Screening and Relative Quantification of Migration from Novel Thermoplastic Starch and PBAT Blend Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Characteristics
2.3. Sample Extraction
2.4. Migration Assays
2.5. Instrument Conditions
2.5.1. GC-MS
2.5.2. SPME-GC-MS
2.5.3. UHPLC-Q-TOF-MS Analysis
3. Results and Discussion
3.1. Identification of Volatile and Semi-Volatile Compounds by GC-MS
3.2. Identification of Non-Volatile Compounds by UHPLC-Q-TOF-MS
3.3. Migration and Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Internal Market, Industry, Entrepreneurship and SMEs. Available online: https://single-market-economy.ec.europa.eu/sectors/biotechnology/bio-based-products_en (accessed on 23 January 2024).
- European Bioplastics. Global Production Capacities of Bioplastics 2023 (by Material Type). Available online: https://www.european-bioplastics.org/bioplastics/materials/ (accessed on 23 January 2024).
- Wongphan, P.; Nerin, C.; Harnkarnsujarit, N. Enhanced compatibility and functionality of thermoplastic cassava starch blended PBAT blown films with erythorbate and nitrite. Food Chem. 2023, 420, 136107. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, M.; Zhang, R.; Wang, W.; Hou, H. Extrusion-blown starch/PBAT biodegradable active films incorporated with high retentions of tea polyphenols and the release kinetics into food simulants. Int. J. Biol. Macromol. 2023, 227, 851–862. [Google Scholar] [CrossRef]
- de Castro, L.; Silva, L.G.L.; Abreu, I.R.; Braz, C.J.F.; Rodrigues, S.C.S.; Moreira-Araujo, R.; Folkersma, R.; de Carvalho, L.H.; Barbosa, R.; Alves, T.S. Biodegradable PBAT/PLA blend films incorporated with turmeric and cinnamomum powder: A potential alternative for active food packaging. Food Chem. 2024, 439, 138146. [Google Scholar] [CrossRef]
- Ibarra, V.G.; De Quirós, A.R.B.; Losada, P.P.; Sendón, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Lin, J.; Wu, W.-L.; Zhong, A.-H.; Xian, Y.-P.; Zhong, H.-N.; Dong, B.; Liang, M.; Hu, J.-P.; Wu, Y.-N.; Yang, X.-F.; et al. Non-targeted analysis and risk assessment of intentionally and non-intentionally added substances migrating from the emerging biodegradable food contact material poly(butylene adipate-co-terephthalate)/modified starch blend film. Food Packag. Shelf Life 2023, 40, 101190. [Google Scholar] [CrossRef]
