Edible Safety Evaluation of Cinnamomum camphora Seed Kernel Oil: Sub-Chronic Toxicity and Teratogenicity Assessments
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Instruments and Equipment
2.3. Test Substance
2.4. Sub-Chronic Toxicity Evaluation of CCSKO
2.4.1. Animals and Housing Environment
2.4.2. Study Design
2.4.3. Animal Feeding
2.4.4. General Clinical Observation of SD Rats
2.4.5. Hematology, Clinical Biochemistry and Urinalysis of SD Rats
2.4.6. Gross Necropsy, Organ Weight and Histopathological Observation of SD Rats
2.5. Teratogenicity Evaluation of CCSKO
2.5.1. Animals and Housing Environment
2.5.2. Study Design
2.5.3. Animal Feeding
2.5.4. General Clinical Observation of Pregnant Rats
2.5.5. Examination of Fetal Rats
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Chronic Toxicity of CCSKO in SD Rats
3.1.1. General Clinical Observations of SD Rats Fed with CCSKO
3.1.2. BW, Food Consumption and Efficiency of SD Rats Fed with CCSKO
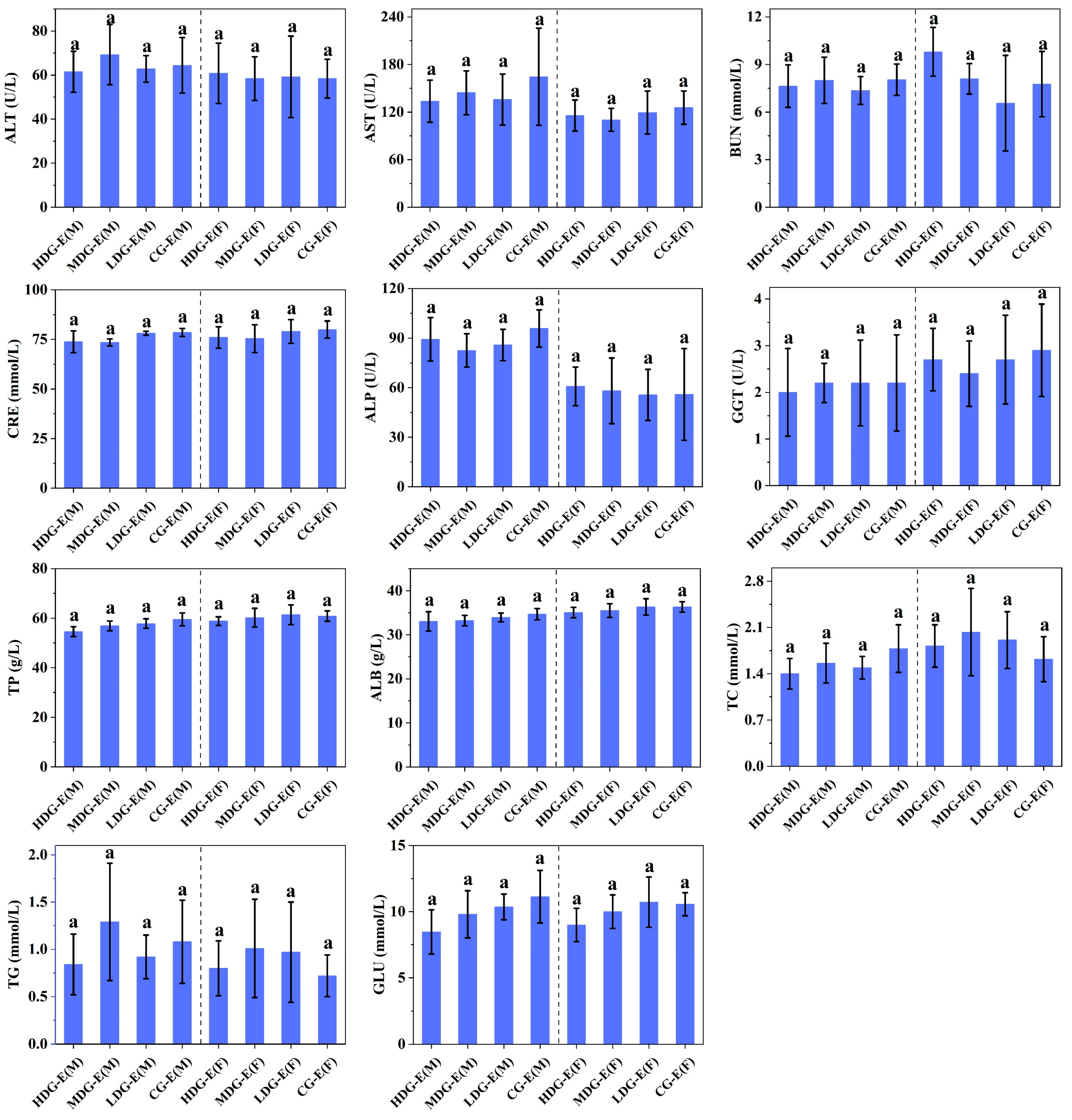
