Biocontrol Ability Against Harmful Microbial Contamination of Vegan Mortadella with an Ingredient of Oat Fermented by Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Microorganisms
2.2. Preparation, Fermentation and Characterization of the Oat Drink Culture Mediums
2.3. Evaluation of the Antimicrobial Properties of the LAB Fermented Oat Drink Culture Mediums
2.4. Production of the Vegan Mortadella Incorporating the Bioactive Ingredient
2.5. Evaluation of the Effect of the Bioactive Ingredient in the Vegan Mortadella Quality
2.6. Preparation of Microbial Inoculum for In Situ Antimicrobial Activity Assays
2.7. Assessment of the Antimicrobial Properties of the Bioactive Ingredient in the Vegan Mortadellas
2.8. Statistical Analysis
3. Results and Discussion
3.1. Assessment of Fermentation Parameters and Antimicrobial Properties of Bioactive Ingredients
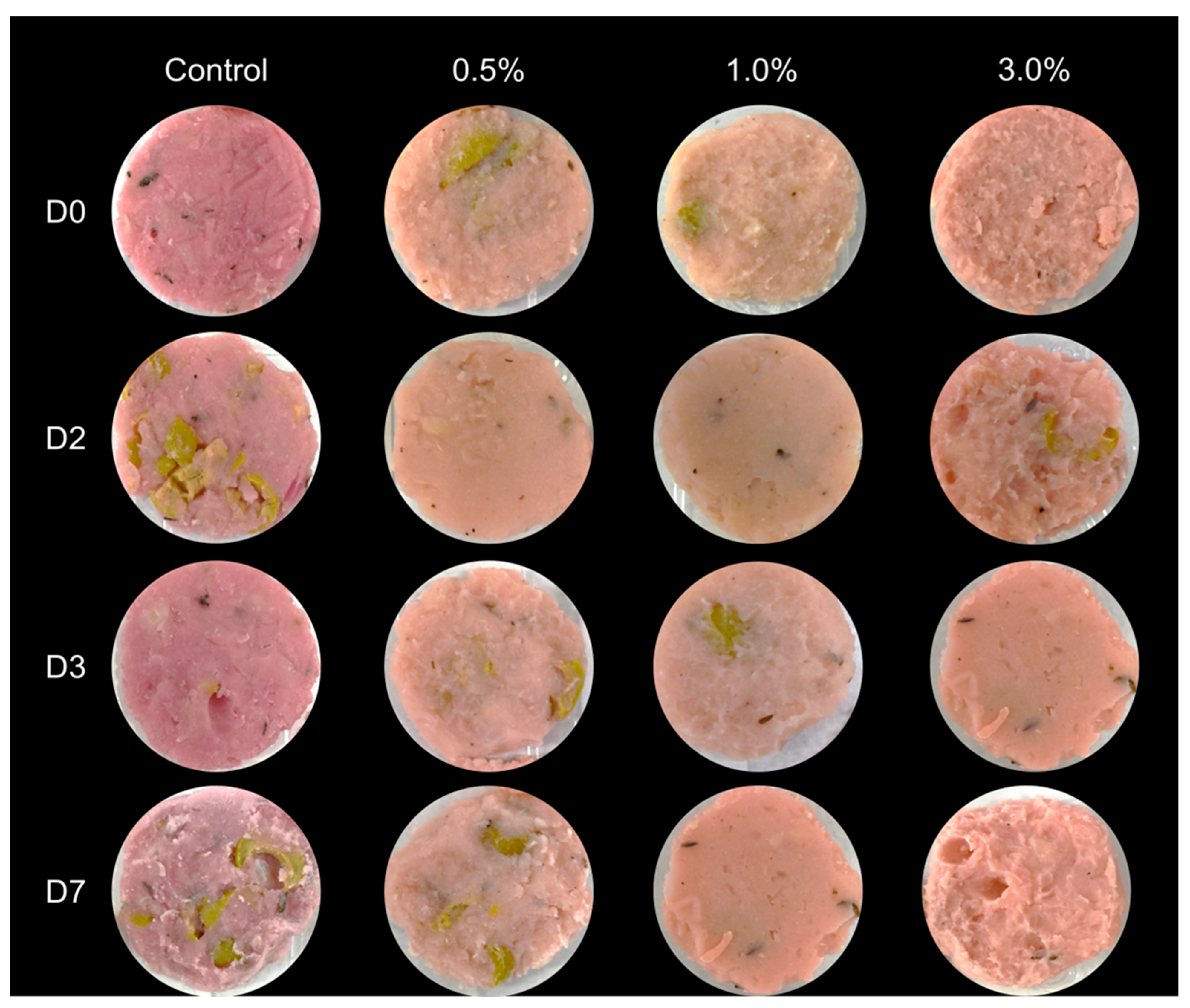
3.2. Assessment of the Effect of the Bioactive Ingredient in the Vegan Mortadella Quality
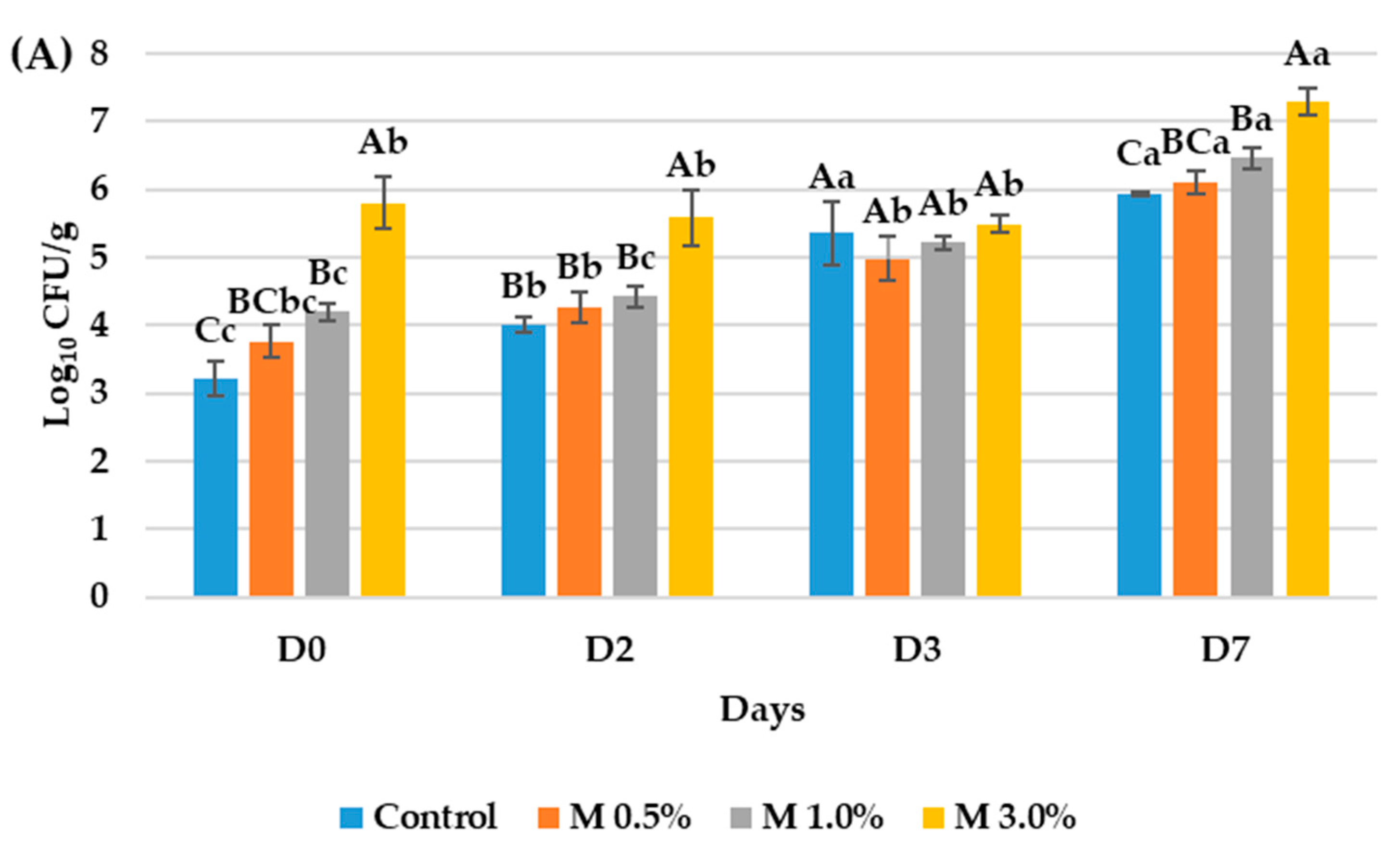
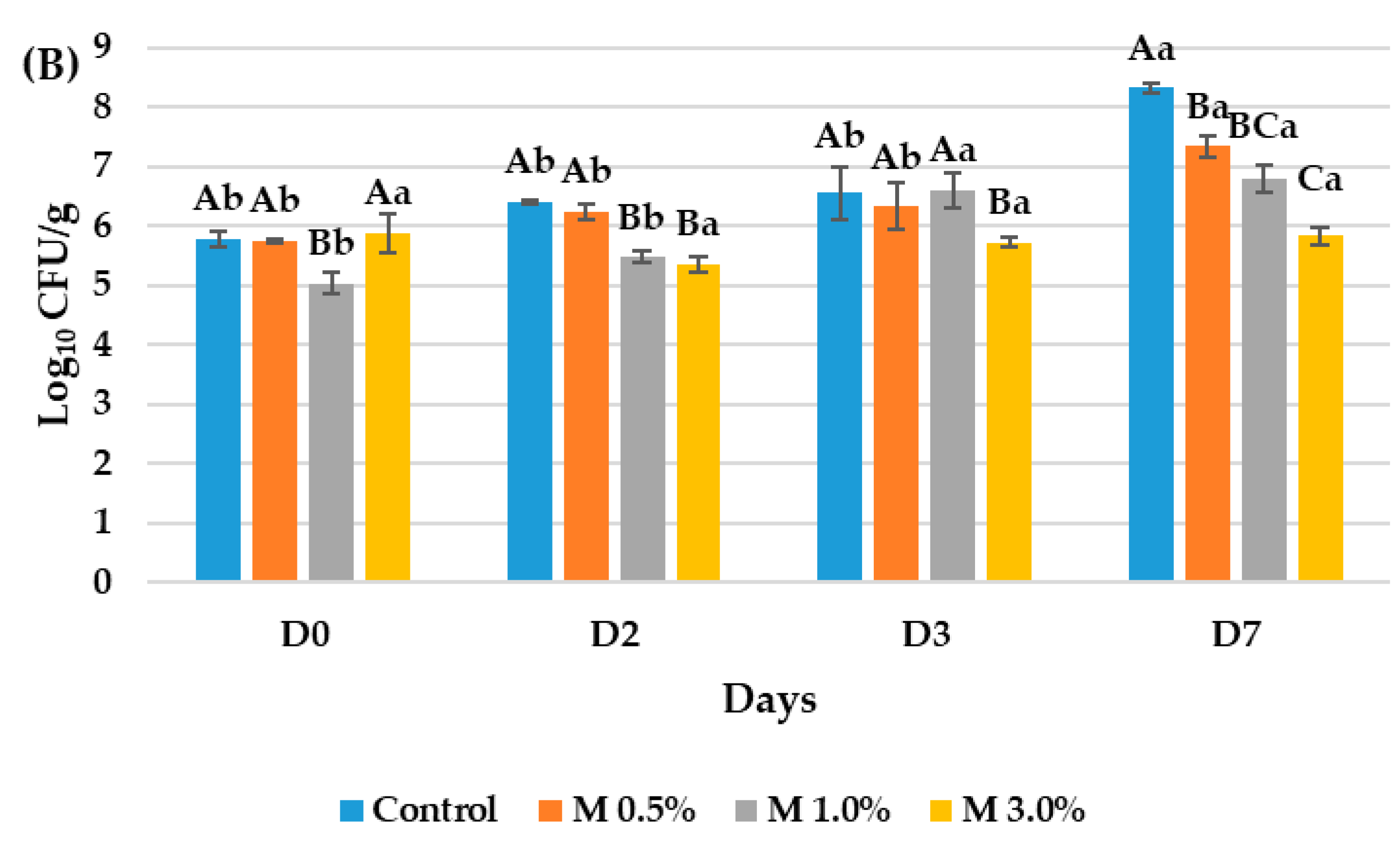
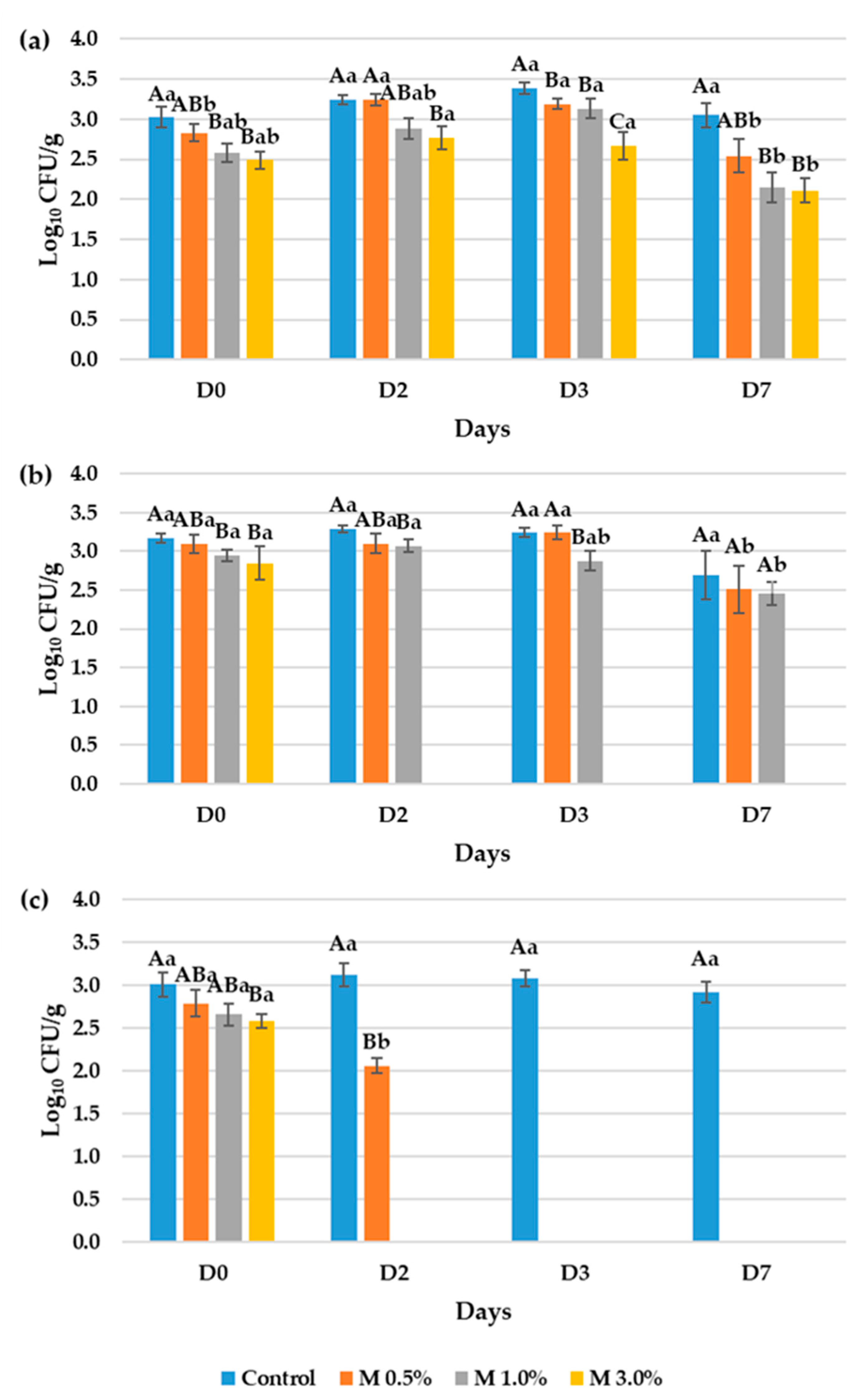
3.3. Evaluation of the Antimicrobial Properties of the Bioactive Ingredient in the Vegan Mortadellas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aw | Water activity |
| BHI | Brain heart infusion |
| CFU/g | Colony forming units per gram of food |
| HSI | Hue, saturation, and intensity |
| LAB | Lactic acid bacteria |
| M 0.5% | Vegan mortadella with a 0.5% of the bioactive ingredient |
| M 1.0% | Vegan mortadella with a 1.0% of the bioactive ingredient |
| M 3.0% | Vegan mortadella with a 3.0% of the bioactive ingredient |
| MRSa | Man, Rogosa and Sharpe agar |
| MRSb | Man, Rogosa and Sharpe broth |
| PCA | Plate count agar |
| PDA | Potato dextrose agar |
| OD | Culture medium oat drink |
| ODG | Culture medium oat drink enriched with glucose |
| ODN | Culture medium oat drink enriched with nutrients |
References
