Abstract
The increasing demand for sustainable protein sources highlights Hermetia illucens (Black Soldier Fly, BSF) as a promising alternative. However, microbiological safety remains a key concern. This study investigated the microbial diversity of BSF larvae, comparing two processing methods: (1) boiling followed by drying and (2) drying alone. Microbial diversity was assessed via 16S rRNA sequencing, while bacterial loads were quantified using culture-based methods on samples from a French company. A systematic review complemented this analysis by synthesizing the existing knowledge on BSF microbiota. The rearing conditions varied, with substrate pH ranging from 4.1 to 9.0 and ambient temperatures between 24.6 °C and 42.7 °C. Mesophilic bacteria, spores, and lactic acid bacteria reached up to 8.6, 7.7, and 8.5 log CFU/g in the substrates and larvae, while yeasts, molds, and sulfite-reducing bacteria remained below 4.8 log CFU/g. Boiling reduced most loads below detection thresholds, particularly for yeasts, molds, and ASR. Salmonella, Listeria monocytogenes, Cronobacter sp., and coagulase-positive staphylococci were absent, whereas Clostridium perfringens and Escherichia coli were variably detected. Metabarcoding showed shifts in composition, with Proteobacteria, Bacteroidota, Actinobacteriota, and Firmicutes (Bacillota and Clostridiota) dominating. Process 1 more effectively reduced the bacterial loads, though Bacillus and Clostridium remained. Campylobacter sp. detection in powders raises food safety concerns.
1. Introduction
The increasing global demand for animal proteins is expected to continue until 2050, raising concerns about the sufficiency of traditional sources and highlighting the need to explore alternatives [1,2,3,4]. To address the need for sustainable food sources and to mitigate the environmental impacts of conventional livestock farming, entomophagy, the practice of consuming insects, presents a promising solution that is gaining attraction in Western countries, both for human consumption and as an alternative feed for animals [5]. It is a valuable source of essential nutrients, with the protein content ranging from 30% to 65% of their total dry matter, and it is abundant in micronutrients, such as iron, zinc, vital amino acids, and other important vitamins and minerals [6]. In addition, insect farming has a significantly lower environmental impact compared to traditional livestock production. Insects require less food and water, occupy less space, and demonstrate superior biomass conversion rates [7].
However, insects naturally harbor diverse microbial communities, including bacteria, virus, yeasts, and fungi, which play pivotal roles in their development, digestion, and overall health [8,9,10]. Additionally, insects may also carry unwanted microorganisms, including foodborne pathogens [11]. Kooh et al. (2019) and Garofalo et al. (2019) [12,13] presented the pathogenic and toxigenic microorganisms that could potentially be transmitted via edible insects, including bacteria such as Salmonella sp., STEC (Shiga toxin E. coli), L. monocytogenes, C. perfringens, S. aureus, and the B. cereus group; viruses such as Norovirus and Hepatitis Virus A; yeasts; and molds, and some studies have examined the microbial communities of edible insects [14,15,16,17,18,19,20,21]. To ensure the safety of insect-based food products, it is crucial to understand the dynamics of microbial communities during the rearing and manufacturing process of insect biomass.
Several factors influence the composition and diversity of microbial communities associated with insects, including insect species, diet, environment, and processing methods employed during biomass transformation [20,22,23]. Three main practices exist: consuming whole insects, processing them into powders or pastes, and extracting specific components, such as protein isolates and oils [24]. Processing steps such as boiling and drying have been shown to reduce microbial loads but residual spore-forming bacteria may persist [16,25]. Moreover, processing parameters such as temperature, duration, and storage conditions can affect microbial dynamics, influencing both product quality and safety [9,26,27,28]. Additional findings from Adamek et al. (2018) indicated that the most effective method for long-term storage involves killing the edible insect with boiling water, drying them at 103 °C for 12 h, and then sealing them in airtight packaging [29].
To evaluate and mitigate potential microbial risks, it is crucial to conduct a comprehensive analysis of these microbial communities throughout the entire process of insect biomass transformation. The study of Gorrens et al. (2021) [30] primarily focused on culture-dependent methods to identify and characterize bacterial, fungal, and yeast species associated with the BSF microbiota. While culture-dependent methods offer valuable insights into lab-culturable microorganisms, these techniques reveal limitations in terms of the detection threshold or assessment of the “true” diversity (e.g., 0.1 to 10% of culturable bacteria) [31]. Recent advancements in molecular microbiology have provided powerful tools for characterizing the microbial ecosystems associated with insects. High-throughput sequencing techniques, such as metabarcoding, enable detailed analysis of microbial community structures [32]. Molecular techniques such as 16S rRNA gene sequencing, metagenomics, and metatranscriptomics are commonly used to analyze the diversity, abundance, and functional potential of microbial taxa associated with the BSF [33]. These methods have revealed previously unknown microbial species and their ecological roles within the insect, for example, a study using 16S rRNA gene sequencing found Campylobacter sp. as one of the prevalent species in the gut of unprocessed BSF [33]. Another study investigated the gut microbiota of BSF larvae using 16S rRNA gene sequencing to assess how the substrate used for larval growth influences microbiota composition [34]. Tegtmeier et al. (2021) conducted a study on the bacterial diversity and culturability using a broad sampling approach over several rearing cycles, combining 16S rRNA amplicon sequencing with a cultivation-dependent approach and genomic fingerprinting and testing selected strains for their ability to inhibit pathogens [35]. These techniques complement traditional culture-based methods, such as plating and biochemical assays, to provide a comprehensive understanding of microbial diversity [9].
The objective of the present study is to analyze the evolution of the microbial dynamics of Hermetia illucens powder considering rearing and processing steps. The microbial diversity was analyzed to identify potential biological hazards, to evaluate the impact of different processing steps (boiling and drying vs. drying alone) on product safety and quality, and to identify strategies to mitigate the risk. To achieve this, a combined approach of culture-dependent and culture-independent methods was applied, based on a series of samples collected across various stages of rearing and processing steps, in collaboration with Cycle Farms, a French company based in Angers. The company employs an optimized two-step substrate approach, where the substrate is fully replaced after 5 days of rearing. In parallel, a systematic review was conducted to synthesize the existing knowledge on the microbial diversity of Hermetia illucens, focusing on data obtained through non-cultural methods to compare findings obtained through metagenomics while culture-dependent results will be discussed in light of the systematic review reported by Brulé et al. (2024) [36].
2. Materials and Methods
2.1. Systematic Review of the Microbial Diversity of BSF Larvae, Substrate, and Frass
Systematic research was performed to collect and synthesize the available information on the microbial diversity of BSF, based on non-cultural methods. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines for systematic reviews were implemented using the PubMed and ScienceDirect databases [37]. The following search was used: PubMed in the title/abstract ((“Black Sol-dier Fly” OR “Hermetia illucens”) AND (“microb*” OR “cultural method*” OR “sequenc*” OR “metagen*” OR “metabarcoding” OR “bacteri*” OR “profil*” OR “dynamic*”)), and Web of Science with two searches (title, abstract, and keywords: (“Hermetia illucens” OR “Black soldier fly”) AND (“microbiol” OR “Microbiological” OR “microbial community” OR “microbiota” OR “sequencing” OR “metagenonic” OR “metabarcoding”)) and (Title, abstract, keywords: (“Hermetia illucens” OR “Black soldier fly”) AND (“bacteria” OR “profile” OR “dynamic”)). The search was achieved on March 21st 2025 without time or language limitations. The software Zotero (6.0.30) was used to remove duplicates. In addition, the list of references of the selected articles was examined to identify additional articles of interest. Articles providing data with non-cultural methods in BSF larvae (raw or processed), substrate, and frass were eligible for data extraction. The primary reasons for exclusion were linked to the report of only culture-dependent results, studies focusing on waste conversion and strain isolation, or articles related only to antibiotic resistance. Three evaluators shared the screening of the articles and discussed any difference or disagreement. The screening of the articles was performed on Zotero (6.0.30). The data extraction was conducted by one evaluator and checked by a second. The data were collected and synthesized in an Excel table, including the author information, publication year, country, analytical methods, sample types (raw larvae, processed larvae, substrate, and frass), and their origins (retail, farming, and processing country). It also examined the processing methods (freezing, heat treatment, drying, and microbial inactivation), rearing conditions (temperature, humidity, and feeding substrates), sample details (number of samples, number of positives, sample weight, and detection limit), and molecular techniques (DNA/RNA extraction kit reference and targeted region). Additional data included OTUs for pathogens and the genus/species identified.
2.2. Experimental Design
The samples were obtained in collaboration with an industrial partner, Cycle Farms (Beaufort en Anjou, France). The rearing steps and the experimental plan are presented in Figure 1.
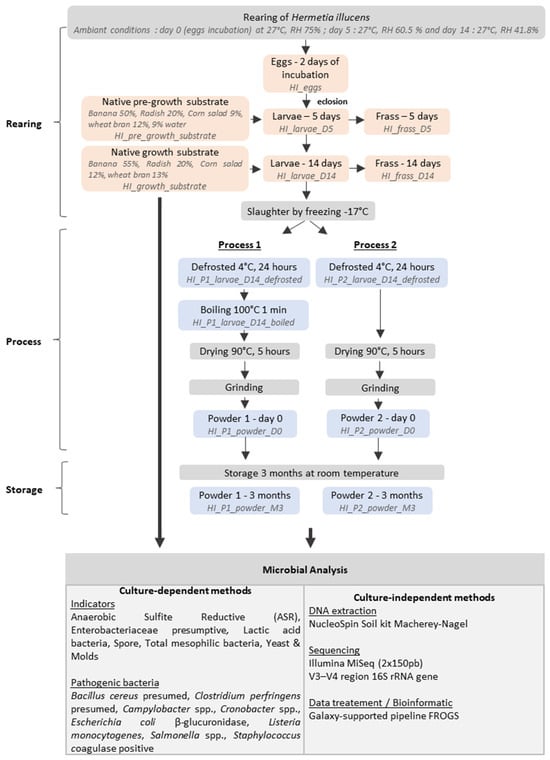
Figure 1.
Experimental plan and sample collected.
Cycle Farm uses a two-step substrate approach. First, a pre-growth substrate composed of 50% banana, 20% radish, 9% corn salad, 12% wheat bran, and 9% water is used for the rearing of 250,000 larvae (3rd instar, 5 days old). Due to larval size rearing constraints, the farm used five batches (dimension 60 cm × 60 cm × 12 cm) for the growth of 5000 larvae until reaching 14 days of rearing (5th instar). This growth substrate is a composition of 55% banana, 20% radish, 12% corn salad, and 13% wheat bran. The incubation occurred under controlled conditions of 27 °C and 75% relative humidity (RH) and was closely monitored and controlled with a precision of 0.1 °C and 0.1%, respectively. The data is continuously logged at hourly intervals. The specific equipment utilized for this purpose is the HB500 H egg incubator (Cimuka, Ankara, Turkey).
The eggs were collected and placed in incubators and represented the start of the rearing process. Sampling was conducted on the 5th day of rearing and on the 14th day (the time at which larvae can be processed into insect powder). For each sampling point, three technical replicates of the eggs, larvae, substrate, and frass were collected. During the rearing, a total of 1381 g of larvae (first rearing), 700 g of frass, 1000 g of pre-growth substrate, 2000 g of the growth substrate, 3276 g of larvae (second rearing), and 1081 g of frass at 5 days and 14 days, respectively, were collected. After the rearing steps, 14-day-reared larvae were killed by frosting (−17 °C) and all the samples were stored at −20 °C.
Two processes described in Figure 1 were investigated to assess the impact of heat treatments: (1) a combined boiling–drying process and (2) a drying-only process. An initial defrosted 14-day-old larvae amount (2200 g) was thawed at 4 °C for 24 h and used equally in both processes. Process 1 (P1) involved two consecutive thermal treatments (boiling and drying), while Process 2 (P2) involved a unique drying process. Before the two processes, one sample of 150 g from each process was collected for microbial enumeration and metabarcoding analysis. In Process 1, a boiling step at 100 °C for 1 min was applied, based on studies demonstrating its efficacy in inactivating non-spore-forming bacteria while preserving product quality [38,39]. In the second step, the boiled larvae were dried in an oven at 90 °C for 5 h. P2 involved a drying step in an oven at 90 °C for 5 h. During the two processes, the weight of the larvae was continuously observed and recorded every 20 min for 5 h. To evaluate the changes in the microbial communities in the insect powder after three months of storage at room temperature, the powders obtained from the larvae processed via P1 or P2 were prepared by grinding the larvae for 10 s three times using a Moulinette DPA1 (Moulinex®, Ecully, France). The resulting powders were divided into two batches: the first was stored at −20 °C for microbial and metabarcoding analysis immediately after processing, while the second was kept at room temperature for three months.