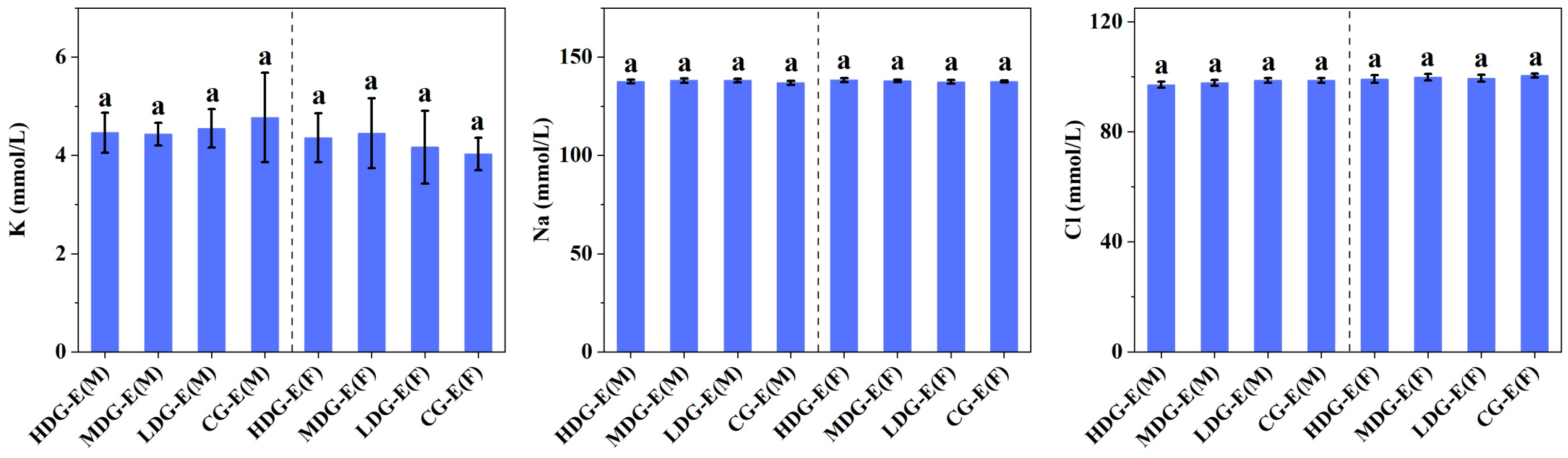
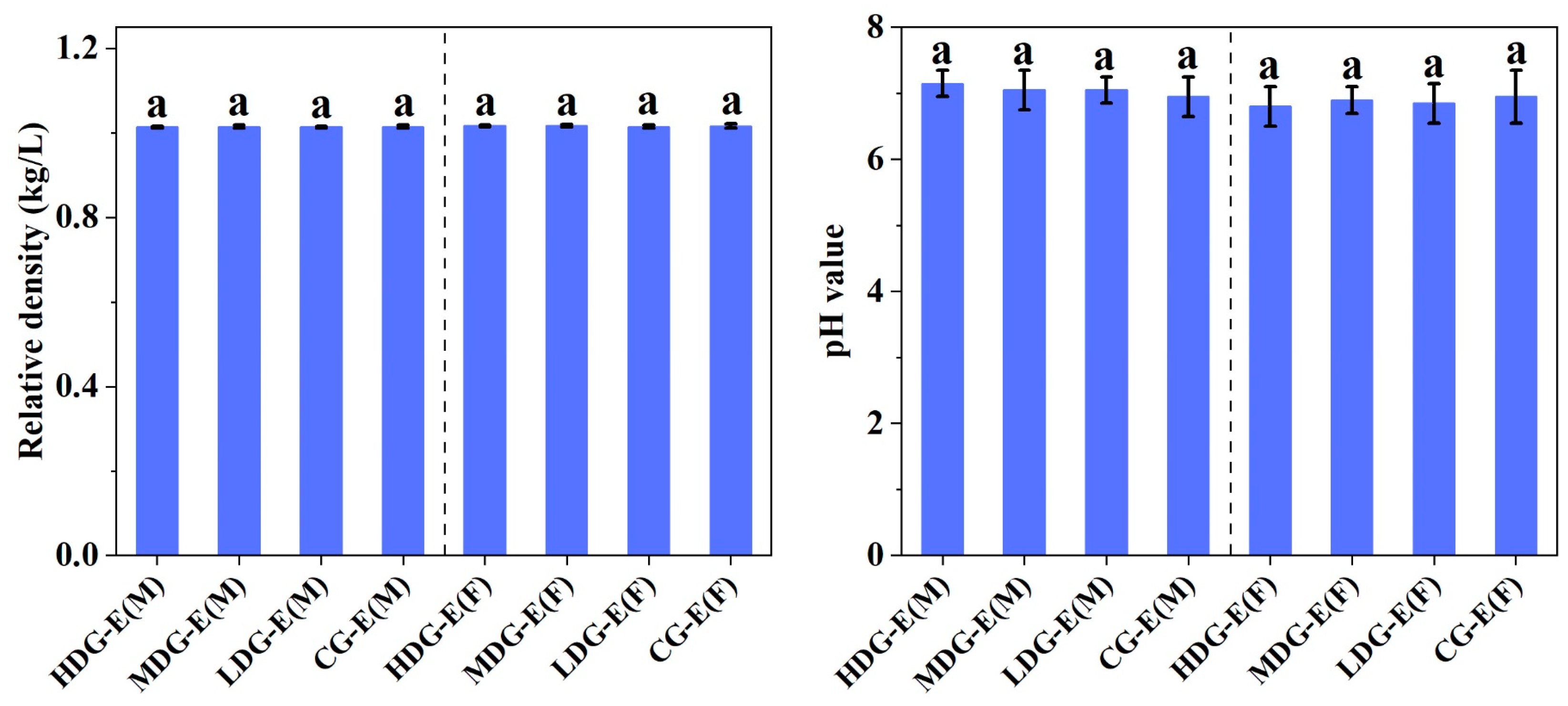
3.1.3. Hematological Properties, Biochemistry, Electrolyte and Urine Parameters of SD Rats Fed with CCSKO
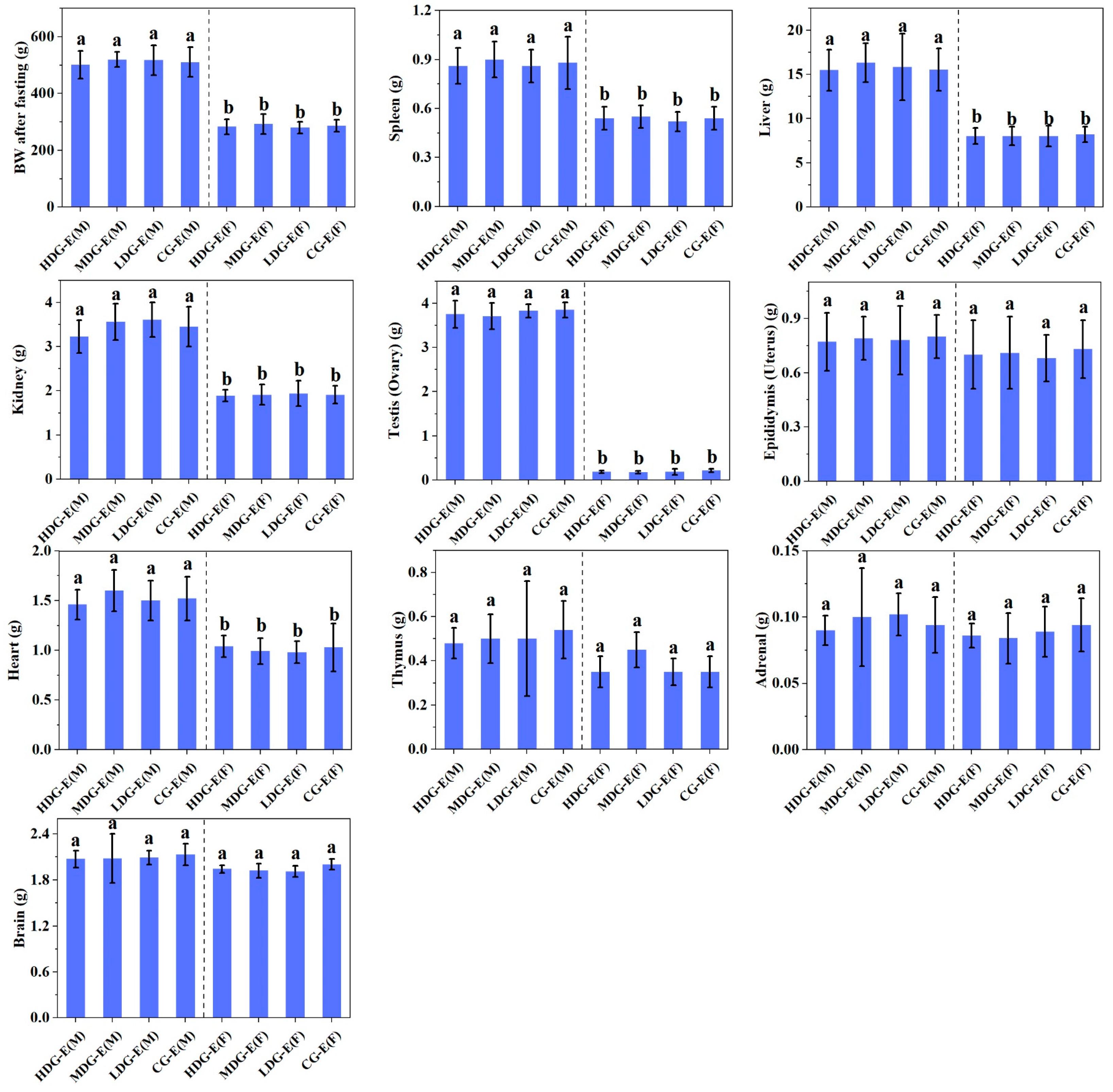
3.1.4. Macroscopic Examinations, Organ Weights and Histopathological Observations of SD Rats Fed with CCSKO
- (A)
- Brain: The neurons in the cerebral cortex were arranged normally in layers, with no hemorrhage and edema, no atrophy in the cerebral cortex, and no degeneration or necrosis of neurons. The molecular layer of the cerebellar cortex, the Purkinje cell layer, and the granular layers were distributed normally, and there were no glial cells in the medulla hyperplasia. The basilar arteries in the hypothalamic–pontine section were normally distributed, the normal distribution of the transverse pontine bundle was observed below the basilar artery, with the normal distribution of the longitudinal pontine bundle and the medial lemniscus on the left and right sides.
