Evaluation of Washing with Sodium Hypochlorite, Ultraviolet Irradiation, and Storage Temperature on Shell Egg Quality During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Samples
2.2. Washing Process
2.3. Microbial Analysis of Eggshell Surface and Egg Internal Contents
- Aa, Ab: colony counts on duplicate plates at dilution level A
- Ba, Bb: colony counts on duplicate plates at dilution level B
- A, B: corresponding dilution factors.
2.4. Measurement of Eggshell Quality
2.5. Ultrastructural Assessment
2.6. Measurement of Egg Internal Quality
- H is albumen height (mm) and w is egg weight (g) [13].
- YI was determined as yolk height/yolk width [33].
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Bacteria Survival on Eggs with Different Treatments During Storage
3.2. Eggshell Quality
3.3. Albumen Quality
3.4. Air Cell Size and Yolk Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seuss-Baum, I.; Nau, F.; Guérin-Dubiard, C. The nutritional quality of eggs. In Improving the Safety and Quality of Eggs and Egg Products: Volume 2: Egg Safety and Nutritional Quality, 1st ed.; Van Immerseel, F., Nys, Y., Bain, M., Eds.; Woodhead Publishing Limite: Cambridge, UK, 2011; Volume 11, pp. 201–209. [Google Scholar]
- Samiullah, S.; Roberts, J.R. The eggshell cuticle of the laying hen. World’s Poult. Sci. J. 2014, 70, 693–708. [Google Scholar] [CrossRef]
- Ratliff, J.; Mutungi, G.; Puglisi, M.J.; Volek, J.S.; Fernandez, M.L. Carbohydrate restriction (with or without additional dietary cholesterol provided by eggs) reduces insulin resistance and plasma leptin without modifying appetite hormones in adult men. Nutr. Res. 2009, 29, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Steffen, L.M.; Loehr, L.R.; Rosamond, W.D.; Folsom, A.R. Incident heart failure is associated with lower whole-grain intake and greater high-fat dairy and egg intake in the Atherosclerosis Risk in Communities (ARIC) study. J. Am. Diet. Assoc. 2008, 108, 1881–1887. [Google Scholar] [CrossRef]
- Barraj, L.; Tran, N.; Mink, P. A comparison of egg consumption with other modifiable coronary heart disease lifestyle risk factors: A relative risk apportionment study. Risk Anal. 2009, 29, 401–415. [Google Scholar] [CrossRef]
- Nys, Y.; Guyot, N. Egg formation and chemistry. In Improving the Safety and Quality of Eggs and Egg Products: Volume 1: Egg Chemistry, Production and Consumption, 1st ed.; Nys, Y., Bain, M., Immerseel, F.V., Eds.; Woodhead Publishing Limite: Cambridge, UK, 2011; Volume 6, pp. 83–126. [Google Scholar]
- Kim, M.; Park, S.Y.; Ha, S.D. Synergistic effect of a combination of ultraviolet–C irradiation and sodium hypochlorite to reduce Listeria monocytogenes biofilms on stainless steel and eggshell surfaces. Food Control 2016, 70, 103–109. [Google Scholar] [CrossRef]
- Turtoi, M.; Borda, D. Decontamination of egg shells using ultraviolet light treatment. World’s Poult. Sci. J. 2014, 70, 265–278. [Google Scholar] [CrossRef]
- Gole, V.C.; Chousalkar, K.K.; Roberts, J.R.; Sexton, M.; May, D.; Tan, J.; Kiermeier, A. Effect of egg washing and correlation between eggshell characteristics and egg penetration by various Salmonella Typhimurium strains. PLoS ONE 2014, 9, e90987. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Gittins, J.; Walker, A.; Moore, A.; Burton, C.; Sparks, N. Washing table eggs: A review of the scientific and engineering issues. World’s Poult. Sci. J. 2003, 59, 233–248. [Google Scholar] [CrossRef]
- Leleu, S.; Messens, W.; De Reu, K.; De Preter, S.; Herman, L.; Heyndrickx, M.; De Baerdemaeker, J.; Michiels, C.W.; Bain, M. Effect of egg washing on the cuticle quality of brown and white table eggs. J. Food Prot. 2011, 74, 1649–1654. [Google Scholar] [CrossRef]
- Samiullah, S.; Chousalkar, K.K.; Roberts, J.R.; Sexton, M.; May, D.; Kiermeier, A. Effects of egg shell quality and washing on Salmonella Infantis penetration. Int. J. Food Microbiol. 2013, 165, 77–83. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, T.H.; Wu, Y.C.; Lee, Y.C.; Tan, F.J. Effects of egg washing and storage temperature on the quality of eggshell cuticle and eggs. Food Chem. 2016, 211, 687–693. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare, Taiwan. Sanitation Standard for Food Cleansers. 2024. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0040070 (accessed on 6 June 2025).
