]
- Ali, M.; Batool, S.; Khalid, N.; Ali, S.; Raza, M.A.; Li, X.; Li, F.; Xinhua, Z. Recent trends in hydrogen-associated treatments for maintaining the postharvest quality of fresh and fresh-cut fruits and vegetables: A review. Food Control 2024, 156, 110114. [Google Scholar] [CrossRef]
- Xu, J.-G.; Huang, X.-N.; Meng, J.; Chen, J.-Y.; Han, B.-Z. Characterization and comparison of the bacterial community on environmental surfaces through a fresh-cut vegetables processing line in China. Food Res. Int. 2022, 155, 111075. [Google Scholar] [CrossRef]
- Ru, X.; You, W.; Zhang, J.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. γ-aminobutyric acid treatment inhibits browning and promotes storage quality by regulating reactive oxygen species and membrane lipid metabolism in fresh-cut stem lettuce. Food Chem. 2024, 459, 140420. [Google Scholar] [CrossRef]
- Hong, C.; Zhao, Y.M.; Zhou, C.; Guo, Y.; Ma, H. Ultrasonic washing as an abiotic elicitor to increase the phenolic content in fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 785–808. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Navina, B.; Keshav Huthaash, K.; Velmurugan, N.K.; Korumilli, T. Insights into recent innovations in anti browning strategies for fruit and vegetable preservation. Trends Food Sci. Technol. 2023, 139, 104128. [Google Scholar] [CrossRef]
- Obadi, M.; Guo, Q.; Sun, J.; Xu, B. Recent developments in the application of physical processing techniques for controlling browning in fresh wet noodles: A review. J. Cereal Sci. 2024, 118, 103951. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michałowska, A. Enzymatic browning in apple products and its inhibition treatments: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5038–5076. [Google Scholar] [CrossRef]
- Hongyang, T.; Daming, H.; Xingyi, H.; Aheto, J.H.; Yi, R.; Yu, W.; Ji, L.; Shuai, N.; Mengqi, X. Detection of browning of fresh-cut potato chips based on machine vision and electronic nose. J. Food Process Eng. 2021, 44, e13631. [Google Scholar] [CrossRef]
- Winters, A.L.; Minchin, F.R.; Michaelson-Yeates, T.P.T.; Lee, M.R.F.; Morris, P. Latent and Active Polyphenol Oxidase (PPO) in Red Clover (Trifolium pratense) and Use of a Low PPO Mutant To Study the Role of PPO in Proteolysis Reduction. J. Agric. Food Chem. 2008, 56, 2817–2824. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Ye, M.; Li, X.W.; Lin, S.B.; Sun, X.-L. The Jasmonic Acid Pathway Positively Regulates the Polyphenol Oxidase-Based Defense against Tea Geometrid Caterpillars in the Tea Plant (Camellia sinensis). J. Chem. Ecol. 2020, 46, 308–316. [Google Scholar] [CrossRef]
- Terefe, N.S.; Delon, A.; Buckow, R.; Versteeg, C. Blueberry polyphenol oxidase: Characterization and the kinetics of thermal and high pressure activation and inactivation. Food Chem. 2015, 188, 193–200. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Pang, L.; Li, Y.; Shao, J. Light exposure reduced browning enzyme activity and accumulated total phenols in cauliflower heads during cool storage. Postharvest Biol. Technol. 2014, 88, 17–20. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Yu, X.; Yagoub, A.E.A.; Zhang, Y.; Ma, H.; Gao, X.; Otu, P.N.Y. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT 2017, 77, 488–496. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Venkitasamy, C.; Wu, B.; Pan, Z.; Ma, H. Effect of pulsed light on activity and structural changes of horseradish peroxidase. Food Chem. 2017, 234, 20–25. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Z.; Wu, D.; Fei, X.; Ei-Seedi, H.R.; Wang, C. High-pressure homogenization influences the functional properties of protein from oyster (Crassostrea gigas). LWT 2021, 151, 112107. [Google Scholar] [CrossRef]
- Xu, B.; Sylvain Tiliwa, E.; Yan, W.; Roknul Azam, S.M.; Wei, B.; Zhou, C.; Ma, H.; Bhandari, B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2022, 152, 110744. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.S.; Bharate, S.B. Non-enzymatic browning in citrus juice: Chemical markers, their detection and ways to improve product quality. J. Food Sci. Technol. 2014, 51, 2271–2288. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Ji, J.; Mou, Y.; Chen, F.; Xiao, Z.; Liao, X.; Hu, X.; Ma, L. Polyphenol mediated non-enzymatic browning and its inhibition in apple juice. Food Chem. 2023, 404, 134504. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Bista, A.; Kebede, B.; Buvé, C.; Hendrickx, M.; Van Loey, A. Insight into non-enzymatic browning of shelf-stable orange juice during storage: A fractionation and kinetic approach. J. Sci. Food Agric. 2020, 100, 3765–3775. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Süfer, Ö.; Özaslan, Z.T.; Gowda, N.A.N.; Pulivarthi, M.K.; Charles, A.P.R.; Ramesh, B.; Ramniwas, S.; Rustagi, S.; Jafari, Z.; et al. Acrylamide in food products: Formation, technological strategies for mitigation, and future outlook. Food Front. 2024, 5, 1063–1095. [Google Scholar] [CrossRef]
- Sacchetti, G.; Ioannone, F.; De Gregorio, M.; Di Mattia, C.; Serafini, M.; Mastrocola, D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2016, 169, 44–52. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Yi, Y.; Wang, S.-Q.; Wang, H.-X.; Xu, W.; Min, T.; Wang, L.-M. Non-enzymatic browning of lotus root during boiling. LWT 2023, 173, 114191. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Kityo, P.; Buvé, C.; Hendrickx, M.E.; Van Loey, A.M. Influence of pH and Composition on Nonenzymatic Browning of Shelf-Stable Orange Juice during Storage. J. Agric. Food Chem. 2020, 68, 5402–5411. [Google Scholar] [CrossRef]
- Peng, H.; Gao, Y.; Zeng, C.; Hua, R.; Guo, Y.; Wang, Y.; Wang, Z. Effects of Maillard reaction and its product AGEs on aging and age-related diseases. Food Sci. Hum. Wellness 2024, 13, 1118–1134. [Google Scholar] [CrossRef]
- Cao, J.; Yang, C.; Zhang, J.; Zhang, L.; Tsao, R. Amadori compounds: Analysis, composition in food and potential health beneficial functions. Crit. Rev. Food Sci. Nutr. 2023, 65, 406–428. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Kontoudakis, N.; Scollary, G.R.; Clark, A.C. Production and Isomeric Distribution of Xanthylium Cation Pigments and Their Precursors in Wine-like Conditions: Impact of Cu(II), Fe(II), Fe(III), Mn(II), Zn(II), and Al(III). J. Agric. Food Chem. 2017, 65, 2414–2425. [Google Scholar] [CrossRef]
- Grant-Preece, P.; Barril, C.; Schmidtke, L.M.; Scollary, G.R.; Clark, A.C. Light-induced changes in bottled white wine and underlying photochemical mechanisms. Crit. Rev. Food Sci. Nutr. 2017, 57, 743–754. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Taip, F.S.; Saifullah, M.; Aziz, N.A.; Talib, R.A. Effect of packaging materials and storage temperature on the retention of physicochemical properties of vacuum packed pink guava powder. Food Packag. Shelf Life 2017, 12, 83–90. [Google Scholar] [CrossRef]
- Barry, C.S. The stay-green revolution: Recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci. 2009, 176, 325–333. [Google Scholar] [CrossRef]
- Queiroz Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Charng, Y.-Y. Chlorophyll dephytylation in chlorophyll metabolism: A simple reaction catalyzed by various enzymes. Plant Sci. 2021, 302, 110682. [Google Scholar] [CrossRef]
