Integral Valorisation of Agri-Food By-Products Through the Production of Food Ingredients Using High-Pressure Thermal Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials Preparation and Processing
2.2. Physico-Chemical Characteristics
2.3. PPO Activity
2.4. Instrumental Color
2.5. Total Phenolic Compounds (TPC)
2.6. Total Carotenoid Content (TCC)
2.7. ABTS Method (2,2′-Azino-bis (3-ethylbenzothiazoline) 6-sulfonicacid)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the By-Product
3.2. Instrumental Color, Color Changes, Nutritional Molecules, and Antioxidant Activity of Procesed By-Products
3.2.1. RP (Capsicum annuum)
| Control | TT (5 min) | HPTT (600 MPa/5 min) | p | |||||
|---|---|---|---|---|---|---|---|---|
| 65 °C | 75 °C | 85 °C | 65 °C | 75 °C | 85 °C | |||
| Instrumental color | ||||||||
| CIE L* | 43.72 ± 1.51 | 41.95 ± 0.81 | 41.89 ± 0.80 | 42.29 ± 1.02 | 40.68 ± 0.44 | 41.13 ± 0.42 | 41.53 ± 1.81 | ns |
| CIE a* | 41.03 ± 1.91 | 40.97 ± 0.40 | 39.81 ± 0.39 | 40.01 ± 0.41 | 39.46 ± 0.38 | 39.64 ± 0.55 | 40.82 ± 2.15 | ns |
| CIE b* | 52.22 a ± 0.32 | 48.29 b ± 1.42 | 45.20c ± 1.12 | 42.96 cd ± 0.76 | 41.28 d ± 1.89 | 40.68 d ± 0.45 | 48.20 b ± 0.54 | *** |
| Color changes | ||||||||
| ΔE | - | 4.69 b ± 1.46 | 3.83 b ± 0.58 | 5.19 ab ± 0.71 | 7.48 a ± 1.89 | 7.80 a ± 0.46 | 3.16 b ± 0.78 | ** |
| Nutritional molecules | ||||||||
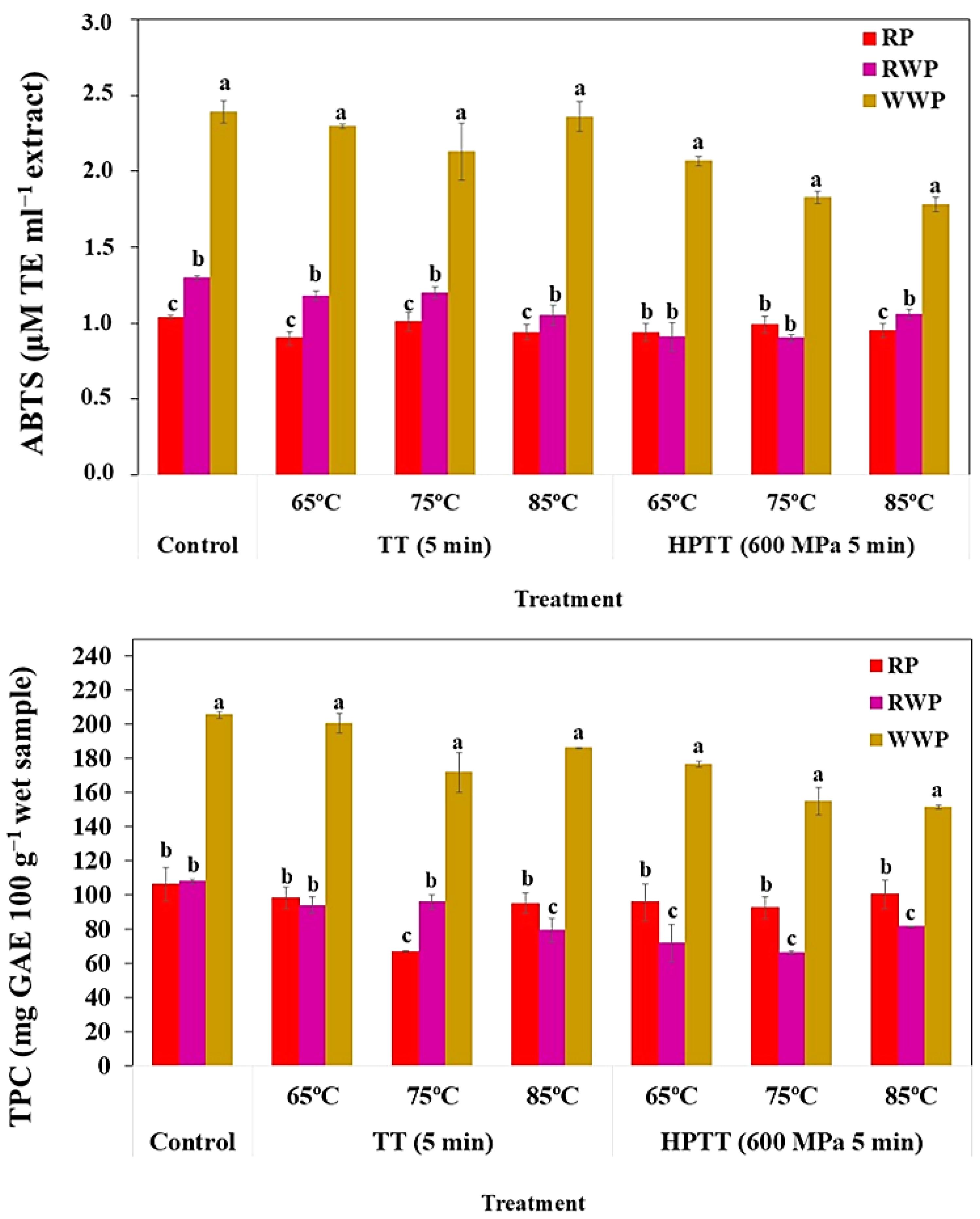
| TPC | 106.40 a ± 9.60 | 98.14 a ± 6.33 | 66.76 b ± 0.73 | 95.14 a ± 6.14 | 95.80 a ± 10.43 | 92.58 a ± 6.27 | 100.44 a ± 8.41 | *** |
| TCC | 146.52 ± 10.52 | 140.15 ± 8.73 | 132.43 ± 6.14 | 157.18 ± 21.01 | 153.31 ± 6.06 | 160.56 ± 17.76 | 139.72 ± 13.07 | ns |
| Antioxidant activity | ||||||||
| ABTS | 1.04 ± 0.01 | 0.90 ± 0.05 | 1.01 ± 0.06 | 0.94 ± 0.05 | 0.94 ± 0.06 | 0.99 ± 0.05 | 0.95 ± 0.05 | ns |
3.2.2. RWP (Tempranillo)
| Control | TT (5 min) | HPTT (600 MPa/5 min) | p | |||||
|---|---|---|---|---|---|---|---|---|
| 65 °C | 75 °C | 85 °C | 65 °C | 75 °C | 85 °C | |||
