Combined UV and Formic Acid Treatment Suppresses Aspergillus flavus and Aflatoxin B1 on Dried Red Chili Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Strain Culture
2.2. Formic Acid and UV Treatment
2.2.1. Formic Acid
2.2.2. Ultraviolet
2.3. Determination of the Inhibitory Effect of Formic Acid on the Growth and AFB1 Synthesis of A. flavus on Dried Red Chili Powder
2.3.1. Determination of the Inhibition Rate of A. flavus Colonies
2.3.2. Determination of AFB1 Content
2.4. Determination of the UV-Formic Acid Combination Approach
2.5. Determination of the Combined Single Factor of Formic Acid and UV
2.6. Determination of Growth Inhibition and AFB1 Synthesis of A. flavus by Combined UV Formic Acid Orthogonal Test
2.7. Determination of the Quality of Dried Red Chili Powder After UV-Formic Acid Treatment
2.7.1. Color, Natural Coloring Matter
2.7.2. Capsaicin Content Analysis
2.7.3. Total Phenolic Content (TPC)
2.7.4. DPPH Radical Scavenging Rate
2.8. Assessing the Impact of UV-Formic Acid Treatment on A. flavus Membrane Integrity
2.8.1. PI and FDA Staining
2.8.2. Leakage of Intracellular Proteins and Nucleic Acids
2.8.3. Observation of Micro-Morphology of A. flavus
2.9. Evaluating the Impact of UV-Formic Acid on A. flavus Antioxidant Response
2.10. Assessment of Energy Metabolic Responses in A. flavus to UV-Formic Acid Treatment
2.11. Experimental Data Processing and Analysis
3. Results
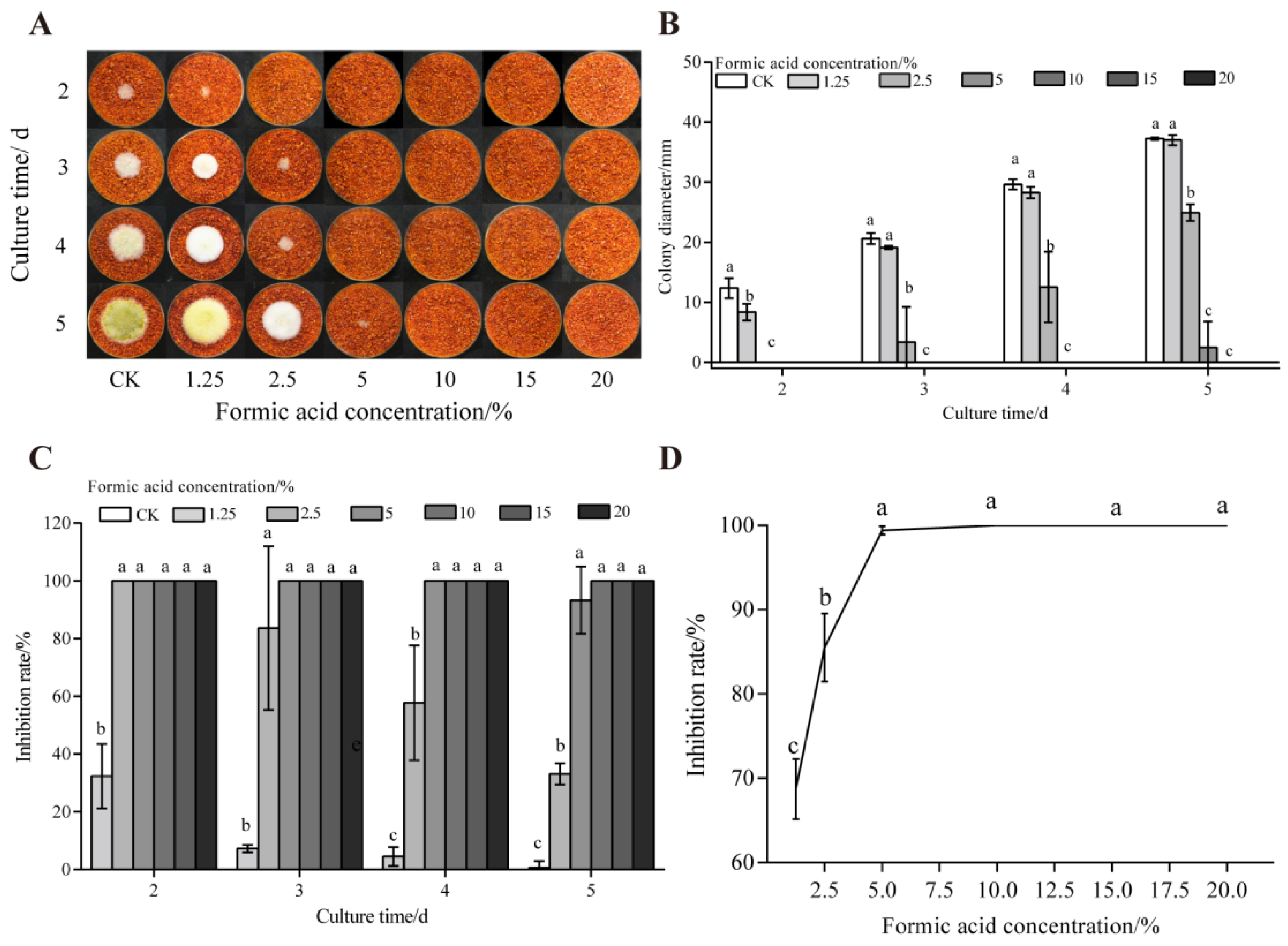
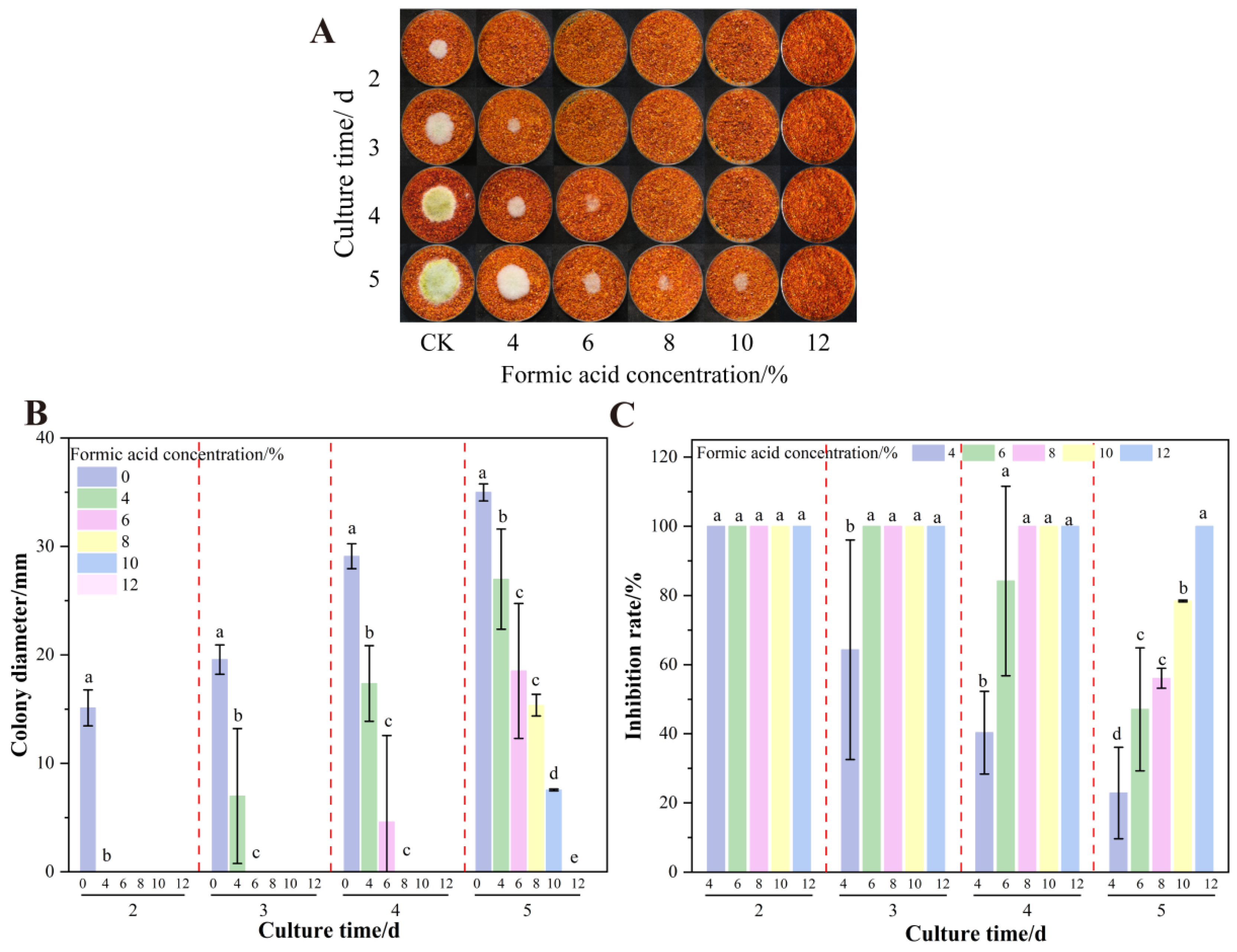
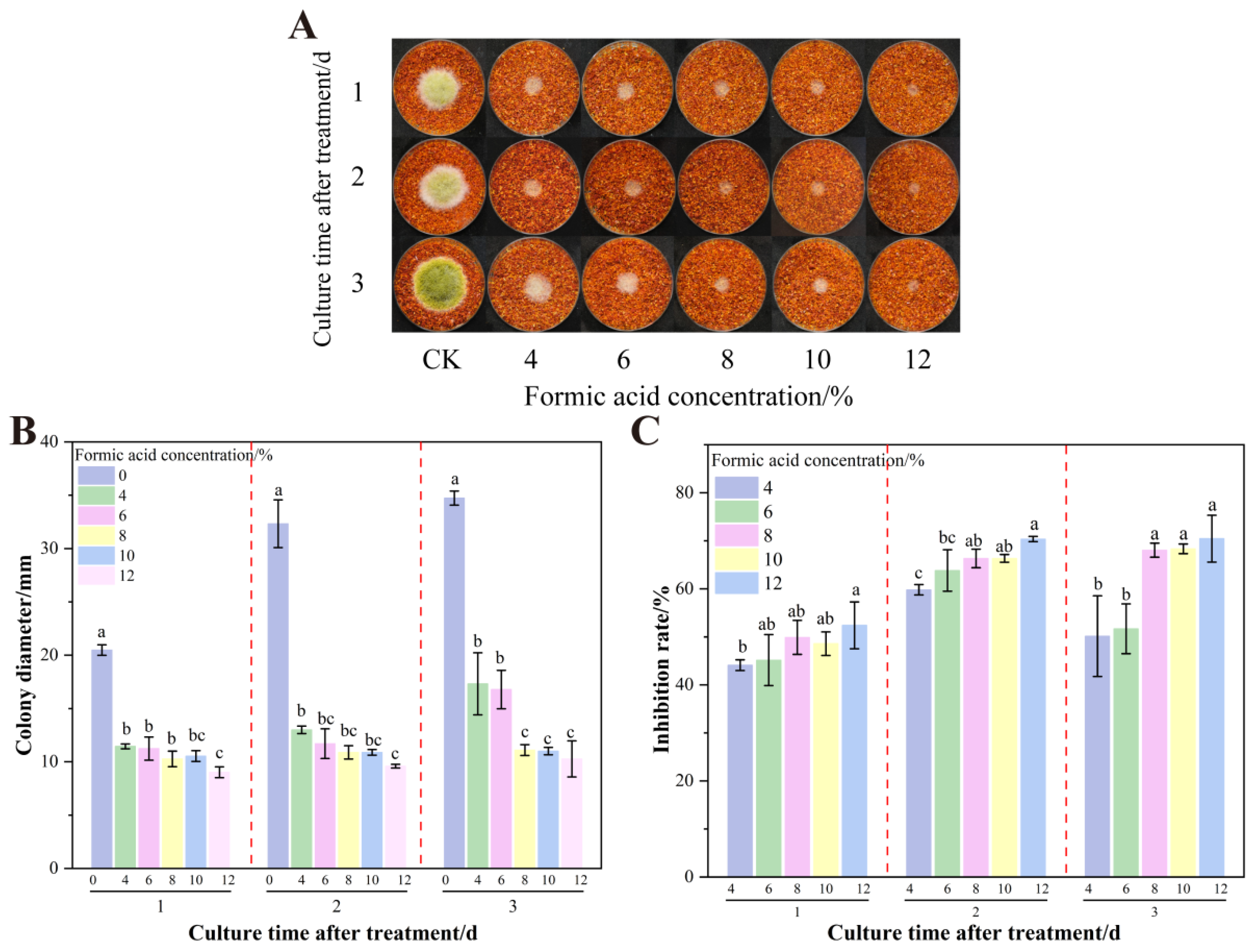
3.1. Effect of Formic Acid on the Growth and AFB1 Synthesis of A. flavus on Dried Red Chili Powder
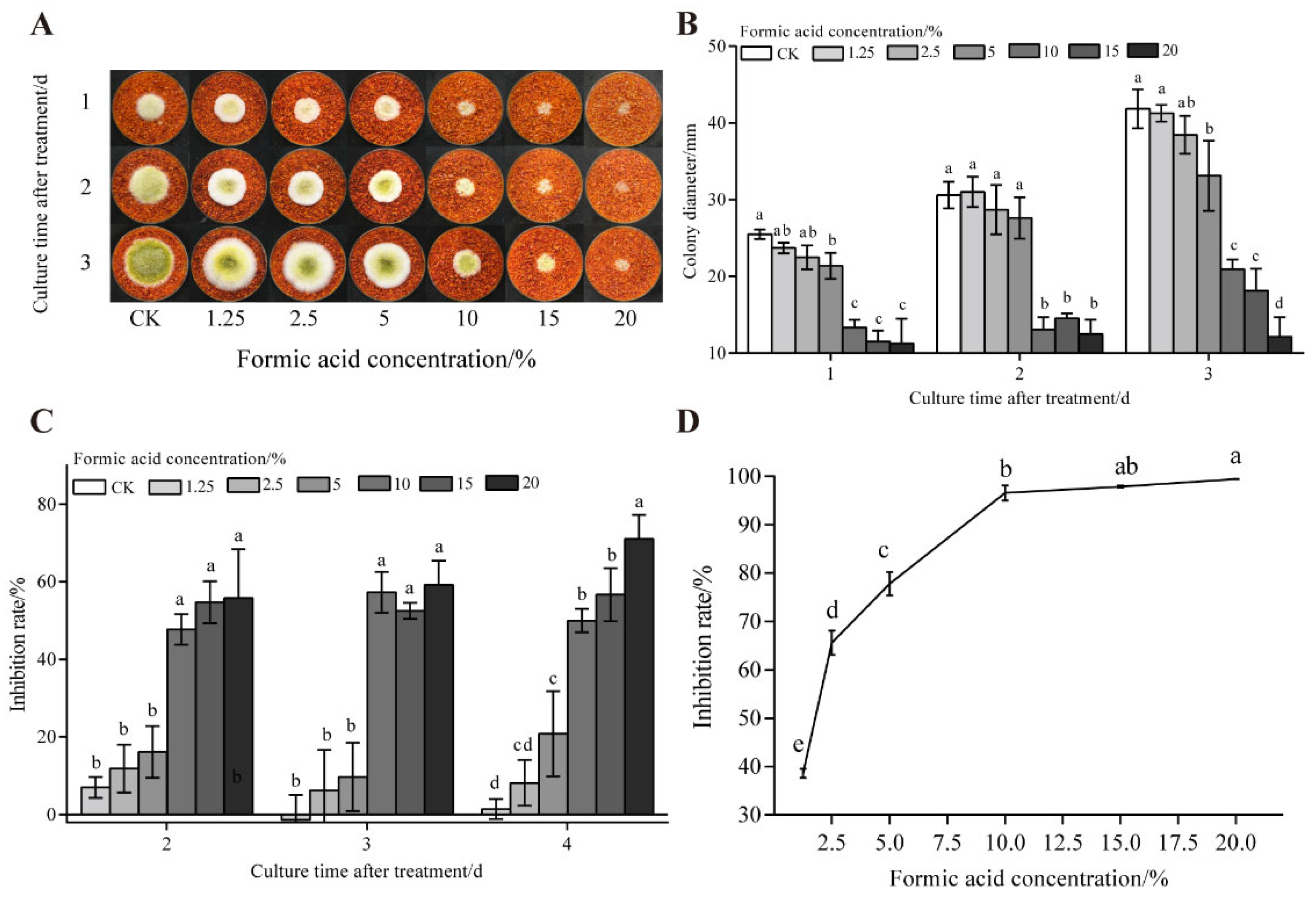
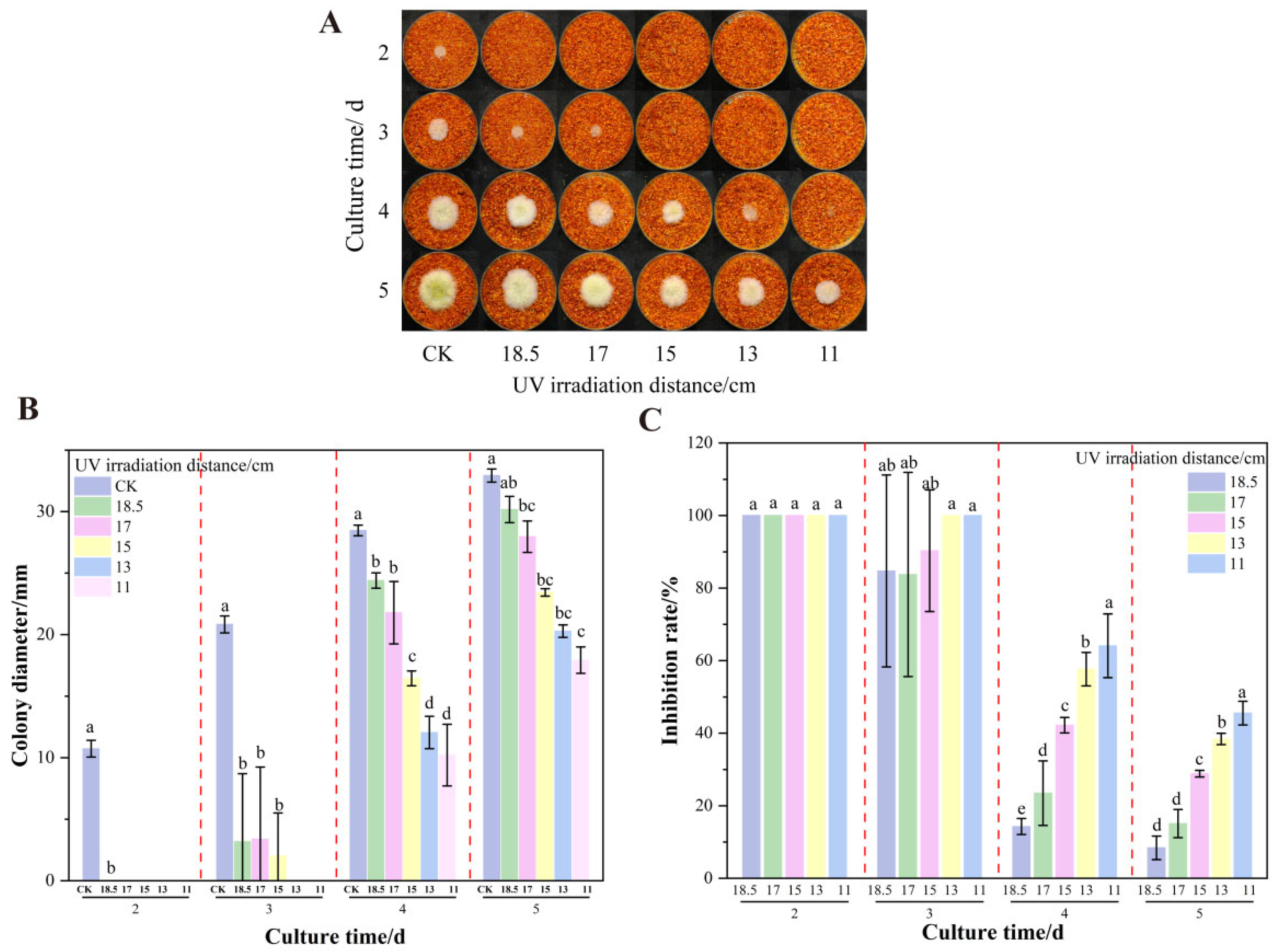
3.2. The Effect of UV on the Growth and AFB1 Synthesis of A. flavus on Dried Red Chili Powder
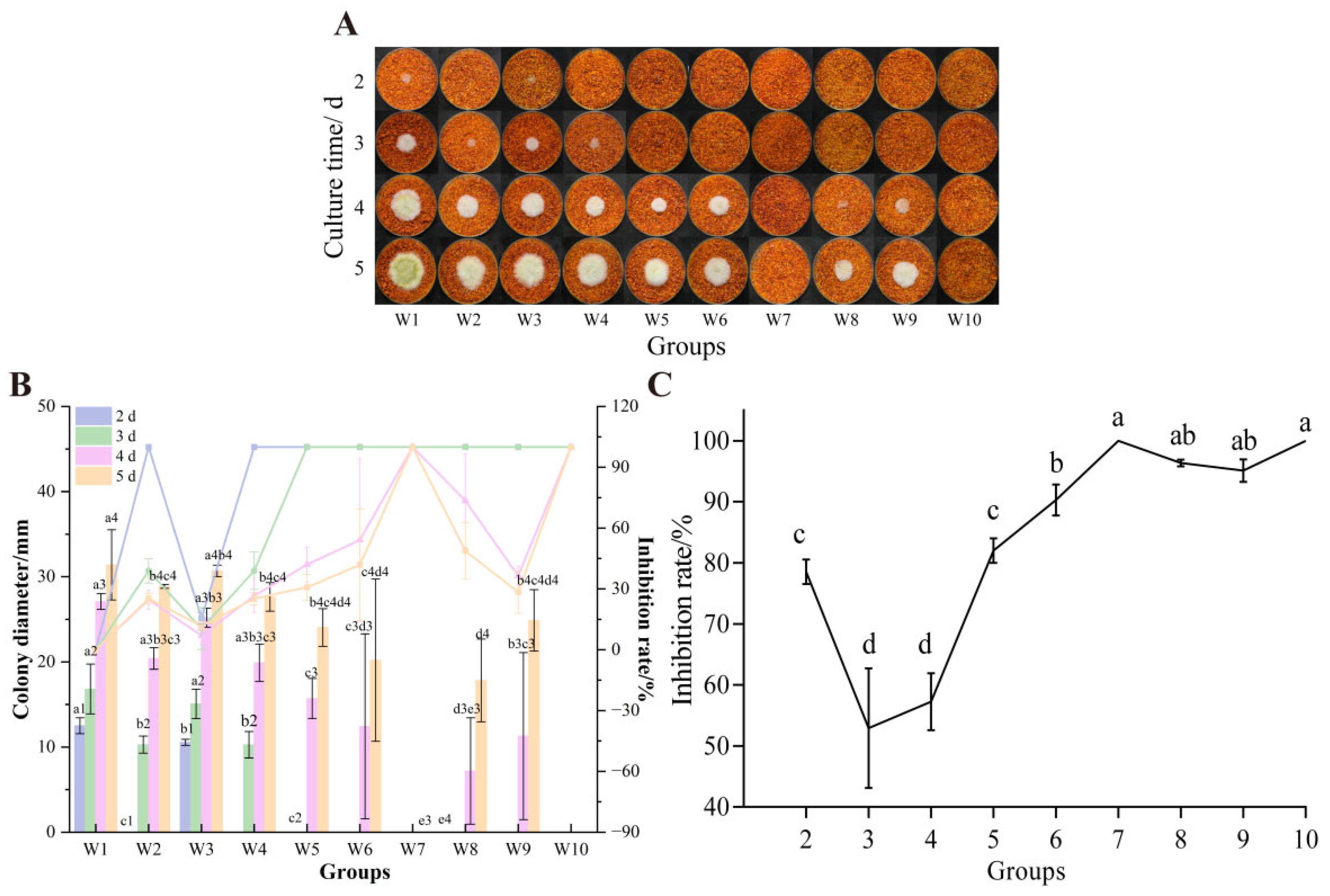
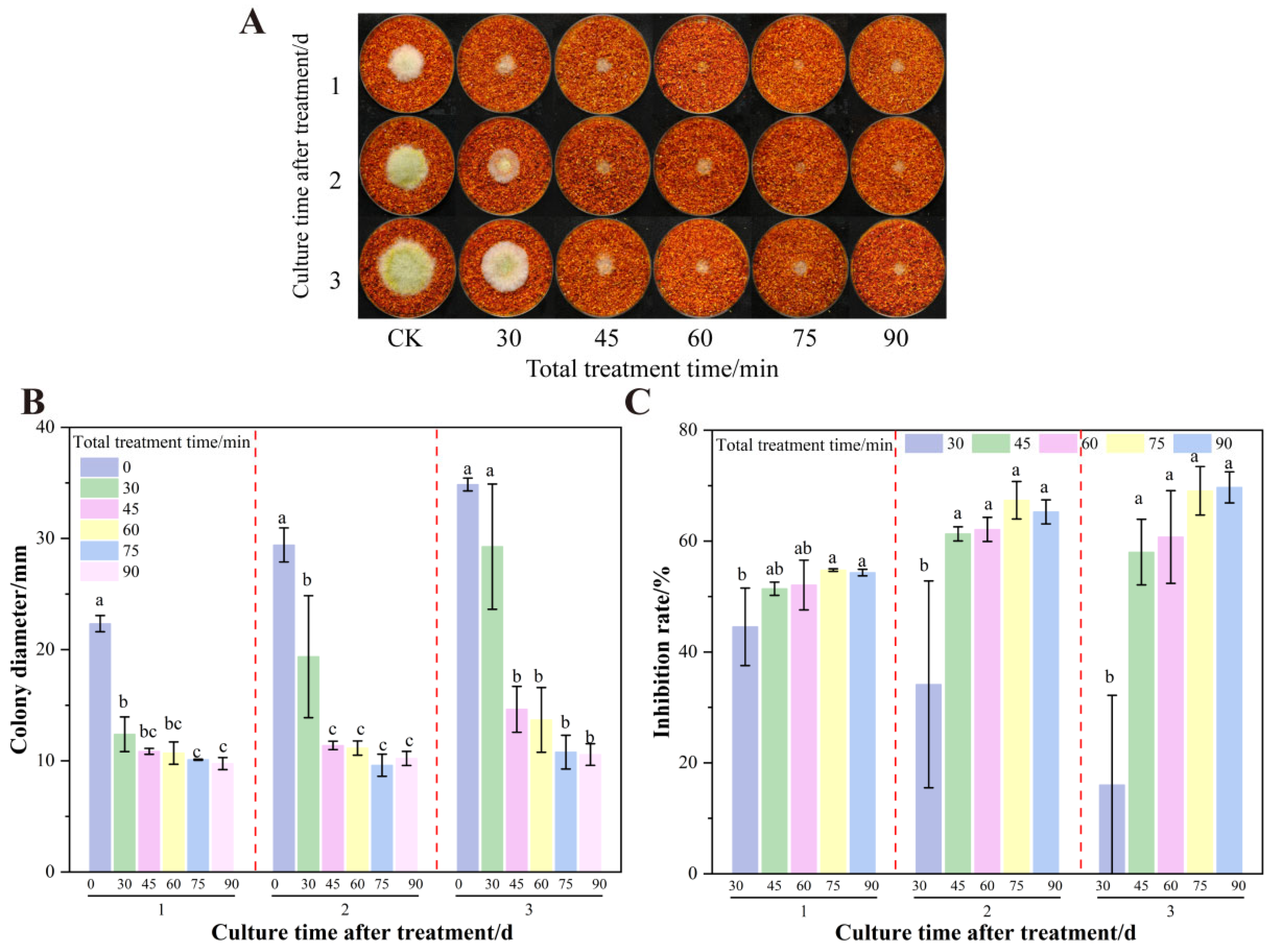
3.3. The Effect of Different UV-Formic Acid Combined Approach on the Growth of A. flavus and AFB1 Synthesis on Dried Red Chili Powders
3.4. The Effect of Formic Acid Concentration on the Growth of A. flavus on Dried Red Chili Powder in UV-Formic Acid Treatment
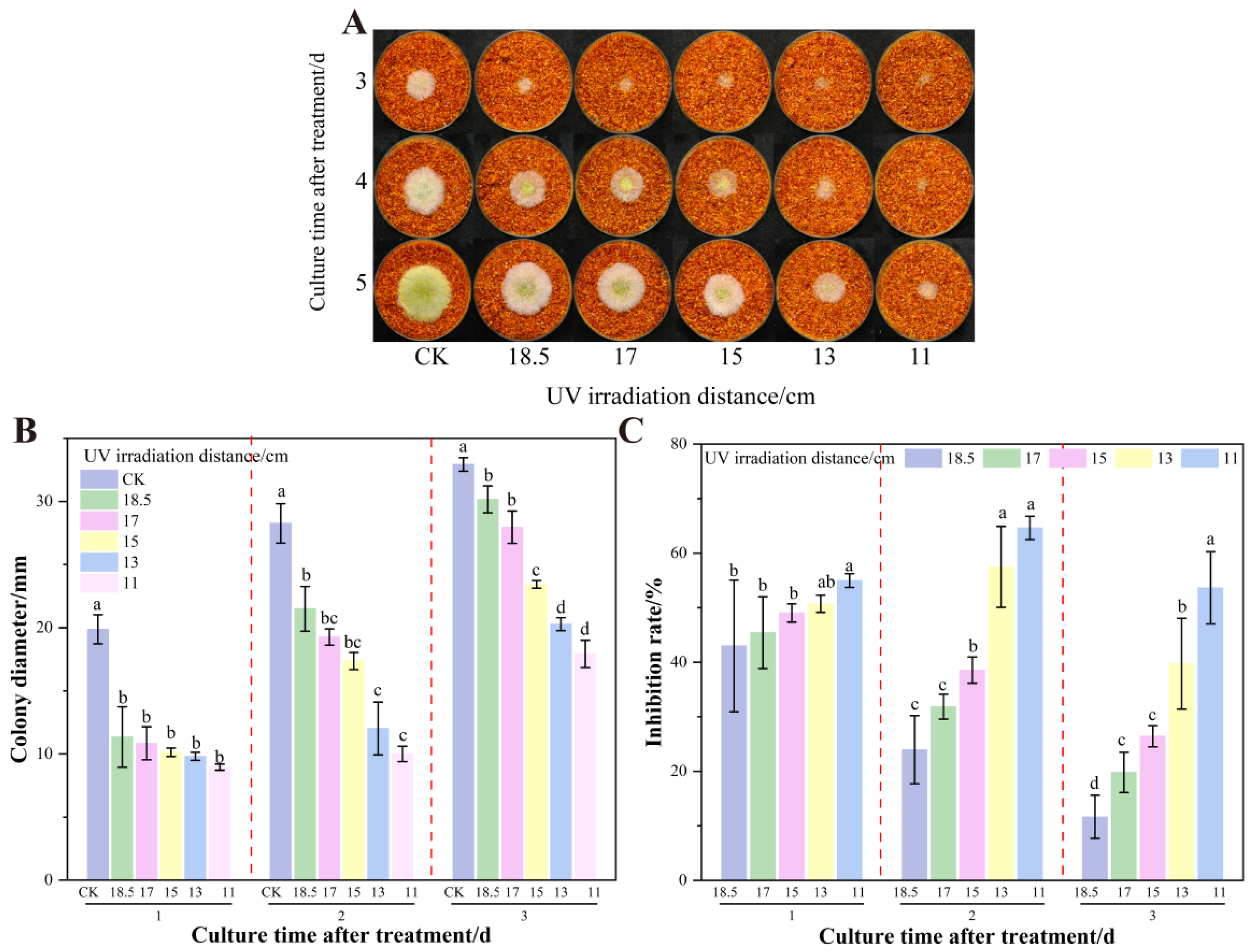
3.5. The Effect of UV Irradiation Distance on the Growth of A. flavus on Dried Red Chili Powder in UV-Formic Acid Treatment
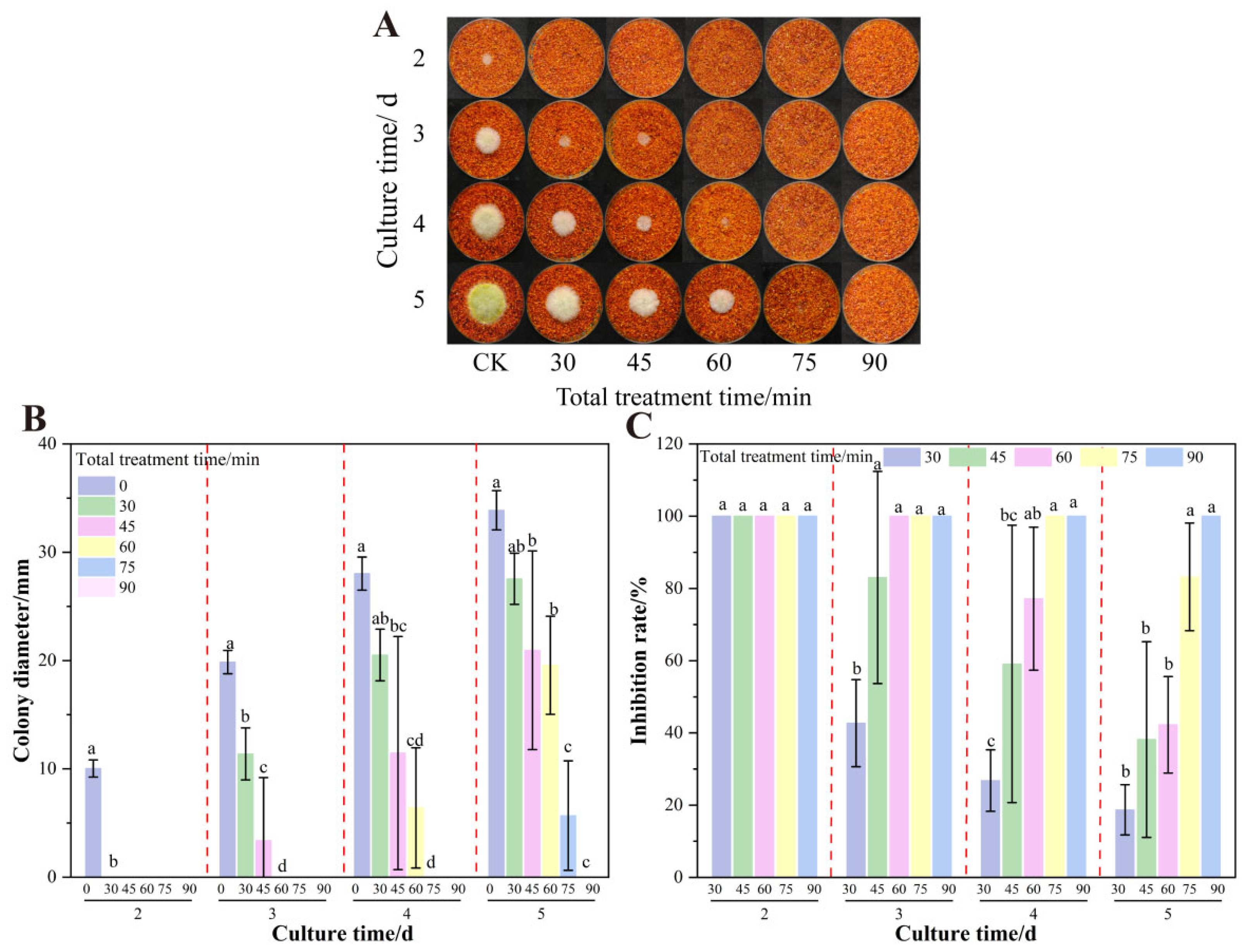
3.6. The Effect of Total Treatment Time on the Growth of A. flavus on Dried Red Chili Powder in UV-Formic Acid Treatment
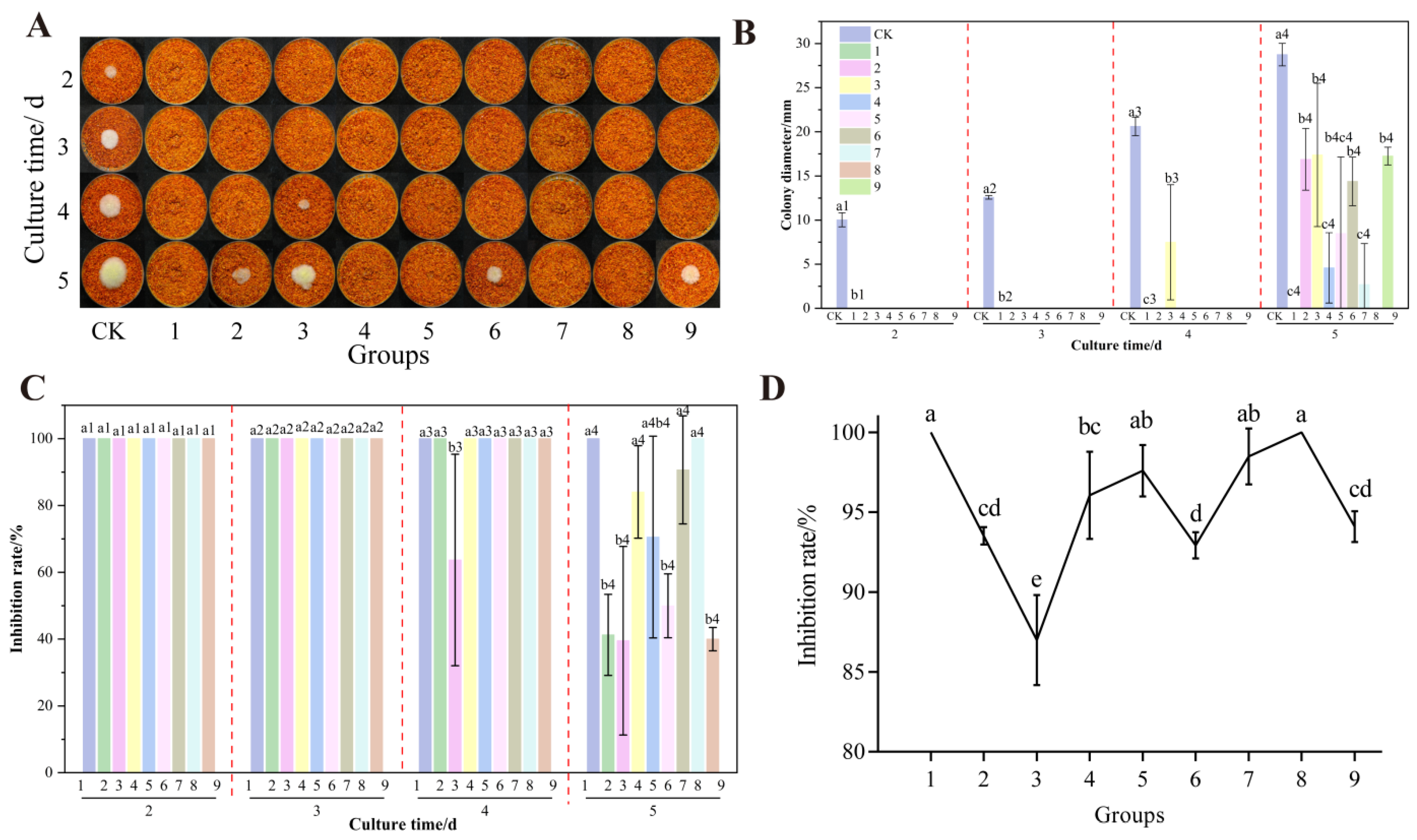
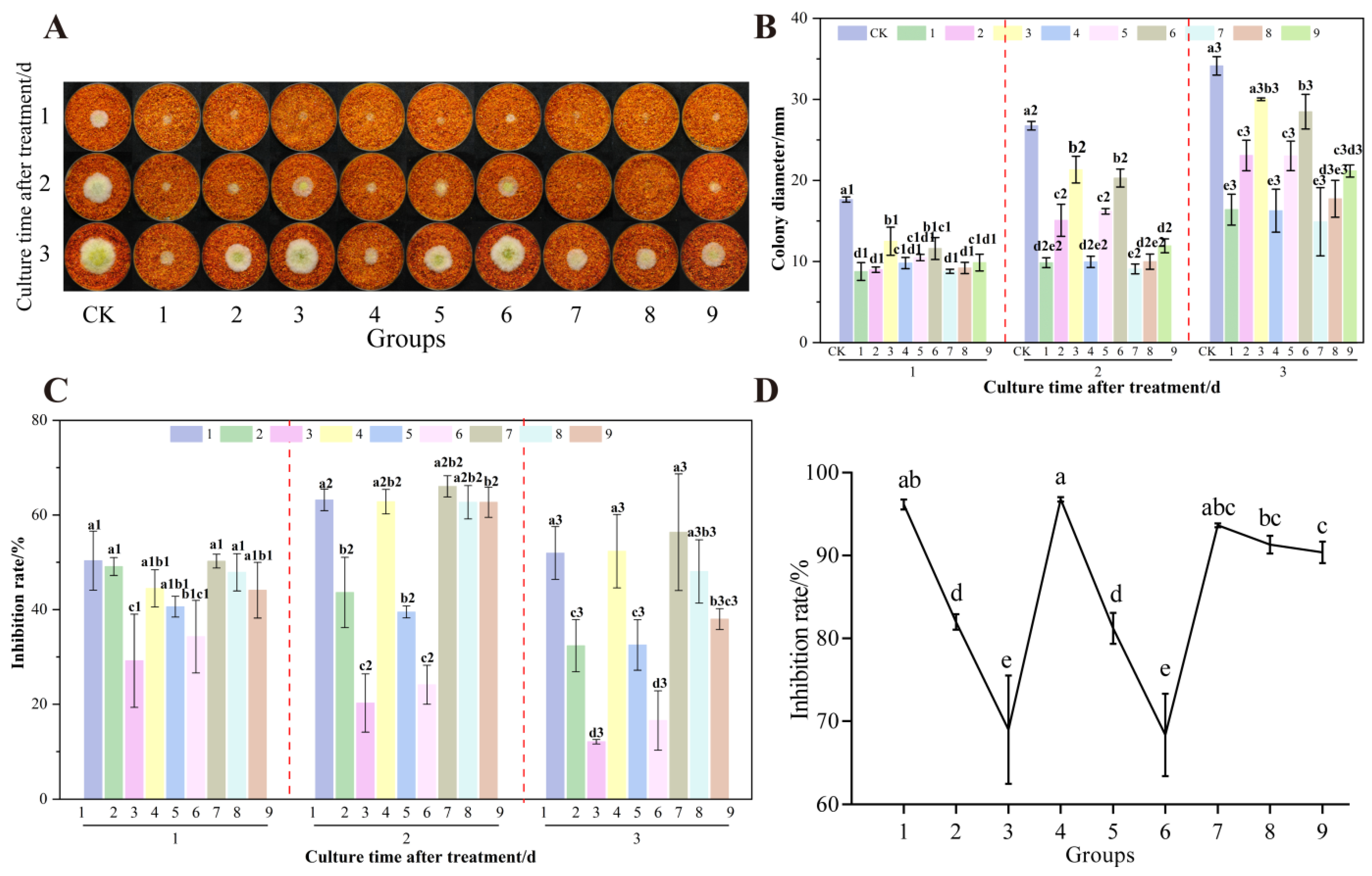
3.7. The Effect of UV-Formic Acid Treatment Orthogonal Test on the Growth of A. flavus and AFB1 Synthesis on Dried Red Chili Powders
3.7.1. Un-Colonized Chili Powder
3.7.2. Already Colonized Chili Powder
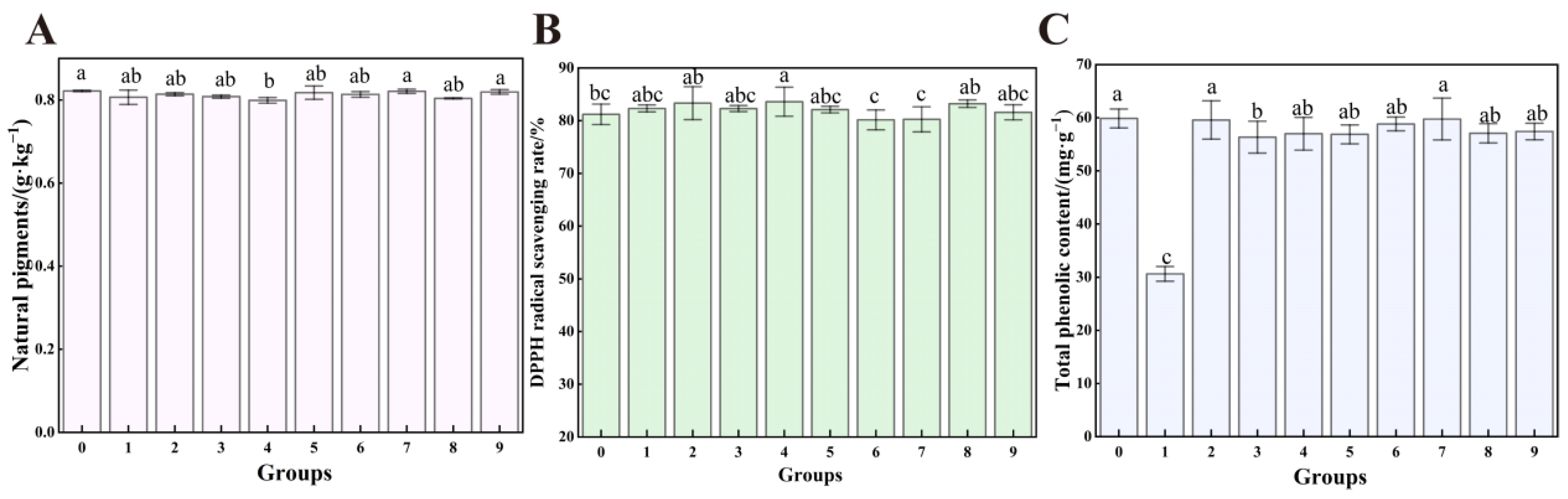