- Zhong, S.; Bird, A.; Kopec, R.E. The Metabolism and Potential Bioactivity of Chlorophyll and Metallo-chlorophyll Derivatives in the Gastrointestinal Tract. Mol. Nutr. Food Res. 2021, 65, 2000761. [Google Scholar] [CrossRef]
- Fang, H.; Luo, F.; Li, P.; Zhou, Q.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, H.; Ji, S. Potential of jasmonic acid (JA) in accelerating postharvest yellowing of broccoli by promoting its chlorophyll degradation. Food Chem. 2020, 309, 125737. [Google Scholar] [CrossRef]
- Bai, J.; Jin, K.; Qin, W.; Wang, Y.; Yin, Q. Proteomic Responses to Alkali Stress in Oats and the Alleviatory Effects of Exogenous Spermine Application. Front. Plant Sci. 2021, 12, 627129. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Zhang, M.; Wang, J.; Cheng, T.; Zhang, Q.; Pan, H. FsHemF is involved in the formation of yellow Forsythia leaves by regulating chlorophyll synthesis in response to light intensity. Plant Physiol. Biochem. 2023, 200, 107746. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Hasan, M.M.; Alotaibi, F.S.; Alabdallah, N.M.; Alharbi, B.M.; Ramadan, K.M.A.; Bendary, E.S.A.; Alshehri, D.; Jabborova, D.; Al-Balawi, D.A.; et al. Exogenous Putrescine Increases Heat Tolerance in Tomato Seedlings by Regulating Chlorophyll Metabolism and Enhancing Antioxidant Defense Efficiency. Plants 2022, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Q.; Ma, X.; Wang, M.; Sun, J.; Wang, S.; Wu, X.; Chen, X.; Wang, C.; Li, Q.; et al. New insight into the effect of riluzole on cadmium tolerance and accumulation in duckweed (Lemna turionifera). Ecotoxicol. Environ. Saf. 2022, 241, 113783. [Google Scholar] [CrossRef]
- Fang, Z.; Bouwkamp, J.C.; Solomos, T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar] [CrossRef]
- Lefebvre, T.; Talbi, A.; Atwi-Ghaddar, S.; Destandau, E.; Lesellier, E. Development of an analytical method for chlorophyll pigments separation by reversed-phase supercritical fluid chromatography. J. Chromatogr. A 2020, 1612, 460643. [Google Scholar] [CrossRef]
- Alabi, K.P.; Zhu, Z.; Sun, D.-W. Transport phenomena and their effect on microstructure of frozen fruits and vegetables. Trends Food Sci. Technol. 2020, 101, 63–72. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, P.; Zhu, Z.; Sun, D.-W. Development of a single/dual-frequency orthogonal ultrasound-assisted rapid freezing technique and its effects on quality attributes of frozen potatoes. J. Food Eng. 2020, 286, 110112. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Murtaza, A.; Iqbal, A.; Zhang, J.; Zhu, L.; Xu, X.; Pan, S.; Hu, W. High-pressure carbon dioxide treatment alleviates browning development by regulating membrane lipid metabolism in fresh-cut lettuce. Food Control 2022, 134, 108749. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Sinrod, A.G.J.; Dao, L.; Takeoka, G.; Williams, T.; Wood, D.; Bridges, D.F.; Powell-Palm, M.J.; Ukpai, G.; Chiou, B.; et al. Preservation of spinach by isochoric (constant volume) freezing. Int. J. Food Sci. Technol. 2020, 55, 2141–2151. [Google Scholar] [CrossRef]
- Nida, S.; Moses, J.A.; Anandharamakrishnan, C. Isochoric Freezing and Its Emerging Applications in Food Preservation. Food Eng. Rev. 2021, 13, 812–821. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Zhao, Y.; Takeoka, G.; Williams, T.; Wood, D.; Chiou, B.; Powell-Palm, M.J.; Wu, V.C.; Rubinsky, B.; McHugh, T. Effect of isochoric freezing on quality aspects of minimally processed potatoes. J. Food Sci. 2020, 85, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhao, M.; Argyropoulos, D. Machine learning based framework for the detection of mushroom browning using a portable hyperspectral imaging system. Postharvest Biol. Technol. 2025, 219, 113247. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, Z.; Jia, Z.; Hou, M.; Yang, X.; Ying, X.; Ji, Z. Artificial neural network-based shelf life prediction approach in the food storage process: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 12009–12024. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Yu, X.; Zhou, C.; Yagoub, A.E.A.; Li, D. A Catalytic Infrared System as a Hot Water Replacement Strategy: A Future Approach for Blanching Fruits and Vegetables to Save Energy and Water. Food Rev. Int. 2024, 40, 641–657. [Google Scholar] [CrossRef]
- You, Y.; Zhou, Y.; Duan, X.; Mao, X.; Li, Y. Research progress on the application of different preservation methods for controlling fungi and toxins in fruit and vegetable. Crit. Rev. Food Sci. Nutr. 2023, 63, 12441–12452. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, B.; Zhou, X.; Wang, S. Effects of combined radio frequency with hot water blanching on enzyme inactivation, color and texture of sweet potato. Innov. Food Sci. Emerg. Technol. 2020, 66, 102513. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Mujumdar, A.S.; Fang, X.-M.; Wang, J.; Pei, Y.-P.; Wu, W.; Zielinska, M.; Xiao, H.-W. High-humidity hot air impingement blanching (HHAIB) efficiently inactivates enzymes, enhances extraction of phytochemicals and mitigates brown actions of chili pepper. Food Control 2020, 111, 107050. [Google Scholar] [CrossRef]
- Alenyorege, E.A. Recent applications of high-humidity hot air impingement blanching (HHAIB) on plant materials: A review. Food Humanit. 2025, 5, 100657. [Google Scholar] [CrossRef]
- Saini, R.; Kaur, S.; Aggarwal, P.; Dhiman, A. The influence of conventional and novel blanching methods on potato granules, phytochemicals, and thermal properties of colored varieties. Front. Nutr. 2023, 10, 1178797. [Google Scholar] [CrossRef]
- Bei, X.; Yu, X.; Li, D.; Sun, Q.; Yu, Y.; Wang, Y.; Okonkwo, C.E.; Zhou, C. Heat source replacement strategy using catalytic infrared: A future for energy saving drying of fruits and vegetables. J. Food Sci. 2023, 88, 4827–4839. [Google Scholar] [CrossRef]
- Mao, C.; Wu, J.; Zhang, X.; Ma, F.; Cheng, Y. Improving the Solubility and Digestibility of Potato Protein with an Online Ultrasound-Assisted PH Shifting Treatment at Medium Temperature. Foods 2020, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Shinali, T.S.; Zhang, Y.; Altaf, M.; Nsabiyeze, A.; Han, Z.; Shi, S.; Shang, N. The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts. Foods 2024, 13, 1163. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-K.; Yang, Y.-T.; Gavahian, M.; Cheng, K.-C.; Hou, C.-Y.; Chen, M.-H.; Santoso, S.P.; Hsieh, C.-W. Prolonging the shelf-life of atemoya (Annona cherimola × Annona squamosa) using pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2023, 88, 103458. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Liu, D.; Fan, J. Effect of heat and pulsed electric field treatment on the physicochemical and nutritional properties of carrots. J. Sci. Food Agric. 2023, 103, 1514–1521. [Google Scholar] [CrossRef]
- Zhang, X.-j.; Zhang, M.; Law, C.L.; Guo, Z. High-voltage electrostatic field-assisted modified atmosphere packaging for long-term storage of pakchoi and avoidance of off-flavors. Innov. Food Sci. Emerg. Technol. 2022, 79, 103032. [Google Scholar] [CrossRef]
- Cukrov, D.; Brizzolara, S.; Tonutti, P. Chapter 20—Physiological and Biochemical Effects of Controlled and Modified Atmospheres. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 425–441. [Google Scholar]