- Garcia, P.S.; Grossmann, M.V.E.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving action of citric acid as compatibiliser in starch/polyester blown films. Ind. Crops Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- González Seligra, P.; Eloy Moura, L.; Famá, L.; Druzian, J.I.; Goyanes, S. Influence of incorporation of starch nanoparticles in PBAT/TPS composite films. Polym. Int. 2016, 65, 938–945. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Moreno, O.; Atares, L.; Chiralt, A. Effect of the incorporation of antimicrobial/antioxidant proteins on the properties of potato starch films. Carbohydr. Polym. 2015, 133, 353–364. [Google Scholar] [CrossRef]
- Fourati, Y.; Tarrés, Q.; Mutjé, P.; Boufi, S. PBAT/thermoplastic starch blends: Effect of compatibilizers on the rheological, mechanical and morphological properties. Carbohydr. Polym. 2018, 199, 51–57. [Google Scholar] [CrossRef]
- Genualdi, S.; Nyman, P.; Begley, T. Updated evaluation of the migration of styrene monomer and oligomers from polystyrene food contact materials to foods and food simulants. Food Addit. Contam. Part A 2014, 31, 723–733. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Huang, H.; Zhang, B.; Ye, Z.; Yu, X.; Shentu, X. Recent advances in non-targeted screening of compounds in plastic-based/paper-based food contact materials. Foods 2023, 12, 4135. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Migration phenomenon in food packaging. Food–package interactions, mechanisms, types of migrants, testing and relative legislation—A review. Food Bioprocess Technol. 2014, 7, 21–36. [Google Scholar] [CrossRef]
- Muzeza, C.; Ngole-Jeme, V.; Msagati, T.A.M. The mechanisms of plastic food-packaging monomers’ migration into food matrix and the implications on human health. Foods 2023, 12, 3364. [Google Scholar] [CrossRef]
- Colombo, G.; Corredig, M.; Ünalan, I.U.; Tsochatzis, E. Untargeted screening of NIAS and cyclic oligomers migrating from virgin and recycled polyethylene terephthalate (PET) food trays. Food Packag. Shelf Life 2024, 41, 101227. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, B.; Fan, Y.; Xu, S.; Liu, H.; Wang, K. Quantification of microplastics released from plastic food containers during rinsing and migration by pyrolysis-gas chromatography/mass spectrometry. Food Chem. 2025, 472, 142934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-C.; Lin, Q.-B.; Xie, C.-H.; Liu, Y.-Q.; Zhong, H.-N.; Gu, W.-Y.; McClements, D.J.; Ma, D. Screening and safety assessment of migrating substances released from biodegradable packaging materials into milk. Food Control 2024, 166, 110755. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Su, Q.-Z.; Mercado, D.; Nerín, C. Migration of volatile substances from recycled high density polyethylene to milk products. Food Packag. Shelf Life 2023, 35, 101020. [Google Scholar] [CrossRef]