3.8. The Effect of UV-Formic Acid Treatment on the Quality of Dried Red Chili Powder
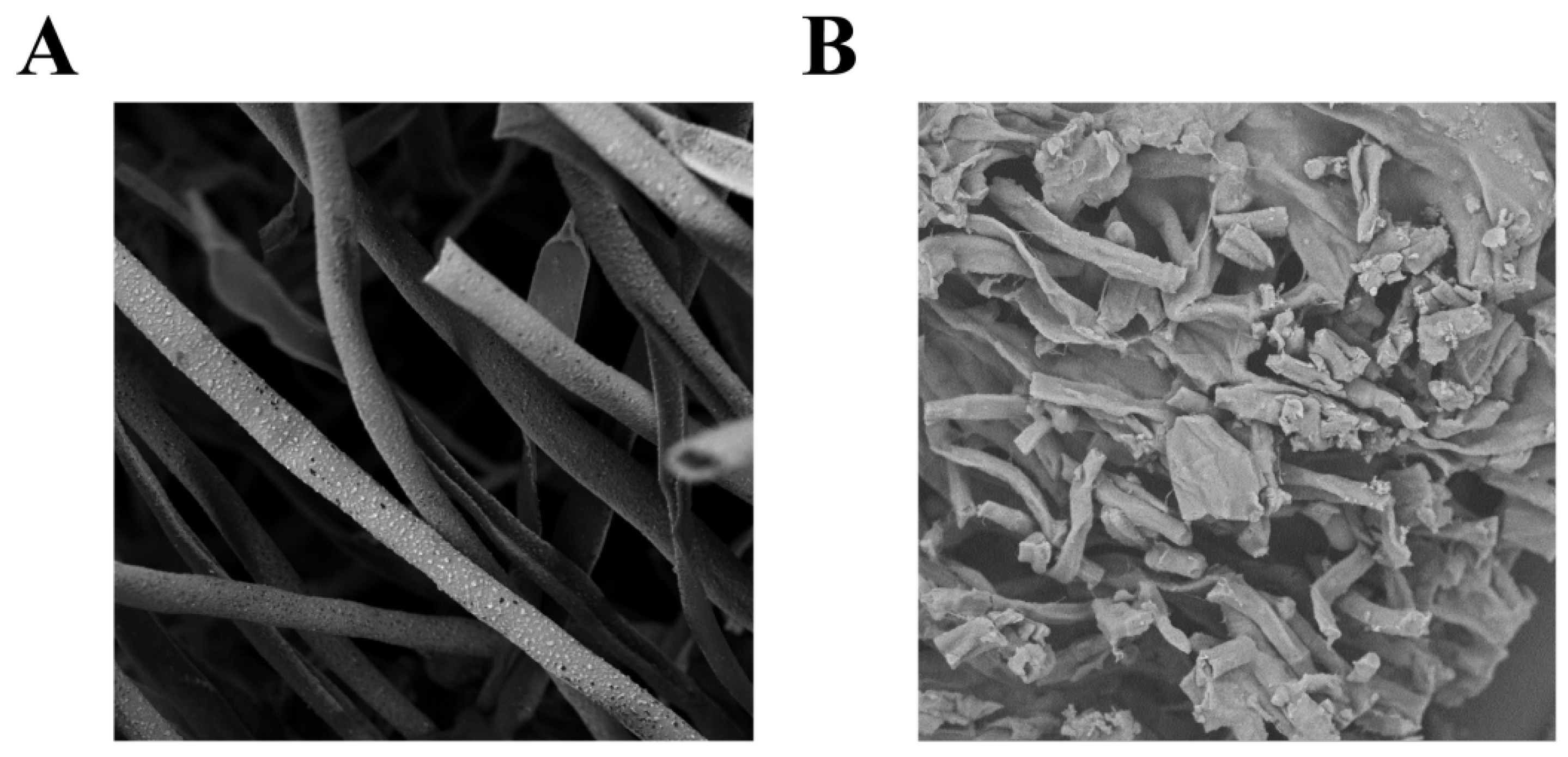
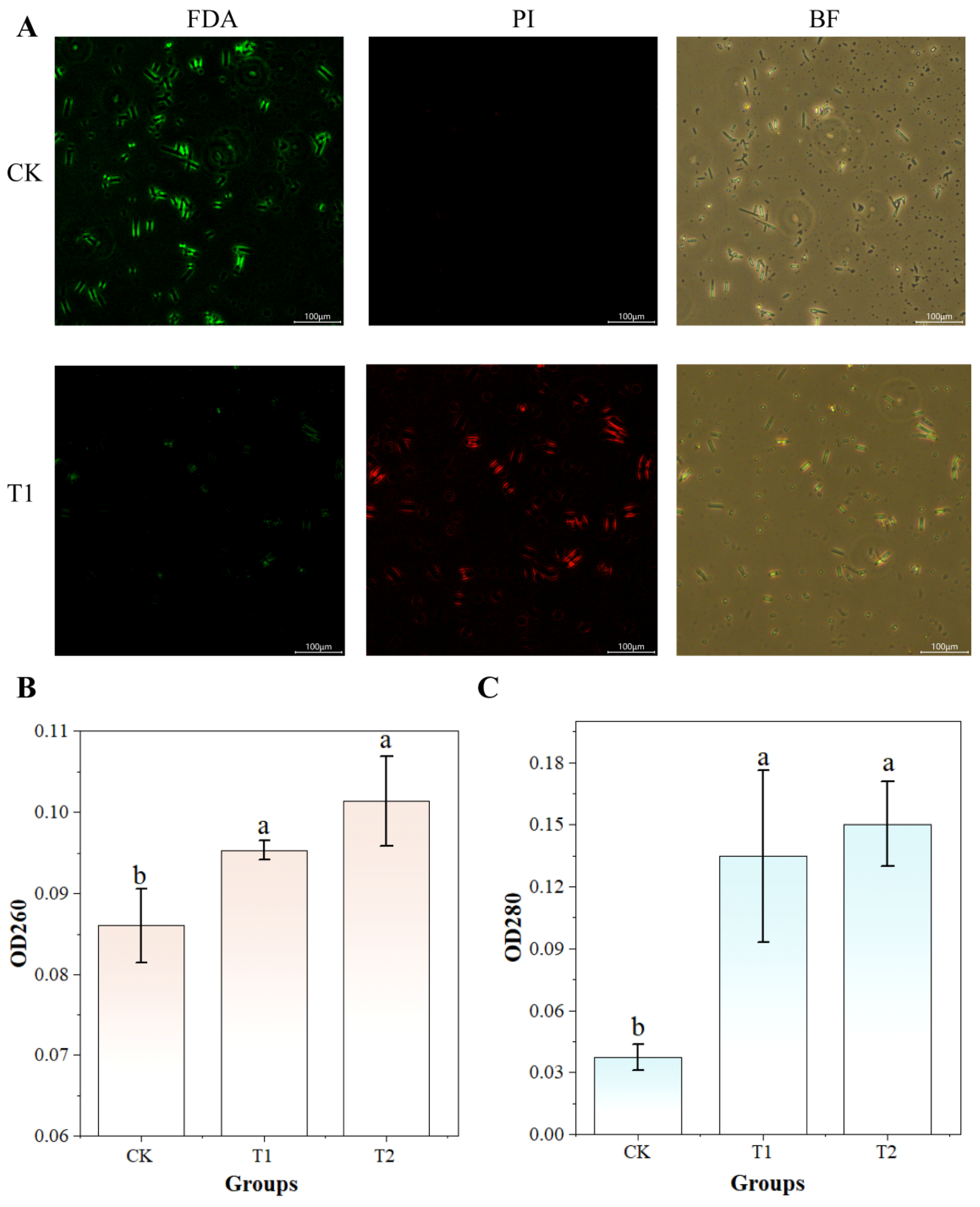
3.9. UV-Formic Acid Treatment Induces Cell Membrane Alterations in A. flavus
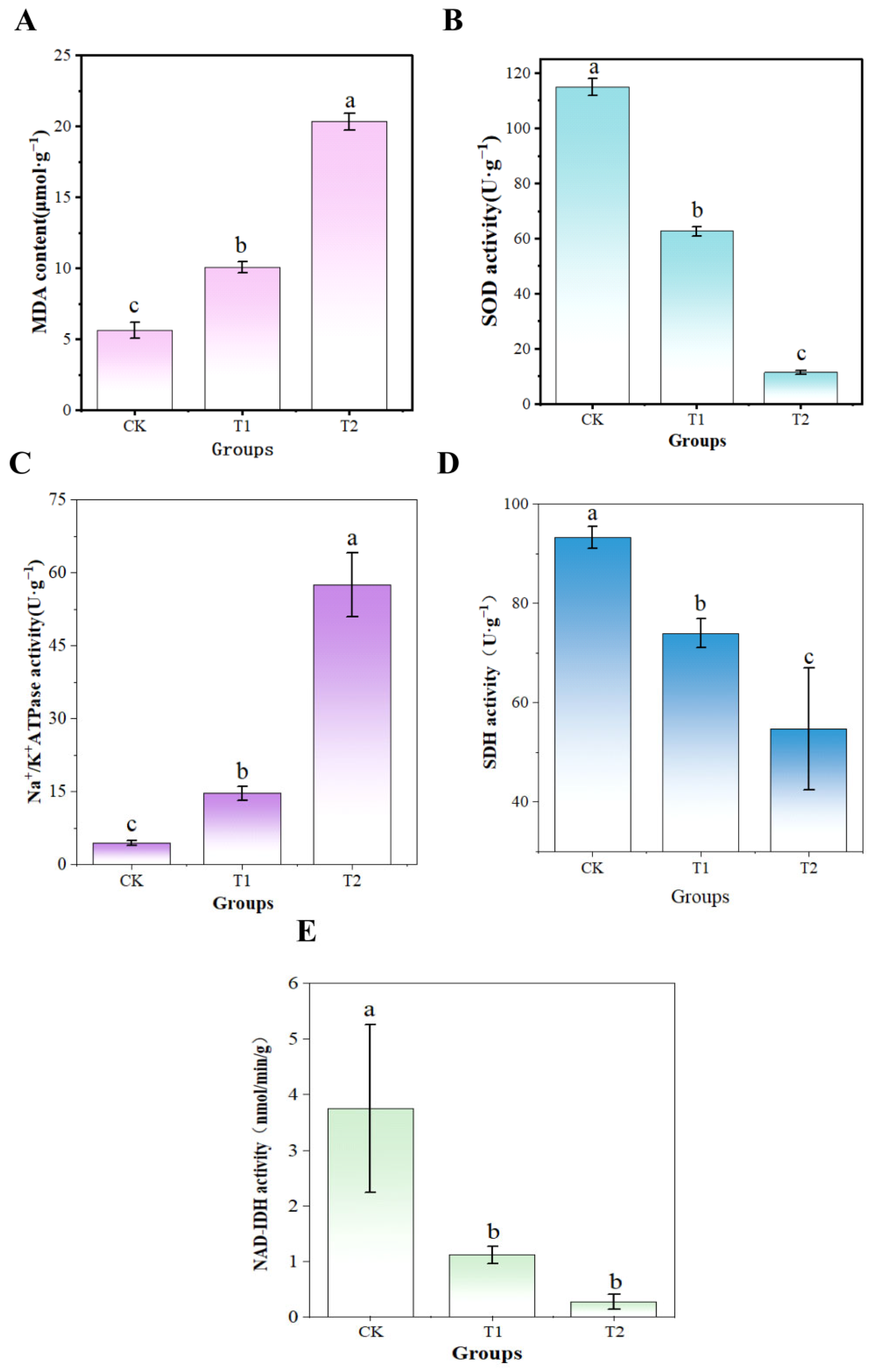
3.10. UV-Formic Acid Induced Antioxidant Response and Energy Metabolic Reprogramming in A. flavus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| AFB1 | Aflatoxin B1 |
| TPC | Total phenolic content |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FDA | Fluorescein diethyl acetate |
| PI | Propidium iodide |
| SEM | Scanning electron microscopy |
| MDA | Malondialdehyde |
| NAD-IDH | NAD-isocitrate dehydrogenase |
| SDH | Succinate dehydrogenase |
Appendix A
References
- Jallow, A.; Xie, H.L.; Tang, X.Q.; Qi, Z.; Li, P.W. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Caceres, I.; Khoury, A.A.; Khoury, R.E.; Lorber, S.; Oswald, I.P.; Khoury, A.E.; Atoui, A.; Puel, O.; Bailly, J. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- Lu, X.X.; Zhu, Y.Y.; Cheng, F.; Wu, T.; Maiwulanjiang, M. Ajwain essential oil inhibits aflatoxin B1 and ergosterol production in Aspergillus flavus: Mechanisms and developmental stages-specific effects. Food Biosci. 2024, 62, 105561. [Google Scholar] [CrossRef]
- Khalil, H.M.A.; Eid, W.A.M.; El-Nablaway, M.; El Nashar, E.M.; Al-tarish, J.S.; El Henafy, H.M.A. Date seeds powder alleviate the aflatoxin B1 provoked heart toxicity in male offspring rat. Sci. Rep. 2024, 14, 30480. [Google Scholar] [CrossRef]
- Korkak, F.A.; Alkac, Z.K.; Arkali, G.; Gungor, I.H.; Yuksel, B.F.; Incili, C.A.; Tanyildizi, S.; Dagoglu, G. Co-administration of rifampicin and Boswellia serrata mitigates testicular toxicity caused by Aflatoxin B1. Toxicon 2025, 255, 108238. [Google Scholar] [CrossRef]
- Pawel, J.; Vladimir, L.V.; Irina, G.M.; Miral, D.; Lloyd, R.S. Aflatoxin B1-induced DNA adduct formation in murine kidney and liver. Environ. Toxicol. Pharmacol. 2025, 114, 104647. [Google Scholar] [CrossRef]
- Tabatabaei-Moradi, L.; Sharifan, A.; Hajizadeh, K.; Bakhoda, H. In Vitro Bioaccessibility, Cytotoxicity Against Liver Cells and Degradation Modeling Aflatoxin B1 in Bread by Cold Atmospheric Pressure Plasma. Food Bioprocess Technol. 2025, 18, 1405–1416. [Google Scholar] [CrossRef]
- Maxwell, L.A.; Callicott, K.A.; Bandyopadhyay, R.; Mehl, H.L.; Orbach, M.J.; Cotty, P.J. Degradation of aflatoxin B1 by atoxigenic Aspergillus flavus biocontrol agents. Plant Dis. 2021, 105, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Taiti, C.; Costa, C.; Migliori, C.A.; Comparini, D.; Figorilli, S.; Mancuso, S. Correlation Between Volatile Compounds and Spiciness in Domesticated and Wild Fresh Chili Peppers. Food Bioprocess Technol. 2019, 12, 1366–1380. [Google Scholar] [CrossRef]