| Instrumental color | ||||||||
| CIE L* | 34.54 a ±1.68 | 33.62 ab ± 0.79 | 33.29 ab ± 0.33 | 32.60 ab ± 0.48 | 34.65 a ± 0.10 | 31.82 b ± 0.24 | 34.22 a ± 0.27 | ns |
| CIE a* | 5.85 a ± 0.23 | 6.77 a ± 0.32 | 6.34 a ± 0.53 | 6.32 a ± 0.11 | 4.45 b ± 0.42 | 4.19 b ± 0.36 | 4.34 b ± 0.37 | ns |
| CIE b* | 3.58 b ± 0.26 | 4.89 a ± 0.36 | 4.63 a ± 0.25 | 4.65 a ± 0.08 | 3.19 ab ± 0.12 | 3.35 ab ± 0.25 | 2.95 b ± 0.05 | *** |
| Color changes | ||||||||
| ΔE | - | 1.91 ab ± 0.72 | 1.75 b ± 0.45 | 2.27 ab ± 0.46 | 1.46 b ± 0.41 | 3.20 a ± 0.36 | 1.68 b ± 0.37 | ** |
| Nutritional molecules | ||||||||
| TPC | 107.78 a ± 1.32 | 93.98 abc ± 4.79 | 95.90 ab ± 4.30 | 79.62 cd ± 6.93 | 72.05 d ± 10.68 | 66.44d ± 1.01 | 81.51bcd ± 0.46 | *** |
| Antioxidant activity | ||||||||
| ABTS | 1.30a ± 0.01 | 1.18ab ± 0.03 | 1.20a ± 0.04 | 1.05b ± 0.07 | 0.91c ± 0.09 | 0.90c ± 0.02 | 1.06b ± 0.03 | ns |
3.2.3. WWP (Cayetana, Pardina, Montúa)
| Control | TT (5 min) | HPTT (600 MPa/5 min) | p | |||||
|---|---|---|---|---|---|---|---|---|
| 65 °C | 75 °C | 85 °C | 65 °C | 75 °C | 85 °C | |||
| Instrumental color | ||||||||
| CIE L* | 38.88 abc ± 0.61 | 39.66 a ± 0.15 | 38.33 bcd ± 0.44 | 38.78 abc ± 0.18 | 39.19 ab ± 0.08 | 37.70 d ± 0.24 | 38.12 cd ± 0.18 | ns |
| CIE a* | 8.78 a ± 0.19 | 8.78 a ± 0.08 | 8.62 a ± 0.06 | 8.39 a ± 0.34 | 6.49 b ± 0.37 | 7.20 b ± 0.23 | 7.34 b ± 0.51 | ns |
| CIE b* | 12.40 a ± 0.33 | 12.92 a ± 0.03 | 11.84 ab ± 0.09 | 11.16 b ± 0.07 | 8.37 b ± 0.17 | 8.60 b ± 0.27 | 9.14 b ± 0.67 | *** |
| Color changes | ||||||||
| ΔE | - | 0.94 b ± 0.11 | 0.82 b ± 0.37 | 1.32 b ± 0.18 | 4.66 a ± 0.11 | 4.29 a ± 0.3 | 3.66 a ± 0.78 | ** |
| Nutritional molecules | ||||||||
| TPC | 205.59 a ± 1.95 | 200.77 ab ± 5.58 | 171.91 cd ± 11.84 | 186.26 bc ± 0.32 | 176.57 c ± 1.78 | 155.07 de ± 7.88 | 151.39 e ± 1.13 | *** |
| Antioxidant activity | ||||||||
| ABTS | 2.39 a ± 0.07 | 2.30 abc ± 0.01 | 2.13 bc ± 0.19 | 2.36 ab ± 0.1 | 2.07 cd ± 0.03 | 1.83 de ± 0.04 | 1.78 e ± 0.05 | ns |