- de Oliveira, L.F.R.; de Medeiros, N.S.; de Assis, C.F.; de Sousa, F.C., Jr. Simultaneous vehiculation of probiotics and yellow mombin (Spondias mombin L.) seed extract to develop a new vegan multifunctional ingredient. LWT 2024, 198, 116018. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Panpipat, W.; Chaijan, M.; Shetty, K.; Rawdkuen, S. Role of plant protein on the quality and structure of meat analogs: A new perspective for vegetarian foods. Future Foods 2023, 8, 100280. [Google Scholar] [CrossRef]
- McLauchlan, J.; Tyler, A.I.I.; Chakrabarti, B.; Orfila, C.; Sarkar, A. Oat protein: Review of structure-function synergies with other plant proteins. Food Hydrocoll. 2024, 154, 110139. [Google Scholar] [CrossRef]
- Mel, R.; Malalgoda, M. Oat protein as a novel protein ingredient: Structure, functionality, and factors impacting utilization. Cereal Chem. 2022, 99, 21–36. [Google Scholar] [CrossRef]
- Holopainen-Mantila, U.; Vanhatalo, S.; Lehtinen, P.; Sozer, N. Oats as a source of nutritious alternative protein. J. Cereal Sci. 2024, 116, 103862. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Jamróz, E.; Zając, M.; Guzik, P.; Derbew Gedif, H.; Turek, K.; Kopeć, M. Antioxidant edible double-layered film based on waste from soybean production as a vegan active packaging for perishable food products. Food Chem. 2023, 400, 134009. [Google Scholar] [CrossRef]
- Tóth, A.J.; Dunay, A.; Battay, M.; Illés, C.B.; Bittsánszky, A.; Süth, M. Microbial spoilage of plant-based meat analogues. Appl. Sci. 2021, 11, 8309. [Google Scholar] [CrossRef]
- Beamonte Vela, B.N.; Garcia-Carretero, R.; Carrasco-Fernandez, B.; Gil-Romero, Y.; Perez-Pomata, M.T. Listeria monocytogenes infections: Analysis of 41 patients. Med. Clin. 2020, 155, 57–62. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 4th ed.; Springer US: New York, NY, USA, 2022; ISBN 9783030856403. [Google Scholar]