- Hadil Mon, V.; John Kennedy, Z.; Paranitharan, V.; Karthikeyan, S. Mycotic contamination and aflatoxin potential of molds in Capsicum annum (chili), and chili powder commercialized in south Indian markets. Toxicon 2022, 210, 109–114. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.S.; Zhu, Q.J.; Wan, J. Ochratoxin A contamination and related high-yield toxin strains in Guizhou dried red chilies. Food Control 2022, 137, 109438. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Deepika; Singh, B.K.; Dubey, N.K. Antimicrobial, Aflatoxin B1 Inhibitory and Lipid Oxidation Suppressing Potential of Anethole-Based Chitosan Nanoemulsion as Novel Preservative for Protection of Stored Maize. Food Bioprocess Technol. 2020, 13, 1462–1477. [Google Scholar] [CrossRef]
- Yuan, S.L.; Wu, Y.J.; Jin, J.; Tong, S.Q.; Zhang, L.C.; Cai, Y.F. Biocontrol Capabilities of Bacillus subtilis E11 against Aspergillus flavus In Vitro and for Dried Red Chili (Capsicum annuum L.). Toxins 2023, 15, 308. [Google Scholar] [CrossRef]
- Halloub, A.; Nekhlaoui, S.; Raji, M.; Essabir, H.; Bensalah, M.; Bouhfid, R.; Qaiss, A.E.K. UV-activated self-healing food packaging: ω-3 based microcapsules in hemicellulose/chitosan blend film for cashew nut preservation. Food Biosci. 2024, 60, 104313. [Google Scholar] [CrossRef]
- Yang, X.D.; Wang, Y. Photocatalytic effect on plasmid DNA damage under different UV irradiation time. Build. Environ. 2008, 43, 253–257. [Google Scholar] [CrossRef]
- Chen, X.M.; Du, M.Y.; Chen, K.W.; Kan, J.Q. Effects of Aspergillus flavus inhibition by using UV-C ultraviolet radiation on dried red pepper quality. Trans. Chin. Soc. Agric. Eng. 2024, 40, 269–278. [Google Scholar] [CrossRef]
- Hu, Y.C.; Zhang, J.M.; Kong, W.J.; Zhao, G.; Yang, M.H. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Mao, J.; He, B.; Zhang, L.X.; Li, P.W.; Zhang, Q.; Ding, X.X.; Zhang, W. A Structure Identification and Toxicity Assessment of the Degradation Products of Aflatoxin B1 in Peanut Oil under UV Irradiation. Toxins 2016, 8, 332. [Google Scholar] [CrossRef]
- Bozidar, U.; Slavica, S.; Nikola, T.; Ilija, D.; Nada, S.; Bojana, S.T.; Dragan, M.; Andreja, R. Evaluation of ultraviolet irradiation effects on Aspergillus flavus and Aflatoxin B1 in maize and peanut using innovative vibrating decontamination equipment. Food Control 2021, 134, 108691. [Google Scholar] [CrossRef]
- Lee, H.; Ryu, J.; Kim, H. Antimicrobial activity of gaseous chlorine dioxide against Aspergillus flavus on green coffee beans. Food Microbiol. 2020, 86, 103308. [Google Scholar] [CrossRef]
- Lee, H.; Kim, N.; Ryu, J.; Kim, H. Inactivation of Aspergillus flavus on green coffee beans by treatments with organic acid vapor. Food Control 2024, 160, 110322. [Google Scholar] [CrossRef]