2.3. Rearing Conditions Measurement
Throughout the rearing process, the pH, temperature, and relative humidity were recorded every three days in the (pre-)growth substrates at five different locations within each rearing batch. The pH values of the rearing batch, including the larvae, substrate, and frass, were measured by a pH-meter checker HI98103 Hanna (Grosseron, France). For temperature measurement, five temperature points were selected within the tank. One temperature point was located at the center of the tank, while the remaining four points were positioned at a distance of 10 cm from each of the four corners. The relative humidity and the temperature were measured using PeakTech Temperature and Humidity Recorders (model 5185), PeakTech®, (Ahrensburg, Germany) according to the manufacturer’s instructions.
2.4. Microbial Enumeration
For each sample (except the eggs), 25 g was collected from a composite sample, homogenized from aseptically taken subsamples across different areas of the batch (e.g., top, middle, and bottom), and diluted tenfold in buffered peptone water (VWR) in a stomacher plastic bag. The samples were stomached 25.5 stroke/s for 2 min with a lab paddle blender (Masticator®, IUL, Barcelona, Spain). After a phase of revival of 1 h at room temperature, serial dilutions were performed in buffered peptone water up to 10−8. The total mesophilic flora and bacterial endospore counts were determined with the pour-plate method, respectively, on Plate Count Agar (PCA, Biokar diagnostics) or PCA with starch 2 g/L at 30 °C for 72 h after a heat treatment at 80 °C for 10 min. For the analysis of other bacterial indicators and pathogens, the samples were processed by the Eurofins laboratory using the reference methods detailed in Table 1.

Table 1.
List of microbiological analysis methods used for culture-dependent analysis.
2.5. DNA Extraction and Sequencing of Bacterial rRNA 16S Amplicons
For each sample, 2 mL was collected and centrifuged at 13,000 rpm for 10 min at 4 °C. Fourteen samples, representing distinct biological conditions across the rearing and processing workflow, were selected for DNA extraction and 16S rRNA gene sequencing. The DNA was extracted using the NucleoSpin Soil-Kit (Macherey Nagel, Hoerdt, France), following the manufacturers protocol. The DNA obtained was quantified by a NanoDrop 2000 (ThermoFischer Scientific, Illkirch-Graffenstaden, France) and was stored at −20 °C until used to send for the sequencing.
The high-throughput sequencing of the V3-V4 hypervariable region of the bacterial 16S rRNA gene of 14 samples was performed by Eurofins Genomics on an Illumina MiSeq platform according to the manufacturer’s instructions. The amplicons were generated using a two-step PCR protocol. The V3-V4 regions were PCR-amplified using template-specific primers (forward: 5′-TACGGGAGGCAGCAG-3′ and reverse: 5′-CCAGGGTATCTAATCC-3′) [40,41] containing a universal overhang. The amplicons were cleaned and set up for the PCR index, with specific primers directed to the universal overhangs. The final amplicon libraries were cleaned, quantified, and pooled equimolar. The resulting final library pool was quantified and sequenced using the v3 chemistry (2 × 300 bp paired-end reads).
2.6. Bioinformatic and Statistical Analyses
Paired-end reads obtained from MiSeq sequencing were analyzed using the Galaxy-supported pipeline named Find Rapidly Operational Taxonomic Units (OTUs) with Galaxy Solution (FROGS) using the dedicated guidelines for 16S data [42]. A taxonomic assignment was performed using Silva132 16S pintail 100 (bacteria, https://www.arb-silva.de, accessed on 15 November 2024). The OTU tables were used for the bioinformatic analyses. The subsequent analyses and data visualization were then performed using the R packages Phyloseq [43], DeSeq2 [44], and Ggplot2 [45]. The distribution of the sampling points (calculated from the OTU contingency table) was displayed in MDS/PCoA ordination by R software (4.2.2). Shannon, Inverse Simpson, and Chao1 estimators, along with the statistical analysis of the alpha and beta diversity indexes, used to evaluate the sample differences in species complexity, were performed using the Phyloseq R package implemented in FROGS (FROGSSTAT) [43]. Interactive sunburst plots were generated using the Plotly library v3.0.1 [46] to illustrate the relative taxonomic composition (up to the genus level) of the bacterial communities identified across the different sample types. These interactive visualizations provide hierarchical insight into microbial abundance profiles in a reader-friendly and navigable format. All the sunburst plots are available online at https://bmisery.github.io/bsf-metabarcoding-visualizations/, accessed on 3 June 2025.
3. Results
3.1. Systematic Review on the Microbial Diversity of BSF
3.1.1. Study Selected
A total of 680 articles were collected (Figure 2) and 564 remained after the duplicates were removed. This selection was screened based on the title, abstract, and full text, following the eligibility criteria, resulting in 27 articles selected. The data extracted are reported in Supplementary File S1.
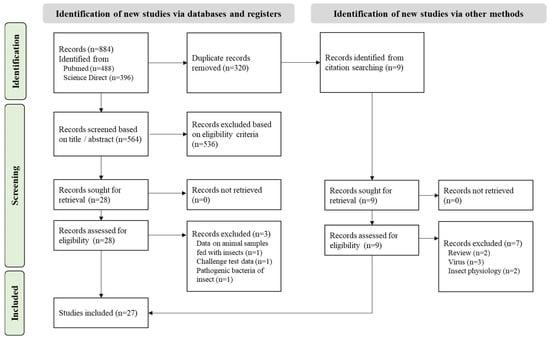
Figure 2.
PRISMA flowchart of the systematic review on BSF microbial diversity.
3.1.2. Study Description
The articles found on the microbial diversity of BSF were published between 2011 and now (Figure 3) in different countries (Figure 4). The samples analyzed included mainly raw larvae (n = 27) resulting from different rearing conditions (Supplementary File S2) and some analyzed substrates (n = 13) and frass (n = 2). None of them explored the effect of the manufacturing process.
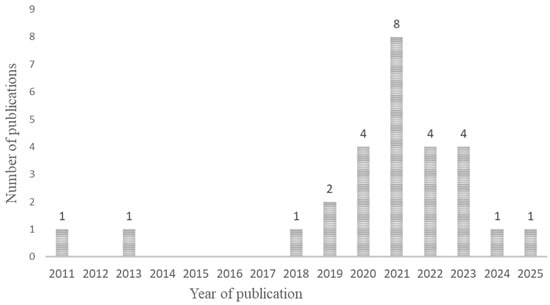
Figure 3.
Chronological distribution of studies included in the systematic review (n = 27).
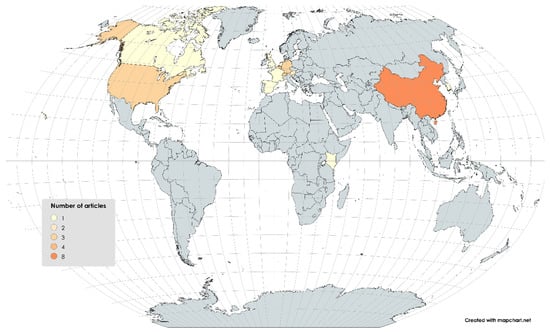
Figure 4.
Geographical distribution of studies included in the systematic review (n = 27).
3.1.3. Microbial Profile of BSF Already Published
The systematic review identified 27 studies based on non-culture-dependent methods, highlighting the extensive microbial diversity associated with Hermetia illucens, mainly in larvae but also in substrates and frass. While most studies focused on raw larvae from various diets and rearing conditions, none directly assessed the effect of industrial processing on microbial diversity.
High-throughput sequencing analyses (mainly 16S rRNA gene) consistently revealed the presence of bacterial genera such as Bacillus, Clostridium, Enterococcus, Providencia, Morganella, and Klebsiella, which were detected in the majority of the reviewed studies (see Table 2). These genera include spore-forming, opportunistic bacteria and potential pathogens, underscoring the importance of targeted monitoring of BSF larvae and resulting products.
Several studies, such as Gorrens et al. (2021, 2022), identified dominant communities of Enterococcus, Providencia, and Morganella in larvae, using extraction kits such as EZNA soil and sequencing of the V3–V4 16S rRNA regions [30,47]. Wynants et al. (2018) reported low levels of Salmonella and Bacillus cereus in larvae and/or residue samples from various European countries, suggesting that, while such pathogens may occasionally be present in raw samples, they are highly influenced by the substrate and rearing conditions [23].
Asian studies such as Ao et al. (2021) and Wu et al. (2022) also reported the persistent detection of Providencia, Klebsiella, Morganella, and Dysgonomonas, commonly associated with the larval gut and indicative of adaptation to nutrient-rich organic substrates [48,49]. Chen et al. (2023) expanded on this by combining 16S and ITS sequencing to detect not only bacteria but also molds such as Diutina, Candida, and Issatchenkia, depending on diet composition, emphasizing the diet’s impact on both bacterial and fungal communities [50].
Geographically, microbial profiles were broadly consistent across studies conducted in Europe (Austria, Belgium, France, Germany, Italy, Netherlands, Slovakia, Spain, and Switzerland), Asia (China and South Korea), and North America (Canada and the United States), despite differences in substrates, rearing conditions, and analytical methods. For instance, Cifuentes et al. (2020) in Germany and Bruno et al. (2019) in Italy detected Clostridium and Bacillus [8,51].
Table 2 confirms the frequent detection of Bacillus and Clostridium across the published studies. These genera were commonly found regardless of the country or method used and were detected not only in larvae but also in substrates and frass. This recurrence suggests a central ecological role for these genera in the BSF microbiome, whether as commensals or as targets for food safety monitoring.
However, several methodological limitations were identified across the existing literature. First, many studies were based on small sample sizes or lacked technical and biological replication, which limits the robustness and generalizability of the findings. Second, most investigations focused solely on taxonomic profiling using 16S rRNA sequencing, with few incorporating functional approaches, such as metabolomics, metagenomics, or metatranscriptomics. As a result, the functional roles of microbial taxa within the insect microbiome, including their potential impact on food safety or nutrient metabolism, remain largely unexplored.
In summary, the microbial profiles described in the literature reveal bacterial communities dominated by enteric Gram-negative genera (Providencia, Morganella, and Klebsiella), spore-forming bacteria (Bacillus and Clostridium), and Gram-positive cocci (Enterococcus), with the variability strongly influenced by the substrate, temperature, and rearing humidity. These findings highlight the need to establish specific microbiological criteria for insect-based products and to combine both culture-dependent and culture-independent approaches for more comprehensive microbial risk assessment.

Table 2.
Summary of bacterial genus identified in the studies from the systematic review.
Table 2.