- Shi, X.; Xiao, H.; Kanamori, K.; Yonezu, A.; Lackner, K.S.; Chen, X. Moisture-Driven CO2 Sorbents. Joule 2020, 4, 1823–1837. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z. Microporous modified atmosphere packaging to extend shelf life of fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 51–65. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Comparative study on nitrogen and argon-based modified atmosphere packaging on microbiological, chemical, and sensory attributes as well as on microbial diversity of Asian sea bass. Food Packag. Shelf Life 2019, 22, 100404. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Wang, M.; Li, R.; Dai, J.; Yan, J.; Qin, W.; Liu, Y. 3D printing of essential oil/β-cyclodextrin/popping candy modified atmosphere packaging for strawberry preservation. Carbohydr. Polym. 2022, 297, 120037. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, M.; Devahastin, S.; Guo, Z. Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biol. Technol. 2019, 149, 159–165. [Google Scholar] [CrossRef]
- Tsikrika, K.; O’Brien, N.; Rai, D.K. The Effect of High Pressure Processing on Polyphenol Oxidase Activity, Phytochemicals and Proximate Composition of Irish Potato Cultivars. Foods 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.B.; Wibisono, R.; Farr, J.; Hallett, I.; Richter, L.; Oey, I.; Wohlers, M.; Zhou, J.; Fletcher, G.C.; Requejo-Jackman, C. Effect of high pressure processing on avocado slices. Innov. Food Sci. Emerg. Technol. 2013, 18, 65–73. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; McClements, D.J.; Liu, X.; Yi, J.; Lyu, J.; Zhou, M.; Verkerk, R.; Dekker, M.; Wu, X.; et al. Impacts of thermal and non-thermal processing on structure and functionality of pectin in fruit- and vegetable-based products: A review. Carbohydr. Polym. 2020, 250, 116890. [Google Scholar] [CrossRef]
- Wen, B.; Cui, S.; Suo, X.; Supapvanich, S. Stress response of fresh-cut potatoes to laser irradiation before processing can prevent discoloration and maintain overall quality. Postharvest Biol. Technol. 2023, 197, 112213. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, X.; Ma, Y.; Zhang, Y.; Wang, D. Reduction of enzymatic browning of fresh-cut Chinese yam (Dioscorea opposita) by UV-C treatment. Int. Food Res. J. 2021, 28, 207–216. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. UVC radiation for food safety: An emerging technology for the microbial disinfection of food products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- Manzocco, L.; Nicoli, M.C. Surface Processing: Existing and Potential Applications of Ultraviolet Light. Crit. Rev. Food Sci. Nutr. 2015, 55, 469–484. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Xu, B.; Yagoub, A.E.A.; Mustapha, A.T.; Zhou, C. Effect of intensive pulsed light on the activity, structure, physico-chemical properties and surface topography of polyphenol oxidase from mushroom. Innov. Food Sci. Emerg. Technol. 2021, 72, 102741. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Trif, M.; Ozogul, F.; Kumar, M.; Chaudhary, V.; Vukic, M.; Tomar, M.; Changan, S. Recent developments in cold plasma-based enzyme activity (browning, cell wall degradation, and antioxidant) in fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1958–1978. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, J.; Wang, Z.; Yang, X.; Jia, L. The influence of gamma irradiation on the storage quality of bamboo shoots. Radiat. Phys. Chem. 2019, 159, 124–130. [Google Scholar] [CrossRef]
- Otero, L.; Pozo, A. Effects of the application of static magnetic fields during potato freezing. J. Food Eng. 2022, 316, 110838. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, W.; Sun, D.-W. Effects of liquid nitrogen quick freezing on polyphenol oxidase and peroxide activities, cell water states and epidermal microstructure of wolfberry. LWT 2020, 120, 108923. [Google Scholar] [CrossRef]
- Qing, S.; Long, Y.; Wu, Y.; Shu, S.; Zhang, F.; Zhang, Y.; Yue, J. Hot-air-assisted radio frequency blanching of broccoli: Heating uniformity, physicochemical parameters, bioactive compounds, and microstructure. J. Sci. Food Agric. 2023, 103, 2664–2674. [Google Scholar] [CrossRef]
- Zhang, J.; Yagoub, A.E.A.; Sun, Y.; Arun, M.S.; Ma, H.; Zhou, C. Role of thermal and non-thermal drying techniques on drying kinetics and the physicochemical properties of shiitake mushroom. J. Sci. Food Agric. 2022, 102, 214–222. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Pan, Z.; Ye, X.; Ma, H. Effectiveness of combined catalytic infrared radiation and holding time for decontamination Aspergillus niger on dried shiitake mushrooms (Lentinus edodes) with different moisture contents. LWT 2023, 176, 114503. [Google Scholar] [CrossRef]
- Nguyen, T.V.L.; Tran, T.Y.N.; Lam, D.T.; Bach, L.G.; Nguyen, D.C. Effects of microwave blanching conditions on the quality of green asparagus (Asparagus officinalis L.) butt segment. Food Sci. Nutr. 2019, 7, 3513–3519. [Google Scholar] [CrossRef]
- Sicari, V.; Romeo, R.; Leporini, M.; Pellicanò, T.M.; Tundis, R.; Loizzo, M.R. Comparison of traditional hot water and vacuum assisted blanching methods on the physico-chemical quality parameters and antioxidant activity of zucchini (Cucurbita pepo L.) slices. J. Food Meas. Charact. 2022, 16, 281–294. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Majumdar, A.S.; Zhou, C. Effect of freeze-thaw pretreatment combined with variable temperature on infrared and convection drying of lotus root. LWT 2022, 154, 112804. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, B.; Guo, X.; Liu, D.; Wu, P.; Ma, H.; Pan, Z. Ultrasonication and thermosonication blanching treatments of carrot at varying frequencies: Effects on peroxidase inactivation mechanisms and quality characterization evaluation. Food Chem. 2021, 343, 128524. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, B.; Guo, X.; Liu, D.; Qiu, C.; Ma, H. Effect of thermosonication on texture degradation of carrot tissue in relation to alterations in cell membrane and cell wall structure. Food Chem. 2022, 393, 133335. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Tuly, J.; Hong, C.; Ma, H. Multi-frequency thermosonication washing treatments of fresh-cut lotus root: Effect on the accumulation of phenolics and storage quality characterization evaluation. Innov. Food Sci. Emerg. Technol. 2024, 93, 103641. [Google Scholar] [CrossRef]
- Wu, B.; Ma, Y.; Guo, X.; Guo, Y.; Qiu, C.; Gao, K.; Ma, H.; Pan, Z. Catalytic infrared blanching and drying of carrot slices with different thicknesses: Effects on surface dynamic crusting and quality characterization. Innov. Food Sci. Emerg. Technol. 2023, 88, 103444. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, B.; Yagoub, A.E.A.; Ma, H.; Sun, Y.; Xu, X.; Yu, X.; Zhou, C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, B.; Guo, X.; Ding, F.; Pan, Z.; Ma, H. Effects of power ultrasound enhancement on infrared drying of carrot slices: Moisture migration and quality characterizations. LWT 2020, 126, 109312. [Google Scholar] [CrossRef]