- Wei, S.; Xu, Q.; Pei, S.; Lv, Y.; Lei, Y.; Zhang, S.; Zhai, H.; Hu, Y. Unraveling the antifungal and anti-aflatoxin B1 mechanisms of piperitone on Aspergillus flavus. Food Microbiol. 2024, 123, 104588. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sadeghi, A.; Mortazavi, S.A. The use of cyclic dipeptide producing lab with potent anti-aflatoxigenic capability to improve techno-functional properties of clean-label bread. Ann. Microbiol. 2020, 70, 24. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Mustafa, M.A.; Hassan, M.A.; Al-Asmari, F. Probiotic-fermentation of oat: Safety, strategies for improving quality, potential food applications and biological activities. Trends Food Sci. Technol. 2024, 151, 104640. [Google Scholar] [CrossRef]
- Zang, J.; Yan, B.; Liu, Z.; Tang, D.; Liu, Y.; Chen, J.; Yin, Z. Current state, challenges and future orientations of the applications of lactic acid bacteria exopolysaccharide in foods. Food Microbiol. 2025, 126, 104678. [Google Scholar] [CrossRef] [PubMed]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P.V. Phenyllactic acid: A green compound for food biopreservation. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Nasrollahzadeh, A.; Mokhtari, S.; Khomeiri, M.; Saris, P.E.J. antifungal preservation of food by lactic acid bacteria. Foods 2022, 11, 395. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of lactic acid bacteria in food preservation and safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Jamuna, M.; Babusha, S.T.; Jeevaratnam, K. Inhibitory efficacy of nisin and bacteriocins from Lactobacillus isolates against food spoilage and pathogenic organisms in model and food systems. Food Microbiol. 2005, 22, 449–454. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.; Wang, Q.; Suo, H.; Hamdy, A.M.; Song, J. Antifungal effect of metabolites from a new strain Lactiplantibacillus plantarum LPP703 isolated from naturally fermented yak yogurt. Foods 2023, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Riolo, M.; Luz, C.; Santilli, E.; Meca, G.; Cacciola, S.O. Antifungal activity of selected lactic acid bacteria from olive drupes. Food Biosci. 2023, 52, 102422. [Google Scholar] [CrossRef]