Summary of bacterial genus identified in the studies from the systematic review.
| Acinetobacter | Actinomyces | Bacillus | Bacteroides | Burkholderia | Campylobacter | Clostridium | Corynebacterium | Dysgonomonas | Enterobacter | Enterobacteriaceae | Enterococcus | Escherichia | Escherichia-Shigella | Ignatzschineria | Klebsiella | Listeria | Lysinibacillus | Morganella | Paenalcaligenes | Proteus | Providencia | Pseudomonas | Rhizobiales | Salmonella | Staphylococcus | Vagococcus | Weissella | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ao et al., 2021 [48] | X | X | X | |||||||||||||||||||||||||
| Auger et al., 2023 [52] | X | X | X | X | X | |||||||||||||||||||||||
| Bruno et al., 2019 [51] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Chen et al., 2023 [50] | X | X | X | X | X | |||||||||||||||||||||||
| Cifuentes et al., 2020 [53] | X | X | X | X | X | |||||||||||||||||||||||
| Cifuentes et al., 2022 [8] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Gorrens et al., 2021 [30] | X | X | X | X | X | |||||||||||||||||||||||
| Gorrens et al., 2022 [47] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Jeon et al., 2011 [54] | X | X | ||||||||||||||||||||||||||
| Jiang et al., 2019 [55] | X | X | X | |||||||||||||||||||||||||
| Klammsteiner et al., 2020 [56] | X | X | X | |||||||||||||||||||||||||
| Klammsteiner et al., 2021 [57] | X | X | X | X | ||||||||||||||||||||||||
| Li et al., 2023 [34] | X | X | X | X | ||||||||||||||||||||||||
| Liu et al., 2020 [58] | X | X | X | X | ||||||||||||||||||||||||
| Mašková et al., 2025 [59] | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| Pei et al., 2023 [60] | X | X | X | |||||||||||||||||||||||||
| Querejeta et al., 2023 [61] | X | X | X | X | X | |||||||||||||||||||||||
| Raimondi et al., 2020 [21] | X | X | X | X | X | |||||||||||||||||||||||
| Schreven et al., 2021 [62] | X | X | X | X | ||||||||||||||||||||||||
| Schreven et al., 2022 [63] | X | X | X | X | X | X | ||||||||||||||||||||||
| Tanga et al., 2021 [64] | X | X | X | X | X | X | ||||||||||||||||||||||
| Tegtmeier et al., 2021 [35] | X | X | X | X | X | |||||||||||||||||||||||
| Van Looveren et al., 2024 [65] | X | X | X | X | X | X | X | |||||||||||||||||||||
| Wu et al., 2022 [49] | X | X | X | X | X | |||||||||||||||||||||||
| Wynants et al., 2018 [23] | X | X | X | X | X | |||||||||||||||||||||||
| Yang et al., 2021 [66] | X | X | X | X | X | X | ||||||||||||||||||||||
| Zheng et al., 2013 [67] | X | X |
3.2. Physico-Chemical Analysis of Rearing Conditions
Table 3 presents the characteristics of the rearing pH, temperature, and relative humidity, recorded over multiple stages of insect development, including incubation, pre-growth, and growth.

Table 3.
Physico-chemical and environmental parameters during the different rearing phases of Hermetia illucens. Data are the mean of replicates ± standard deviation. * Each growth stage (pre-growth and growth) had a distinct substrate, which was changed between these two stages.
The environmental and substrate conditions were closely monitored throughout rearing, with incubation maintained at 27.0 ± 0.1 °C and 75.0 ± 0.3% relative humidity; during the pre-growth phase, the substrate pH increased from 5.2 to 7.3, the internal temperature rose from 24.7 ± 0.09 °C to 42.7 ± 0.97 °C, and the humidity rose from 52.7 ± 8.4% to 63.5 ± 8.8%, while in the growth phase, the pH ranged from 4.1 to 9.0, internal temperatures from 24.6 ± 0.43 °C to 32.1 ± 0.81 °C, and humidity from 36.2 ± 2.1% to 44.2 ± 2.8%, with rearing room conditions remaining stable between 26.8 and 28.0 °C and 36–75% relative humidity.
3.3. Quantification of Microbial Indicators and Pathogens
The quantification results of the microbial counts of the bacterial indicators in the larvae and resulting powders (Figure 5) as well as the frass and substrate (Table 4) highlight the microbial diversity and evolution in the total mesophilic bacteria, spores, lactic acid bacteria, yeast, mold, and anaerobic sulfite-reductive bacteria.
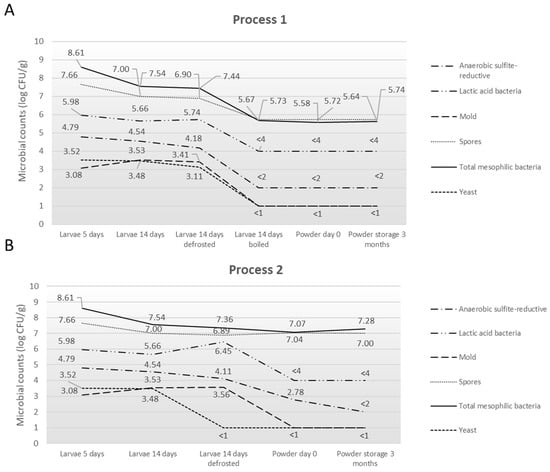
Figure 5.
Summary of microorganism levels found in larvae and resulting powders following (A) Process 1 and (B) Process 2.

Table 4.
Summary of microorganism levels (log CFU/g) found in the substrate and frass during the rearing of Hermetia illucens. Data are the mean of replicates ± standard deviation. a,b, respectively, one sample of less than 2 and 4 log cfu/g.
In the pre-growth and growth substrates, mesophilic bacteria ranged from 7.19 to 7.86 log CFU/g and spores from 4.97 to 5.39 log CFU/g, and lactic acid bacteria reached 8.48 log CFU/g in both substrates. Yeasts and molds were quantified between 2.56 and 4.60 log CFU/g and 2.60 to 3.60 log CFU/g, respectively. In larvae at D5, the counts reached 8.61 log CFU/g for mesophilic bacteria, 7.66 log CFU/g for spores, 5.98 log CFU/g for lactic acid bacteria, 3.52 log CFU/g for yeasts, and 3.08 log CFU/g for molds. At D14, the values decreased to 7.54 log CFU/g for mesophilic bacteria and 7.00 log CFU/g for spores, while lactic acid bacteria were measured at 5.66 log CFU/g. Yeasts and molds were quantified at 3.48 and 3.53 log CFU/g, respectively. Anaerobic sulfite-reducing bacteria (ASR) ranged from 4.54 to 4.79 log CFU/g. After defrosting, mesophilic bacteria were quantified at 7.44 log CFU/g, spores at 6.90 log CFU/g, lactic acid bacteria at 5.74 log CFU/g, yeasts at 3.11 log CFU/g, molds at 3.41 log CFU/g, and ASR at 4.18 log CFU/g, showing levels comparable to those measured at day 14. Following the boiling step, mesophilic bacteria were measured at 5.67 log CFU/g, spores at 5.73 log CFU/g, lactic acid bacteria at <4 log CFU/g, yeasts and molds at <1 log CFU/g, and ASR at <2 log CFU/g. The powder samples showed similar values, with mesophilic bacteria and spores around 5.58–5.74 log CFU/g and lactic acid bacteria stable at 4 log CFU/g. Yeasts, molds, and ASR remained below detection thresholds (<1 or <2 log CFU/g) during storage.
The quantification of pathogenic bacteria provided insight on Bacillus cereus presumed, Clostridium perfringens presumed, Cronobacter sp., Enterobacteriaceae presumed, Listeria monocytogenes, Salmonella sp., and Staphylococcus coagulase-positive (Table 5).

Table 5.
Summary of microorganism levels (log cfu/g) found during the rearing of Hermetia illucens (substrates, larvae, and frass) and after processing (powder). Substrate and frass at days 5 and 14; larvae at day 5, 14, and defrosted; and powder at day 0 and after 3 months of storage. Data are the mean of replicates ± standard deviation or all values are reported when at least one sample was below the limit of quantification. * at least one sample less than 1 log cfu/g. ND is not detected in 25 g.
The results obtained highlighted that presumptive Bacillus cereus, Clostridium perfringens, Cronobacter sp., presumptive Enterobacteriaceae, Listeria monocytogenes, Salmonella sp., and coagulase-positive staphylococci were not quantified or detected in any of the sample types analyzed using culture-dependent methods. Clostridium perfringens was only detected in the larval sample at D5, with counts up to 4.28 CFU/g. E. coli was not detected in the pre-growth and growth substrates (<1 log CFU/g) but was found at 2.85 log CFU/g for larvae at D5 and 2.52 log CFU/g at D14. The frass samples showed E. coli contamination levels of 3.51 log CFU/g at D0 and <1 log CFU/g at D14. E. coli contamination in insect powders was below the quantification threshold (<1 log CFU/g) after Processes 1 and 2 and during storage.
3.4. Distribution of Global Phylum Taxonomic
To describe bacterial diversity, the high-throughput sequencing of the V3–V4 hypervariable region of the bacterial 16S rRNA gene came to a total of 885,675 sequences with an average length of 300 nts (length filter with minimum 100 nts and maximum 580 nts). The sequence abundance and presence were filtered in multiple samples. The sequence proportion in the samples was kept at a minimum of 0.0005% and the Amplicon Sequence Variants (ASVs), present in at least three samples, were preserved. In this study, 84 families, 184 genera, and 362 species were detected and the final ASV table contained 599 bacterial ASVs. Figure 6 illustrates the relative abundance of the microbial phyla across the different sample types, providing a comparative overview of the taxonomic composition.
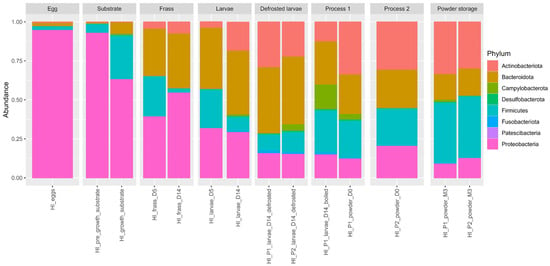
Figure 6.
Global phylum taxonomic distribution according to the type of sample.
The predominant bacterial taxa in the samples were Proteobacteria and Bacteroidota, each accounting for approximately 32.42% and 24.83% of the total bacterial composition, respectively. Firmicutes (according to classical taxonomy) represented 21.23% of the overall bacterial distribution, while Actinobacteriota accounted for 5.91%. According to the revised taxonomy proposed by the Genome Taxonomy Database (GTDB), Firmicutes are now split into several phyla, mainly Bacillota and Clostridiota. In our dataset, the dominant genera formerly grouped under Firmicutes—such as Bacillus sp., Enterococcus sp., Lactococcus sp., Latilactobacillus sp., and Paenibacillus sp.—are now classified within Bacillota, while Clostridium sp., Lachnoclostridium sp., and Sedimentibacter sp. belong to Clostridiota. Campylobacterota constituted a smaller proportion (0.46%) of this community. The remaining bacterial taxa represent 15.55% of the composition.
At the phylum level, the egg samples were dominated by Proteobacteria (95.01%), followed by Firmicutes (2.45%), Bacteroidota (2.10%), and Actinobacteriota (0.44%). The substrate samples were similarly dominated by Proteobacteria (80.68%), followed by Firmicutes (15.26%) and Bacteroidota (3.58%). In the frass, Proteobacteria (47.73%), Bacteroidota (32.93%), Firmicutes (13.36%), and Actinobacteriota (5.78%) were most represented. In the larvae samples, the most represented phyla were Bacteroidota (41.32%), Actinobacteriota (17.55%), Proteobacteria (23.96%), and Firmicutes (14.59%). In the boiled larvae, Bacteroidota and Firmicutes accounted for 27.66% and 27.11%, respectively, followed by Campylobacterota (15.84%), Proteobacteria (15.22%), and Actinobacteriota (12.31%). In the powder samples, Actinobacteriota ranged from 30.15% to 33.38%, Firmicutes from 30.03% to 31.07%, Bacteroidota from 20.98% to 21.54%, Proteobacteria from 10.99% to 17.55%, and Campylobacterota from 0.42% to 2.55%. Fusobacteriota, Patescibacteria, and Desulfobacterota were present at ≤0.03%.
3.5. Taxonomic Identification of Potential Pathogenic Bacteria
The microbial composition across various samples, including substrate, egg, frass, larvae, defrosted larvae, processing steps, and powder storage, provides valuable insights into the presence of potential pathogens, as shown in Figure 7 and Supplementary File S3.
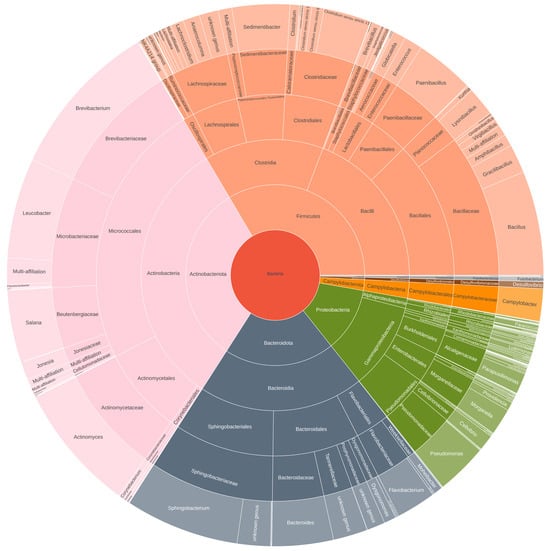
Figure 7.