- Waseem, M.; Akhtar, S.; Ahmad, N.; Ismail, T.; Lazarte, C.E.; Hussain, M.; Manzoor, M.F.; Al-Farga, A. Effect of Microwave Heat Processing on Nutritional Indices, Antinutrients, and Sensory Attributes of Potato Powder-Supplemented Flatbread. J. Food Qual. 2022, 2022, 2103884. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Wang, Y. Electromagnetic fields assisted blanching—Effect on the dielectric and physicochemical properties of cabbage. J. Food Process Eng. 2019, 42, e13294. [Google Scholar] [CrossRef]
- Osae, R.; Zhou, C.; Xu, B.; Tchabo, W.; Tahir, H.E.; Mustapha, A.T.; Ma, H. Effects of ultrasound, osmotic dehydration, and osmosonication pretreatments on bioactive compounds, chemical characterization, enzyme inactivation, color, and antioxidant activity of dried ginger slices. J. Food Biochem. 2019, 43, e12832. [Google Scholar] [CrossRef]
- Mashabela, M.; Mahajan, P.V.; Sivakumar, D. Influence of different types of modified atmosphere packaging films and storage time on quality and bioactive compounds in fresh-cut cauliflower. Food Packag. Shelf Life 2019, 22, 100374. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wang, X.; Liu, X.; Zhu, X.; Zhang, J. Integration of Metabolome and Transcriptome Profiling Reveals the Effect of Modified Atmosphere Packaging (MAP) on the Browning of Fresh-Cut Lanzhou Lily (Lilium davidii var. unicolor) Bulbs during Storage. Foods 2023, 12, 1335. [Google Scholar] [CrossRef]
- Jin, S.; Ding, Z.; Xie, J. Modified Atmospheric Packaging of Fresh-Cut Amaranth (Amaranthus tricolor L.) for Extending Shelf Life. Agriculture 2021, 11, 1016. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, X.; Ma, Y.; Guan, H.; Liang, H.; Wang, D. Inhibitory effect of modified atmosphere packaging on Escherichia coli O157:H7 in fresh-cut cucumbers (Cucumis sativus L.) and effectively maintain quality during storage. Food Chem. 2022, 369, 130969. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Machado, T.B.; Alves, M.R.; Oliveira, M.B.P.P. Fresh-Cut Bell Peppers in Modified Atmosphere Packaging: Improving Shelf Life to Answer Food Security Concerns. Molecules 2020, 25, 2323. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest application of hydrogen-rich water not only affects daylily bud yield but also contributes to the alleviation of bud browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Elwan, M.W.M.; Nasef, I.N.; El-Seifi, S.K.; Hassan, M.A.; Ibrahim, R.E. Storability, shelf-life and quality assurance of sugar snap peas (cv. super sugar snap) using modified atmosphere packaging. Postharvest Biol. Technol. 2015, 100, 205–211. [Google Scholar] [CrossRef]
- Yang, T.-D.; Chen, Y.-l.; Zeng, F.-K.; Ye, M.-Q.; Wang, L.; Luo, Z.; Qi, Y.-W.; Chen, F.-P. Effects of modified atmosphere packaging on the postharvest quality of mulberry leaf vegetable. Sci. Rep. 2022, 12, 10893. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, J.; Li, Z.; Zhang, J.; Pan, Y. Ethanol fumigation combined with modified atmosphere packaging delays potato greening under light. Plant Physiol. Biochem. 2023, 202, 107962. [Google Scholar] [CrossRef]
- Almuhayawi, S.M.; Almuhayawi, M.S.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.A. Effect of Laser Light on Growth, Physiology, Accumulation of Phytochemicals, and Biological Activities of Sprouts of Three Brassica Cultivars. J. Agric. Food Chem. 2021, 69, 6240–6250. [Google Scholar] [CrossRef]
- Ufuk Kasım, M.; Kasım, R. Yellowing of fresh-cut spinach (Spinacia oleracea L.) leaves delayed by UV-B applications. Inf. Process. Agric. 2017, 4, 214–219. [Google Scholar] [CrossRef]
- Valerga, L.; González, R.E.; Pérez, M.B.; Concellón, A.; Cavagnaro, P.F. Differential and Cultivar-Dependent Antioxidant Response of Whole and Fresh-Cut Carrots of Different Root Colors to Postharvest UV-C Radiation. Plants 2023, 12, 1297. [Google Scholar] [CrossRef]
- Zhang, J.; Yagoub, A.E.A.; Sun, Y.; Mujumdar, A.S.; Ma, H.; Wahia, H.; Zhou, C. Intensive pulsed light pretreatment combined with controlled temperature and humidity for convection drying to reduce browning and improve quality of dried shiitake mushrooms. J. Sci. Food Agric. 2021, 101, 5608–5617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Antimicrobial mechanism of pulsed light for the control of Escherichia coli O157:H7 and its application in carrot juice. Food Control 2019, 106, 106751. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; He, R.; Cui, H. Action mechanism of pulsed magnetic field against E. coli O157:H7 and its application in vegetable juice. Food Control 2019, 95, 150–156. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, B.; Lu, D.; Pan, Z.; Ma, H. Tri-frequency ultrasound as pretreatment to infrared drying of carrots: Impact on enzyme inactivation, color changes, nutrition quality parameters and microstructures. Int. J. Food Eng. 2021, 17, 275–284. [Google Scholar] [CrossRef]
- Osae, R.; Zhou, C.; Tchabo, W.; Xu, B.; Bonah, E.; Alenyorege, E.A.; Ma, H. Optimization of osmosonication pretreatment of ginger (Zingiber officinale Roscoe) using response surface methodology: Effect on antioxidant activity, enzyme inactivation, phenolic compounds, and physical properties. J. Food Process Eng. 2019, 42, e13218. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, D.; Ma, H.; Chen, X. Ultrasound accelerated γ-aminobutyric acid accumulation in coffee leaves through influencing the microstructure, enzyme activity, and metabolites. Food Chem. 2022, 385, 132646. [Google Scholar] [CrossRef]
- Faisal Manzoor, M.; Ahmed, Z.; Ahmad, N.; Karrar, E.; Rehman, A.; Aadil, R.M.; Al-Farga, A.; Iqbal, M.W.; Rahaman, A.; Zeng, X. Probing the combined impact of pulsed electric field and ultra-sonication on the quality of spinach juice. J. Food Process. Preserv. 2021, 45, e15475. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, B.; Xu, J. Selective enzyme inactivation in a simulated system and in cabbage juice using electrospray technology. Innov. Food Sci. Emerg. Technol. 2022, 75, 102875. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.; Aheto, J.H.; Agyekum, A.A.; Zhou, C. Effect of sequential multi-frequency ultrasound washing processes on quality attributes and volatile compounds profiling of fresh-cut Chinese cabbage. LWT 2020, 117, 108666. [Google Scholar] [CrossRef]
- Gong, W.; Shi, B.; Zeng, F.K.; Dong, N.; Lei, Z.; Liu, J. Evaluation of cooking, nutritional, and quality characteristics of fresh-cut potato slice pretreated with acetic acid. J. Food Sci. 2022, 87, 427–437. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef] [PubMed]
- Gérard, V.; Ay, E.; Graff, B.; Morlet-Savary, F.; Galopin, C.; Mutilangi, W.; Lalevée, J. Ascorbic Acid Derivatives as Potential Substitutes for Ascorbic Acid To Reduce Color Degradation of Drinks Containing Ascorbic Acid and Anthocyanins from Natural Extracts. J. Agric. Food Chem. 2019, 67, 12061–12071. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.-N.; Zhou, Y.-Y.; Yang, Y.-N. Kinetics of browning and correlations between browning degree and pyrazine compounds in l-ascorbic acid/acidic amino acid model systems. Food Chem. 2017, 221, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Šamec, D.; Pavlović, I.; Radojčić Redovniković, I.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, X.; Kitazawa, H.; Wang, X.; Guo, Y.; Li, L.; Liu, H.; Wang, Y.; Wang, J. Characterization of bioactive films loaded with melatonin and regulation of postharvest ROS scavenging and ascorbate-glutathione cycle in Agaricus bisporus. Postharvest Biol. Technol. 2022, 194, 112107. [Google Scholar] [CrossRef]