- Lopez-Malo, A.; Palou, E. Ultraviolet light and food preservation. In Novel Food Processing Technologies; Barbosa-Cánovas, G.V., Tapia, M.S., Cano, M.P., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 405–422. [Google Scholar]
- Keklik, N.M.; Demirci, A.; Patterson, P.H.; Puri, V.M. Pulsed UV light inactivation of Salmonella Enteritidis on eggshells and its effects on egg quality. J. Food Prot. 2010, 73, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture (MOA), Taiwan. Egg Industry Management and Improvement Policy. 2018. Available online: https://www.moa.gov.tw/redirect_files.php?id=25549 (accessed on 19 May 2025).
- Ministry of Agriculture (MOA), Taiwan. Traceable Chicken Eggs Ensure Sanitation and Safety. 2021. Available online: https://eng.moa.gov.tw/ws.php?id=2505640 (accessed on 2 May 2025).
- Poultry Association, Republic of China. Taiwan Poultry Production Statistics. 2023. Available online: https://www.poultry.org.tw/page/16 (accessed on 2 May 2025).
- Hannah, J.F.; Wilson, J.L.; Cox, N.A.; Cason, J.A.; Bourassa, D.V.; Musgrove, M.T.; Buhr, R.J. Comparison of shell bacteria from unwashed and washed table eggs harvested from caged laying hens and cage-free floor-housed laying hens. Poult. Sci. 2011, 90, 1586–1593. [Google Scholar] [CrossRef]
- Ministry of Agriculture (MOA), Taiwan. Egg Production and Marketing Information. 2025. Available online: https://www.moa.gov.tw/ws.php?id=2513467 (accessed on 28 May 2025).
- European Commission (EC). Commission Delegated Regulation (EU) 2023/2465 of 17 August 2023 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Eggs, and Repealing Commission Regulation (EC) No 589/2008. Off. J. Eur. Union 2023. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32023R2465 (accessed on 19 May 2025).
- Chousalkar, K.K.; Khan, S.; McWhorter, A.R. Microbial quality, safety and storage of eggs. Curr. Opin. Food Sci. 2021, 38, 91–95. [Google Scholar] [CrossRef]
- Jones, D.R.; Ward, G.E.; Regmi, P.; Karcher, D.M. Impact of egg handling and conditions during extended storage on egg quality. Poult. Sci. 2018, 97, 716–723. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Hwang, Y.; Hwang, S.; Kwon, H.; Gu, H.; Park, K.; Choi, C. Comparative Evaluation of Egg Quality in Response to Temperature Variability: From Farm to Table Exposure Scenarios. Food Sci. Anim. Resour. 2023, 43, 1002–1016. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA), Taiwan. Methods of Test for Food Microorganisms Test of Standard Plate Count (Aerobic Plate Count). 2023. Available online: https://www.fda.gov.tw/tc/includes/GetFile.ashx?mid=189&id=28686&t=s (accessed on 8 June 2025).