Microbial composition of the larvae powder and the presence of potential pathogens.
In our study, the main potential pathogens detected through the 16S metabarcoding approach were Bacillus sp., Campylobacter sp., Staphylococcus sp., Acinetobacter sp., Clostridium sp., and Escherichia-Shigella sp. No Salmonella sp., Cronobacter sp., or Listeria monocytogenes were detected by this approach in our study.
In the substrate samples, the presence of Campylobacter sp. and Staphylococcus sp. was not detected in either the pre-growth or growth substrate samples. Bacillus sp. was observed at 0.01% in the pre-growth substrate and not detected in the growth substrate. Clostridium sp. abundance was at 0.19% in the pre-growth substrate and 0.12% in the growth substrate. Enterobacteriaceae species (Escherichia and Shigella sp.) were present at 0.04% in the pre-growth substrate and absent in the growth substrate. Acinetobacter sp. abundance was 1.10% in the pre-growth substrate and 2.63% in the growth substrate.
For the egg sample, Campylobacter sp. was not detected, while Bacillus sp. was at 0.99%. Staphylococcus sp. was found at 0.01%, and Clostridium sp. was present at 0.02%. Enterobacteriaceae species (Escherichia and Shigella sp.) were at 0.02%, and Acinetobacter sp. was detected at 0.20%.
In the frass samples, Campylobacter sp. was not detected at D5 but was present at 0.26% on D14. Bacillus sp. abundance decreased from 6.35% on D5 to 0.17% on D14. Staphylococcus sp. abundance remained low but present at 0.02% (D5) and 0.01% (D14). Clostridium sp. was consistently low at 0.04% on both days. Enterobacteriaceae species (Escherichia and Shigella sp.) were present at 0.24% on D5 and 0.10% on D14. Acinetobacter sp. abundance showed a drop from 4.43% on D5 to 0.27% on D14.
In the larvae samples, Campylobacter sp. showed an increase from 0.20% on D5 to 1.24% on D14. Bacillus sp. decreased from 4.22% on D5 to 0.54% on D14, while Staphylococcus sp. remained at low levels (1.09% on D5 and 0.13% on D14). Clostridium sp. was present at 0.30% on D5 and increased to 0.61% on D14. Enterobacteriaceae species (Escherichia and Shigella sp.) abundance ranged from 0.51% on D5 to 5.06% on D14. Acinetobacter sp. abundance was 0.62% on D5 and dropped to 0.02% on D14.
For the defrosted larvae, Campylobacter sp. was detected at 0.37% in P1 and 4.25% in P2. Bacillus sp. was observed at 1.20% in both P1 and P2. Staphylococcus sp. remained at low levels, with 0.18% in P1 and 0.11% in P2. Clostridium sp. was found at 1.17% in P1 and 1.64% in P2. Enterobacteriaceae species (Escherichia and Shigella sp.) abundance ranged from 1.21% in P1 to 2.46% in P2. Acinetobacter sp. was present at 0.05% in P1 and 0.04% in P2.
In Process 1 (boiling and drying step), Campylobacter sp. was found at 15.84%, and Bacillus sp. abundance was at 4.52%. Staphylococcus sp. was present at 0.11%, and Clostridium sp. increased to 6.55%. Enterobacteriaceae species (Escherichia and Shigella sp.) were present at 0.43%, and Acinetobacter sp. was not detected at the end of this process.
For Process 2 (drying step), Campylobacter sp. was also present at 3.55%, and Bacillus sp. remained at 3.82%. Staphylococcus sp. stayed at low levels (0.15%), and Clostridium sp. was found at 2.88%. Enterobacteriaceae species (Escherichia and Shigella sp.) dropped to 0.29%, and Acinetobacter sp. remained low at 0.17%.
In powder storage after Process 1, Campylobacter sp. was present at 3.55% on D0 and decreased to 1.51% after 3 months of storage (M3). Bacillus sp. rose from 3.82% on D0 to 8.11% on M3. Staphylococcus sp. abundance remained at a low level (0.15% on D0 and 0.24% on M3). Clostridium sp. increased from 2.88% on D0 to 5.70% on M3. Enterobacteriaceae species (Escherichia and Shigella sp.) were low at 0.29% on D0 and decreased to 0.14% on M3. Acinetobacter sp. abundance dropped from 0.17% on D0 to 0.02% on M3.
In powder storage after Process 2, Campylobacter sp. was present at 0.40% on D0 and 0.45% after 3 months of storage (M3). Bacillus sp. rose from 4.53% on D0 to 9.10% on M3. Staphylococcus sp. remained low at 0.22% on D0 and 0.37% on M3. Clostridium sp. increased from 3.86% on D0 to 6.47% on M3. Enterobacteriaceae species (Escherichia and Shigella sp.) were very low at 0.29% on D0 and decreased to 0.12% on M3. Acinetobacter sp. abundance was from 0.09% on D0 to 0.04% on M3.
3.6. Alpha Diversity Analysis
Figure 8 illustrates the diversity indices (Observed, Chao1, Shannon, and InvSimpson) across the various sample types, providing a comprehensive comparison of the microbial diversity within the different sample groups. The Chao1 index reflected the microbial community richness and the InvSimpson and Shannon indexes reflected the microbial community diversity.
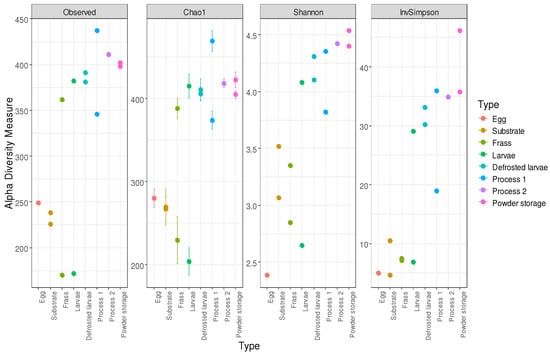
Figure 8.
Bacterial alpha diversity measurement according to sample types.
The alpha diversity metrics varied across the sample types. In the egg sample, the observed richness was 249, the Chao1 was 280.1, the Shannon index was 2.39, and the Inverse Simpson index was 5.05. The pre-growth substrate showed an observed richness of 238, a Chao1 of 267.1, a Shannon index of 3.07, and an Inverse Simpson index of 4.65. The growth substrate had an observed richness of 226, a Chao1 of 269.5, a Shannon index of 3.52, and an Inverse Simpson index of 10.49. For the frass, the values increased from D5 (observed richness 170, Chao1 229.5, Shannon index 2.85, and Inverse Simpson index 7.12) to D14 (observed richness 362, Chao1 388.1, Shannon index 3.35, and Inverse Simpson index 7.54). For the larvae, the D5 values were observed richness 172, Chao1 203.9, Shannon index 3.31, and Inverse Simpson index 11.78, while the D14 values were observed richness 382, Chao1 415.2, Shannon index 4.08, and Inverse Simpson index 29.1. In Process 1, the defrosted larvae had observed richness 391, Chao1 406.1, Shannon index 4.31, and Inverse Simpson index 33.15. The boiled larvae showed observed richness 346, Chao1 374.2, Shannon index 3.82, and Inverse Simpson index 18.95. The powder at D0 presented observed richness 437, Chao1 469.3, Shannon index 4.36, and Inverse Simpson index 35.94. In Process 2, the defrosted larvae had observed richness 381, Chao1 411.0, Shannon index 4.10, and Inverse Simpson index 30.22. The powder at D0 showed observed richness 411, Chao1 418.6, Shannon index 4.27, and Inverse Simpson index 32.06. At M1, the values were observed richness 402, Chao1 422.7, Shannon index 4.40, and Inverse Simpson index 30.38; at M3, they were observed richness 398, Chao1 405.1, Shannon index 4.54, and Inverse Simpson index 36.33.
The ANOVA performed on the Chao’s alpha, Shannon’s diversity, and Inverse Simpson’s indices revealed differences in the bacterial composition between the larvae (processed and unprocessed) and frass samples at D14 compared to D5 (p-value < 0.005). The ANOVA results also indicate that there is significant effect of the sample type (egg, substrate, frass, and larvae) on the observed species count (p-value < 0.005). The performance of the ANOVA test on the Shannon and Chao’s indexes highlighted a similar bacterial composition between the larvae (processed and unprocessed) and the insect powder after 3 months of storage.
3.7. Abundance of Microbial Communities
The microbial composition was analyzed using 16S rRNA gene sequencing to identify and characterize the bacterial communities in Hermetia illucens larvae during rearing and two distinct processing methods, referred to as Process 1 and Process 2. The microbial diversity across the rearing and processing stages of Hermetia illucens larvae is shown in Supplementary File S4.
The substrates used for the rearing Hermetia illucens larvae were characterized by a high abundance of Enterobacter sp., Latilactobacillus sp., and Enterococcus sp. In the pre-growth substrate, Enterobacter sp. dominated the bacterial community, representing approximately 50.60%, followed by Comamonas sp. (3.11%), Enterococcus sp. (1.96%), Pseudomonas sp. (2.15%), and Latilactobacillus sp. (0.38%). In the growth substrate, Enterobacter sp. remained predominant at 28.87%, with Pseudomonas sp. (3.85%), Comamonas sp. (3.05%), and Dysgonomonas sp. (4.53%) also present. Additionally, the growth substrate showed a higher abundance of Latilactobacillus sp. (19.90%) compared to the pre-growth substrate.
The most abundant genus in the eggs was Pseudomonas sp. (76.20%), followed by Enterobacter (5.23%), Comamonas sp. (1.48%), Bacillus sp. (0.98%), and Enterococcus sp. (0.11%).
In the frass samples, Sphingobacterium sp. and Comamonas sp. were the dominant genera. Sphingobacterium sp. accounted for 27.16% at day 5 (D5) and increased to 30.45% at day 14 (D14), while Comamonas sp. decreased from 31.52% at D5 to 2.42% at D14. Pseudomonas sp. became more prominent at D14, comprising 32.94% of the bacterial community, up from 0.27% at D5. Bacillus sp. was present at 6.34% at D5 but decreased to 0.17% at D14. Other bacteria detected in the frass at D14 included Leucobacter sp. (0.84%), Salana sp. (0.45%), Actinomyces sp. (2.11%), Brevibacterium sp. (0.26%), and Campylobacter sp. (0.26%).
At day 5, the larvae exhibited a diverse bacterial community, including Sphingobacterium sp. (39.15%), Comamonas sp. (27.09%), Enterococcus sp. (15.53%), Bacillus sp. (4.22%), Leucobacter sp. (0.96%), and Campylobacter sp. (0.20%). At day 14, the bacterial community exhibited increased diversity, with dominant genera including Sphingobacterium sp. (17.89%), Actinomyces sp. (6.00%), and Morganella sp. (7.99%), followed by Tannerellaceae species (7.31%), and Porphyromonadaceae (8.74%). Other genera present were Brevibacterium sp. (3.58%), Leucobacter sp. (2.37%), Enterococcus sp. (3.93%), Salana sp. (1.86%), Campylobacter sp. (1.24%), and Dysgonomonas sp. (3.59%). Bacteroides sp. was also detected at 0.80%.
The microbial composition of Hermetia illucens larvae was analyzed during the two processing methods, Process 1 and Process 2, to assess the dynamic changes in the bacterial communities across the different stages of processing.
The microbial communities of the larvae at day 14 post-defrosting for Process 1 and Process 2 shared many similarities but with varying abundances. Both samples contained Sphingobacterium sp. (13.11–23.85%), Tannerellaceae sp. (5.68–13.86%), Salana sp. (3.25–5.36%), Dysgonomonas sp. (3.37–3.91%), Leucobacter sp. (2.38–3.23%), Morganella sp. (1.93–6.72%), Bacillus sp. (1.20% in both), Escherichia-Shigella sp. (1.21–2.46%), Paracoccus sp. (0.80–1.88%), and Fusobacterium sp. (0.97–1.62%). The differences in the abundance between the two processes included Campylobacter sp., which is more abundant in Process 2 (4.25%) compared to Process 1 (0.37%); Morganella sp. (6.72% in Process 2, 1.93% in Process 1); and Tannerellaceae sp. (13.86% in Process 2, 5.68% in Process 1).