- Sruthi, S.; Millot, N.; Mohanan, P.V. Zinc oxide nanoparticles mediated cytotoxicity, mitochondrial membrane potential and level of antioxidants in presence of melatonin. Int. J. Biol. Macromol. 2017, 103, 808–818. [Google Scholar] [CrossRef]
- Fan, X. Chemical inhibition of polyphenol oxidase and cut surface browning of fresh-cut apples. Crit. Rev. Food Sci. Nutr. 2023, 63, 8737–8751. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Y.; Jiang, Y.; Zhang, Z. Advances in control technologies and mechanisms to treat peel browning in postharvest fruit. Sci. Hortic. 2023, 311, 111798. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Azooz, M.M. Concentration-dependent effects of tungstate on germination, growth, lignification-related enzymes, antioxidants, and reactive oxygen species in broccoli (Brassica oleracea var. italica L.). Environ. Sci. Pollut. Res. 2019, 26, 36441–36457. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zhai, S.; Xu, X.-T.; Lu, Y.-T.; Yuan, T.-T. Hydrogen peroxide participates in leaf senescence by inhibiting CHLI1 activity. Plant Cell Rep. 2024, 43, 258. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Pochodylo, A.L.; Aristilde, L. Molecular dynamics of stability and structures in phytochelatin complexes with Zn, Cu, Fe, Mg, and Ca: Implications for metal detoxification. Environ. Chem. Lett. 2017, 15, 495–500. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, H.; Tu, J.; He, Y.; Zhou, Z.; Wang, R.; Jin, W.; Han, J.; Liu, W. Effect of pH, protein/polysaccharide ratio and preparation method on the stability of lactoferrin-polysaccharide complexes. Food Chem. 2024, 456, 140056. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Hussain, S. Effect of postharvest oxalic acid application on enzymatic browning and quality of lotus (Nelumbo nuciferaGaertn.) root slices. Food Chem. 2020, 312, 126051. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ejaz, S.; Sardar, H. Effect of pre-storage ascorbic acid and Aloe vera gel coating application on enzymatic browning and quality of lotus root slices. J. Food Biochem. 2020, 44, e13136. [Google Scholar] [CrossRef]
- Chung, Y.B.; Song, H.; Jo, K.; Suh, H.J. Effect of ascorbic acid and citric acid on the quality of salted Chinese cabbage during storage. Food Sci. Biotechnol. 2021, 30, 227–234. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Nawaz, A.; Naz, S.; Ejaz, S.; Ullah, S. Glutathione application delays surface browning of fresh-cut lotus (Nelumbo nucifera Gaertn.) root slices during low temperature storage. Postharvest Biol. Technol. 2023, 200, 112311. [Google Scholar] [CrossRef]
- Li, F.; Han, Q.; Wang, W.; Wu, S. Carboxymethyl chitosan-based coatings loaded with glutathione extend the shelf-life of harvested enoki mushrooms (Flammulina velutipes). LWT 2022, 166, 113807. [Google Scholar] [CrossRef]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Beneficial impact of exogenous arginine, cysteine and methionine on postharvest senescence of broccoli. Food Chem. 2021, 338, 128055. [Google Scholar] [CrossRef]
- Wen, B.; Li, D.; Tang, D.; Huang, Z.; Kedbanglai, P.; Ge, Z.; Du, X.; Supapvanich, S. Effects of simultaneous ultrasonic and cysteine treatment on antibrowning and physicochemical quality of fresh-cut lotus roots during cold storage. Postharvest Biol. Technol. 2020, 168, 111294. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, D.; Zhao, L.; Liu, Y.; Wang, C.; Farid, M.S.; Gu, Y.; Li, J.; Li, T.; Sun, Y.; et al. l-Cysteine Treatment Delayed the Quality Deterioration of Fresh-Cut Button Mushrooms by Regulating Oxygen Metabolism, Inhibiting Water Loss, and Stimulating Endogenous H2S Production. J. Agric. Food Chem. 2023, 71, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Shekari, A.; Hassani, R.N.; Aghdam, M.S.; Rezaee, M.; Jannatizadeh, A. The effects of melatonin treatment on cap browning and biochemical attributes of Agaricus bisporus during low temperature storage. Food Chem. 2021, 348, 129074. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Hu, H.; Wang, Y.; Zhou, H.; Zhang, Y.; Zhang, L.; Li, P. The role of melatonin in alleviating the postharvest browning of lotus seeds through energy metabolism and membrane lipid metabolism. Postharvest Biol. Technol. 2020, 167, 111243. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Ding, Z.; Xie, J. Prolong the postharvest shelf life of spinach through the antioxidative ability of melatonin. Food Chem. X 2023, 19, 100769. [Google Scholar] [CrossRef]
- Sun, L.; Luo, S.; Huali, H.; Zhou, H.; Zhang, Y.; An, R.; Ling, J.; Li, P. Melatonin promotes the normal cellular mitochondrial function of lotus seeds through stimulating nitric oxide production. Postharvest Biol. Technol. 2022, 185, 111814. [Google Scholar] [CrossRef]
- Hu, H.; Luo, S.; An, R.; Li, P. Endogenous melatonin delays sepal senescence and extends the storage life of broccoli florets by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 2022, 188, 111894. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Ma, H.; Ayim, I.; Aheto, J.H.; Hong, C.; Zhou, C. Reduction of Listeria innocua in fresh-cut Chinese cabbage by a combined washing treatment of sweeping frequency ultrasound and sodium hypochlorite. LWT 2019, 101, 410–418. [Google Scholar] [CrossRef]
- He, M.; Fan, M.; Liu, W.; Li, Y.; Wang, G. Design, synthesis, molecular modeling, and biological evaluation of novel kojic acid derivatives containing bioactive heterocycle moiety as inhibitors of tyrosinase and antibrowning agents. Food Chem. 2021, 362, 130241. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, D.; Sun, Y.; Meng, Z.; Malik, A.U.; Zhang, S.; Yan, H.; Wang, Q. A novel anti-browning agent S-furfuryl thioacetate for fresh-cut potato screened from food-derived sulfur compounds. Postharvest Biol. Technol. 2022, 192, 112007. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, Y.; Meng, Z.; Sui, X.; Zhang, D.; Yan, H.; Wang, Q. S-Ethyl thioacetate as a natural anti-browning agent can significantly inhibit the browning of fresh-cut potatoes by decreasing polyphenol oxidase activity. Sci. Hortic. 2022, 305, 111427. [Google Scholar] [CrossRef]
- Wang, G.; He, M.; Huang, Y.; Peng, Z. Synthesis and biological evaluation of new kojic acid-1,3,4-oxadiazole hybrids as tyrosinase inhibitors and their application in the anti-browning of fresh-cut mushrooms. Food Chem. 2023, 409, 135275. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Mo, L.; Zou, Y.; Zhao, G. Tyrosinase inhibitory mechanism and the anti-browning properties of piceid and its ester. Food Chem. 2022, 390, 133207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, Y.; Meng, W.; Pei, L.; Zhang, X. Browning inhibition of seabuckthorn leaf extract on fresh-cut potato sticks during cold storage. Food Chem. 2022, 389, 133076. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, L.; Sun, Y.; Yu, K.; Yu, W.; Tian, Y.; Liu, J.; Zou, L.; Liu, W. Polyphenol oxidase inhibited by 4-hydroxycinnamic acid and naringenin: Multi-spectroscopic analyses and molecular docking simulation at different pH. Food Chem. 2022, 396, 133662. [Google Scholar] [CrossRef]