- (B)
- Lung: The bronchial wall and alveolar septum at all levels of the lung tissue were intact, and there was no pulmonary edema or hemorrhage, no necrosis of lung tissue cells, no inflammatory cell infiltration and no interstitial fibrosis.
- (C)
- Liver: The hepatic lobules were intact; the hepatocytes were neatly arranged and the bile canaliculi and capillary bile ducts were normal.
- (D)
- Spleen: The structure of the white pulp and red pulp of the spleen parenchyma was clear, and there was no abnormal proliferation or decrease in lymphocytes.
- (E)
- Stomach: The gastric mucosa was intact, and there was no mucosal epithelial necrosis, shedding or hemorrhage.
- (F)
- Intestine: The mucosal surface of the intestine was flat, with no villi, isolated lymph nodes in the lamina propria, or intestine hemorrhage, necrosis, ulceration and inflammatory cell infiltration.
- (G)
- Testis and ovary: All levels of spermatogenic cells and mature sperm were observed in the testis, and there was no abnormality in the interstitium. The ovaries were well developed, with follicles and mature corpus luteum at all levels, with no ovarian cysts, no ovarian hemorrhage and no interstitial abnormalities.
- (H)
- Epididymis and uterus: Many efferent tubules and epididymal ducts were observed in the epididymis; a large number of sperm cells and spermatocytes were seen in the official cavity, and there was no abnormality in the interstitium. The endometrium was normal, with no hyperplasia and inflammatory cell infiltration, no bleeding in the myometrium, and no abnormality in the outer membrane.
- (I)
- Heart: There was no degeneration or necrosis of myocardial fibers, no inflammatory cell infiltration in the endocardium and epicardium, no hypertrophy and atrophy of myocardial cells and no interstitial vascular hemorrhage.
- (J)
- Kidneys: The structure of the renal cortex and medulla was clear, and the distribution of nephrons was uniform. No abnormal pathological changes were observed in the epithelial cells of the glomerular capillary bundles, renal vesicles and renal tubules. No vascular dilation or inflammatory exudation was observed in renal interstitium, with no transitional epithelium of renal pelvis mucosa, no squamous metaplasia and no inflammatory cell infiltration.
3.2. The Teratogenicity Evaluation of CCSKO in Wistar Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. BBA-Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, P.; Matern, D.; Bennett, M.J. Fatty acid oxidation disorders. Annu. Rev. Physiol. 2002, 64, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Daull, P.; Paterson, C.A.; Kuppermann, B.D.; Garrigue, J.S. A preliminary evaluation of dexamethasone palmitate emulsion: A novel intravitreal sustained delivery of corticosteroid for treatment of macular edema. J. Ocul. Pharmacol. Th. 2013, 29, 258–269. [Google Scholar] [CrossRef]
- Hayasaka, K.; Numakura, C.; Yamakawa, M.; Mitsui, T.; Watanabe, H.; Haga, H.; Yazaki, M.; Ohira, H.; Ochiai, Y.; Tahara, T.; et al. Medium-chain triglycerides supplement therapy with a low-carbohydrate formula can supply energy and enhance ammonia detoxification in the hepatocytes of patients with adult–onset type II citrullinemia. J. Inherit. Metab. Dis. 2018, 41, 777–784. [Google Scholar] [CrossRef]
- Soler, V.J.; Laurent, C.; Sakr, F.; Regnier, A.; Tricoire, C.; Cases, O.; Kozyraki, R.; Douet, J.Y.; Pagot-Mathis, V. Preliminary study of the safety and efficacy of medium-chain triglycerides for use as an intraocular tamponading agent in minipigs. Graef. Arch. Clin. Exp. 2017, 255, 1593–1604. [Google Scholar] [CrossRef]