3.3. Global Effect of Processing on the By-Products
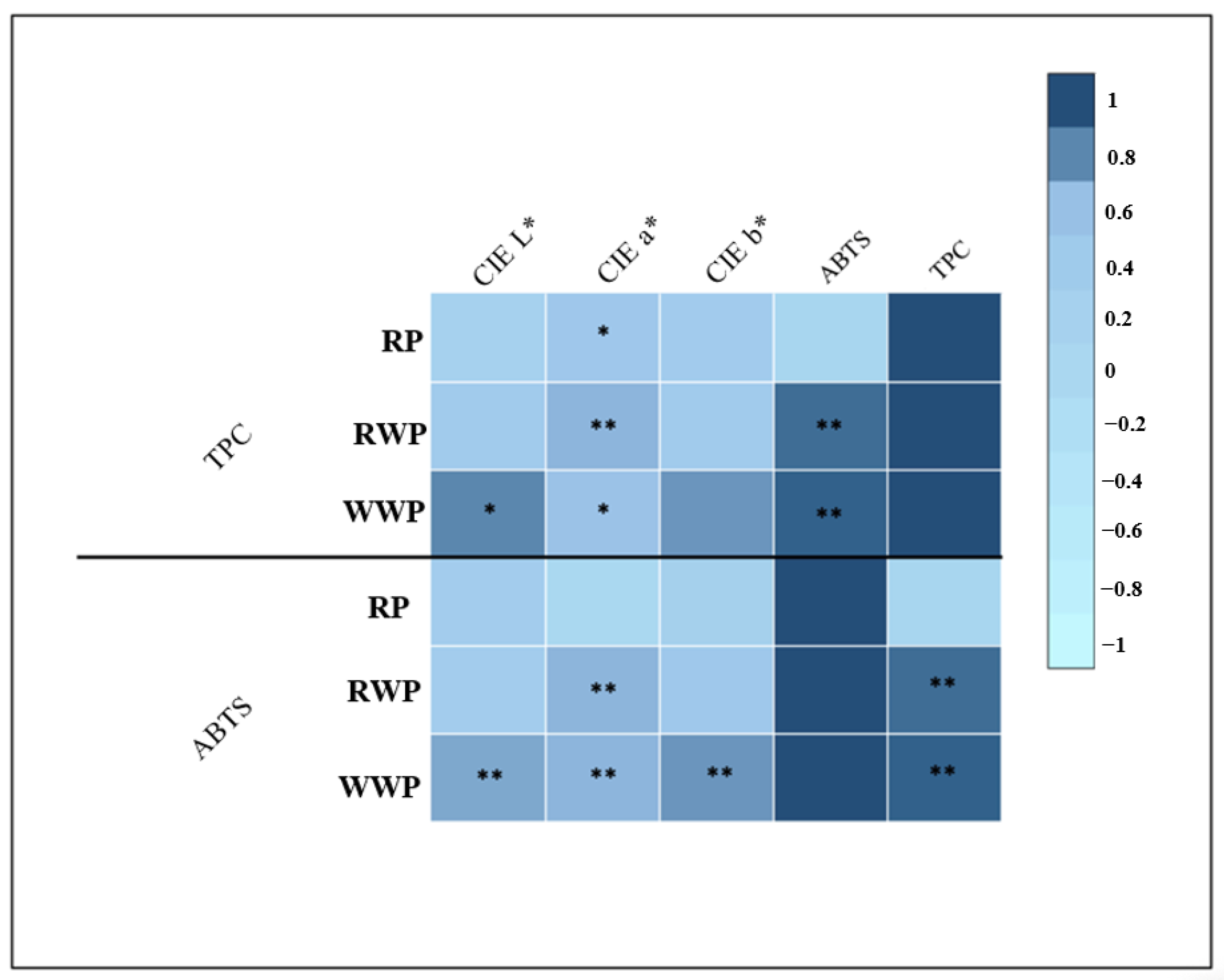
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPTT | High-pressure thermal treatment |
| HHP | Hydrostatic high pressure |
| TT | Thermal treatment |
| RP | Red pepper |
| RWP | Red wine pomace |
| WWP | White wine pomace |
| TPC | Total phenolic content |
| PPO | Polyphenol oxidase enzyme |
| aw | Water activity |
| TCC | Total carotenoid content |
References
- Soares Mateus, A.R.; Pena, A.; Sendón, R.; Almeida, C.; Nieto, G.A.; Khwaldia, K.; Sanches Silva, A. By-Products of Dates, Cherries, Plums and Artichokes: A Source of Valuable Bioactive Compounds. Trends Food Sci. Technol. 2023, 131, 220–243. [Google Scholar] [CrossRef]
- EUROSTAT. Food Waste and Food Waste Prevention—Estimates—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 4 April 2025).
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Câmara, J.S.; Lourenço, S.; Silva, C.; Lopes, A.; Andrade, C.; Perestrelo, R. Exploring the Potential of Wine Industry By-Products as Source of Additives to Improve the Quality of Aquafeed. Microchem. J. 2020, 155, 104758. [Google Scholar] [CrossRef]
- Cheikh Rouhou, M.; Abdelmoumen, S.; Atrous, H.; Lung, A.; Vaca-Medina, G.; Raynaud, C.; Ghorbel, D. Pilot Scale Production of Dietary Fibers from Tunisian Tomato and Red Pepper By-Products. Sustain. Chem. Pharm. 2024, 39, 101521. [Google Scholar] [CrossRef]
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (Hot Pepper): An Ancient Latin-American Crop with Outstanding Bioactive Compounds and Nutraceutical Potential. A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical Characteristics and Compositions of Red Pepper Seed Oils Extracted by Different Methods. Ind. Crops Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sevgi Arsunar, E.; Aydeniz, B.; Güneşer, O. Cold Pressed Capia Pepperseed (Capsicum annuum L.) Oils: Composition, Aroma, and Sensory Properties. Eur. J. Lipid Sci. Technol. 2015, 117, 1016–1026. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape Extract Activates Brown Adipose Tissue Through Pathway Involving the Regulation of Gut Microbiota and Bile Acid. Mol. Nutr. Food Res. 2020, 64, 2000149. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of White Grape Pomace from Winemaking as Source of Bioactive Compounds, and Its Antiproliferative Activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Timón, M.L.; Andrés, A.I.; Petrón, M.J. Antioxidant Activity of Aqueous Extracts Obtained from By-Products of Grape, Olive, Tomato, Lemon, Red Pepper and Pomegranate. Foods 2024, 13, 1802. [Google Scholar] [CrossRef] [PubMed]
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M.L. Food By-Products as Potential Antioxidant and Antimicrobial Additives in Chill Stored Raw Lamb Patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Delgado-Adámez, J.; Rocha-Pimienta, J.; Valdés-Sánchez, M.E.; Ramírez-Bernabé, M.R. Integral Use of Red Wine Pomace after Hydrostatic High Pressure: Application of Two Consecutive Cycles of Treatment. Foods 2024, 13, 149. [Google Scholar] [CrossRef]
- Ramírez, R.; Delgado, J.; Rocha-Pimienta, J.; Valdés, M.E.; Martín-Mateos, M.J.; Ayuso-Yuste, M.C. Preservation of White Wine Pomace by High Hydrostatic Pressure. Heliyon 2023, 9, e21199. [Google Scholar] [CrossRef]
- Cerdán-Calero, M.; Izquierdo, L.; Sentandreu, E. Valencia Late Orange Juice Preserved by Pulp Reduction and High Pressure Homogenization: Sensory Quality and Gas Chromatography–Mass Spectrometry Analysis of Volatiles. LWT-Food Sci. Technol. 2013, 51, 476–483. [Google Scholar] [CrossRef]
- Queirós, R.P.; Serment-Moreno, V.; Tonello-Samson, C. High Pressure Thermal Processing Systems. In High Pressure Thermal Processing; Academic Press: Cambridge, MA, USA, 2023; pp. 205–241. [Google Scholar] [CrossRef]
- Ardia, A.; Knorr, D.; Heinz, V. Adiabatic Heat Modelling for Pressure Build-up During High-Pressure Treatment in Liquid-Food Processing. Food Bioprod. Process. 2004, 82, 89–95. [Google Scholar] [CrossRef]