- Ortiz de Elguea-Culebras, G.; Bourbon, A.I.; Costa, M.J.; Muñoz-Tebar, N.; Carmona, M.; Molina, A.; Sánchez-Vioque, R.; Berruga, M.I.; Vicente, A.A. Optimization of a chitosan solution as potential carrier for the incorporation of Santolina chamaecyparissus L. solid by-product in an edible vegetal coating on ‘Manchego’ cheese. Food Hydrocoll. 2019, 89, 272–282. [Google Scholar] [CrossRef]
- Nacef, M.; Chevalier, M.; Chollet, S.; Drider, D.; Flahaut, C. MALDI-TOF mass spectrometry for the identification of lactic acid bacteria isolated from a french cheese: The maroilles. Int. J. Food Microbiol. 2017, 247, 2–8. [Google Scholar] [CrossRef]
- Demir, H.; Simsek, M.; Yıldırım, G. Effect of oat milk pasteurization type on the characteristics of yogurt. LWT 2021, 135, 110271. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Revalorization by lactic acid bacterial fermentation of goat whey from cheese industry as a potential antifungal agent. Food Biosci. 2023, 53, 102586. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey as a bio-preservation ingredient. LWT 2023, 174, 114427. [Google Scholar] [CrossRef]
- De Muynck, C.; Leroy, A.I.J.; De Maeseneire, S.; Arnaut, F.; Soetaert, W.; Vandamme, E.J. Potential of Selected lactic acid bacteria to produce food compatible antifungal metabolites. Microbiol. Res. 2004, 159, 339–346. [Google Scholar] [CrossRef]
- Illueca, F.; Moreno, A.; Calpe, J.; Nazareth, T.d.M.; Dopazo, V.; Meca, G.; Quiles, J.M.; Luz, C. Bread biopreservation through the addition of lactic acid bacteria in sourdough. Foods 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.C.F.; Franca, A.S.; Oliveira, L.S. A Comparative evaluation of methodologies for water content determination in green coffee. LWT 2007, 40, 1300–1303. [Google Scholar] [CrossRef]
- Luz, C.; Quiles, J.M.; Romano, R.; Blaiotta, G.; Rodríguez, L.; Meca, G. Application of whey of mozzarella di bufala campana fermented by lactic acid bacteria as a bread biopreservative agent. Int. J. Food Sci. Technol. 2021, 56, 4585–4593. [Google Scholar] [CrossRef]