- Musgrove, M.T.; Jones, D.R.; Northcutt, J.K.; Cox, N.A.; Harrison, M.A. Shell rinse and shell crush methods for the recovery of aerobic microorganisms and Enterobacteriaceae from shell eggs. J. Food Prot. 2005, 68, 2144–2148. [Google Scholar] [CrossRef]
- Samli, H.E.; Agma, A.; Senkoylu, N. Effects of storage time and temperature on egg quality in old laying hens. J. Appl. Poult. Res. 2005, 14, 548–553. [Google Scholar] [CrossRef]
- Van Den Brand, H.; Parmentier, H.K.; Kemp, B. Effects of housing system (outdoor vs. cages) and age of laying hens on egg characteristics. Br. Poult. Sci. 2004, 45, 745–752. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.B.; Domínguez-Gasca, N.; Muñoz, A.; Ortega-Huertas, M. Change in the chicken eggshell cuticle with hen age and egg freshness. Poult. Sci. 2013, 92, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Mahato, P.L.; Weatherby, T.; Ewell, K.; Jha, R.; Mishra, B. Scanning electron microscope-based evaluation of eggshell quality. Poult. Sci. 2024, 103, 103428. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Qiu, N.; Ma, M.H.; Jin, Y.G.; Yang, H.; Geng, F.; Sun, S.H. Estimation of egg freshness using S-ovalbumin as an indicator. Poult. Sci. 2012, 91, 739–743. [Google Scholar] [CrossRef]
- Ragni, L.; Al-Shami, A.; Mikhaylenko, G.; Tang, J. Dielectric characterization of hen eggs during storage. J. Food Eng. 2007, 82, 450–459. [Google Scholar] [CrossRef]
- Caner, C.; Yüceer, M. Efficacy of various protein-based coatings on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015, 94, 1665–1677. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the AOAC International, 11th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Wells, J.B.; Coufal, C.D.; Parker, H.M.; McDaniel, C.D. Disinfection of eggshells using ultraviolet light and hydrogen peroxide independently and in combination. Poult. Sci. 2010, 89, 2499. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V. Pulsed light processing of foods: An overview. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar]