- Setlow, P.; Doona, C.J. High Pressure Thermal Sterilization Technology for Bacterial Spore Inactivation and the Production of Sterile, Ambient-Stable, Low-Acid Foods. In High Pressure Thermal Processing; Academic Press: Cambridge, MA, USA, 2023; pp. 41–53. [Google Scholar] [CrossRef]
- González-Cebrino, F.; Durán, R.; Delgado-Adámez, J.; Contador, R.; Ramírez, R. Changes after High-Pressure Processing on Physicochemical Parameters, Bioactive Compounds, and Polyphenol Oxidase Activity of Red Flesh and Peel Plum Purée. Innov. Food Sci. Emerg. Technol. 2013, 20, 34–41. [Google Scholar] [CrossRef]
- Gupta, R.; Balasubramaniam, V.M.; Schwartz, S.J.; Francis, D.M. Storage Stability of Lycopene in Tomato Juice Subjected to Combined Pressure-Heat Treatments. J. Agric. Food Chem. 2010, 58, 8305–8313. [Google Scholar] [CrossRef] [PubMed]
- Terefe, N.S.; Tepper, P.; Ullman, A.; Knoerzer, K.; Juliano, P. High Pressure Thermal Processing of Pears: Effect on Endogenous Enzyme Activity and Related Quality Attributes. Innov. Food Sci. Emerg. Technol. 2016, 33, 56–66. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Delgado, J.; Cava, R.; Ramírez, R. High Pressure Assisted Thermal Processing of Pumpkin Purée: Effect on Microbial Counts, Color, Bioactive Compounds and Polyphenoloxidase Enzyme. Food Bioprod. Process. 2016, 98, 124–132. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 20th Edition. 2016. Available online: https://www.techstreet.com/standards/official-methods-of-analysis-of-aoac-international-20th-edition-2016?product_id=1937367 (accessed on 25 April 2023).
- Folch, J.; Lees, M.; Sloane-Stanley, G. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Villanueva, M.J.; Barragán, R. Determinación Cuantitativa de La Fracción Hidrocarbonada En Alimentos. Anal. Bromatol. 1985, 37, 61–77. [Google Scholar]
- ISO 937:1978; Meat and Meat Products—Determination of Nitrogen Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 1978.