- Vignes, S.; Bellanger, J. Primary intestinal lymphangiectasia (Waldmann’s disease). Orphanet, J. Rare Dis. 2008, 3, 5. [Google Scholar] [CrossRef]
- Du, Y.; Chen, S.; Zhu, H.; Niu, X.; Li, J.; Fan, Y.; Deng, Z. Consumption of interesterified medium- and long-chain triacylglycerols improves lipid metabolism and reduces inflammation in high-fat diet-induced obese rats. J. Agric. Food Chem. 2020, 68, 8255–8262. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Tang, T.K.; Chan, E.S.; Phuah, E.T.; Lai, O.M.; Tan, C.P.; Wang, Y.; Ab Karim, N.A.; Mat Dian, N.H.; Tan, J.S. Medium chain triglyceride and medium-and long chain triglyceride: Metabolism, production, health impacts and its applications—a review. Crit. Rev. Food Sci. 2022, 62, 4169–4185. [Google Scholar] [CrossRef]
- Nimbkar, S.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Medium chain triglycerides (MCT): State-of-the-art on chemistry, synthesis, health benefits and applications in food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 843–867. [Google Scholar] [CrossRef]
- Roopashree, P.G.; Shetty, S.S.; Suchetha Kumari, N. Effect of medium chain fatty acid in human health and disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Thevenet, J.; De Marchi, U.; Domingo, J.S.; Christinat, N.; Bultot, L.; Lefebvre, G.; Sakamoto, K.; Descombes, P.; Masoodi, M.; Wiederkehr, A. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J. 2016, 30, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yu, P.; Zeng, Z.; Ma, M.; Yan, X.; Zhao, J.; Gong, D.; Zhang, G.; Wang, J. Effects of medium chain triglycerides on lipid metabolism in high-fat diet induced obese rats. Food Funct. 2022, 13, 8998–9009. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gao, L.; Chen, C. Role of medium-chain fatty acids in healthy metabolism: A clinical perspective. Trends Endocrin. Met. 2021, 32, 351–366. [Google Scholar] [CrossRef]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef]
- Chang, P.; Augustin, K.; Boddum, K.; Williams, S.; Sun, M.; Terschak, J.A.; Hardege, J.R.D.; Chen, P.E.; Walker, M.C.; Williams, R.S.B. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016, 139, 431–443. [Google Scholar] [CrossRef]
- Giannos, P.; Prokopidis, K.; Lidoriki, I.; Triantafyllidis, K.K.; Kechagias, K.S.; Celoch, K.; Candow, D.G.; Ostojic, S.M.; Forbes, S.C. Medium-chain triglycerides may improve memory in non-demented older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2022, 22, 817. [Google Scholar] [CrossRef]
- Warren, E.C.; Walker, M.C.; Williams, R.S.B. All you need is fats—For seizure control: Using amoeba to advance epilepsy research. Front. Cell Neurosci. 2018, 12, 199. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, L.; Zhu, X.; Hu, J.; Li, H.; Luo, L.; Lei, L.; Deng, Z. Enzymatic production of zero-trans plastic fat rich in ɑ-linolenic acid and medium-chain fatty acids from highly hydrogenated soybean oil, Cinnamomum camphora seed oil, and Perilla oil by Lipozyme TL IM. J. Agric. Food Chem. 2013, 61, 1189–1195. [Google Scholar] [CrossRef]
- Zou, X.; Hu, J.; Zhao, M.; Zhu, X.; Li, H.; Liu, X.; Liu, R.; Deng, Z. Lipozyme RM IM-catalyzed acidolysis of Cinnamomum camphora seed oil with oleic acid to produce human milk fat substitutes enriched in medium-chain fatty acids. J. Agric. Food Chem. 2014, 62, 10594–10603. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Amin, I. Virgin coconut oil: Emerging functional food oil. Trends Food Sci. Technol. 2009, 20, 481–487. [Google Scholar] [CrossRef]