In Process 1, boiled larvae at day 14 (D14) and powdered larvae at day 0 (D0) and after three months (M3) showed a dynamic shift in the bacterial community. For the boiled larvae, Campylobacter sp. was the dominant genus, comprising 15.84% of the bacterial community, followed by Tannerellaceae sp. (6.26%), Actinomyces sp. (4.77%), and Salana sp. (4.14%). Other genera included Bacillus sp. (4.52%), Dysgonomonas sp. (1.84%), Enterococcus sp. (2.81%), Sphingobacterium sp. (1.74%), Brevibacterium sp. (1.02%), Flaviflexus sp. (0.48%), Corynebacterium sp. (0.32%), and Lysinimicrobium sp. (0.26%). The powdered larvae at D0 showed Brevibacterium sp. (10.18%), Leucobacter sp. (9.81%), and Sphingobacterium sp. (9.41%) as the most abundant genera. Other genera present included Actinomyces sp. (7.10%), Comamonas sp. (3.55%), Bacillus sp. (3.82%), Flavobacterium sp. (2.70%), Bacteroides sp. (2.44%), Tannerellaceae sp. (2.54%), Dysgonomonas sp. (0.84%), and Campylobacter sp. (0.88%). After 3 months of storage (M3), Leucobacter sp. (7.32%), Sphingobacterium sp. (7.20%), and Bacillus sp. (8.11%) remained the most prominent genera. Brevibacterium sp. (12.34%) and Actinomyces sp. (6.44%) were still present, along with Enterococcus sp. (1.37%). Other genera such as Salana sp. (4.07%), Pseudoclavibacter sp. (1.08%), and Dysgonomonas sp. (0.60%) were also found.
In Process 2, the powdered larvae at D0 and after three months of storage (M3) displayed distinct microbial profiles. At D0, the community was dominated by Sphingobacterium sp. (15.07%), followed by Leucobacter sp. (6.23%), Brevibacterium sp. (5.40%), and Bacillus sp. (4.53%). Other genera included Dysgonomonas sp. (1.37%), Lysinibacillus sp. (1.06%), and Enterococcus sp. (0.99%). Also present were Tannerellaceae sp. (2.05%), Amphibacillus sp. (0.52%), and Gordonia sp. (0.25%). After three months of storage, the microbial community of the powdered larvae in Process 2 remained dominated by Bacillus sp. (9.10%) and Sphingobacterium sp. (10.12%), while Lysinibacillus sp. (2.30%) increased and Leucobacter sp. (4.76%) decreased compared to their levels at D0. Enterococcus sp. (1.52%) was also detected, along with Gracilibacillus sp. (0.43%), Moheibacter sp. (0.34%), and Lysinimicrobium sp. (0.67%). Dysgonomonas sp. (0.90%), Tannerellaceae sp. (2.26%), and Amphibacillus sp. (1.10%) were also present.
3.8. Comparison of Microbial Diversity (Process/Rearing)
To examine the differences in the bacterial composition between the sample types, the microbial profiles were analyzed using MDS/PCoA, providing a visual representation of the distribution of bacterial ASVs across the different categories (egg, substrate, frass, larvae, processing, and powder), as shown in Figure 9.
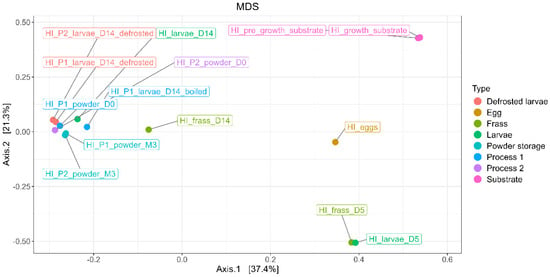
Figure 9.
MDS/PCoA representing bacterial ASV distribution according to the type of samples (egg, substrate, frass, larvae, processing, and powder).
The results show that the first axis (PC1) explains 37.4% of the variance, while the second axis (PC2) explains 21.3%, collectively accounting for 58.7% of the total variance. The bacterial composition of the egg and substrate samples was significantly distinct from the larvae and frass samples. The bacterial composition of the egg samples exhibited the most distinct clustering in the MDS/PCoA analysis, reflecting their unique microbial community. Similarly, the bacterial profiles of the substrate samples, including the pre-growth and growth substrates, were significantly different from all the other sample types, forming a distinct cluster. The bacterial compositions of the samples from day 5 (D5), including both the frass and larvae samples, showed similar microbial profiles. These samples were distinctly separated from those collected at day 14 (D14), particularly the larvae samples, which exhibited a different microbial composition.
The larvae samples at D14 (processed and unprocessed) formed a separate and distinct cluster, highlighting significant shifts in the microbial composition over time. The frass and larvae samples from D5 and D14 also exhibited clear separation, indicating dynamic changes in the bacterial communities as the rearing progressed.
The MDS/PCoA plot in Figure 10 illustrates the distribution of the samples based on the abundance of the observed OTUs and shows the distribution of ASVs according to the larvae processed by Process 1 and 2, as well as the associated powder samples.
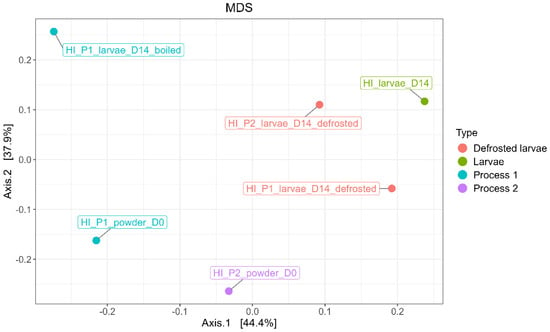
Figure 10.
MDS/PCoA representing bacterial ASV distribution according to the type of samples (processed larvae and powder).
A variation in the bacterial composition between the powder samples and the larvae (both processed and unprocessed) was observed. Similarly, the boiled sample exhibited a distinct bacterial composition compared to the final powder samples and the larvae samples. The microbial communities associated with Process 1 and Process 2 also differed. No significant difference in bacterial composition was found between the powder samples at D0 and after 3 months of storage (M3).
The R packages DeSeq2 was used to underline the significant fold change in the bacterial taxa between the larvae (processed and unprocessed) and insect powder. All the differences in the microbial proportions between the tanks quoted in this paragraph correspond to a fold change > 2 with a p-value < 0.001. The powder samples were enriched with ASVs affiliated to Bacillus species (three ASVs), Paenibacillus sp., Clostridium sp., and Anaerocolumna sp., while the larvae samples were enriched with Acinetobacter sp., Sphingobacterium sp., Comamonas sp., Enterococcus sp., and Enterobacteriaceae species. We performed the same analysis comparing the larvae (processed and unprocessed) with the substrates. The substrate samples were enriched with ASVs affiliated with species from the Enterobacteriaceae family (e.g., Enterobacter sp. and Rhanella sp.) and Lactobacillus sp. (e.g., Lactobacillus sakei), while the larvae samples showed an enrichment of bacteria commonly associated with the gut microbiome of larvae, including Sphingobacterium sp., Flavobacterium sp., Olivibacter sp., Parapedobacter sp., Porphyromonadaceae species, Prevotella sp., Dysgonomonas sp., Candidatus sp., Bacteroides species, Moheibacter sp., Myroides sp., Klebsiella sp., Proteus sp., Providencia sp., and Morganella sp.
4. Discussion
Understanding the microbial dynamics of Hermetia illucens throughout rearing and processing is crucial for optimizing production methods and ensuring food safety. Our findings confirm previous research while also highlighting key differences that can be attributed to specific rearing conditions, microbial interactions, and processing methods.
The composition of the substrate played a central role in shaping the microbial communities of H. illucens. The pre-growth substrate was dominated by Enterobacter sp. and Latilactobacillus sp., taxa frequently associated with organic matter decomposition and lactic acid fermentation [23,63]. This microbial profile aligns with previous studies reporting that carbohydrate-rich substrates promote lactic acid bacteria, which contribute to microbial stabilization by outcompeting potential pathogens [25]. In contrast, the eggs displayed a distinct microbiota dominated by Pseudomonas sp. (76.2%), a genus often associated with environmental persistence and biofilm formation [51]. The presence of Pseudomonas sp. in eggs suggests that microbial colonization may occur early in development, possibly through vertical transmission, as proposed in other insect models [62,68]. Such early associations may contribute to the establishment of a core microbiota involved in substrate decomposition, thereby enhancing the ecological plasticity of the species [62,65,68].
As larvae developed, a notable shift in microbial diversity was observed. At day 5 (D5), the community was dominated by Sphingobacterium sp. and Comamonas sp., two genera previously identified in H. illucens larvae and known for their involvement in organic matter degradation [22]. By day 14 (D14), the microbiota became more diverse, with an increase in Morganella sp. and Actinomyces sp., mirroring findings from Schreven et al. (2022) that demonstrated microbiota shifts in response to larval metabolic activity and substrate composition [63]. This transition also supports previous research showing that gut microbiota diversity increases with larval age as new taxa colonize the digestive tract [23,51,69]. Unlike Schreven et al. (2022), who reported a higher prevalence of lactic acid bacteria in larvae, we observed a dominance of Sphingobacterium sp. and Comamonas sp. at early developmental stages, suggesting that microbial composition is highly dependent on rearing conditions [63]. Recent metabarcoding analysis further supports this notion, demonstrating clear stage-specific shifts in microbial diversity and composition in H. illucens, suggesting that microbial communities dynamically respond to environmental and nutritional inputs rather than being randomly distributed [61]. The microbial composition of frass closely resembled that of larvae, reinforcing the hypothesis that gut-associated bacteria are continuously excreted into the rearing environment, a process described in Cifuentes et al. (2020) [53]. The detection of Bacillus sp. and Clostridium sp. in frass suggests that sporulated bacteria persist throughout the rearing cycle, as also reported by Vandeweyer et al. (2017) [10].
Processing significantly altered the microbial composition of H. illucens, with notable differences between the two processing methods. Boiling followed by drying (Process 1) was effective in reducing microbial loads, particularly mesophilic bacteria, but had a limited effect on spore-forming bacteria, consistent with previous findings on heat-treated edible insects [24,70]. However, metabarcoding analysis revealed the persistence of Bacillus sp. and Clostridium sp. post-processing, while culture-dependent methods revealed spore counts of 5.74 CFU/g, indicating that thermal treatment alone might be insufficient to completely eradicate these resilient taxa [16]. Notably, the increased relative abundance of Campylobacter sp. detected in boiled larvae (15.84%) likely results from the enhanced release and detection of bacterial DNA following heat-induced cell disruption. In contrast, drying alone (Process 2) preserved a higher microbial diversity, particularly mesophilic bacteria, supporting findings by Wynants et al. (2019) that drying alone is insufficient for complete microbial inactivation [23]. While Process 2 did not select for Campylobacter sp., spore-forming bacteria such as Bacillus sp. and Clostridium sp. remained abundant, highlighting the need for additional decontamination strategies. Microbial diversity remained stable in the powders stored for three months, suggesting that processed powders are microbiologically resilient under controlled conditions. However, the relative abundance of Bacillus sp. increased during storage, a trend also noted in Fasolato et al. (2018), emphasizing the importance of optimizing storage conditions to prevent spore germination [16].