- Terefe, N.S.; Matthies, K.; Simons, L.; Versteeg, C. Combined High Pressure-Mild Temperature Processing for Optimal Retention of Physical and Nutritional Quality of Strawberries (Fragaria × ananassa). Innov. Food Sci. Emerg. Technol. 2009, 10, 297–307. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Cava, R.; Ramírez, R. Effect of a Different High Pressure Thermal Processing Compared to a Traditional Thermal Treatment on a Red Flesh and Peel Plum Purée. Innov. Food Sci. Emerg. Technol. 2014, 26, 26–33. [Google Scholar] [CrossRef]
- Lima, V.L.A.G.; Mélo, E.A.; Maciel, M.I.S.; Prazeres, F.G.; Musser, R.S.; Lima, D.E.S. Total Phenolic and Carotenoid Contents in Acerola Genotypes Harvested at Three Ripening Stages. Food Chem. 2005, 90, 565–568. [Google Scholar] [CrossRef]
- Bohoyo-Gil, D.; Dominguez-Valhondo, D.; García-Parra, J.J.; González-Gómez, D. UHPLC as a Suitable Methodology for the Analysis of Carotenoids in Food Matrix. Eur. Food Res. Technol. 2012, 235, 1055–1061. [Google Scholar] [CrossRef]
- Turoli, D.; Testolin, G.; Zanini, R.; Bellù, R. Determination of Oxidative Status in Breast and Formula Milk. Acta Paediatr. 2004, 93, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Saraiva, J.A.; Domingues, F.M.J.; Delgadillo, I. Effect of Mild Pressure Treatments and Thermal Blanching on Yellow Bell Peppers (Capsicum annuum L.). LWT-Food Sci. Technol. 2011, 44, 363–369. [Google Scholar] [CrossRef]
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; Van Loey, A.; Smout, C.; Hendrickx, M. Effect of Thermal Blanching and of High Pressure Treatments on Sweet Green and Red Bell Pepper Fruits (Capsicum annuum L.). Food Chem. 2008, 107, 1436–1449. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of Grape Pomace: Drying Behavior and Ultrasound Extraction of Phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red Pepper (Capsicum annuum) Carotenoids as a Source of Natural Food Colors: Analysis and Stability—A Review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef]
- Silva, L.R.; Azevedo, J.; Pereira, M.J.; Valentão, P.; Andrade, P.B. Chemical Assessment and Antioxidant Capacity of Pepper (Capsicum annuum L.) Seeds. Food Chem. Toxicol. 2013, 53, 240–248. [Google Scholar] [CrossRef]
- Wang, C.; You, Y.; Huang, W.; Zhan, J. The High-Value and Sustainable Utilization of Grape Pomace: A Review. Food Chem. 2024, 24, 101845. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, H.W.; Emire, S.A.; Teshome, P.G.; Töpfl, S.; Aganovic, K. Microbial Inactivation and Quality Impact Assessment of Red Pepper Paste Treated by High Pressure Processing. Heliyon 2022, 8, e12441. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Nakamura, H.; Chino, M.; Takeda, M. Antioxidant Activity of Capsanthin and the Fatty Acid Esters in Paprika (Capsicum annuum). J. Agric. Food Chem. 1998, 46, 3468–3472. [Google Scholar] [CrossRef]
- Mengistu, H.K.; Beri, G.B. Cooking Effect on Bioactive Compounds and Antioxidant Capacity of Red Pepper (Capsicum annuum L.). Heliyon 2024, 10, e35418. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Zhu, Q.; Xie, C.; Yan, Y. Alterations in Physico-Chemical Properties, Microstructure, Sensory Characteristics, and Volatile Compounds of Red Pepper (Capsicum annuum var. conoides) during Various Thermal Drying Durations. Food Chem. 2024, 23, 101566. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Hornero-Méndez, D. Comparative Study of the Effect of Paprika Processing on the Carotenoids in Peppers (Capsicum annuum) of the Bola and Agridulce Varieties. J. Agric. Food Chem. 1994, 42, 1555–1560. [Google Scholar] [CrossRef]
- Butz, P.; Edenharder, R.; García, A.F.; Fister, H.; Merkel, C.; Tauscher, B. Changes in Functional Properties of Vegetables Induced by High Pressure Treatment. Food Res. Int. 2002, 35, 295–300. [Google Scholar] [CrossRef]