- Wang, B.; Yang, H.; Yang, C.; Lu, F.; Wang, X.; Liu, D. Prediction of total volatile basic nitrogen (TVB-N) and 2-thiobarbituric acid (TBA) of smoked chicken thighs using computer vision during storage at 4 °C. Comput. Electron. Agric. 2022, 199, 107170. [Google Scholar] [CrossRef]
- Riolo, M.; Villena, A.M.; Calpe, J.; Luz, C.; Meca, G.; Tuccitto, N.; Cacciola, S.O. A Circular economy approach: A new formulation based on a lemon peel medium activated with Lactobacilli for sustainable control of post-harvest fungal rots in fresh citrus fruit. Biol. Control 2024, 189, 105443. [Google Scholar] [CrossRef]
- Rovetto, E.I.; La Spada, F.; El boumlasy, S.; Conti Taguali, S.; Riolo, M.; Pane, A.; Cacciola, S.O. Biological control of green mold in simulated post-harvest chain of citrus fruit: Efficacy of Candida oleophila strain o and molecular insight into elicitation of host immune system. Biol. Control 2024, 193, 105531. [Google Scholar] [CrossRef]
- Dopazo, V.; Luz, C.; Calpe, J.; Vila-Donat, P.; Rodríguez, L.; Meca, G. Antifungal Properties of whey fermented by lactic acid bacteria in films for the preservation of cheese slices. Int. J. Dairy. Technol. 2022, 75, 619–629. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Ponce, A.G.; Del Valle, C.E.; Roura, S.I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT 2005, 38, 565–570. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Mariod, A.A. Effect of essential oils on organoleptic (smell, taste, and texture) properties of food essential oils in food preservation. In Flavor and Safety, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 131–137. [Google Scholar] [CrossRef]
- Sorathiya, K.B.; Melo, A.; Hogg, M.C.; Pintado, M. Organic acids in food preservation: Exploring synergies, molecular insights, and sustainable applications. Sustainability 2025, 17, 3434. [Google Scholar] [CrossRef]
- Russo, P.; Fares, C.; Longo, A.; Spano, G.; Capozzi, V. Lactobacillus plantarum with broad antifungal activity as a protective starter culture for bread production. Foods 2017, 6, 110. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial potential of polyphenols: Mechanisms of action and microbial responses—A narrative review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446. [Google Scholar] [CrossRef]