- Kaya, E.D.; Türkhan, A.; Gür, F.; Gür, B. A novel method for explaining the product inhibition mechanisms via molecular docking: Inhibition studies for tyrosinase from Agaricus bisporus. J. Biomol. Struct. Dyn. 2022, 40, 7926–7939. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, G.; Tang, J.; Yao, C.; Li, P.; Song, Q.; Wang, C. Inhibitory effect of chlorogenic acid on polyphenol oxidase and browning of fresh-cut potatoes. Postharvest Biol. Technol. 2020, 168, 111282. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Yang, Q.; Yang, H.; Li, Y.; Zhou, B.; Li, T.; Gao, Y.; Qiao, L. Cod peptides inhibit browning in fresh-cut potato slices: A potential anti-browning agent of random peptides for regulating food properties. Postharvest Biol. Technol. 2018, 146, 36–42. [Google Scholar] [CrossRef]
- Rivero-Pino, F. Bioactive food-derived peptides for functional nutrition: Effect of fortification, processing and storage on peptide stability and bioactivity within food matrices. Food Chem. 2023, 406, 135046. [Google Scholar] [CrossRef]
- Arias, E.; González, J.; Peiró, J.M.; Oria, R.; Lopez-Buesa, P. Browning Prevention by Ascorbic Acid and 4-Hexylresorcinol: Different Mechanisms of Action on Polyphenol Oxidase in the Presence and in the Absence of Substrates. J. Food Sci. 2007, 72, C464–C470. [Google Scholar] [CrossRef]
- de OG Silva, C.; Sun, P.; Barrett, K.; Sanders, M.G.; van Berkel, W.J.; Kabel, M.A.; Meyer, A.S.; Agger, J.W. Polyphenol oxidase activity on guaiacyl and syringyl lignin units. Angew. Chem. 2024, 136, e202409324. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Bi, J.; Li, X.; Xin, G. The roles of soluble poly and insoluble tannin in the enzymatic browning during storage of dried persimmon. Food Chem. 2022, 366, 130632. [Google Scholar] [CrossRef] [PubMed]
- de Chiara, M.L.V.; Castagnini, J.M.; Capozzi, V. Cutting-edge physical techniques in postharvest for fruits and vegetables: Unveiling their power, inclusion in ‘hurdle’ approach, and latest applications. Trends Food Sci. Technol. 2024, 151, 104619. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Wagle, A.; Min, B.S.; Jung, H.A.; Choi, J.S. Antioxidant and anti-browning property of 2-arylbenzofuran derivatives from Morus alba Linn root bark. Food Chem. 2020, 309, 125739. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Cheun, C.F.; Wong, C.W. Inhibition of enzymatic browning in sweet potato (Ipomoea batatas (L.)) with chemical and natural anti-browning agents. J. Food Process. Preserv. 2019, 43, e14195. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, X.; Zheng, J.; Wang, T.; You, X.; Liu, H.; Liu, X. The effect of ultrasonic on reducing anti-browning minimum effective concentration of purslane extract on fresh-cut potato slices during storage. Food Chem. 2021, 343, 128401. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Wang, Q.; Liu, J.; Lu, Y.; Shi, Y. Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots. Molecules 2021, 26, 1369. [Google Scholar] [CrossRef]
- Tanhaş, E.; Martin, E.; Korucu, E.N.; Dirmenci, T. Effect of aqueous extract, hydrosol, and essential oil forms of some endemic Origanum L. (Lamiaceae) taxa on polyphenol oxidase activity in fresh-cut mushroom samples. J. Food Process. Preserv. 2020, 44, e14726. [Google Scholar] [CrossRef]
- Xiao, Y.; He, J.; Zeng, J.; Yuan, X.; Zhang, Z.; Wang, B. Application of citronella and rose hydrosols reduced enzymatic browning of fresh-cut taro. J. Food Biochem. 2020, 44, e13283. [Google Scholar] [CrossRef]
- Jirasuteeruk, C.; Theerakulkait, C. Ultrasound-Assisted Extraction of Phenolic Compounds from Mango (Mangifera indica cv. Chok Anan) Peel and Its Inhibitory Effect on Enzymatic Browning of Potato Puree. Food Technol. Biotechnol. 2019, 57, 350–357. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, N.; Yagoub, A.E.A.; Chen, L.; Mustapha, A.T.; Yu, X.; Zhou, C. Ultrasound-assisted probiotics fermentation suspension treatment under mild heat to improve the storage quality of freshly cut lotus root. Food Chem. 2022, 397, 133823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Yagoub, A.E.A.; Xia, G.; Zhou, C. Effect of vacuum impregnation assisted probiotics fermentation suspension on shelf life quality of freshly cut lotus root. Food Chem. 2022, 381, 132281. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, W.; Li, C.; Chen, X.; Cui, H.; Lin, L. Pickering emulsion stabilized by gliadin/soybean polysaccharide composite colloidal nanoparticle: Physicochemical properties and its application on washing of fresh-cut cabbage. Food Res. Int. 2022, 161, 111886. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Yuan, L.; Surendhiran, D.; Lin, L. Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int. J. Food Microbiol. 2018, 268, 1–9. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Cui, H. Synergistic efficacy of pulsed magnetic fields and Litseacubeba essential oil treatment against Escherichia coli O157:H7 in vegetable juices. Food Control 2019, 106, 106686. [Google Scholar] [CrossRef]
- Duan, L.; Jiang, T.; Zhou, Y.; Bai, X.; Wang, Y.; Lü, X.; Xia, X.; Lin, L.; Shi, C. The inactivation of Shigella flexneri by synergistic effect of ultrasound combined with basil essential oil nanoemulsion and application in cabbage cleaning. Food Control 2024, 156, 110142. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157:H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef]
- Song, L.; Yang, H.; Cheng, S.; Zhang, Z.; Zhang, L.; Su, R.; Li, Y.; Zhan, X.; Yang, B.; Lin, L.; et al. Combination effects of ultrasound and citral nanoemulsion against Shigella flexneri and the preservation effect on fresh-cut carrots. Food Control 2024, 155, 110069. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Li, Y.; Huang, X.; Li, Z.; Zhai, X.; Shi, J.; Zou, X.; Xiao, J.; Sun, Y.; et al. Sodium alginate/guar gum based nanocomposite film incorporating β-Cyclodextrin/persimmon pectin-stabilized baobab seed oil Pickering emulsion for mushroom preservation. Food Chem. 2024, 437, 137891. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Bacha, S.A.S.; Liang, Q.; Liu, Y.; Kang, L.; Chi, Z.; Chi, R.; Han, X.; Ekumah, J.-N.; et al. Preparation and functional characterization of pullulan-sodium alginate composite film enhanced with ultrasound-assisted clove essential oil Nanoemulsions for effective preservation of cherries and mushrooms. Food Chem. 2024, 457, 140048. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition of Escherichia coli O157:H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT 2020, 117, 108656. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Abdel-Samie, M.A.; Cui, H.; Lin, L. Unraveling the inhibitory mechanism of clove essential oil against Listeria monocytogenes biofilm and applying it to vegetable surfaces. LWT 2020, 134, 110210. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Li, C.; Abdel-Samie, M.A.; Siva, S.; Cui, H. Cold nitrogen plasma modified cuminaldehyde/β-cyclodextrin inclusion complex and its application in vegetable juices preservation. Food Res. Int. 2021, 141, 110132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, Y.; Wang, J.; Dhanasekaran, S.; Yue, Q.; Feng, F.; Gu, X.; Li, B.; Zhao, L.; Zhang, H. Characterization of a Bacillus velezensis strain as a potential biocontrol agent against soft rot of eggplant fruits. Int. J. Food Microbiol. 2024, 410, 110480. [Google Scholar] [CrossRef]