- Lu, W.; Maidannyk, V.A.; Lim, A.S.L. Carotenoids Degradation and Precautions during Processing. In Carotenoids: Properties, Processing and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 223–258. [Google Scholar] [CrossRef]
- Abbeddou, S.; Petrakis, C.; Pérez-Gálvez, A.; Kefalas, P.; Hornero-Méndez, D. Effect of Simulated Thermo-Degradation on the Carotenoids, Tocopherols and Antioxidant Properties of Tomato and Paprika Oleoresins. J. Am. Oil Chem. Soc. 2013, 90, 1697–1703. [Google Scholar] [CrossRef]
- Sánchez, C.; Baranda, A.B.; De Marañón, I.M. The Effect of High Pressure and High Temperature Processing on Carotenoids and Chlorophylls Content in Some Vegetables. Food Chem. 2014, 163, 37–45. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of Carotenoid Concentrations in Red Peppers (Capsicum annuum) under Domestic Refrigeration for Three Weeks as Determined by HPLC-DAD. Food Chem. 2020, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.H.; Sil, H.Y. Antioxidant Activities of Red Pepper (Capsicum annuum) Pericarp and Seed Extracts. Int. J. Food Sci. Technol. 2008, 43, 1813–1823. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive Characteristics and Antioxidant Activities of Nine Peppers. J. Funct. Foods 2012, 4, 331–338. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant Capacity and Major Phenolic Compounds of Spices Commonly Consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of Chemical Profile and Antioxidant Activity of Twenty Cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A Comparison between Fresh and Processed Peppers. LWT-Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Phytochemicals in Capsicum oleoresin from Different Varieties of Hot Chilli Peppers with their Antidiabetic and Antioxidant Activities Due to Some Phenolic Compounds. Ultrason. Sonochem. 2017, 38, 629–639. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Jin, J.S.; Cho, Y.A.; Lee, J.H.; Park, S.; Jeong, S.W.; Kim, Y.H.; Lim, C.S.; Abd El-Aty, A.M.; Kim, G.S.; et al. Determination of Polyphenols in Three Capsicum annuum L. (Bell Pepper) Varieties Using High-Performance Liquid Chromatography-Tandem Mass Spectrometry: Their Contribution to Overall Antioxidant and Anticancer Activity. J. Sep. Sci. 2011, 34, 2967–2974. [Google Scholar] [CrossRef]
- Fernandez Garcia, A.; Butz, P.; Tauscher, B. Effects of High-Pressure Processing on Carotenoid Extractability, Antioxidant Activity, Glucose Diffusion, and Water Binding of Tomato Puree (Lycopersicon Esculentum Mill.). J. Food Sci. 2001, 66, 1033–1038. [Google Scholar] [CrossRef]
- Indrawati; Van Loey, A.; Hendrickx, M. Pressure and Temperature Stability of Water-Soluble Antioxidants in Orange and Carrot Juice: A Kinetic Study. Eur. Food Res. Technol. 2004, 219, 161–166. [Google Scholar] [CrossRef]
- Xu, Y.; Sismour, E.; Abraha-Eyob, Z.; McKinney, A.; Jackson, S. Physicochemical, Microstructural, and Antioxidant Properties of Skins from Pomaces of Five Virginia-Grown Grape Varieties and their Response to High Hydrostatic Pressure Processing. J. Food Meas. Charact. 2021, 15, 5547–5555. [Google Scholar] [CrossRef]
- de Jesus, A.L.T.; Leite, T.S.; Cristianini, M. High Isostatic Pressure and Thermal Processing of Açaí Fruit (Euterpe oleracea Martius): Effect on Pulp Color and Inactivation of Peroxidase and Polyphenol Oxidase. Food Res. Int. 2018, 105, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Fortea, M.I.; López-Miranda, S.; Serrano-Martínez, A.; Carreño, J.; Núñez-Delicado, E. Kinetic Characterisation and Thermal Inactivation Study of Polyphenol Oxidase and Peroxidase from Table Grape (Crimson Seedless). Food Chem. 2009, 113, 1008–1014. [Google Scholar] [CrossRef]