- Chen, G.Q.; Liu, X. On the future fermentation. Microb. Biotechnol. 2021, 14, 18–21. [Google Scholar] [CrossRef]
- Ojo, A.O.; de Smidt, O. Lactic acid: A comprehensive review of production to purification. Processes 2023, 11, 688. [Google Scholar] [CrossRef]
- Gabriel, A.A. Estimation of water activity from pH and °Brix values of some food products. Food Chem. 2008, 108, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Tangyu, M.; Fritz, M.; Tan, J.P.; Ye, L.; Bolten, C.J.; Bogicevic, B.; Wittmann, C. Flavour by design: Food-grade lactic acid bacteria improve the volatile aroma spectrum of oat milk, sunflower seed milk, pea milk, and faba milk towards improved flavour and sensory perception. Microb. Cell Factories 2023, 22, 133. [Google Scholar] [CrossRef]
- Dagnas, S.; Gauvry, E.; Onno, B.; Membré, J.M. Quantifying effect of lactic, acetic, and propionic acids on growth of molds isolated from spoiled bakery products. J. Food Prot. 2015, 78, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Landim Neves, M.I. Color solutions in plant-based foods. In Handbook of Plant-Based Food and Drinks Design, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 319–334. [Google Scholar] [CrossRef]
- Kale, P.; Mishra, A.; Annapure, U.S. development of vegan meat flavour: A review on sources and techniques. Future Foods 2022, 5, 100149. [Google Scholar] [CrossRef]
- Peters, J.C.; Breen, J.A.; Pan, Z. Effects of Culinary spices on liking and consumption of protein rich foods in community-dwelling older adults. Nutrients 2023, 15, 1172. [Google Scholar] [CrossRef]
- Gajowniczek-Ałasa, D.; Baranowska-Wójcik, E.; Szwajgier, D. Vegan and vegetarian soups are excellent sources of cholinesterase inhibitors. Nutrients 2024, 16, 2025. [Google Scholar] [CrossRef]
- Craig, W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef]
- EFSA Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union 2008, 354, 16–33.