- Tan, C.P.; Nehdi, I.A. 13—The physicochemical properties of palm oil and its components. In Palm Oil; Lai, O.M., Tan, C.P., Akoh, C.C., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 377–391. [Google Scholar]
- Yan, X.; Gong, X.; Zeng, Z.; Xia, J.; Ma, M.; Zhao, J.; Zhang, G.; Wang, P.; Wan, D.; Yu, P.; et al. Geographic pattern of variations in chemical composition and nutritional value of Cinnamomum camphora seed kernels from China. Foods 2023, 12, 2630. [Google Scholar] [CrossRef]
- Wang, P.; Wan, D.; Peng, T.; Yang, Y.; Wen, X.; Yan, X.; Xia, J.; Zhu, Q.; Yu, P.; Gong, D.; et al. Acute oral toxicity and genotoxicity test and evaluation of Cinnamomum camphora seed kernel oil. Foods 2023, 12, 293. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, C.; Zeng, Z.; Wang, B.; Gong, D. Cinnamomum camphora seed kernel oil ameliorates oxidative stress and inflammation in diet-induced obese rats. J. Food Sci. 2016, 81, H1295–H1300. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, B.; Gong, D.; Zeng, C.; Jiang, Y.; Zeng, Z. Camphor tree seed kernel oil reduces body fat deposition and improves blood lipids in rats. J. Food Sci. 2015, 80, H1912–H1917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jiang, C.; Jin, J.; Jin, Q.; Akoh, C.C.; Wei, W.; Wang, X. Medium- and long-chain triacylglycerol: Preparation, health benefits, and food utilization. Annu. Rev. Food Sci. T. 2024, 15, 381–408. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission of the People’s Republic of China. National Food Safety Standard-90 Days Oral Toxicity Test (GB 15193.13-2015). Available online: http://down.foodmate.net/standard/yulan.php?itemid=46911 (accessed on 7 October 2015).
- National Health and Family Planning Commission of the People’s Republic of China. National Food Safety Standard-Teratogenicity Test (GB 15193.14-2015). Available online: http://down.foodmate.net/standard/yulan.php?itemid=46912 (accessed on 7 October 2015).
- Le Bars, G.; Dion, S.; Gauthier, B.; Mhedhbi, S.; Pohlmeyer-Esch, G.; Comby, P.; Vivan, N.; Ruty, B. Oral toxicity of Miglyol 812(®) in the Göttingen(®) minipig. Regul. Toxicol. Pharm. 2015, 73, 930–937. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Guan, J.; Xie, Q.; Zhao, Y. The anti-tussive, anti-inflammatory effects and sub-chronic toxicological evaluation of Perilla seed oil. J. Sci. Food Agr. 2021, 101, 1419–1427. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Tech. 2023, 60, 2143–2152. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Phuah, E.T.; Karim, N.a.A.; Alitheen, N.B.M.; Tan, C.P.; Razak, I.S.A.; Lai, O.M. Structural difference of palm based medium- and long-chain triacylglycerol (MLCT) further reduces body fat accumulation in DIO C57BL/6J mice when consumed in low fat diet for a mid-term period. Food Res. Int. 2018, 103, 200–207. [Google Scholar] [CrossRef]