- Capolupo, M.; Rafiq, A.; Coralli, I.; Alessandro, T.; Valbonesi, P.; Fabbri, D.; Fabbri, E. Bioplastic leachates characterization and impacts on early larval stages and adult mussel cellular, biochemical and physiological responses. Environ. Pollut. 2023, 319, 120951. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. UPLC–ESI-Q-TOF-MS E and GC–MS identification and quantification of non-intentionally added substances coming from biodegradable food packaging. Anal. Bioanal. Chem. 2015, 407, 6781–6790. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of volatile compounds and their sensory impact in a biopolymer based on polylactic acid (PLA) and polyester. Food Chem. 2019, 294, 171–178. [Google Scholar] [CrossRef]
- Brocca, D.; Arvin, E.; Mosbæk, H. Identification of organic compounds migrating from polyethylene pipelines into drinking water. Water Res. 2002, 36, 3675–3680. [Google Scholar] [CrossRef]
- Felix, J.S.; Isella, F.; Bosetti, O.; Nerin, C. Analytical tools for identification of non-intentionally added substances (NIAS) coming from polyurethane adhesives in multilayer packaging materials and their migration into food simulants. Anal. Bioanal. Chem. 2012, 403, 2869–2882. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, OJ L 12, 1–89. [Google Scholar]
- Pack, E.C.; Lee, K.Y.; Jung, J.S.; Jang, D.Y.; Kim, H.S.; Koo, Y.J.; Lee, H.G.; Kim, Y.S.; Lim, K.M.; Lee, S.H.; et al. Determination of the migration of plastic additives and non-intentionally added substances into food simulants and the assessment of health risks from convenience food packaging. Food Packag. Shelf Life 2021, 30, 100736. [Google Scholar] [CrossRef]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Muncke, J. Endocrine disrupting chemicals and other substances of concern in food contact materials: An updated review of exposure, effect and risk assessment. J. Steroid Biochem. Mol. Biol. 2011, 127, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Groh, K.J.; Geueke, B.; Martin, O.; Maffini, M.; Muncke, J. Overview of intentionally used food contact chemicals and their hazards. Environ. Int. 2021, 150, 106225. [Google Scholar] [CrossRef]
- Isella, F.; Canellas, E.; Bosetti, O.; Nerin, C. Migration of non intentionally added substances from adhesives by UPLC-Q-TOF/MS and the role of EVOH to avoid migration in multilayer packaging materials. J. Mass. Spectrom. 2013, 48, 430–437. [Google Scholar] [CrossRef]
- Garcia, P.S.; Baron, A.M.; Yamashita, F.; Mali, S.; Eiras, D.; Grossmann, M.V.E. Compatibilization of starch/poly (butylene adipate-co-terephthalate) blown films using itaconic acid and sodium hypophosphite. J. Appl. Polym. Sci. 2018, 135, 46629. [Google Scholar] [CrossRef]