- Park, M.; Kang, D. Antibacterial Activity of Caffeic Acid Combined with Ultraviolet-A Light against Escherichia coli O157:H7, Salmonella Typhimurium and Listeria monocytogenes. Appl. Environ. Microbiol. 2021, 87, e00631-21. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Tiwari, S.; Maurya, A.; Das, S.; Singh, V.K.; Dubey, N.K. Chitosan-Based Nanoencapsulation of Ocimum americanum Essential Oil as Safe Green Preservative Against Fungi Infesting Stored Millets, Aflatoxin B1 Contamination, and Lipid Peroxidation. Food Bioprocess Technol. 2023, 16, 1851–1872. [Google Scholar] [CrossRef]
- Wang, K.M.; Wang, H.; Wu, Y.; Yi, C.; Lv, Y.X.; Luo, H.Y.; Yang, T. The antibacterial mechanism of compound preservatives combined with low voltage electric fields on the preservation of steamed mussels (Mytilus edulis) stored at ice-temperature. Front. Nutr. 2023, 10, 1126456. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, H.S.; El-Sayed, S.M.; Fouly, M.; Abd El-Aziz, M.E. Novel Bionanocomposites Based on Cinnamon Nanoemulsion and TiO2-NPs for Preserving Fresh Chicken Breast Fillets. Food Bioprocess Technol. 2023, 16, 356–367. [Google Scholar] [CrossRef]
- Zhang, C.H.; Hou, W.F.; Zhao, W.T.; Zhao, S.; Wang, P.; Zhao, X.; Wang, D. Effect of Ultrasound Combinated with Sodium Hypochlorite Treatment on Microbial Inhibition and Quality of Fresh-Cut Cucumber. Foods 2023, 12, 754. [Google Scholar] [CrossRef]
- Fan, L.L.; Li, L.L.; Shang, F.F.; Xie, Y.H.; Duan, Z.H.; Cheng, Q.W.; Zhang, Y.Q. Study on antibacterial mechanism of electron beam radiation on Aspergillus flavus. Food Biosci. 2023, 51, 102197. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.R.; Yang, G.; Huang, S.; Liao, S.L.; Chen, K.W.; Du, M.Y.; Zalán, Z.; Hegyi, F.; Kan, J.Q. Biocontrol potential of 1-pentanal emitted from lactic acid bacteria strains against Aspergillus flavus in red pepper (Capsicum annuum L.). Food Control 2022, 142, 109261. [Google Scholar] [CrossRef]
- Zhao, X.B.; Wang, N.N.; Lu, Y.Y.; Li, Y.X.; Zhao, T.; Xu, J.P.; Liu, B.; Shao, K.; Wang, Z.L.; Yuan, Y.H.; et al. Effects of cold plasma on the growth and aflatoxin production of Aspergillus flavus. Food Biosci. 2024, 61, 104552. [Google Scholar] [CrossRef]
- GB 5009.22-2016; National Food Safety Standard—Determination of Aflatoxins B and G in Foods. National Health Commission of the People’s Republic of China (NHC): Beijing, China, 2016. (In Chinese)
- Aguilera, J.M.; Del Valle, J.M.; Karel, M. Caking phenomena in amorphous food powders. Trends Food Sci. Technol. 1995, 6, 149–155. [Google Scholar] [CrossRef]
- GB/T 21266-2007; Determination of Capsaicinoids in Chilli and Chilli Products and Scoville Index. Standardization Administration of China (SAC); General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ); National Standard of the People’s Republic of China: Beijing, China, 2008. (In Chinese)