- Rocchetti, G.; Galimberti, S.; Callegari, M.L.; Lucini, L. Metabolomics and proteomics approaches provide a better understanding of non-enzymatic browning and pink discoloration in dairy products: A mini review. Food Biosci. 2023, 56, 103328. [Google Scholar] [CrossRef]
- Huang, J.; Li, M.; Han, C.; Zhang, Z.; Liu, X.; Ying, Z.; Yin, P.; Yang, L. Structural and mechanistic insights into the anti-tyrosinase, anti-melanogenesis, and anti-browning effect of proanthocyanidins from seed coats of Acer truncatum Bunge. Int. J. Biol. Macromol. 2025, 284, 138246. [Google Scholar] [CrossRef]
- Shi, J.; Xie, W.; Li, S.; Wang, Y.; Wang, Q.; Li, Q. Prohibitin StPHB3 affects the browning of fresh-cut potatoes via influencing antioxidant capacity and polyphenol oxidase activation. Postharvest Biol. Technol. 2024, 207, 112598. [Google Scholar] [CrossRef]
| Methods | Applications | Conclusions | References | |
|---|---|---|---|---|
| Cold treatment | Orthogonal ultrasound-assisted freezing | Potato | Sample tissue structure integrity, and significant increase in phenolic content | [47] |
| Static magnetic fields freezing | Potato | SMF does not significantly affect enzymatic browning | [82] | |
| Isometric freezing | Spinach | Thawed samples had similar cellular integrity and lysis as fresh spinach | [49] | |
| Potato | The total phenolic content and antioxidant capacity of the samples increased and browning was delayed by 1 week | [51] | ||
| Liquid nitrogen spray freezing | Chinese matrimony vine | PPO activity was significantly reduced | [83] | |
| Heat treatment | High-humidity hot air impingement blanching | Chili peppers | Reduce the residual activity (0.52%) and Browning index (7.09) of POD | [57] |
| Hot air-assisted radio-frequency heating | Red bell pepper | Higher ascorbic acid retention and free radical scavenging activity | [84] | |
| Infrared hot bleaching | Mushroom | IRHAD provides samples with the highest oxidation resistance and the lowest total color difference. | [85] | |
| Catalytic infrared heating coupled with holding time (CIRH) | Mushroom | CIRH enhanced the maintenance of microstructure and increased the retention of total phenols | [86] | |
| Microwave ironing and bleaching | Asparagus | The highest free radical scavenging capacity and total phenol content were found in samples blanched at 300 W for 4 min | [87] | |
| Vacuum-assisted blanching | Zucchini slice | Less color difference with hot water rinsing, TFC reduced to 39.91%. | [88] | |
| Infrared and convection drying | Lotus root | Inhibits the activity of PPO and POD enzymes | [89] | |
| Thermosonication blanching | Carrot | 22/40 kHz downregulated POD genes expression | [90] | |
| Carrot | Thermal sonication at 60 °C significantly increased the hardness of the sample tissue. | [91] | ||
| Lotus root | TS decreased the browning degree, POD activity and MDA content | [92] | ||
| Catalytic infrared heating | Carrot | 90% inactivation of POD enzyme activity by short-term CIR blanching | [93] | |
| Infrared hot air drying | Garlic | IRHAD retains nutrients and antioxidant activity. | [94] | |
| Bitter Gourd | BPS-I exhibits enhanced antioxidant activity in vitro | [95] | ||
| Ultrasound infrared drying | Carrot | US-IR drying does not adversely affect the color of the sample | [96] | |
| Microwave heat | Potato | 1.1 kW microwave heating can reduce the anti-nutrient load of potato | [97] | |
| Electromagnetic field-assisted blanching | Cabbage | The dielectric properties of blanched cabbage were significantly improved and color retention was increased | [98] | |
| Osmosonication drying | Ginger | Significantly improves the efficiency of enzyme inactivation | [99] | |
| Gas-conditioning treatment | Map | Cauliflower | 6.06% O2; 11.43% CO2 can inhibit browning most effectively | [100] |
| Lily | 10% O2, 5% CO2, 85% N2 inhibits the synthesis and accumulation of phenolics, as well as oxidative reactions | [101] | ||
| Amaranth | 10% O2, 10% CO2 and 80% N2 are effective in maintaining chlorophyll and ascorbic acid content and antioxidant enzyme activities | [102] | ||
| Cucumber | 2% O2, 7% CO2, and 91% N2 reduced the ability of bacterial biofilm formation | [103] | ||
| Bell pepper | 10% O2; 45% CO2 slows down metabolism | [104] | ||
| Daylily bud yield | HRW treatment maintained redox balance by inhibiting O2•− and H2O2 accumulation | [105] | ||
| Microporous MAP | Bean pods | MPPP12 (14% O2 and 4% CO2) effectively preserved chlorophyll content | [106] | |
| Mulberry leaf lettuce | Modified polyethylene packaging reduced the respiration intensity of the samples, and the inhibition intensity was positively correlated with the enzyme activity inhibition rate | [107] | ||
| Ethanol fumigation MAP | Potato | Effectively prevents the conversion of branched-chain starch to chloroplasts | [108] | |
| High-pressure treatment | High pressure inert gases | Potato | 4 MPa high pressure effectively inhibited respiration rate and biofilm oxidation | [71] |
| Irradiation treatment | Diode laser irradiation (450 nm) | Potato | Inhibition of PPO and POD expression and enhancement of antioxidant activity | [75] |
| He-Ne laser irradiation | Shepherd’s purse Cauliflower Turnip | Enhanced antioxidant, antibacterial, anti-inflammatory, and anticancer activities | [109] | |
| UV-B | Spinach | High-dose UV-B irradiation reduced leaf yellowing | [110] | |
| UV-C | Carrot | Increased total phenolic content and antioxidant capacity | [111] | |
| Intensive pulsed light | Mushroom | LPL altered the secondary and tertiary structure of PPO leading to inactivation | [79] | |
| Shiitake mushroom | After 25 pulses of IPL energy of 400 J, the PPO activity was reduced by 42.83% | [112] | ||
| Carrot juice | The production of hydroxyl radicals accelerates the death of bacteria | [113] | ||
| Other methods | Pulsed magnetic field | Cucumber Juice Lettuce Juice Carrot Juice Tomato Juice | Maintain the color and flavor of vegetable juice while controlling pathogenic bacteria | [114] |
| Tri-frequency ultrasound | Carrot | POD activity decreased by 81.43% | [115] | |
| Osmosonication | Ginger | The highest phenol content and antioxidant activity were obtained at 50 kHz | [116] | |
| Ultrasound | Coffee leaf | Ultrasonic regulation of phenolic metabolism | [117] | |
| Pulsed electric field and ultra-sonication | Spinach juice | The inactivation rates of POD and PPO increased from 0.85 and 0.025 Abs/min to 0.18 and 0.011 Abs/min | [118] | |
| Electrospray | Cabbage juice | The secondary and tertiary structures of PPO and POD are destroyed | [119] | |
| Multi-frequency ultrasound | Chinese cabbage | Dual-frequency ultrasound showed more positive sensory properties | [120] | |
| Method | Applications | Conclusions | References | |
|---|---|---|---|---|
| Acidifying agents | Acetic acid | Potatoes | Delayed ascorbic acid degradation and enhanced cell wall integrity | [121] |