- Aznar, M.; Ubeda, S.; Dreolin, N.; Nerín, C. Determination of non-volatile components of a biodegradable food packaging material based on polyester and polylactic acid (PLA) and its migration to food simulants. J. Chromatogr. A 2019, 1583, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, Q.Z.; Shang, G.Q.; Weng, Y.X.; Zhu, L. Elucidation of Non-Intentionally Added Substances from Plant Fiber/Plastic Composites by UPLC-QTOF/MS. Foods 2023, 12, 678. [Google Scholar] [CrossRef]
- Riboni, N.; Bianchi, F.; Cavazza, A.; Piergiovanni, M.; Mattarozzi, M.; Careri, M. Mass spectrometry-based techniques for the detection of non-intentionally added substances in bioplastics. Separations 2023, 10, 222. [Google Scholar] [CrossRef]
- Osorio, J.; Aznar, M.; Nerín, C.; Elliott, C.; Chevallier, O. Comparison of LC-ESI, DART, and ASAP for the analysis of oligomers migration from biopolymer food packaging materials in food (simulants). Anal. Bioanal. Chem. 2022, 414, 1335–1345. [Google Scholar] [CrossRef]
- Argyri, A.A.; Mallouchos, A.; Panagou, E.Z.; Nychas, G.J. The dynamics of the HS/SPME-GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015, 193, 51–58. [Google Scholar] [CrossRef]
- Committee, E.S.; More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Hougaard Bennekou, S.; Koutsoumanis, K.P.; Machera, K. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA J. 2019, 17, e05708. [Google Scholar]
- Vera, P.; Canellas, E.; Barknowitz, G.; Goshawk, J.; Nerin, C. Ion-Mobility Quadrupole Time-of-Flight Mass Spectrometry: A Novel Technique Applied to Migration of Nonintentionally Added Substances from Polyethylene Films Intended for Use as Food Packaging. Anal. Chem. 2019, 91, 12741–12751. [Google Scholar] [CrossRef]
- Diamantidou, D.; Tsochatzis, E.; Kalogiannis, S.; Alberto Lopes, J.; Theodoridis, G.; Gika, H. Analysis of Migrant Cyclic PET Oligomers in Olive Oil and Food Simulants Using UHPLC-qTOF-MS. Foods 2023, 12, 2739. [Google Scholar] [CrossRef]
- Hoppe, M.; Fornari, R.; de Voogt, P.; Franz, R. Migration of oligomers from PET: Determination of diffusion coefficients and comparison of experimental versus modelled migration. Food Addit. Contam. Part A 2017, 34, 1251–1260. [Google Scholar] [CrossRef]
| Sample Name | Sample Code | Film Properties | |||
|---|---|---|---|---|---|
| Oxygen Permeability (cm3·mm/m2·day·atm) | Water Vapor Permeability (g·mm/m2·day·KPa) | Tensile Strength (MPa) | Elongation at Break (%) | ||
| PBAT Resins | R | - | - | - | - |
| PBAT Films | F | - | - | - | - |
| PBAT/TPS | C | 16.69 ± 0.08 | 4.20 ± 0.14 | 14.43 ± 0.51 | 656.11 ± 11.50 |