- Jones, D.R.; Musgrove, M.T.; Northcutt, J.K. Variations in external and internal microbial populations in shell eggs during extended storage. J. Food Prot. 2004, 67, 2657–2660. [Google Scholar] [CrossRef]
- Tilki, M.; Saatci, M. Effects of storage time on external and internal characteristics in partridge (Alectoris graeca) eggs. Rev. Méd. Vét. 2004, 155, 561–564. [Google Scholar]
- Pan, D.; Li, R.; Li, Y.; Gao, X.; Fan, X.; Du, Q.; Zhou, C. Effects of manual washing with three alkaline sterilizing agent solutions on egg quality during storage. Food Chem. 2022, 396, 133733. [Google Scholar] [CrossRef] [PubMed]
- Ketta, M.; Tůmová, E. Eggshell structure, measurements, and quality-affecting factors in laying hens: A review. Czech J. Anim. Sci. 2016, 61, 299–309. [Google Scholar] [CrossRef]
- Favier, G.I.; Escudero, M.E.; Velázquez, L.; De Guzmán, A.M.S. Reduction of Yersinia enterocolitica and mesophilic aerobic bacteria in egg-shell by washing with surfactants and their effect on the shell microstructure. Food Microbiol. 2000, 17, 73–81. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Rodriguez-Navarro, A.; Sanchez-Rodriguez, E.; Diep, T.; Hincke, M.T. Cuticle and pore plug properties in the table egg. Poult. Sci. 2018, 97, 1382–1390. [Google Scholar] [CrossRef]
- USDA (U.S. Department of Agriculture). USDA Egg Grading Manual. 2000. Available online: https://www.ams.usda.gov/publications/content/egg-grading-manual (accessed on 16 June 2025).