- Almusallam, I.A.; Mohamed Ahmed, I.A.; Babiker, E.E.; Al Juhaimi, F.Y.; Fadimu, G.J.; Osman, M.A.; Al Maiman, S.A.; Ghafoor, K.; Alqah, H.A.S. Optimization of ultrasound-assisted extraction of bioactive properties from date palm (Phoenix dactylifera L.) spikelets using response surface methodology. Lwt Food Sci. Technol. 2020, 140, 110816. [Google Scholar] [CrossRef]
- Zeb, A.; Rahman, F. Phenolic profile, total bioactive contents, and antioxidant activity of pear fruits. Food Chem. Adv. 2024, 5, 100780. [Google Scholar] [CrossRef]
- Mangiapelo, L.; Blasi, F.; Ianni, F.; Suvieri, C.; Sardella, R.; Volpi, C.; Cossignani, L. Optimization of a Simple Analytical Workflow to Characterize the Phenolic Fraction from Grape Pomace. Food Bioprocess Technol. 2024, 17, 1942–1957. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, H.D.; Yang, N.; Xie, H.J.; Liang, W.D.; Lin, J.Y.; Zhu, H.F.; Zhou, Y.; Wang, N.; Tan, X.Y.; et al. Fascaplysin derivatives binding to DNA via unique cationic five-ring coplanar backbone showed potent antimicrobial/antibiofilm activity against MRSA In Vitro and In Vivo. Eur. J. Med. Chem. 2022, 230, 114099. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Zhu, X.M.; Xie, Y.L. Antifungal efficacy of paeonol on Aspergillus flavus and its mode of action on cell walls and cell membranes. Lwt Food Sci. Technol. 2021, 149, 111985. [Google Scholar] [CrossRef]
- Li, M.H.; Yao, B.B.; Meng, X.M. Inhibitory effect and possible mechanism of phenyllactic acid on Aspergillus flavus spore germination. J. Basic Microbiol. 2022, 62, 1457–1466. [Google Scholar] [CrossRef]
- Liao, L.; Dong, T.T.; Qiu, X.; Rong, Y.; Sun, G.C.; Wang, Z.H.; Zhu, J. Antioxidant enzyme activity and growth responses of Huangguogan citrus cultivar to nitrogen supplementation. Biosci. Biotechnol. Biochem. 2019, 83, 1924–1936. [Google Scholar] [CrossRef]
- Moon, Y.; Kim, H.; Chun, H.S.; Lee, S. Organic acids suppress aflatoxin production via lowering expression of aflatoxin biosynthesis-related genes in Aspergillus flavus. Food Control 2018, 88, 207–216. [Google Scholar] [CrossRef]
- Shen, M.; Singh, R.K. Effect of rotating peanuts on aflatoxin detoxification by ultraviolet C light and irradiation uniformity evaluated by AgCl-based dosimeter. Food Control 2021, 120, 107533. [Google Scholar] [CrossRef]
- Hassan, A.B. Effect of UV-C followed by storage on the fungal growth and aflatoxins content and storability characteristics of sesame (Sesamum indicum) seeds. Heliyon 2023, 9, e12779. [Google Scholar] [CrossRef]
- Wu, X.L.; Guan, W.Q.; Yan, R.X.; Lei, J.; Xu, L.X.; Wang, Z.D. Effects of UV-C on antioxidant activity, total phenolics and main phenolic compounds of the melanin biosynthesis pathway in different tissues of button mushroom. Postharvest Biol. Technol. 2016, 118, 51–58. [Google Scholar] [CrossRef]