| PBAT/TPS + Sodium Nitrite | N | 4.65 ± 0.26 | 4.51 ± 0.18 | 11.64 ± 0.66 | 794.62 ± 17.45 |
| PBAT/TPS + Sodium Erythorbate | E | 5.26 ± 0.18 | 3.54 ± 0.18 | 12.82 ± 0.58 | 651.94 ± 18.46 |
| PBAT/TPS + Sodium Tripolyphosphate | T | 21.42 ± 0.73 | 4.01 ± 0.06 | 10.60 ± 0.24 | 571.37 ± 16.18 |
| PBAT/TPS + Sodium Hexametaphosphate | H | 11.22 ± 1.89 | 3.11 ± 0.14 | 10.42 ± 8.78 | 595.95 ± 8.78 |
| PBAT/TPS + Tetrasodium Pyrophosphate | P | 29.07 ± 3.81 | 4.30 ± 0.20 | 9.31 ± 0.31 | 521.30 ± 6.19 |
| No. | Rt | Candidate | CAS Number | Molecular Formular | Mass | Cramer Class | Samples | Remark | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | F | C | N | E | T | H | P | ||||||||
| 1 | 5.35 | Cyclopentanone | 120-92-3 | C5H8O | 84.06 | II | 1 | 1 | 1 | 1 | 1 | 1 | nd | nd | Surfactant |
| 2 | 7.73 | Butyrolactone | 96-48-0 | C4H6O2 | 86.04 | I | nd | nd | nd | 1 | 1 | nd | nd | nd | By product |
| 3 | 8.86 | 1,4-Butanediol | 110-63-4 | C4H10O2 | 90.07 | I | nd | nd | nd | 1 | 1 | 1 | nd | nd | Monomer |
| 4 | 9.10 | ni | nd | nd | 1 | 1 | 1 | 1 | 1 | 1 | Fragments; 61.0/75.0/108.0/117.0/133.0 | ||||
| 5 | 9.28 | Tetraethyl silicate | 78-10-4 | C8H20O4Si | 208.11 | III | 1 | 1 | nd | nd | nd | nd | nd | nd | Processing aids |
| 6 | 12.85 | Cyclopentanecarboxylic acid, 2-oxo-, ethyl ester | 611-10-9 | C8H12O3 | 156.18 | - | nd | 1 | nd | 1 | nd | nd | nd | nd | By product |
| 7 | 16.38 | Glycerin | 56-81-5 | C3H8O3 | 92.05 | I | nd | nd | 4 | 4 | 4 | 4 | 4 | 4 | Plasticizer |
| 8 | 17.16 | Butylated Hydroxytoluene | 128-37-0 | C15H24O | 220.18 | II | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Antioxidants |
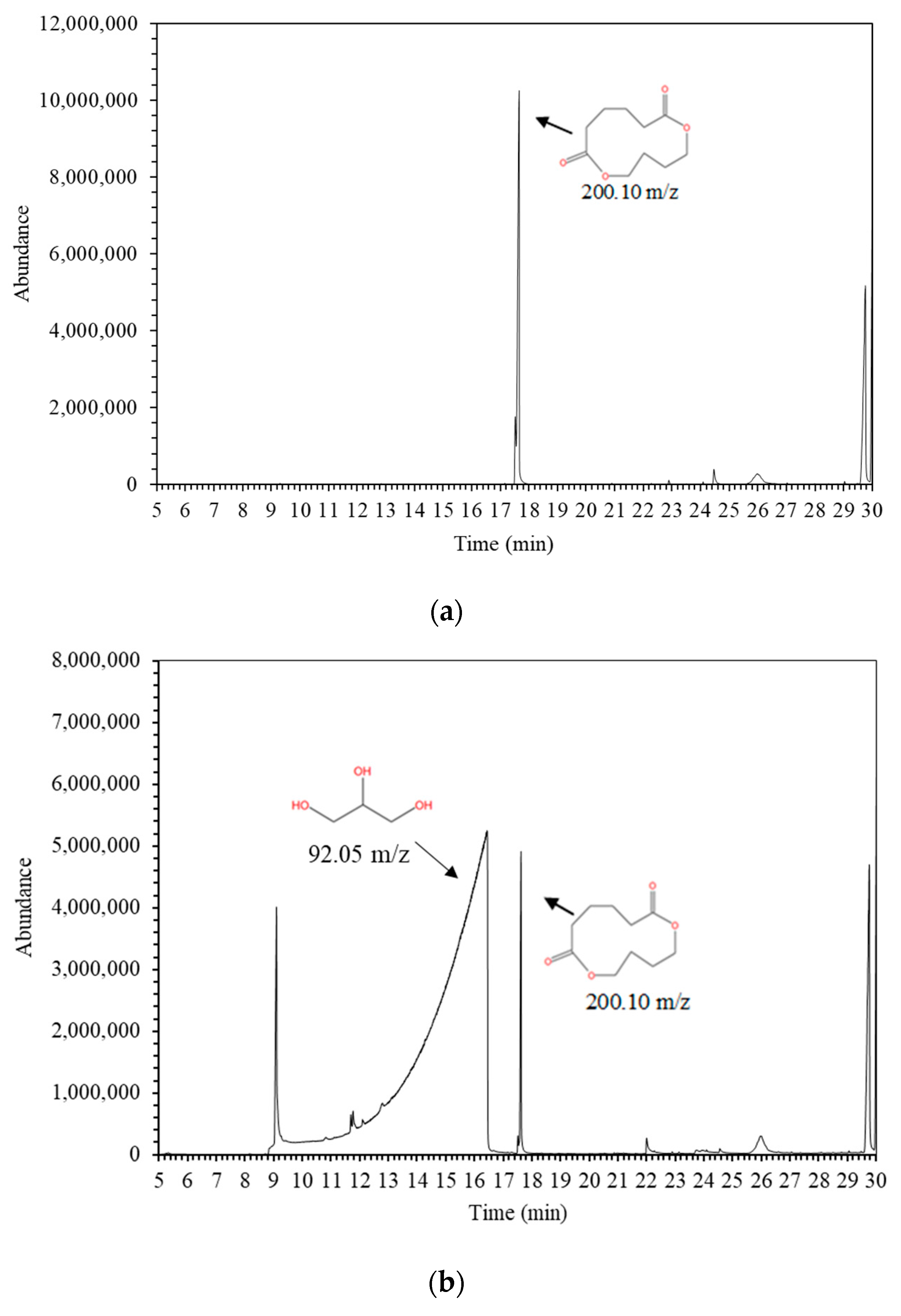
| 9 | 17.55 | 1,6-Dioxacyclododecane-7,12-dione | 777-95-7 | C10H16O4 | 200.10 | - | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | Oligomer |
| 10 | 17.99 | 1,4-Benzenedicarboxylic acid, ethyl methyl ester | 22163-52-6 | C11H12O4 | 208.07 | - | nd | nd | nd | 1 | 1 | 1 | nd | nd | By product |
| 11 | 20.66 | 2-Ethylhexyl salicylate | 118-60-5 | C15H22O3 | 250.16 | I | 1 | 1 | nd | nd | nd | 1 | 1 | nd | Processing aids |
| 12 | 21.99 | Palmitic acid | 57-10-3 | C16H32O2 | 256.24 | I | nd | nd | 1 | nd | nd | 1 | 1 | 1 | FDA Inventory of Effective Food Contact Substance Notifications |
| 13 | 23.50 | Methyl stearate | 112-61-8 | C19H38O2 | 298.29 | I | 1 | 1 | 1 | nd | 1 | nd | nd | nd | By product |
| 14 | 24.09 | Ethyl stearate | 111-61-5 | C20H40O2 | 312.30 | I | 1 | 1 | 1 | nd | 1 | 1 | nd | nd | By product |
| 15 | 26.00 | ni | nd | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Fragments; 54.0/104.0/132.0/149.0/369.1 | ||||