The absence of Salmonella sp., Listeria monocytogenes, and Cronobacter sp. aligns with previous studies, suggesting that these pathogens are not commonly associated with H. illucens when rearing and processing conditions are properly managed [25]. However, given the variability observed across different studies, establishing microbiological criteria specific to insect-based foods would enhance safety and ensure regulatory compliance. The identification of Campylobacter sp., Bacillus sp., and Clostridium sp. in the final powders raises concerns regarding food safety. Campylobacter sp. are major foodborne pathogens, though their role in edible insects remains underexplored. Although Campylobacter spp. were not detected in some previous studies on processed H. illucens [14,17], the occurrence of Campylobacter sp. in larvae samples has also been documented by Gorrens et al. (2022), who reported the consistent detection of a Campylobacter-like OTU across multiple insect rearing cycles, highlighting potential microbiological risks and underscoring the necessity for stringent hygiene practices during rearing and processing [47]. In our study, their detection may be linked to the presence of cells in a viable but non-culturable (VBNC) state induced by thermal stress, which can impair cultivation on selective media while allowing detection through DNA-based methods, such as metabarcoding. This finding may also reflect the presence of Campylobacter sp. in a viable but non-culturable (VBNC) state, a stress response known to occur after heat exposure in some Campylobacter species [71,72]. Previous risk assessments, including Kooh et al. (2020), suggested that Campylobacter sp. exhibits low resistance to processing in T. molitor [38]. The detection of Campylobacter sp. in BSF larvae aligns with prior studies demonstrating the presence and potential transmission of Campylobacter jejuni in other insect vectors, such as Musca domestica and Alphitobius diaperinus, which act as mechanical carriers within agricultural environments [60,73,74]. These findings contribute to the growing evidence that insects can harbor and potentially disseminate Campylobacter spp., reinforcing observations from previous research on house flies and lesser mealworms, where viable Campylobacter cells were recovered from both external surfaces and excreta, suggesting a possible role in pathogen resilience and transmission [60,74,75,76]. To better assess the presence of stressed or VBNC Campylobacter cells, future studies could benefit from the application of enrichment protocols such as the Park and Sanders method, which has proven effective in recovering sublethally injured cells, particularly in complex matrices [77,78].
While most Bacillus sp. species are not pathogenic, some within the B. cereus group could pose risks, particularly in products stored under suboptimal conditions [16]. Spore-forming bacteria such as Bacillus sp. and Clostridium sp. remained detectable after processing, consistent with previous reports [7,38]. The persistence of Clostridium sp. spores further highlights the need for additional decontamination strategies, such as high-pressure processing (HPP) or controlled humidity drying, as suggested by Osimani et al. (2021) [70]. It is crucial to acknowledge that the detection of these genera does not inherently imply the presence of pathogenic species. Numerous Bacillus species act as environmental decomposers and exhibit mutualistic or symbiotic relationships with insects [79]. Moreover, Bacillus species, commonly detected in the microbiota of Hermetia illucens, play significant ecological roles such as substrate decomposition and nutrient cycling, thereby promoting insect health and development [61,80]. Likewise, several Clostridium species are commensals in animal gastrointestinal tracts, distinct from pathogenic strains such as C. perfringens [81]. Recent evidence shows that intestinal Clostridium populations in insects may actively contribute to digestion processes, immune modulation, and the maintenance of intestinal homeostasis rather than posing a health risk [60,82]. This could explain discrepancies between culture-based and molecular detection methods, highlighting the need for further investigations into its survival mechanisms in insect-based products. These findings underscore the importance of species-specific risk assessments to optimize decontamination strategies and ensure microbial safety in edible insect production.
The culture-dependent analyses in our study did not detect B. cereus, and Clostridium perfringens was found only transiently at day 5, suggesting limitations in the applicability of traditional foodborne microbial standards designed for food matrices to insect matrices, which typically possess high microbial loads capable of obscuring targeted pathogen detection [15,36]. Indeed, insects such as H. illucens harbor a complex microbiome that can competitively exclude pathogens and reduce their culturability, emphasizing the need for methodological adaptations in microbial risk assessments specific to insect matrices [83]. Moreover, culture-based methods might not capture novel or less characterized pathogenic strains of Bacillus or Clostridium, emphasizing the necessity for comprehensive characterization and risk assessment of potentially pathogenic taxa in edible insects [84]. The persistence of Bacillus sp. and Clostridium sp. after processing corroborates previous research, emphasizing the necessity of targeted microbial risk management strategies in edible insect production [16,20,23]. Interestingly, while amplicon-based 16S rRNA profiling revealed the presence of these genera in post-processing samples, they were not detected using culture-dependent methods, raising questions about the viability of the detected taxa or the potential presence of non-culturable or sublethally injured cells. This discrepancy might be partly explained by the ecological role of insect-produced antimicrobial peptides (AMPs). BSF larvae naturally synthesize antimicrobial peptides (AMPs) as a critical component of their innate immune system, modulating microbial populations within their gut environment. Such peptides can inhibit or reduce the viability and culturability of sensitive bacterial taxa, thereby contributing to observed differences between culture-based and molecular detection methods [85,86,87]. Moreover, the limited taxonomic resolution of short-read 16S sequencing (e.g., V3–V4) complicates species-level identification, thereby precluding definitive assessment of pathogenicity and necessitating reference to these taxa as putative pathogenic bacteria pending further detailed characterization [88]. This underscores the need for a deeper taxonomic resolution to distinguish between innocuous and potentially hazardous species. Future research should focus on optimizing decontamination strategies, such as vacuum-assisted drying or fermentation, while leveraging full-length 16S metabarcoding and metagenomic sequencing to assess microbial dynamics and processing effects on food safety [89,90,91,92]. Full-length 16S rRNA sequencing offers superior taxonomic resolution over targeted V3-V4 regions, particularly at the species level [93], while metagenomics provides a broader perspective by detecting low-abundance taxa and revealing functional traits [92]. In parallel, fungal communities were only assessed using culture-based methods in our study. To better capture the full microbial ecology of insect matrices, future research should incorporate ITS-based sequencing to characterize fungal diversity and evaluate the impact of processing on these communities and potential safety concerns. Advances in sequencing technologies refine these methods, with Oxford Nanopore R10.4.1 and PacBio Sequel II improving species-level resolution in environmental microbiomes [94]. Additionally, optimized primer sets and bioinformatics pipelines enhance classification accuracy and mitigate PCR bias, allowing for better characterization of complex microbial communities [95,96]. Future studies should focus on overcoming methodological limitations and integrating multi-omics approaches, such as metatranscriptomics, to better assess microbial activity and its role in the safety and quality of insect-based products [33,60,97,98].
This study provides a comprehensive analysis of the microbial dynamics in Hermetia illucens during rearing and processing, highlighting the influence of the substrate composition, developmental stage, and processing method on microbial diversity. While thermal processing effectively reduced the overall microbial loads, certain taxa—including spore-forming bacteria such as Bacillus sp. and Clostridium sp.—remained detectable in processed samples due to their heat-resistant spores. Additionally, although Campylobacter sp. was not detected post-processing, the continuous detection of these microorganisms highlights the importance of developing more sensitive and targeted microbial monitoring strategies. These findings contribute to the growing body of knowledge on the microbial safety of edible insects and underscore the importance of tailored decontamination strategies for their safe commercialization. Future research should focus on refining processing parameters and developing predictive models to assess microbial risks in insect-based food production.
5. Conclusions
In conclusion, this study provides valuable insights into the microbiota characterization of the edible insect species Hermetia illucens (Black Soldier Fly). The microbial analysis revealed high microbial loads and a diverse bacterial community, including spore-forming bacteria such as Clostridium spp. and Bacillus spp. While human foodborne pathogens like Salmonella spp., Listeria monocytogenes, and Cronobacter spp. were not detected, the presence of certain bacterial taxa, particularly spore-forming bacteria, may represent a potential risk under specific conditions. Furthermore, this study demonstrated that processing steps significantly influence microbial reduction, with the combination of boiling and drying (Process 1) being more effective than drying alone (Process 2). Future research should investigate alternative decontamination strategies to ensure microbial safety in insect-derived products. Emerging techniques such as high-pressure processing (HPP) or controlled fermentation may offer effective solutions. Despite the growing interest in the microbiota of BSF larvae, there is still a lack of comprehensive data on the microbial safety of processed insect powders. In particular, studies combining culture-dependent and culture-independent approaches remain scarce, although such integration is essential to obtain a more complete and robust picture of microbial risks. Our results highlight the need to further explore these complementary tools, especially in the context of insect-based food products. Beyond identifying risks, this study underlines the importance of building operational solutions to support producers in controlling microbial hazards. Establishing insect-specific microbiological criteria, designing effective monitoring strategies across the production chain, and identifying critical control points adapted to insect farming are key priorities. Moreover, strengthening collaborations between researchers, food safety authorities, and insect producers is crucial to co-develop pragmatic guidelines and risk management practices. These should aim to improve both the microbial safety and the consumer acceptability of insect-based foods. As Europe moves towards the approval of insects for a potential protein alternative, particularly Hermetia illucens, the anticipated increase in consumption underscores the importance of further studies to assess and manage microbial risks across various processing methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14132161/s1, Supplementary File S1. Synthesis of samples included in the systematic review; Supplementary File S2. Characteristics and methodologies of included studies in the PRISMA systematic review on microbial risk in insect-based products; Supplementary File S3. Distribution of potentially pathogenic bacteria across different rearing and processing matrices of insects; Supplementary File S4. Phylogenetic taxonomy for bacterial communities showing dynamic changes at the genus level during Hermetia illucens rearing and processing.