- Shen, M.; Singh, R.K. Detoxification of aflatoxins in foods by ultraviolet irradiation, hydrogen peroxide, and their combination—A review. Lwt Food Sci. Technol. 2021, 142, 110986. [Google Scholar] [CrossRef]
- Bang, H.; Park, S.Y.; Kim, S.E.; Md Furkanur Rahaman, M.; Ha, S. Synergistic effects of combined ultrasound and peroxyacetic acid treatments against Cronobacter sakazakii biofilms on fresh cucumber. Lwt 2017, 84, 91–98. [Google Scholar] [CrossRef]
- Sagong, H.; Lee, S.; Chang, P.; Heu, S.; Ryu, S.; Choi, Y.; Kang, D. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011, 145, 287–292. [Google Scholar] [CrossRef]
- Bu, Y.; Lv, Y.Y.; Tan, G.Z.; Zhu, W.H.; Li, J.R. Optimization of bacteria-reducing of oyster by ozone combined with slightly acidic electrolytic water. Iop Conf. Ser. Earth Environ. Sci. 2021, 792, 12019. [Google Scholar] [CrossRef]
- Yoon, J.; Oh, M.; Lee, S. Effectiveness of organic acids for inactivating pathogenic bacteria inoculated in laboratory media and foods: An updated minireview. Food Sci. Biotechnol. 2024, 33, 2715–2728. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Lopusiewicz, L. Recent progress on UV-light barrier food packaging films—A systematic review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103550. [Google Scholar] [CrossRef]
- McGuiggan, P.M.; Hall, A.K.I.; McGath, M.K.; Pasternack, L. The Permeability of PET by Formic and Acetic Acid Vapors. Stud. Conserv. 2022, 68, 458–473. [Google Scholar] [CrossRef]
- Kaidbey, K.H.; Kligman, A.M. Cumulative effects from repeated exposures to ultraviolet radiation. J. Investig. Dermatol. 1981, 76, 352–355. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhang, M.; Mujumdar, A.S.; Liu, Y.P. Application advantages of new non-thermal technology in juice browning control: A comprehensive review. Food Rev. Int. 2022, 39, 4102–4123. [Google Scholar] [CrossRef]
- Sharad, B.; Hideki, A. Thermal and UV Degradation Kinetics of Water-Soluble Extracellular Pigment Produced by Talaromyces purpurogenus. Food Bioprocess Technol. 2022, 15, 606–619. [Google Scholar] [CrossRef]
- Lv, X.R.; Li, L.L.; Lu, X.M.; Wang, W.X.; Sun, J.F.; Liu, Y.Q.; Mu, J.L.; Ma, Q.Y.; Wang, J. Effects of organic acids on color intensification, thermodynamics, and copigmentation interactions with anthocyanins. Food Chem. 2022, 396, 133691. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.; Shah, M.A.; Nawaz, M.A.; Pervez, S.; Noreen, N.; Vargas-de La Cruz, C.; Khan, F.; Blundell, R.; Briffa, J.; Azzopardi, J.; et al. Chapter 6—Analysis of other phenolics (capsaicin, gingerol, and alkylresorcinols). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 255–271. ISBN 978-0-12-816455-6. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Hamishehkar, H.; Ehsani, A.; Ghasempour, Z.; Moghaddas Kia, E. Applications of capsaicin in food industry: Functionality, utilization and stabilization. Crit. Rev. Food Sci. Nutr. 2021, 63, 4009–4025. [Google Scholar] [CrossRef]