| No | Rt | m/z | Adduct | Molecular Formula | Candidates | CAS No | Sample | Remark (Detected Similar by) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | F | C | N | E | T | H | P | ||||||||
| 1 | 4.69 | 165.0552 | 1H+ | C9H9O3 | Methyl phenylglyoxylate | 15206-55-0 | x | Lin et al. (2023) [7] | |||||||
| 2 | 5.13 | 203.0919 | 1H+ | C9H15O5 | Triacetin | 102-76-1 | x | x | x | x | x | Lin et al. (2023) [7] | |||
| 3 | 6.08 | 111.0446 | 1H+ | C6H6O2 | Resorcinol | 108-46-3 | x | x | x | x | x | x | x | x | Lin et al. (2023) [7] |
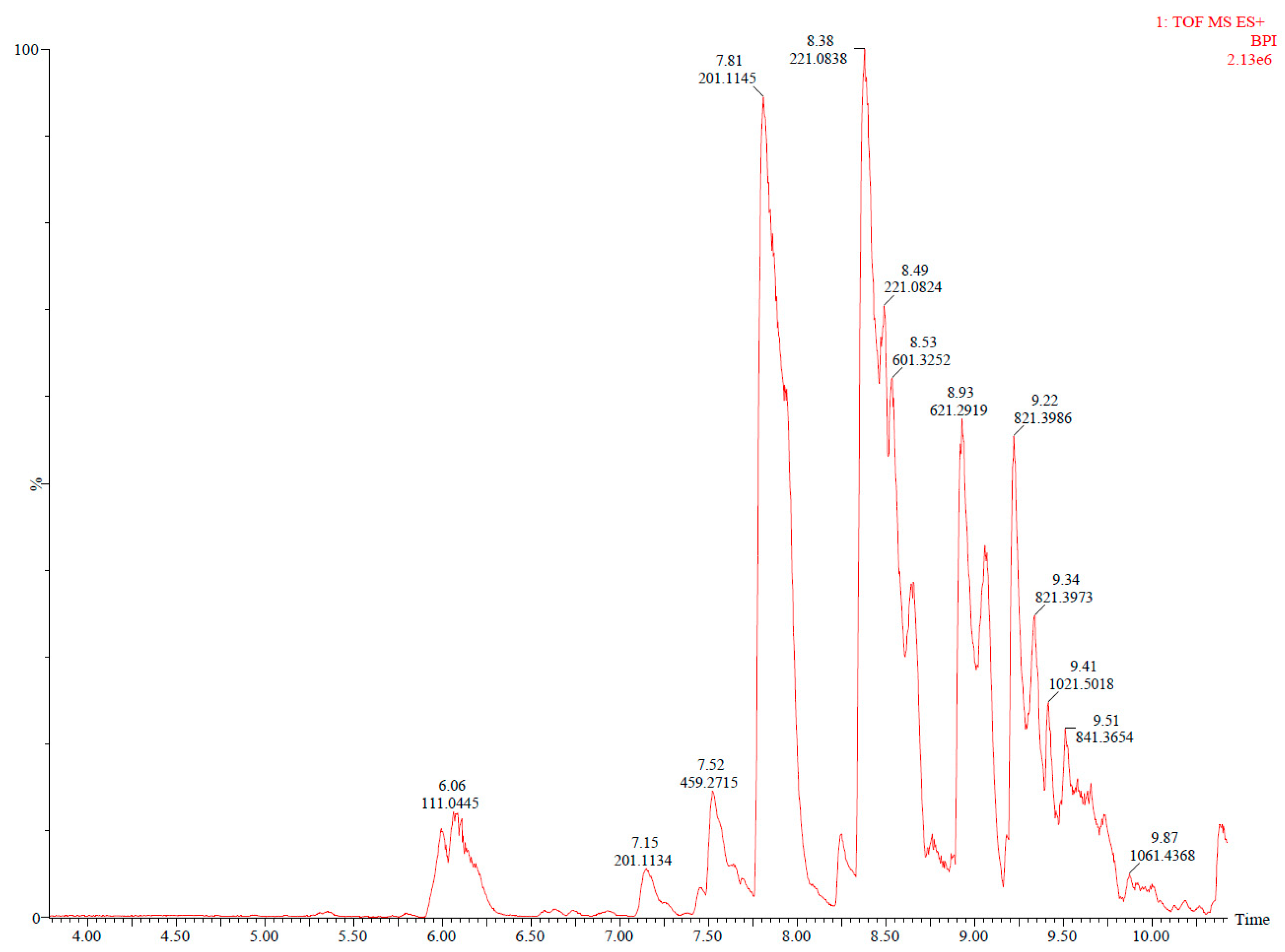
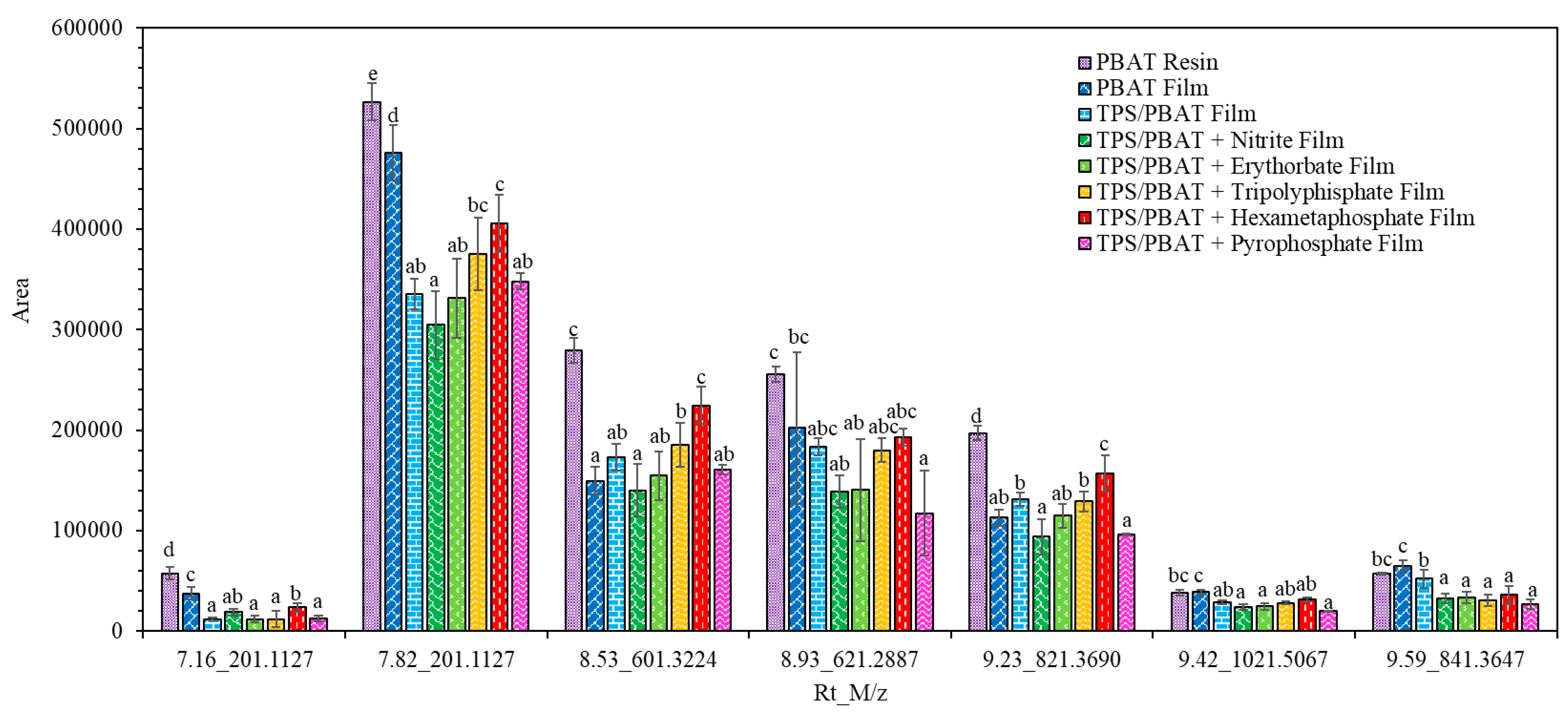
| 4 | 7.16 | 201.1127 | 1H+ | C10H16O4 | Cyclic [AA-BD] | 777-95-7 | x | x | x | x | x | x | x | x | Canellas, Vera & Nerín (2015) [22] and Aznar, Ubeda, Dreolin & Nerín, 2019 [34] |
| 5 | 7.82 | 201.1127 | 1H+ | C10H16O4 | Cyclic [AA-BD] | 777-95-7 | x | x | x | x | x | x | x | x | Canellas, Vera & Nerín (2015) [22] and Aznar, Ubeda, Dreolin & Nerín, 2019 [34] |
| 6 | 8.53 | 601.3224 | 1H+ | C30H48O12 | Cyclic [AA-BD]3 | x | x | x | x | x | x | x | x | Zhang, Su, Shang, Weng & Zhu (2023) [35] | |
| 7 | 8.93 | 621.2887 | 1H+ | C32H44O12 | Cyclic [TPA-AA2-BD2] | x | x | x | x | x | x | x | x | Osorio, Aznar, Nerín, Elliott & Chevallier (2022) [37] and Zhang, Su, Shang, Weng & Zhu (2023) [35] | |
| 8 | 9.23 | 821.396 | 1H+ | C42H60O16 | Cyclic [TPA-AA3-BD3] | x | x | x | x | x | x | x | x | Osorio, Aznar, Nerín, Elliott & Chevallier (2022) [37] and Zhang, Su, Shang, Weng & Zhu (2023) [35] | |
| 9 | 9.42 | 1021.5067 | 1H+ | C52H76O20 | Cyclic [TPA-AA4-BD4] | x | x | x | x | x | x | x | x | Osorio, Aznar, Nerín, Elliott & Chevallier (2022), [37] and Zhang, Su, Shang, Weng & Zhu (2023) [35] | |
| 10 | 9.59 | 841.3647 | 1H+ | C44H56O16 | Cyclic [TPA2-AA2-BD4] | x | x | x | x | x | x | x | x | Zhang, Su, Shang, Weng & Zhu (2023) [35] | |
| No | Rt | Migrants | CAS No. | Molecular Formular | Mass | Cramer Class | LOD (μg/g) | LOQ (μg/g) | Specific Migration (mg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPS/PBAT | N | E | T | H | P | |||||||||
| 1 | 10.33 | Glycerin | 56-81-5 | C3H8O3 | 92.05 | I | 0.20 | 0.70 | 1.20 | 1.30 | 1.20 | 1.10 | 1.10 | 1.40 |
| 2 | 18.47 | 1,6-Dioxacyclododecane-7,12-dione | 777-95-7 | C10H16O4 | 200.10 | I | 1.16 | 3.51 | 9.08 ± 0.77 | 9.14 ± 1.16 | 4.07 ± 0.06 | <3.51 | <3.51 | <3.51 |
| No. | Formular | Compound Name | Retention Time (min) | CAS | Adduct | m/z | Cramer Class | LOD (μg g−1) | LOQ (μg g−1) | Specific Migration in EtOH (μg g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPS/PBAT | N | E | T | H | P | ||||||||||