Author Contributions
Conceptualization, B.M., L.B., R.D., G.B. (Guillaume Baudouin), M.F. and G.B. (Géraldine Boué); Methodology, B.M., L.B., R.D., G.B. (Guillaume Baudouin) and G.B. (Géraldine Boué); Software, B.M.; Validation, B.M., L.B., R.D., X.Y., G.B. (Guillaume Baudouin), M.F. and G.B. (Géraldine Boué); Formal analysis, B.M., L.B. and G.B. (Géraldine Boué); Investigation, B.M., R.D., X.Y., G.B. (Guillaume Baudouin) and G.B. (Géraldine Boué); Resources, B.M., L.B., G.B. (Guillaume Baudouin) and G.B. (Géraldine Boué); Data curation, B.M. and G.B. (Géraldine Boué); Writing—original draft, B.M. and G.B. (Géraldine Boué); Writing—review & editing, B.M., L.B., X.Y., V.L.C., G.B. (Guillaume Baudouin), M.F. and G.B. (Géraldine Boué); Visualization, B.M., X.Y. and G.B. (Géraldine Boué); Supervision, G.B. (Géraldine Boué); Project administration, G.B. (Géraldine Boué); Funding acquisition, G.B. (Géraldine Boué). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Godfray, H.C.J.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Nisbett, N.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The Future of the Global Food System. Phil. Trans. R. Soc. B 2010, 365, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spiegel, M.; Noordam, M.Y.; Van Der Fels-Klerx, H.J. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Comp. Rev. Food Sci. Food Safe 2013, 12, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed. Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Gill, K.; Chenier, K.A.; Free, A.; Goff, J.; Pitchford, J.L.; Cressman, K.; Posten, M.; Brunden, E.; Shelton, M.; Swanson, K.; et al. Research Needs, Environmental Concerns, and Logistical Considerations for Incorporating Livestock Grazing into Coastal Upland Habitat Management. J. Environ. Manag. 2023, 329, 117119. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and Hurdles of Edible Insects for Food and Feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Söderqvist, K.; Bakeeva, A.; Vaga, M.; Dicksved, J.; Vagsholm, I.; Jansson, A.; Boqvist, S. Microbial Communities and Food Safety Aspects of Crickets (Acheta Domesticus) Reared under Controlled Conditions. J. Insects Food Feed. 2020, 6, 429–440. [Google Scholar] [CrossRef]
- Cifuentes, Y.; Vilcinskas, A.; Kämpfer, P.; Glaeser, S.P. Isolation of Hermetia illucens Larvae Core Gut Microbiota by Two Different Cultivation Strategies. Antonie Van. Leeuwenhoek 2022, 115, 821–837. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Metagenetic Analysis of the Bacterial Communities of Edible Insects from Diverse Production Cycles at Industrial Rearing Companies. Int. J. Food Microbiol. 2017, 261, 11–18. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Microbial Counts of Mealworm Larvae (Tenebrio molitor) and Crickets (Acheta domesticus and Gryllodes sigillatus) from Different Rearing Companies and Different Production Batches. Int. J. Food Microbiol. 2017, 242, 13–18. [Google Scholar] [CrossRef]
- Stoops, J.; Crauwels, S.; Waud, M.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial Community Assessment of Mealworm Larvae (Tenebrio molitor) and Grasshoppers (Locusta Migratoria Migratorioides) Sold for Human Consumption. Food Microbiol. 2016, 53, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kooh, P.; Ververis, E.; Tesson, V.; Boué, G.; Federighi, M. Entomophagy and Public Health: A Review of Microbiological Hazards. Health 2019, 11, 1272–1290. [Google Scholar] [CrossRef]
- Garofalo, C.; Milanović, V.; Cardinali, F.; Aquilanti, L.; Clementi, F.; Osimani, A. Current Knowledge on the Microbiota of Edible Insects Intended for Human Consumption: A State-of-the-Art Review. Food Res. Int. 2019, 125, 108527. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The Microbiota of Marketed Processed Edible Insects as Revealed by High-Throughput Sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of Processed Edible Insect Products – Results of a Preliminary Survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Carraro, L.; Fontana, F.; Novelli, E.; Balzan, S. Edible Processed Insects from E-Commerce: Food Safety with a Focus on the Bacillus Cereus Group. Food Microbiol. 2018, 76, 296–303. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; et al. The Bacterial Biota of Laboratory-Reared Edible Mealworms (Tenebrio molitor L.): From Feed to Frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Greenhalgh, J.P.; Amund, D. Examining the Presence of Cronobacter Spp. in Ready-to-Eat Edible Insects. Food Saf. 2019, 7, 74–78. [Google Scholar] [CrossRef]
- Mancini, S.; Paci, G.; Ciardelli, V.; Turchi, B.; Pedonese, F.; Fratini, F. Listeria Monocytogenes Contamination of Tenebrio molitor Larvae Rearing Substrate: Preliminary Evaluations. Food Microbiol. 2019, 83, 104–108. [Google Scholar] [CrossRef]
- Wynants, E.; Frooninckx, L.; Van Miert, S.; Geeraerd, A.; Claes, J.; Van Campenhout, L. Risks Related to the Presence of Salmonella Sp. during Rearing of Mealworms (Tenebrio molitor) for Food or Feed: Survival in the Substrate and Transmission to the Larvae. Food Control 2019, 100, 227–234. [Google Scholar] [CrossRef]
- Raimondi, S.; Spampinato, G.; Macavei, L.I.; Lugli, L.; Candeliere, F.; Rossi, M.; Maistrello, L.; Amaretti, A. Effect of Rearing Temperature on Growth and Microbiota Composition of Hermetia illucens. Microorganisms 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- De Smet, J.; Wynants, E.; Cos, P.; Van Campenhout, L. Microbial Community Dynamics during Rearing of Black Soldier Fly Larvae (Hermetia illucens) and Impact on Exploitation Potential. Appl. Env. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the Microbiota of Black Soldier Fly Larvae (Hermetia illucens) Reared on Organic Waste Streams on Four Different Locations at Laboratory and Large Scale. Microb. Ecol. 2019, 77, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Klunder, H.C.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.J.R. Microbiological Aspects of Processing and Storage of Edible Insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Osimani, A.; Ferrocino, I.; Corvaglia, M.R.; Roncolini, A.; Milanović, V.; Garofalo, C.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Jamshidi, E.; et al. Microbial Dynamics in Rearing Trials of Hermetia illucens Larvae Fed Coffee Silverskin and Microalgae. Food Res. Int. 2021, 140, 110028. [Google Scholar] [CrossRef]
- Cacchiarelli, C.; Fratini, F.; Puccini, M.; Vitolo, S.; Paci, G.; Mancini, S. Effects of Different Blanching Treatments on Colour and Microbiological Profile of Tenebrio molitor and Zophobas Morio Larvae. LWT Food Sci. Technol. 2022, 157, 113112. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Tuccinardi, T.; Turchi, B.; Nuvoloni, R.; Paci, G. Effects of Different Blanching Treatments on Microbiological Profile and Quality of the Mealworm (Tenebrio molitor). J. Insects Food Feed. 2019, 5, 225–234. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Marques, J.P.; Fernandes, T.R.; Pintado, M.E.; Carvalho, S.M.P.; Cunha, L.M. Effect of Blanching, Storage and Drying Conditions on the Macro-Composition, Color and Safety of Mealworm Tenebrio molitor Larvae. LWT 2024, 191, 115646. [Google Scholar] [CrossRef]
- Adamek, M.; Mlček, J.; Adámková, A.; Suchánková, J.; Janalíková, M.; Borkovcová, M.; Bednářová, M. Effect of Different Storage Conditions on the Microbiological Characteristics of Insect. Potravinarstvo Slovak J. Food Sci. 2018, 12, 248–253. [Google Scholar] [CrossRef]
- Gorrens, E.; Van Moll, L.; Frooninckx, L.; De Smet, J.; Van Campenhout, L. Isolation and Identification of Dominant Bacteria From Black Soldier Fly Larvae (Hermetia illucens) Envisaging Practical Applications. Front. Microbiol. 2021, 12, 665546. [Google Scholar] [CrossRef]
- Juste, A.; Thomma, B.; Lievens, B. Recent Advances in Molecular Techniques to Study Microbial Communities in Food-Associated Matrices and Processes. Food Microbiol. 2008, 25, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Staats, M.; Arulandhu, A.J.; Gravendeel, B.; Holst-Jensen, A.; Scholtens, I.; Peelen, T.; Prins, T.W.; Kok, E. Advances in DNA Metabarcoding for Food and Wildlife Forensic Species Identification. Anal. Bioanal. Chem. 2016, 408, 4615–4630. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, E.G.; Kibet, C.; Paredes, J.C.; Mboowa, G.; Mwaura, O.; Njogu, J.; Masiga, D.; Bugg, T.D.H.; Tanga, C.M. Metatranscriptomic Analysis of the Gut Microbiome of Black Soldier Fly Larvae Reared on Lignocellulose-Rich Fiber Diets Unveils Key Lignocellulolytic Enzymes. Front. Microbiol. 2023, 14, 1120224. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Mei, C.; Luo, X.-Y.; Wulamu, D.; Zhan, S.; Huang, Y.-P.; Yang, H. Dynamics of the Intestinal Bacterial Community in Black Soldier Fly Larval Guts and Its Influence on Insect Growth and Development. Insect Sci. 2023, 30, 947–963. [Google Scholar] [CrossRef]
- Tegtmeier, D.; Hurka, S.; Mihajlovic, S.; Bodenschatz, M.; Schlimbach, S.; Vilcinskas, A. Culture-Independent and Culture-Dependent Characterization of the Black Soldier Fly Gut Microbiome Reveals a Large Proportion of Culturable Bacteria with Potential for Industrial Applications. Microorganisms 2021, 9, 1642. [Google Scholar] [CrossRef]
- Brulé, L.; Misery, B.; Baudouin, G.; Yan, X.; Guidou, C.; Trespeuch, C.; Foltyn, C.; Anthoine, V.; Moriceau, N.; Federighi, M.; et al. Evaluation of the Microbial Quality of Hermetia illucens Larvae for Animal Feed and Human Consumption: Study of Different Type of Rearing Substrates. Foods 2024, 13, 1587. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Kooh, P.; Jury, V.; Laurent, S.; Audiat-Perrin, F.; Sanaa, M.; Tesson, V.; Federighi, M.; Boué, G. Control of Biological Hazards in Insect Processing: Application of HACCP Method for Yellow Mealworm (Tenebrio molitor) Powders. Foods 2020, 9, 1528. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Lenaerts, S.; Callens, A.; Van Campenhout, L. Effect of Blanching Followed by Refrigerated Storage or Industrial Microwave Drying on the Microbial Load of Yellow Mealworm Larvae (Tenebrio molitor). Food Control 2017, 71, 311–314. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.W.; Palmer, J.D. Investigating Deep Phylogenetic Relationships among Cyanobacteria and Plastids by Small Subunit rRNA Sequence Analysis1. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Kisand, V.; Cuadros, R.; Wikner, J. Phylogeny of Culturable Estuarine Bacteria Catabolizing Riverine Organic Matter in the Northern Baltic Sea. Appl. Environ. Microbiol. 2002, 68, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Gómez-Rubio, V. Ggplot2—Elegant Graphics for Data Analysis (2nd Edition). J. Stat. Soft. 2017, 77. [Google Scholar] [CrossRef]
- Plotly Technologies Inc. Collaborative Data Science; Plotly Technologies Inc.: Montreal, QC, Canada, 2015. [Google Scholar]
- Gorrens, E.; Smet, J.D.; Vandeweyer, D.; Bossaert, S.; Crauwels, S.; Lievens, B.; Campenhout, L.V. The Bacterial Communities of Black Soldier Fly Larvae (Hermetia illucens) during Consecutive, Industrial Rearing Cycles. J. Insects Food Feed. 2022, 8, 1061–1076. [Google Scholar] [CrossRef]
- Ao, Y.; Yang, C.; Wang, S.; Hu, Q.; Yi, L.; Zhang, J.; Yu, Z.; Cai, M.; Yu, C. Characteristics and Nutrient Function of Intestinal Bacterial Communities in Black Soldier Fly (Hermetia illucens L.) Larvae in Livestock Manure Conversion. Microb. Biotechnol. 2021, 14, 886–896. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.; Mao, Z.; Liang, J.; Liu, X.; Xu, X. Bioconversion of Chicken Meat and Bone Meal by Black Soldier Fly Larvae: Effects of Straw Addition on the Quality and Microbial Profile of Larval Frass. J. Environ. Manag. 2022, 307, 114579. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, K.; Tang, W.; Li, Y.; Pang, J.; Yuan, X.; Song, X.; Jiang, L.; Yu, X.; Zhu, H.; et al. Feed Nutritional Composition Affects the Intestinal Microbiota and Digestive Enzyme Activity of Black Soldier Fly Larvae. Front. Microbiol. 2023, 14, 1184139. [Google Scholar] [CrossRef]