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Nazareth, T.d.M.; Luz, C.; Luciano, F.B.; Mañes, J.; Meca, G. Development of an antifungal and antimycotoxigenic device containing allyl isothiocyanate for silo fumigation. Toxins 2019, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. Essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- Lu, Y.; Joerger, R.; Wu, C. Similar reduction of Salmonella enterica Typhimurium on grape tomatoes and its cross-contamination in wash water by washing with natural antimicrobials as compared with chlorine treatment. Food Bioprocess Technol. 2014, 7, 661–670. [Google Scholar] [CrossRef]
- Lima, G.; Spina, A.M.; Castoria, R.; De Curtis, F.; De Cicco, V. Integration of Biocontrol agents and food-grade additives for enhancing protection of stored apples from Penicillium expansum. J. Food Prot. 2005, 68, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L. Essential Oils: What they are and how the terms are used and defined essential oils in food preservation. In Flavor and Safety, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–10. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A review of regulatory standards and advances in essential oils as antimicrobials in foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Aguilar, F.; Crebelli, R.; Domenico, A.; Dusemund, B.; Frutos, M.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.; et al. Scientific Opinion on the re-evaluation of propionic acid (E 280), sodium propionate (E 281), calcium propionate (E 282) and potassium propionate (E 283) as food additives. EFSA J. 2014, 12, 3779. [Google Scholar] [CrossRef]
- Sutoh, M.; Imura, T.; Tsukada, H.; Yamada, A. Flavour preference conditioned by postabsorptive propionate and acetate in wethers. Appl. Anim. Behav. Sci. 2008, 111, 274–285. [Google Scholar] [CrossRef]
- El-Nezami, H.S.; Chrevatidis, A.; Auriola, S.; Salminen, S.; Mykkänen, H. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 2002, 19, 680–686. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Paulauskienė, A.; Baltušnikienė, A.; Kłębukowska, L.; Czaplicki, S.; Konopka, I. Changes in selected quality indices in microbially fermented commercial almond and oat drinks. Appl. Sci. 2022, 12, 9983. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Samples | |||
|---|---|---|---|---|
| Control | M 0.5% | M 1.0% | M 3.0% | |
| Bean mash | 538.0 | 534.5 | 531.0 | 517.0 |
| Water | 135.0 | 135.0 | 135.0 | 135.0 |
| Olive | 50.0 | 50.0 | 50.0 | 50.0 |
| Agar | 24.4 | 24.4 | 24.4 | 24.4 |
| Salt | 2.0 | 2.0 | 2.0 | 2.0 |
| Thyme | 0.4 | 0.4 | 0.4 | 0.4 |
| Garlic | 0.2 | 0.2 | 0.2 | 0.2 |
| Bioactive ingredient | - | 3.5 | 7.0 | 21.0 |
| Samples | pH | Bacterial Count | Lactic Acid | Phenyllactic Acid | |
|---|---|---|---|---|---|
| Control | OD | 7.39 ± 0.01 a | nd | nd | nd |
| ODG | 6.58 ± 0.01 b | nd | nd | nd | |
| ODN | 6.41 ± 0.01 c | nd | nd | nd | |
| L. plantarum VC7 | OD | 3.44 ± 0.01 d | 7.37 ± 0.20 b | 14.84 ± 0.01 b | 2.61 ± 0.09 c |
| ODG | 3.41 ± 0.02 e | 8.20 ± 0.09 a | 17.27 ± 0.01 a | 5.42 ± 0.21 b | |
| ODN | 3.52 ± 0.02 e | 8.32 ± 0.12 a | 17.34 ± 0.01 a | 9.23 ± 0.13 a | |
| Contaminant Microorganism | Control | L. plantarum VC7 | ||||
|---|---|---|---|---|---|---|
| OD | ODG | ODN | OD | ODG | ODN | |
| A. flavus | - | - | - | - | ++ | +++ |