| 1 | C10H16O4 | Cyclic [AA-BD] | 6.19 | 777-95-7 | 1H+ | 201.1126 | I | 0.08 | 0.23 | <LOQ | <LOQ | 0.23 ± 0.01 | <LOQ | <LOQ | <LOQ |
| 2 | C14H26O6 | Linear [AA-BD2] | 6.25 | 20985-13-1 | 23Na+ | 313.1623 | I | 0.08 | 0.23 | <LOQ | <LOQ | 1.04 ± 0.04 | <LOQ | 0.91 ± 0.04 | 0.26 ± 0.02 |
| 3 | C12H22O5 | CH3CH2OH-Linear [AA-BD] | 6.77 | 925-06-4 | 23Na+ | 269.1365 | I | 0.08 | 0.23 | <LOQ | 0.24 ± | ||||
| 4 | C10H18O4 | Sebacic acid | 7.37 | 141-28-6 | 23Na+ | 225.1103 | I | 0.11 | 0.34 | <LOQ | <LOQ | <LOQ | |||
| 5 | C24H42O10 | Linear [BD3-AA2] | 7.56 | 23Na+ | 513.2667 | I | 0.08 | 0.23 | 0.29 ± 0.01 | 0.25 ± 0.04 | 0.26 ± 0.01 | ||||
| 6 | C10H10O4 | CH3CH2OH-TPA | 7.68 | 131-11-3 | 1H+ | 195.065 | I | 0.17 | 0.50 | ||||||
| 7 | C22H30O9 | Linear [TPA-AA-BD2] | 7.81 | 23Na+ | 461.1788 | I | 0.17 | 0.50 | <LOD | ||||||
| 8 | C20H32O8 | Cyclic [AA2-BD2] | 7.93 | 78837-87-3 | 23Na+ | 423.1995 | I | 0.08 | 0.23 | 0.88 ± 0.02 | 0.98 ± 0.02 | 0.94 ± 0.04 | 0.87 ± 0.04 | 0.84 ± 0.02 | 0.84 ± 0.01 |
| 9 | C22H38O9 | CH3CH2OH-Linear [AA2-BD2] | 7.98 | 23Na+ | 469.2414 | I | 0.08 | 0.23 | |||||||
| 10 | C26H38O10 | Linear [TPA-AA-BD3] | 8.05 | 23Na+ | 533.2363 | I | 0.17 | 0.50 | <LOQ | <LOD | <LOD | <LOD | <LOD | ||
| 11 | C34H58O14 | Linear [AA3-BD4] | 8.22 | 23Na+ | 713.3724 | I | 0.08 | 0.23 | <LOQ | <LOQ | <LOQ | ||||
| 12 | C24H34O9 | CH3CH2OH-Linear [TPA-AA-BD2] | 8.45 | 21259–20–1 | 23Na+ | 489.2101 | I | 0.17 | 0.50 | ||||||
| 13 | C22H28O8 | Cyclic [TPA-BD2-AA] | 8.49 | 1H+ | 421.1862 | I | 0.17 | 0.50 | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | |
| 14 | C20H26O8 | CH3CH2OH-linear [AA-BB-TPA] | 8.54 | 1H+ | 395.1706 | I | 0.17 | 0.50 | |||||||
| 15 | C30H48O12 | Cyclic [AA3-BD3] | 8.63 | 1135871-5-6 | 23Na+ | 623.3043 | I | 0.08 | 0.23 | 0.26 ± 0.02 | 0.33 ± 0.02 | 0.35 ± 0.02 | 0.28 ± 0.02 | 0.32 ± 0.02 | 0.27 ± 0.00 |
| 16 | C24H24O8 | Cyclic [TPA-BD2] | 8.86 | 23Na+ | 441.1558 | I | 0.17 | 0.50 | <LOQ | ||||||
| 17 | C32H44O12 | Cyclic [TPA-BD3-AA2] | 9.02 | 23Na+ | 643.273 | I | 0.17 | 0.50 | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | |
| 18 | C44H66O17 | Cyclic [TPA-BD3-AA4] | 9.19 | 23Na+ | 889.4198 | I | 0.17 | 0.50 | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | |
| 19 | C42H60O16 | Cyclic [TPA-BD4-AA3] | 9.30 | 23Na+ | 843.3779 | I | 0.17 | 0.50 | <LOQ | <LOD | <LOD | <LOD | <LOD | <LOD | |
| 20 | C22H28O8 | Cyclic [TPA-BD3-AA2] | 9.42 | 1H+ | 443.1688 | I | 0.17 | 0.50 | <LOD | <LOD | |||||
| 21 | C34H40O12 | Cyclic [TPA2-BD3-AA1] | 9.44 | 23Na+ | 663.2417 | I | 0.17 | 0.50 | <LOQ | <LOD | <LOD | <LOD | |||