- Shin, D.; Narciso-Gaytán, C.; Regenstein, J.M.; Sánchez-Plata, M.X. Effect of various refrigeration temperatures on quality of shell eggs. J. Sci. Food Agric. 2012, 92, 1341–1345. [Google Scholar] [CrossRef]

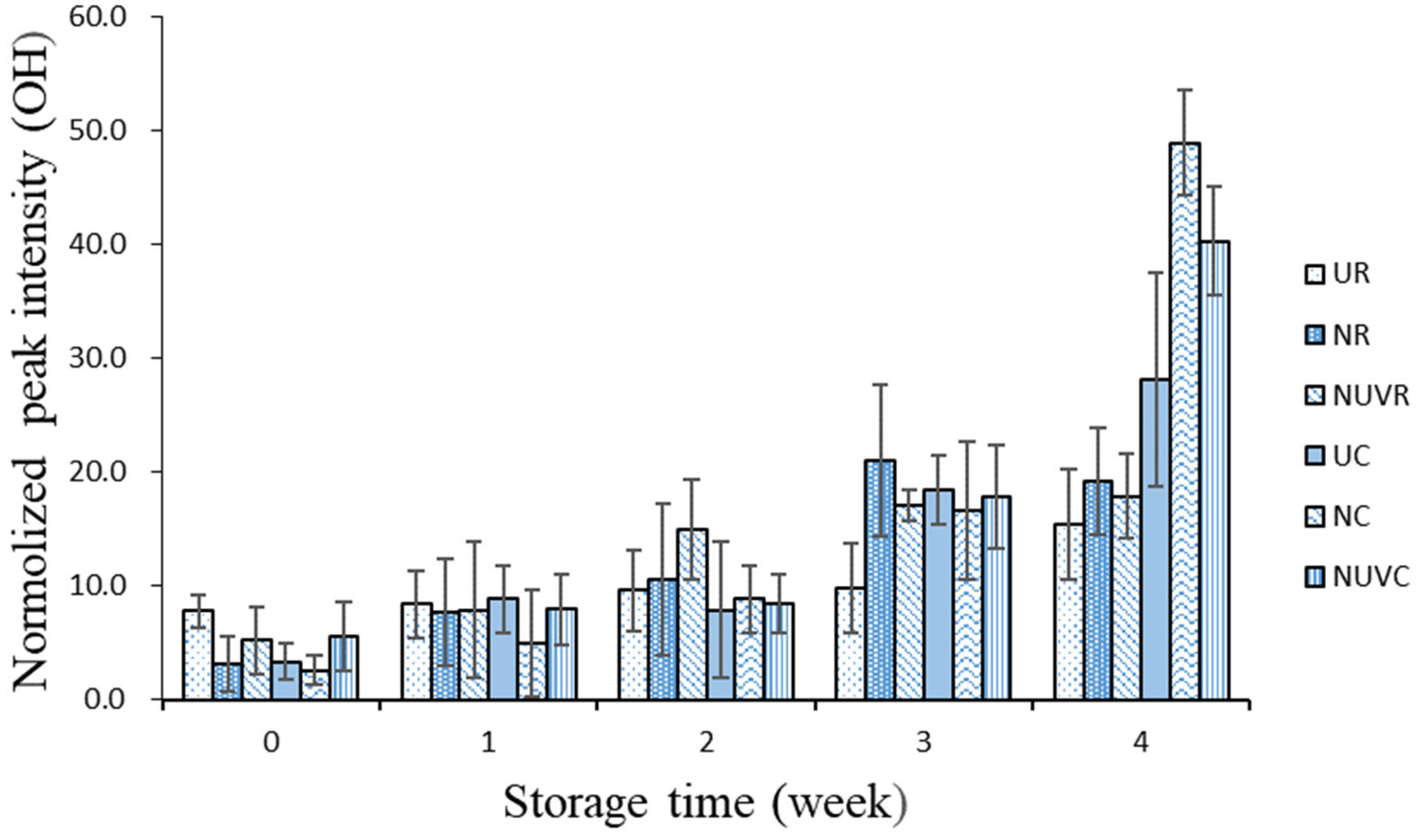




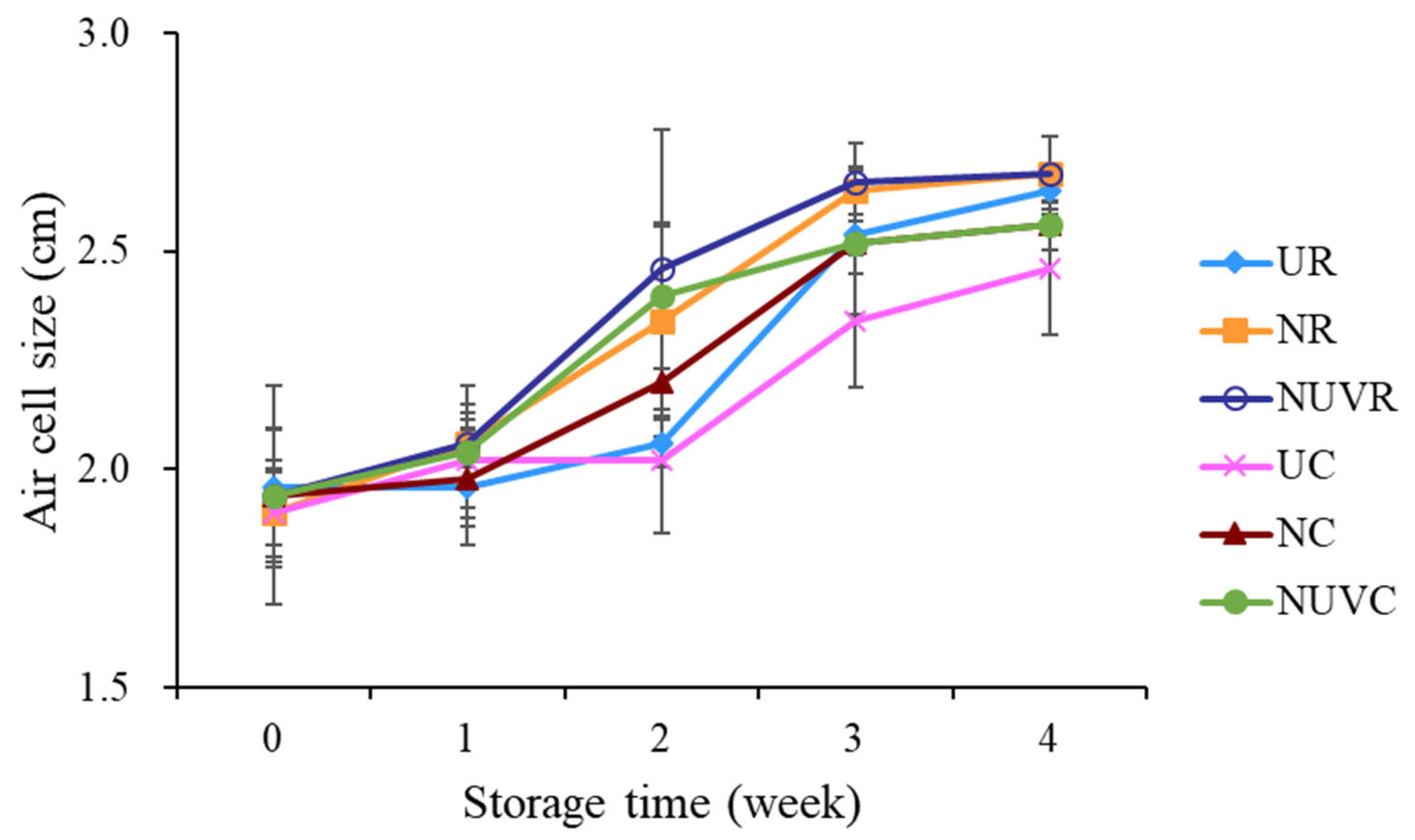


| Treatment * | Eggshell ** (log CFU/mL) | Egg Content ** (log CFU/g) | ||||
|---|---|---|---|---|---|---|
| Week 0 | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | |
| Unwashed, 25 °C (UR) | 4.48 ± 0.24 a | <1 | <1 | <1 | 0 | 0 |
| NaOCl-washed, 25 °C (NR) | 3.25 ± 0.26 b | <1 | 0 | 0 | <1 | <1 |
| NaOCl + UV, 25 °C (NUVR) | 3.00 ± 0.22 c | <1 | 0 | 0 | 0 | <1 |
| Unwashed, 7 °C (UC) | - | <1 | <1 | 0 | 0 | <1 |
| NaOCl-washed, 7 °C (NC) | - | <1 | 0 | <1 | 0 | 0 |
| NaOCl + UV, 7 °C (NUVC) | - | <1 | <1 | <1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-C.; Chen, I.-C.; Tan, F.-J. Evaluation of Washing with Sodium Hypochlorite, Ultraviolet Irradiation, and Storage Temperature on Shell Egg Quality During Storage. Foods 2025, 14, 2156. https://doi.org/10.3390/foods14132156
Yu H-C, Chen I-C, Tan F-J. Evaluation of Washing with Sodium Hypochlorite, Ultraviolet Irradiation, and Storage Temperature on Shell Egg Quality During Storage. Foods. 2025; 14(13):2156. https://doi.org/10.3390/foods14132156
Chicago/Turabian StyleYu, Hui-Chuan, I-Chi Chen, and Fa-Jui Tan. 2025. "Evaluation of Washing with Sodium Hypochlorite, Ultraviolet Irradiation, and Storage Temperature on Shell Egg Quality During Storage" Foods 14, no. 13: 2156. https://doi.org/10.3390/foods14132156
APA StyleYu, H.-C., Chen, I.-C., & Tan, F.-J. (2025). Evaluation of Washing with Sodium Hypochlorite, Ultraviolet Irradiation, and Storage Temperature on Shell Egg Quality During Storage. Foods, 14(13), 2156. https://doi.org/10.3390/foods14132156