| Oxalic acid | Lotus Root | Inhibited H2O2 and O2− production and reduced POD and PPO activities | [136] | |
| Citric acid | Lotus root | Reduced production of H2O2 and O2− while decreasing malondialdehyde, PPO and POD levels | [137] | |
| Antioxidants | Citric acid | Cabbage | Reduced residual PPO activity | [138] |
| Glutathione | Lotus root | Increasing PAL activity to stimulate total phenol accumulation while inhibiting PPO and POD activity | [139] | |
| Flammulina velutipes | Increase soluble solids content | [140] | ||
| L-arginine L-cysteine L-methionine | Broccoli | Reduced metabolism by inhibiting endogenous ethylene production led to a lower browning rate | [141] | |
| L-cysteine | Lotus root | Conversion of phenolic compounds to cysteine adducts as competitive inhibitors of PPO | [142] | |
| Flammulina velutipes | Regulates ROS metabolism and stimulates endogenous H2S production | [143] | ||
| Melatonin | Sweet potato | Induces the expression of genes related to antioxidant pathway and reduces enzyme activity, ROS content and membrane lipid peroxidation | [91] | |
| Mushroom | 100 μM melatonin decreased PPO gene expression and enzyme activity | [144] | ||
| Lotus seed | Inhibited oxidase activity and increased endogenous MT | [145] | ||
| Spinach | 0.20 mg/mL melatonin retarded chlorophyll degradation but increased POD activity | [146] | ||
| Lotus seeds | Reduction in membrane oxidative damage in mitochondria by stimulating antioxidant enzymes to scavenge ROS | [147] | ||
| Broccoli | Endogenous melatonin homeostasis led to downregulation of the expression of chlorophyll degradation-related and ethylene synthesis-related genes | [148] | ||
| Naclo | Cabbage | The synergistic effect of multi-frequency ultrasound in a sweeping mode combined with naclo is effective in sterilization | [149] | |
| Chelating agents | Oxalic acid | Lotus root | Inhibition of H2O2 and O2− production and reduction in POD and PPO activity | [136] |
| Kojic acid derivatives | Potato | Competitive inhibitor of tyrosinase (IC50 = 3.23 ± 0.26 μM) | [150] | |
| S-Furfuryl thioacetate | Potato Eggplant Lettuce | Changing the conformation of PPO by chelating Cu2+ | [151] | |
| Potato | Decreased PPO activity by chelating Cu2+ and acting on the residues of PPO | [152] | ||
| Kojic acid-1,3,4-oxadiazole derivatives | Mushrooms | Binds to Cu2+ in the active region, altering the secondary structure of tyrosinase | [153] | |
| Fern 6’-O-azelate | Potato | The inhibition of monophenolase was 31.4 ± 1.36% | [154] | |
| Natural Additives | Applications | Conclusions | References |
|---|---|---|---|
| Γ-aminobutyric acid | Stem lettuce | Inhibition of PPO activity by delaying the expression of lsppo | [7] |
| Sea buckthorn leaf extract | Potato | Competitive inhibitor of PPO (IC50 = 0.7 mg/mL) | [155] |
| Chlorogenic acid | Potato | Rearrangement of PPO secondary structure by hydrophobic interaction | [158] |
| 4-Hydroxycinnamic acid | Interaction with PPO through hydrogen bonding and hydrophobic interactions converted α-helix into β-sheet | [156] | |
| Cod peptide | Potato | High concentration of Cod peptide decreased the total phenol content, PPO, POD and PAL activities | [159] |
| Mulberry root bark 2-arylbenzofuran derivatives | The active site interacted with Cu2+ and peroxide ions to enhance antioxidant activity and inhibit tyrosinase activity | [165] | |
| Pineapple extract Onion extract Pepper extract Honey | Sweet potato | All of them inhibited PPO, among which honey had the highest inhibition rate of 41.39–48.0% | [166] |
| Purslane extract | Potato | Ultrasound-coupled extract treatment is more effective in maintaining cell membrane integrity, inhibiting PPO and POD activities, and improving antioxidant capacity | [167] |
| Golden ginkgo tannins | Lotus root | Acted as a reversible mixed competitive inhibitor of tyrosinase (IC50 = 123.90 ± 0.140 μg/mL) | [168] |
| Beef oregano extract | Mushroom | The greatest inhibitory effect was observed on PPO with 64.50% inhibition | [169] |
| Citronella hydrosol Rose hydrosol | Taro | Terpenoids were effective in reducing PAL, POD and PPO activities | [170] |
| Mango peel extract | Mashed potato | Competitive inhibition of PPO (IC50 = 0.3 mg/mL) | [171] |
| Probiotics fermentation suspension | Lotus root | Decreased activities of PPO, POD and PAL, reduced TPC and soluble quinones | [172] |
| Lotus root | Slowed down physiological responses and inhibits enzymatic browning-related enzyme activities | [173] | |
| Eucalyptus citriodora essential oil | Cabbage | Essential oil-based Pickering emulsion maintained color, chlorophyll content | [174] |
| Bacteriophages | Lettuce, Cucumber Carrot | Improvement of organoleptic quality of vegetables by sterilization (Disrupts the cellular structural properties of bacteria) | [175] |
| Litseacubeba essential oil | Cucumber juice Carrot juice Spinach juice | The optimal synergistic effects were found using PMF (3 times under 8 T, 60 pulses) treatments combined with 1.5 mg/ml | [176] |
| Basil essential oil | Cabbage | Ultrasound and basil essential oil nanoemulsion disrupted the increase in intracellular ROS and extracellular MDA leading to bacterial sterilization | [177] |
| Litsea cubeba essential oil | Bitter gourd Juice Carrot juice Cucumber juice Spinach juice | Inhibit bacterial respiratory tract metabolism, hinder bacterial nucleic acid replication | [178] |
| Citral | Carrot | US and CLON reduced the amount of Sh. Flexneri adhering to the sample surface while retaining important quality attributes | [179] |
| Baobab seed oil | Mushroom | Not only improved the freshness of mushrooms but also maintained the structural stability | [180] |
| Clove essential oil | Mushroom | Ultrasound-assisted clove essential oil nanoemulsions effectively maintained the quality characteristics of mushroom | [181] |
| Cucumber Lettuce | Enhances vegetable color by killing bacteria | [182] [183] | |
| Cumin | Cucumber juice Tomato juice | Cold nitrogen plasma-modified cumin aldehyde/β-cyclodextrin inclusion complexes for effective color retention in vegetable juices | [184] |
| Bacillus velezensis | Eggplant | The activity of ROS scavenging enzyme was enhanced and the antioxidant capacity was improved | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, J.; Zhang, L.; Xue, Y.; Zhang, K. Mechanistic Insights into Vegetable Color Stability: Discoloration Pathways and Emerging Protective Strategies. Foods 2025, 14, 2222. https://doi.org/10.3390/foods14132222
Zhang J, Zhang J, Zhang L, Xue Y, Zhang K. Mechanistic Insights into Vegetable Color Stability: Discoloration Pathways and Emerging Protective Strategies. Foods. 2025; 14(13):2222. https://doi.org/10.3390/foods14132222
Chicago/Turabian StyleZhang, Jianing, Junjun Zhang, Lidan Zhang, Yuhong Xue, and Ke Zhang. 2025. "Mechanistic Insights into Vegetable Color Stability: Discoloration Pathways and Emerging Protective Strategies" Foods 14, no. 13: 2222. https://doi.org/10.3390/foods14132222
APA StyleZhang, J., Zhang, J., Zhang, L., Xue, Y., & Zhang, K. (2025). Mechanistic Insights into Vegetable Color Stability: Discoloration Pathways and Emerging Protective Strategies. Foods, 14(13), 2222. https://doi.org/10.3390/foods14132222