| 22 | C44H56O16 | Cyclic [TPA2-BD4-AA2] | 9.57 | 23Na+ | 863.3466 | I | 0.17 | 0.50 | <LOD | <LOD | |||||
| Specific Migration in 10% EtOH (μg g−1) | |||||||||||||||
| 23 | C10H18O5 | Linear [AA-BD] | 5.42 | 777-95-7 | 23Na+ | 241.1036 | I | 0.08 | 0.23 | <LOQ | <LOQ | <LOQ | |||
| 24 | C14H26O6 | Linear [AA-BD2] | 6.25 | 20985-13-1 | 23Na+ | 313.1628 | I | 0.08 | 0.23 | <LOQ | <LOQ | ||||
| 25 | C14H17O7 | CH3CH2OH-Linear TPA-BD | 6.70 | 1H+ | 299.1102 | I | 0.17 | 0.50 | <LOQ | ||||||
| 26 | C16H26O8 | Linear [AA2-BD] | 6.83 | 23Na+ | 396.1526 | I | 0.08 | 0.23 | <LOQ | ||||||
| 27 | C20H34O9 | Linear [AA2-BD2] | 7.24 | 23Na+ | 441.2095 | I | 0.08 | 0.23 | <LOQ | 0.25 ± 0.08 | <LOQ | <LOQ | |||
| 28 | C12H120O4 | Cyclic [TPA-BD] | 7.48 | H+ | 221.0809 | I | 0.17 | 0.50 | <LOQ | <LOQ | <LOQ | <LOQ | |||
| 29 | C24H42O10 | Linear [AA2-BD3] | 7.54 | 23Na+ | 513.2678 | I | 0.08 | 0.23 | <LOQ | ||||||
| 30 | C20H32O8 | Cyclic [AA2-BD2] | 7.91 | 78837-87-3 | 23Na+ | 423.1971 | I | 0.08 | 0.23 | 0.35 ± 0.11 | 0.37 ± 0.10 | 0.57 ± 0.08 | 0.31 ± 0.08 | ||
| Specific Migration in 3% HAc (μg g−1) | |||||||||||||||
| 31 | C6H6O2 | Resorcinol | 3.91 | 108-46-3 | 1H+ | 111.0435 | I | - | - | x | x | x | x | x | - |
| 32 | C10H18O5 | Linear [AA-BD] | 5.42 | 777-95-7 | 23Na+ | 241.1030 | I | 0.08 | 0.23 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 33 | C16H28O7 | HAc-Linear [AA-BD] | 7.00 | 1H+ | 333.1317 | I | 0.08 | 0.23 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongphan, P.; Canellas, E.; Nerín, C.; Estremera, C.; Harnkarnsujarit, N.; Vera, P. Screening and Relative Quantification of Migration from Novel Thermoplastic Starch and PBAT Blend Packaging. Foods 2025, 14, 2171. https://doi.org/10.3390/foods14132171
Wongphan P, Canellas E, Nerín C, Estremera C, Harnkarnsujarit N, Vera P. Screening and Relative Quantification of Migration from Novel Thermoplastic Starch and PBAT Blend Packaging. Foods. 2025; 14(13):2171. https://doi.org/10.3390/foods14132171
Chicago/Turabian StyleWongphan, Phanwipa, Elena Canellas, Cristina Nerín, Carlos Estremera, Nathdanai Harnkarnsujarit, and Paula Vera. 2025. "Screening and Relative Quantification of Migration from Novel Thermoplastic Starch and PBAT Blend Packaging" Foods 14, no. 13: 2171. https://doi.org/10.3390/foods14132171
APA StyleWongphan, P., Canellas, E., Nerín, C., Estremera, C., Harnkarnsujarit, N., & Vera, P. (2025). Screening and Relative Quantification of Migration from Novel Thermoplastic Starch and PBAT Blend Packaging. Foods, 14(13), 2171. https://doi.org/10.3390/foods14132171