- Bruno, D.; Bonelli, M.; De Filippis, F.; Di Lelio, I.; Tettamanti, G.; Casartelli, M.; Ercolini, D.; Caccia, S. The Intestinal Microbiota of Hermetia illucens Larvae Is Affected by Diet and Shows a Diverse Composition in the Different Midgut Regions. Appl. Env. Microbiol. 2019, 85, e01864-18. [Google Scholar] [CrossRef]
- Auger, L.; Deschamps, M.-H.; Vandenberg, G.; Derome, N. Microbiota Is Structured by Gut Regions, Life Stage, and Diet in the Black Soldier Fly (Hermetia illucens). Front. Microbiol. 2023, 14, 1221728. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, Y.; Glaeser, S.P.; Mvie, J.; Bartz, J.-O.; Müller, A.; Gutzeit, H.O.; Vilcinskas, A.; Kämpfer, P. The Gut and Feed Residue Microbiota Changing during the Rearing of Hermetia illucens Larvae. Antonie Van. Leeuwenhoek 2020, 113, 1323–1344. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Park, S.; Choi, J.; Jeong, G.; Lee, S.-B.; Choi, Y.; Lee, S.-J. The Intestinal Bacterial Community in the Food Waste-Reducing Larvae of Hermetia illucens. Curr. Microbiol. 2011, 62, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-L.; Jin, W.-Z.; Tao, X.-H.; Zhang, Q.; Zhu, J.; Feng, S.-Y.; Xu, X.-H.; Li, H.-Y.; Wang, Z.-H.; Zhang, Z.-J. Black Soldier Fly Larvae (Hermetia illucens) Strengthen the Metabolic Function of Food Waste Biodegradation by Gut Microbiome. Microb. Biotechnol. 2019, 12, 528–543. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The Core Gut Microbiome of Black Soldier Fly (Hermetia illucens) Larvae Raised on Low-Bioburden Diets. Front. Microbiol. 2020, 11, 993. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Insam, H. Impact of Processed Food (Canteen and Oil Wastes) on the Development of Black Soldier Fly (Hermetia illucens) Larvae and Their Gut Microbiome Functions. Front. Microbiol. 2021, 12, 619112. [Google Scholar] [CrossRef]
- Liu, C.; Yao, H.; Chapman, S.J.; Su, J.; Wang, C. Changes in Gut Bacterial Communities and the Incidence of Antibiotic Resistance Genes during Degradation of Antibiotics by Black Soldier Fly Larvae. Environ. Int. 2020, 142, 105834. [Google Scholar] [CrossRef]
- Mašková, Z.; Medo, J.; Kolesár, E.; Tančinová, D.; Ivanišová, E.; Urminská, D.; Hleba, L.; Urminská, J.; Mrvová, M.; Barboráková, Z. Hermetia illucens in the Process of Kitchen Waste Biodegradation: The Effect of Different Approaches to Waste Storage on the Microbiological Profile and Nutritional Parameters of the Larvae. Insects 2025, 16. [Google Scholar] [CrossRef]
- Pei, Y.; Sun, M.; Zhang, J.; Lei, A.; Chen, H.; Kang, X.; Ni, H.; Yang, S. Comparative Metagenomic and Metatranscriptomic Analyses Reveal the Response of Black Soldier Fly (Hermetia illucens) Larvae Intestinal Microbes and Reduction Mechanisms to High Concentrations of Tetracycline. Toxics 2023, 11. [Google Scholar] [CrossRef]
- Querejeta, M.; Hervé, V.; Perdereau, E.; Marchal, L.; Herniou, E.A.; Boyer, S.; Giron, D. Changes in Bacterial Community Structure Across the Different Life Stages of Black Soldier Fly (Hermetia illucens). Microb. Ecol. 2023, 86, 1254–1267. [Google Scholar] [CrossRef]
- Schreven, S.J.J.; de Vries, H.; Hermes, G.D.A.; Smidt, H.; Dicke, M.; Jvan Loon, J.J.A. Relative Contributions of Egg-Associated and Substrate-Associated Microorganisms to Black Soldier Fly Larval Performance and Microbiota. FEMS Microbiol. Ecol. 2021, 97. [Google Scholar] [CrossRef]
- Schreven, S.J.J.; de Vries, H.; Hermes, G.D.A.; Zeni, G.; Smidt, H.; Dicke, M.; van Loon, J.J.A. Black Soldier Fly Larvae Influence Internal and Substrate Bacterial Community Composition Depending on Substrate Type and Larval Density. Appl. Env. Microbiol. 2022, 88, e0008422. [Google Scholar] [CrossRef] [PubMed]
- Tanga, C.M.; Waweru, J.W.; Tola, Y.H.; Onyoni, A.A.; Khamis, F.M.; Ekesi, S.; Paredes, J.C. Organic Waste Substrates Induce Important Shifts in Gut Microbiota of Black Soldier Fly (Hermetia illucens L.): Coexistence of Conserved, Variable, and Potential Pathogenic Microbes. Front. Microbiol. 2021, 12, 635881. [Google Scholar] [CrossRef] [PubMed]
- Van Looveren, N.; IJdema, F.; van der Heijden, N.; Van Der Borght, M.; Vandeweyer, D. Microbial Dynamics and Vertical Transmission of Escherichia Coli across Consecutive Life Stages of the Black Soldier Fly (Hermetia illucens). Anim. Microbiome 2024, 6, 29. [Google Scholar] [CrossRef]
- Yang, F.; Tomberlin, J.K.; Jordan, H.R. Starvation Alters Gut Microbiome in Black Soldier Fly (Diptera: Stratiomyidae) Larvae. Front. Microbiol. 2021, 12, 601253. [Google Scholar] [CrossRef]
- Zheng, L.; Crippen, T.L.; Singh, B.; Tarone, A.M.; Dowd, S.; Yu, Z.; Wood, T.K.; Tomberlin, J.K. A Survey of Bacterial Diversity from Successive Life Stages of Black Soldier Fly (Diptera: Stratiomyidae) by Using 16S rDNA Pyrosequencing. J. Med. Entomol. 2013, 50, 647–658. [Google Scholar] [CrossRef]
- Eke, M.; Tougeron, K.; Hamidovic, A.; Tinkeu, L.S.N.; Hance, T.; Renoz, F. Deciphering the Functional Diversity of the Gut Microbiota of the Black Soldier Fly (Hermetia illucens): Recent Advances and Future Challenges. Anim. Microbiome 2023, 5, 40. [Google Scholar] [CrossRef]
- Bruno, D.; Bonelli, M.; Cadamuro, A.G.; Reguzzoni, M.; Grimaldi, A.; Casartelli, M.; Tettamanti, G. The Digestive System of the Adult Hermetia illucens (Diptera: Stratiomyidae): Morphological Features and Functional Properties. Cell Tissue Res. 2019, 378, 221–238. [Google Scholar] [CrossRef]
- Osimani, A.; Aquilanti, L. Spore-Forming Bacteria in Insect-Based Foods. Curr. Opin. Food Sci. 2021, 37, 112–117. [Google Scholar] [CrossRef]
- Chaisowwong, W.; Kusumoto, A.; Hashimoto, M.; Harada, T.; Maklon, K.; Kawamoto, K. Physiological Characterization of Campylobacter Jejuni under Cold Stresses Conditions: Its Potential for Public Threat. J. Vet. Med. Sci. 2012, 74, 43–50. [Google Scholar] [CrossRef]
- İzgördü, Ö.K.; Darcan, C.; Kariptaş, E. Overview of VBNC, a Survival Strategy for Microorganisms. 3 Biotech. 2022, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.; Bahrndorff, S.; Lowenberger, C. Campylobacter Jejuni in Musca Domestica: An Examination of Survival and Transmission Potential in Light of the Innate Immune Responses of the House Flies. Insect Sci. 2017, 24, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Strother, K.O.; Steelman, C.D.; Gbur, E.E. Reservoir Competence of Lesser Mealworm (Coleoptera: Tenebrionidae) for Campylobacter Jejuni (Campylobacterales: Campylobacteraceae). J. Med. Entomol. 2005, 42, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Bahrndorff, S.; Gill, C.; Lowenberger, C.; Skovgård, H.; Hald, B. The Effects of Temperature and Innate Immunity on Transmission of Campylobacter Jejuni (Campylobacterales: Campylobacteraceae) Between Life Stages of Musca Domestica (Diptera: Muscidae). J. Med. Entomol. 2014, 51, 670–677. [Google Scholar] [CrossRef]
- Jensen, A.N.; Hald, B. Campylobacter Contamination Level in Houseflies after Exposure to Materials Containing Campylobacter. J. Insects Food Feed. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Carrillo, C.D.; Plante, D.; Iugovaz, I.; Kenwell, R.; Bélanger, G.; Boucher, F.; Poulin, N.; Trottier, Y.-L. Method-Dependent Variability in Determination of Prevalence of Campylobacter Jejuni and Campylobacter Coli in Canadian Retail Poultry. J. Food Prot. 2014, 77, 1682–1688. [Google Scholar] [CrossRef]
- Magajna, B.; Schraft, H. Evaluation of Propidium Monoazide and Quantitative PCR To Quantify Viable Campylobacter Jejuni Biofilm and Planktonic Cells in Log Phase and in a Viable but Nonculturable State. J. Food Prot. 2015, 78, 1303–1311. [Google Scholar] [CrossRef]
- Nicholson, W.L. Roles of Bacillus Endospores in the Environment. Cell. Mol. Life Sci. (CMLS) 2002, 59, 410–416. [Google Scholar] [CrossRef]
- Heussler, C.D.; Klammsteiner, T.; Stonig, K.T.; Insam, H.; Schlick-Steiner, B.C.; Steiner, F.M. Microbial Influences on Black Soldier Fly Reproduction: A Focus on Egg Surface Colonization. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Wells, C.; Wilkins, T. Clostridia: Sporeforming Anaerobic Bacilli. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 18. [Google Scholar]
- Zeng, T.; Jaffar, S.; Xu, Y.; Qi, Y. The Intestinal Immune Defense System in Insects. Int. J. Mol. Sci. 2022, 23, 15132. [Google Scholar] [CrossRef]
- Vogel, M.; Shah, P.N.; Voulgari-Kokota, A.; Maistrou, S.; Aartsma, Y.; Beukeboom, L.W.; Salles, J.F.; Van Loon, J.J.A.; Dicke, M.; Wertheim, B. Health of the Black Soldier Fly and House Fly under Mass-Rearing Conditions: Innate Immunity and the Role of the Microbiome. J. Insects Food Feed. 2022, 8, 857–878. [Google Scholar] [CrossRef]
- Van Huis, A.; Rumpold, B.A.; Van Der Fels-Klerx, H.J.; Tomberlin, J.K. Advancing Edible Insects as Food and Feed in a Circular Economy. J. Insects Food Feed. 2021, 7, 935–948. [Google Scholar] [CrossRef]
- Nakagawa, A.; Sakamoto, T.; Kanost, M.R.; Tabunoki, H. Development of New Methods to Stimulate the Production of Antimicrobial Peptides in the Larvae of the Black Soldier Fly Hermetia illucens. Int. J. Mol. Sci. 2023, 24, 15765. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A Bioinformatic Study of Antimicrobial Peptides Identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Van Moll, L.; De Smet, J.; Paas, A.; Tegtmeier, D.; Vilcinskas, A.; Cos, P.; Van Campenhout, L. In Vitro Evaluation of Antimicrobial Peptides from the Black Soldier Fly (Hermetia illucens) against a Selection of Human Pathogens. Microbiol. Spectr. 2022, 10, e01664-21. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA Gene Sequencing for Species and Strain-Level Microbiome Analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Parniakov, O.; Mikhrovska, M.; Wiktor, A.; Alles, M.; Ristic, D.; Bogusz, R.; Nowacka, M.; Devahastin, S.; Mujumdar, A.; Heinz, V.; et al. Insect Processing for Food and Feed: A Review of Drying Methods. Dry. Technol. 2022, 40, 1500–1513. [Google Scholar] [CrossRef]
- Kewuyemi, Y.O.; Kesa, H.; Chinma, C.E.; Adebo, O.A. Fermented Edible Insects for Promoting Food Security in Africa. Insects 2020, 11, 283. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, E.; Ibarra-Herrera, C.C.; Pérez-Carrillo, E. Effect of Incorporation of Solid-State Fermented Edible Insects Tenebrio molitor and Sphenarium Purpurascens with Aspergillus Oryzae in the Elaboration of Bread. LWT 2023, 184, 115003. [Google Scholar] [CrossRef]
- Rubiola, S.; Macori, G.; Civera, T.; Fanning, S.; Mitchell, M.; Chiesa, F. Comparison Between Full-Length 16S rRNA Metabarcoding and Whole Metagenome Sequencing Suggests the Use of Either Is Suitable for Large-Scale Microbiome Studies. Foodborne Pathog. Dis. 2022, 19, 495–504. [Google Scholar] [CrossRef]
- Olivier, S.A.; Bull, M.K.; Strube, M.L.; Murphy, R.; Ross, T.; Bowman, J.P.; Chapman, B. Long-Read MinIONTM Sequencing of 16S and 16S-ITS-23S rRNA Genes Provides Species-Level Resolution of Lactobacillaceae in Mixed Communities. Front. Microbiol. 2023, 14, 1290756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, H.; Ma, S.; Cao, J.; Liao, H.; Huang, Q.; Chen, W. The Newest Oxford Nanopore R10.4.1 Full-Length 16S rRNA Sequencing Enables the Accurate Resolution of Species-Level Microbial Community Profiling. Appl. Environ. Microbiol. 2023, 89, e00605-23. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Komiya, S.; Yasumizu, Y.; Yasuoka, Y.; Mizushima, K.; Takagi, T.; Kryukov, K.; Fukuda, A.; Morimoto, Y.; Naito, Y.; et al. Full-Length 16S rRNA Gene Amplicon Analysis of Human Gut Microbiota Using MinIONTM Nanopore Sequencing Confers Species-Level Resolution. BMC Microbiol. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.; Valido, E.; Stoyanov, J. Optimized Bacterial Community Characterization through Full-Length 16S rRNA Gene Sequencing Utilizing MinION Nanopore Technology. BMC Microbiol. 2024, 24, 58. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics Insights into Food Fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef]
- Butowski, C.F.; Dixit, Y.; Reis, M.M.; Mu, C. Metatranscriptomics for Understanding the Microbiome in Food and Nutrition Science. Metabolites 2025, 15, 185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).