| A. fumigatus | - | - | - | - | ++ | ++++ |
| A. niger | - | - | - | - | - | ++ |
| A. steynii | - | - | - | - | ++ | +++ |
| P. camemberti | - | - | - | - | - | +++ |
| P. commune | - | - | - | + | ++ | +++ |
| P. digitatum | - | - | - | + | ++ | +++ |
| P. expansum | - | - | - | + | +++ | ++++ |
| P. italicum | - | - | - | - | +++ | ++++ |
| P. nordicum | - | - | - | - | +++ | ++++ |
| P. roqueforti | - | - | - | + | + | ++ |
| P. verrucosum | - | - | - | + | +++ | +++ |
| L. monocytogenes | - | - | - | ++ | ++ | ++++ |
| (a) pH | ||||
| Samples | Days | |||
| D0 | D2 | D3 | D7 | |
| Control | 5.98 ± 0.03 BCa | 6.22 ± 0.06 Aa | 6.03 ± 0.01 Ba | 5.93 ± 0.04 Ca |
| M 0.5% | 5.70 ± 0.02 ABb | 5.90 ± 0.09 Ab | 5.68 ± 0.04 ABb | 5.65 ± 0.12 Bb |
| M 1.0% | 5.10 ± 0.06 Cc | 5.50 ± 0.13 Ac | 5.41 ± 0.05 Bc | 5.40 ± 0.03 Bc |
| M 3.0% | 4.60 ± 0.03 Bd | 4.85 ± 0.09 Ad | 4.73 ± 0.02 ABd | 4.74 ± 0.03 ABd |
| (b) Aw | ||||
| Samples | Days | |||
| D0 | D2 | D3 | D7 | |
| Control | 0.975 ± 0.001 Ba | 0.905 ± 0.001 Da | 0.985 ± 0.001 Aa | 0.954 ± 0.001 Ca |
| M 0.5% | 0.975 ± 0.002 Aa | 0.907 ± 0.001 Da | 0.968 ± 0.002 Bb | 0.935 ± 0.001 Cb |
| M 1.0% | 0.925 ± 0.001 Bb | 0.858 ± 0.001 Db | 0.948 ± 0.001 Ad | 0.918 ± 0.001 Cc |
| M 3.0% | 0.874 ± 0.001 Cc | 0.862 ± 0.001 Db | 0.958 ± 0.001 Ac | 0.908 ± 0.001 Bd |
| (c) Moisture | ||||
| Samples | Days | |||
| D0 | D2 | D3 | D7 | |
| Control | 25.22 ± 0.76 Bb | 25.61 ± 0.66 Bc | 26.34 ± 0.40 ABb | 28.53 ± 0.52 Aa |
| M 0.5% | 27.34 ± 0.38 Ab | 26.34 ± 0.31 Aab | 23.76 ± 1.25 Bb | 24.69 ± 0.97 Bc |
| M 1.0% | 27.31 ± 0.76 Ab | 27.74 ± 0.66 Aba | 24.46 ± 0.30 Bb | 25.01 ± 0.31 Ab |
| M 3.0% | 30.57 ± 0.19 Aa | 29.55 ± 0.18 Aa | 28.76 ± 0.96 Ba | 26.46 ± 0.25 Cb |
| (d) HSI | ||||
| Samples | Days | |||
| D0 | D2 | D3 | D7 | |
| Control | 25.39 ± 4.67 ABc | 26.33 ± 3.85 Ab | 20.21 ± 0.53 Bc | 19.23 ± 6.54 ABb |
| M 0.5% | 49.86 ± 0.93 Ab | 53.09 ± 5.10 Aa | 43.36 ± 6.68 Aa | 44.17 ± 3.67 Aa |
| M 1.0% | 58.27 ± 2.91 Aa | 59.89 ± 4.95 Aa | 48.27 ± 0.46 Ba | 47.31 ± 5.32 Ba |
| M 3.0% | 48.13 ± 1.97 Ab | 52.79 ± 5.68 Aa | 47.01 ± 1.50 Aa | 44.38 ± 3.70 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.; Gonçalves, A.; Riolo, M.; Dopazo, V.; Calpe, J.; Meca, G. Biocontrol Ability Against Harmful Microbial Contamination of Vegan Mortadella with an Ingredient of Oat Fermented by Lactiplantibacillus plantarum. Foods 2025, 14, 2195. https://doi.org/10.3390/foods14132195
Moreno A, Gonçalves A, Riolo M, Dopazo V, Calpe J, Meca G. Biocontrol Ability Against Harmful Microbial Contamination of Vegan Mortadella with an Ingredient of Oat Fermented by Lactiplantibacillus plantarum. Foods. 2025; 14(13):2195. https://doi.org/10.3390/foods14132195
Chicago/Turabian StyleMoreno, Ana, Alberto Gonçalves, Mario Riolo, Victor Dopazo, Jorge Calpe, and Giuseppe Meca. 2025. "Biocontrol Ability Against Harmful Microbial Contamination of Vegan Mortadella with an Ingredient of Oat Fermented by Lactiplantibacillus plantarum" Foods 14, no. 13: 2195. https://doi.org/10.3390/foods14132195
APA StyleMoreno, A., Gonçalves, A., Riolo, M., Dopazo, V., Calpe, J., & Meca, G. (2025). Biocontrol Ability Against Harmful Microbial Contamination of Vegan Mortadella with an Ingredient of Oat Fermented by Lactiplantibacillus plantarum. Foods, 14(13), 2195. https://doi.org/10.3390/foods14132195