- Bae, J.; Lee, D.; Oh, K.; Jeong, D.; Lee, D.; Kim, J. Photochemical advanced oxidative process treatment effect on the pesticide residues reduction and quality changes in dried red peppers. Sci. Rep. 2023, 13, 4444. [Google Scholar] [CrossRef] [PubMed]
- Ami, N.; Honami, N.; Fumi, T.; Ayumu, K.; Akira, Y.; Munetaka, H. Night-time ultraviolet B treatment can prevent edible rose flowers from plant disease without changes in appearance and polyphenol content. Sci. Hortic. 2024, 338, 113724. [Google Scholar] [CrossRef]
- Xu, X.Q.; Zuo, J.; Wan, Q.Q.; Cao, R.H.; Xu, H.N.; Li, K.; Huang, T.L.; Wen, G.; Ma, J. Effective Inactivation of Fungal Spores by the Combined UV/PAA: Synergistic Effect and Mechanisms. J. Hazard. Mater. 2022, 430, 128515. [Google Scholar] [CrossRef]
- Hu, Y.M.; Wang, Y.R.; Zhao, W.B.; Ding, Y.Y.; Wu, Z.R.; Wang, G.H.; Deng, P.; Zhang, S.Y.; An, J.X.; Zhang, Z.J.; et al. Efficacy of pterostilbene suppression on Aspergillus flavus growth, aflatoxin B1 biosynthesis and potential mechanisms. Int. J. Food Microbiol. 2023, 404, 110318. [Google Scholar] [CrossRef]
- Jaiswal, J.; Kumari, N.; Gupta, A.; Singh, A.P.; Sinha, R.P. Impacts of ultraviolet and photosynthetically active radiations on photosynthetic efficiency and antioxidant systems of the cyanobacterium Spirulina subsalsa HKAR-19. Folia Microbiol. 2023, 69, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Chkadua, G.; Nozadze, E.; Tsakadze, L.; Shioshvili, L.; Leladze, M.; Arutinova, N.; Dzneladze, S.; Javakhishvili, M.; Jariashvili, T. Cytochrome c and Ouabain Binding Site of Na,K-ATPase. Cell Biochem. Biophys. 2025. Epub ahead of printing. [Google Scholar] [CrossRef]
- Bauer, T.M.; Murphy, E. Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ. Res. 2020, 126, 316309. [Google Scholar] [CrossRef]
- Chen, X.C.; Ding, J.P. Molecular insights into the catalysis and regulation of mammalian NAD-dependent isocitrate dehydrogenases. Curr. Opin. Struct. Biol. 2023, 82, 102672. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, L.; Li, W.X.; Xu, Y.Q.; Han, X.Y.; Bao, N.N.; Sheng, Z.W.; Yuan, Y.Y.; Zhang, X.T.; Luo, Z.S. Elevated O2 alleviated anaerobic metabolism in postharvest winter jujube fruit by regulating pyruvic acid and energy metabolism. Postharvest Biol. Technol. 2023, 203, 112397. [Google Scholar] [CrossRef]
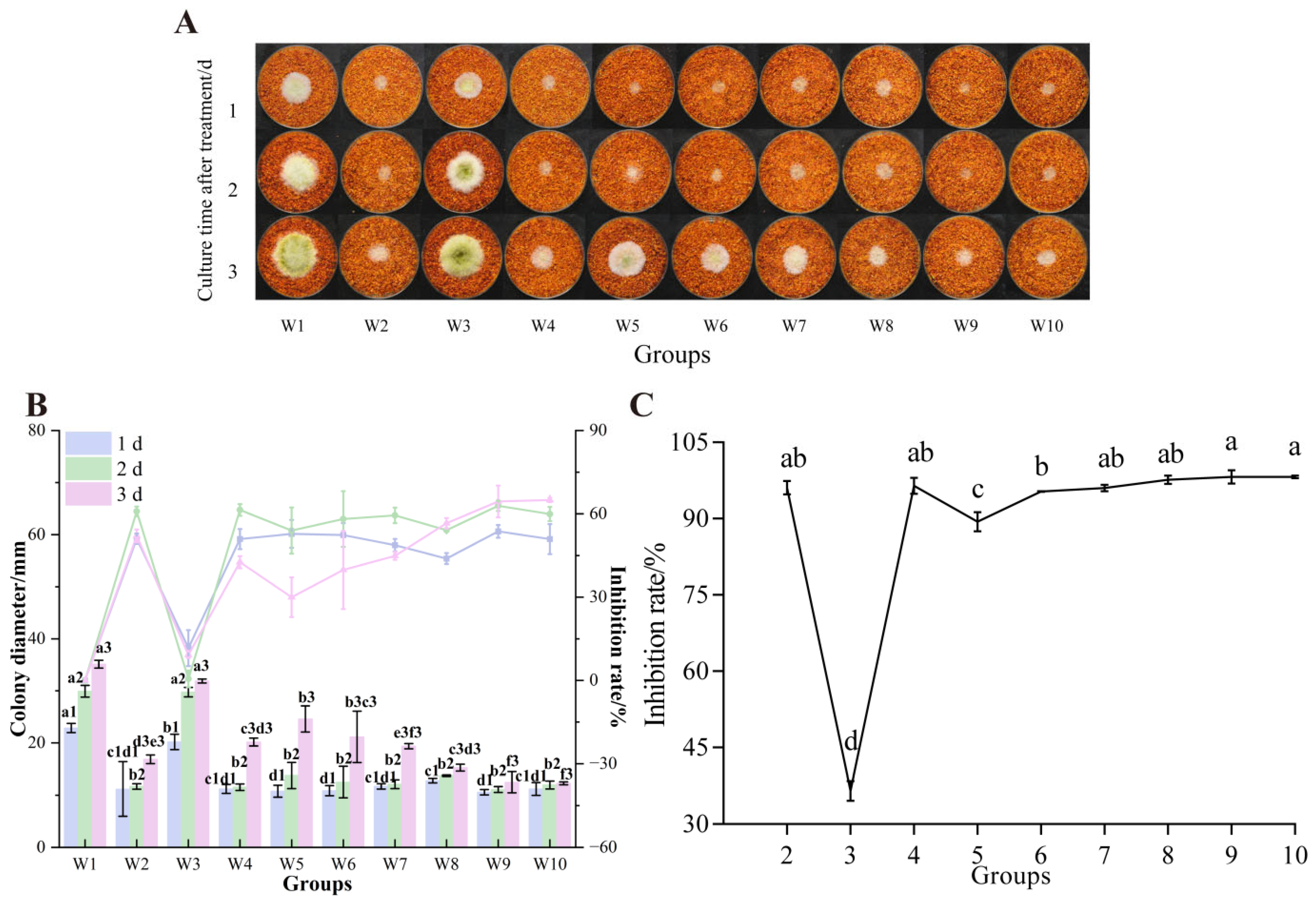
| Combined Approach | Groups | Time Distribution/min |
|---|---|---|
| CK * | W1 | 0 |
| Single | W2 | 90 UV |
| W3 | 90 FA | |
| Link together | W4 | 45 FA + 45 UV |
| W5 | 45 UV + 45 FA | |
| First single, then parallel | W6 | 45 FA + 45 FA/UV |
| W7 | 45 UV + 45 FA/UV | |
| First parallel, then single | W8 | 45 FA/UV + 45 FA |
| W9 | 45 FA/UV + 45 UV | |