- O’Donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of Ultrasonic Processing on Food Enzymes of Industrial Importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of Polyphenol Oxidase and Peroxidase and Influence on Browning of Cold Stored Strawberry Fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Dos Santos Gomes, F.; Cabral, L.M.C.; Tonon, R.V. Effect of Temperature on the Degradation of Bioactive Compounds of Pinot noir Grape Pomace during Drying. Braz. J. Food Technol. 2017, 21. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Francia-Aricha, E.; Rivas-Gonzalo, J.C. Formation of Anthocyanin-Derived Pigments in Experimental Red Wines [Formación de Pigmentos Derivados de Antocianos en Vinos Tintos Experimentales]. Food Sci. Technol. Int. 1999, 5, 347–352. [Google Scholar] [CrossRef]
- Pinelo, M.; Manzocco, L.; Nuñez, M.J.; Nicoli, M.C. Interaction among Phenols in Food Fortification: Negative Synergism on Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 1177–1180. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Martín-Mateos, M.J.; Delgado-Adámez, J.; Moreno-Cardona, D.; Valdés-Sánchez, M.E.; Ramírez-Bernabé, M.R. Application of White-Wine-Pomace-Derived Ingredients in Extending Storage Stability of Fresh Pork Burgers. Foods 2023, 12, 4468. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Delgado-Adámez, J.; Cava, R.; Martín-Belloso, O.; Elez-Martínez, P.; Ramírez, R. Application of Innovative Technologies, Moderate-Intensity Pulsed Electric Fields and High-Pressure Thermal Treatment, to Preserve and/or Improve the Bioactive Compounds Content of Pumpkin. Innov. Food Sci. Emerg. Technol. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Shirahigue, L.D.; Plata-Oviedo, M.; de Alencar, S.M.; d’Arce, M.A.B.R.; de Souza Vieira, T.M.F.; Oldoni, T.L.C.; Contreras-Castillo, C.J. Wine Industry Residue as Antioxidant in Cooked Chicken Meat. Int. J. Food Sci. Technol. 2010, 45, 863–870. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Antioxidant Activity and Dietary Fibre of Prensal Blanc White Grape (Vitis vinifera) by-Products. Int. J. Food Sci. Technol. 2008, 43, 1953–1959. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
| Lot | Treatment Type | Pressure (MPa) | Time (min) | Temperature (°C) |
|---|---|---|---|---|
| 1 | Control | 0.1 | 0 | 0 |
| 2 | TT | 0.1 | 5 | 65 |
| 3 | TT | 0.1 | 5 | 75 |
| 4 | TT | 0.1 | 5 | 85 |
| 5 | HPTT | 600 | 5 | 65 (83) * |
| 6 | HPTT | 600 | 5 | 75 (93) * |
| 7 | HPTT | 600 | 5 | 85 (103) * |
| RP | RWP | WWP | p-Value | |
|---|---|---|---|---|
| pH | 5.18 a ± 0.00 | 3.48 b ± 0.01 | 3.31 c ± 0.01 | *** |
| aw | 0.979 a ± 0.00 | 0.954 b ± 0.00 | 0.944 c ± 0.00 | *** |
| Moisture (g 100 g−1) | 91.42 a ± 0.55 | 49.16 b ± 0.43 | 44.11 c ± 0.23 | *** |
| Fat (g 100 g−1) | 0.59 c ± 0.18 | 3.26 a ± 0.02 | 2.33 b ± 0.03 | *** |
| Fiber (g 100 g−1) | 1.94 c ± 0.21 | 38.14 a ± 0.29 | 34.46 b ± 0.22 | *** |
| Protein (g 100 g−1) | 1.13 b ± 0.01 | 2.92 a ± 0.16 | 2.40 a ± 0.04 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ordóñez, M.; Saraiva, J.A.; Pinto, C.A.; Delgado-Adámez, J.; Ramírez-Bernabé, M.R. Integral Valorisation of Agri-Food By-Products Through the Production of Food Ingredients Using High-Pressure Thermal Treatments. Foods 2025, 14, 2214. https://doi.org/10.3390/foods14132214
Sánchez-Ordóñez M, Saraiva JA, Pinto CA, Delgado-Adámez J, Ramírez-Bernabé MR. Integral Valorisation of Agri-Food By-Products Through the Production of Food Ingredients Using High-Pressure Thermal Treatments. Foods. 2025; 14(13):2214. https://doi.org/10.3390/foods14132214
Chicago/Turabian StyleSánchez-Ordóñez, Miriam, Jorge A. Saraiva, Carlos A. Pinto, Jonathan Delgado-Adámez, and M. Rosario Ramírez-Bernabé. 2025. "Integral Valorisation of Agri-Food By-Products Through the Production of Food Ingredients Using High-Pressure Thermal Treatments" Foods 14, no. 13: 2214. https://doi.org/10.3390/foods14132214
APA StyleSánchez-Ordóñez, M., Saraiva, J. A., Pinto, C. A., Delgado-Adámez, J., & Ramírez-Bernabé, M. R. (2025). Integral Valorisation of Agri-Food By-Products Through the Production of Food Ingredients Using High-Pressure Thermal Treatments. Foods, 14(13), 2214. https://doi.org/10.3390/foods14132214