| Parallel | W10 | 90 FA/UV |
| Groups | Treatment | ||
|---|---|---|---|
| Formic Acid Concentration/% | Total Treatment Time/min | UV Distance/cm | |
| 1 | 12 | 90 | 11 |
| 2 | 12 | 75 | 13 |
| 3 | 12 | 60 | 15 |
| 4 | 10 | 90 | 15 |
| 5 | 10 | 75 | 11 |
| 6 | 10 | 60 | 13 |
| 7 | 8 | 90 | 13 |
| 8 | 8 | 75 | 15 |
| 9 | 8 | 60 | 11 |
| Index | CK | Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| L | 48.336 ± 0.890 a | 47.473 ± 1.194 a | 48.326 ± 0.649 a | 48.273 ± 1.063 a | 47.879 ± 0.479 a | 47.386 ± 0.603 a | 47.659 ± 0.362 a | 47.691 ± 0.421 a | 48.061 ± 0.260 a | 47.919 ± 0.163 a |
| a | 19.467 ± 0.208 a | 16.867 ± 0.513 d | 18.533 ± 1.365 abc | 18.600 ± 0.436 abc | 17.867 ± 0.252 bcd | 16.567 ± 0.379 d | 18.767 ± 0.802 ab | 17.633 ± 0.289 cd | 18.333 ± 0.153 abc | 18.767 ± 0.058 ab |
| b | 21.302 ± 0.795 a | 20.034 ± 1.726 a | 20.438 ± 1.313 a | 21.077 ± 1.091 a | 20.176 ± 0.968 a | 20.806 ± 1.219 a | 20.608 ± 0.945 a | 20.308 ± 0.937 a | 20.315 ± 1.292 a | 20.541 ± 0.980 a |
| Capsaicin/(g·kg−1) | 0.944 ± 0.039 a | 0.998 ± 0.089 a | 0.966 ± 0.007 a | 0.958 ± 0.044 a | 0.944 ± 0.045 a | 0.972 ± 0.006 a | 0.982 ± 0.008 a | 0.947 ± 0.033 a | 0.966 ± 0.045 a | 0.939 ± 0.055 a |
| Dihydrocapsaicin/(g·kg−1) | 0.362 ± 0.018 a | 0.384 ± 0.031 a | 0.364 ± 0.005 a | 0.360 ± 0.012 a | 0.353 ± 0.012 a | 0.365 ± 0.003 a | 0.366 ± 0.005 a | 0.354 ± 0.010 a | 0.364 ± 0.017 a | 0.354 ± 0.018 a |
| Total capsaicin/(g·kg−1) | 1.451 ± 0.064 a | 1.535 ± 0.134 a | 1.478 ± 0.014 a | 1.463 ± 0.063 a | 1.441 ± 0.064 a | 1.485 ± 0.010 a | 1.498 ± 0.014 a | 1.446 ± 0.047 a | 1.478 ± 0.070 a | 1.437 ± 0.081 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, G.; Zhang, Y.; Su, Y.; Huang, J.; Li, A.; Chen, K.; Du, M.; Zalán, Z.; Awad, S.; et al. Combined UV and Formic Acid Treatment Suppresses Aspergillus flavus and Aflatoxin B1 on Dried Red Chili Powder. Foods 2025, 14, 2194. https://doi.org/10.3390/foods14132194
Chen X, Yang G, Zhang Y, Su Y, Huang J, Li A, Chen K, Du M, Zalán Z, Awad S, et al. Combined UV and Formic Acid Treatment Suppresses Aspergillus flavus and Aflatoxin B1 on Dried Red Chili Powder. Foods. 2025; 14(13):2194. https://doi.org/10.3390/foods14132194
Chicago/Turabian StyleChen, Xiaoman, Gang Yang, Yi Zhang, Yaoyao Su, Jun Huang, Aijun Li, Kewei Chen, Muying Du, Zsolt Zalán, Sameh Awad, and et al. 2025. "Combined UV and Formic Acid Treatment Suppresses Aspergillus flavus and Aflatoxin B1 on Dried Red Chili Powder" Foods 14, no. 13: 2194. https://doi.org/10.3390/foods14132194
APA StyleChen, X., Yang, G., Zhang, Y., Su, Y., Huang, J., Li, A., Chen, K., Du, M., Zalán, Z., Awad, S., & Kan, J. (2025). Combined UV and Formic Acid Treatment Suppresses Aspergillus flavus and Aflatoxin B1 on Dried Red Chili Powder. Foods, 14(13), 2194. https://doi.org/10.3390/foods14132194