- Lewis, K.D.; Huang, W.; Zheng, X.; Jiang, Y.; Feldman, R.S.; Falk, M.C. Toxicological evaluation of arachidonic acid (ARA)-rich oil and docosahexaenoic acid (DHA)-rich oil. Food Chem. Toxicol. 2016, 96, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Matulka, R.A.; Larry Thompson, D.V.M.; Burdock, G.A. Lack of toxicity by medium chain triglycerides (MCT) in canines during a 90-day feeding study. Food Chem. Toxicol. 2009, 47, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Traul, K.A.; Driedger, A.; Ingle, D.L.; Nakhasi, D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Terada, S.; Sekine, S.; Aoyama, T. Dietary intake of medium- and long-chain triacylglycerols prevents the progression of hyperglycemia in diabetic ob/ob mice. J. Oleo Sci. 2015, 64, 683–688. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Katapodis, P.; Sharma, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprospecting of thraustochytrids for omega-3 fatty acids: A sustainable approach to reduce dependency on animal sources. Trends Food Sci. Technol. 2021, 115, 433–444. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Luo, X.; Zhao, M.; Liu, T.; Feng, F. Synthesis and characterization of medium- and long-chain structural lipid rich in α-linolenic acid and lauric acid. Food Biosci. 2023, 52, 102363. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, X.; Ma, X.; Xiong, H.; Zeng, Z.; Peng, H.; Hu, J. Enzymatic production of trans-free shortening from coix seed oil, fully hydrogenated palm oil and Cinnamomum camphora seed oil. Food Biosci. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Z.; Mao, J.; Xu, Y.; Zhu, X.; Xiong, H. Synthesis of cocoa butter substitutes from Cinnamomum camphora seed oil and fully hydrogenated palm oil by enzymatic interesterification. J. Food Sci. Tech. 2019, 56, 835–845. [Google Scholar] [CrossRef]
- Kupongsak, S.; Sathitvorapojjana, S. Properties and storage stability of O/W emulsion replaced with medium-chain fatty acid oil. Pol. J. Food Nutr. Sci. 2017, 67, 107–115. [Google Scholar] [CrossRef]
- Zhu, M.; Li, X. Meta-analysis of structured triglyceride versus other lipid emulsions for parenteral nutrition. Nutrition 2013, 29, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Matulka, R.A.; Noguchi, O.; Nosaka, N. Safety evaluation of a medium- and long-chain triacylglycerol oil produced from medium-chain triacylglycerols and edible vegetable oil. Food Chem. Toxicol. 2006, 44, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Y.; Jiang, Y.; Yu, L. Safety assessment of medium- and long-chain triacylglycerols containing 30% (w/w) medium-chain fatty acids in mice and rats. Regul. Toxicol. Pharm. 2017, 86, 42–48. [Google Scholar] [CrossRef] [PubMed]

| Groups | Number of Pregnant Rats | BW (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 9 | Day 12 | Day 15 | Day 20 | BW Gain | Net BW Gain | ||
| CG-T | 16 | 282.4 ± 19.1 | 317.0 ± 18.2 | 326.1 ± 19.4 | 347.1 ± 19.9 | 367.2 ± 19.1 | 433.5 ± 32.2 | 116.5 ± 26.3 | 35.5 ± 15.8 |
| LDG-T | 16 | 282.4 ± 11.4 | 318.9 ± 13.0 | 329.4 ± 15.7 | 349.3 ± 14.5 | 368.9 ± 16.8 | 444.5 ± 26.5 | 125.5 ± 22.4 | 38.9 ± 22.0 |
| MDG-T | 17 | 281.6 ± 11.8 | 318.8 ± 15.4 | 329.4 ± 17.5 | 344.9 ± 18.8 | 369.7 ± 24.1 | 443.6 ± 29.4 | 124.8 ± 22.5 | 32.0 ± 16.3 |
| HDG-T | 16 | 281.5 ± 13.7 | 317.9 ± 14.1 | 328.9 ± 15.5 | 346.9 ± 15.5 | 367.5 ± 20.0 | 439.6 ± 28.2 | 121.7 ± 18.2 | 39.6 ± 12.2 |
| Groups | Number of Corpora Luteum | Number of Implantations | Number of Live Fetuses | Sex Ratio (Female/ Male) | Number of Stillbirths | Number of Absorbed Fetuses | Stillbirth Rate (%) | Absorbed Fetal Rate (%) |
|---|---|---|---|---|---|---|---|---|
| CG-T | 301 | 219 | 212 | 106/106 | 0 | 7 | 0.00 | 3.20 |
| LDG-T | 314 | 244 | 232 | 100/132 | 0 | 12 | 0.00 | 4.92 |
| MDG-T | 339 | 257 | 252 | 120/132 | 0 | 5 | 0.00 | 1.95 |
| HDG-T | 306 | 230 | 220 | 103/117 | 0 | 10 | 0.00 | 4.35 |
| Groups | Number of Fetal Rats | Weight of the Uterus with the Fetus (g) | BW (g) | Body Length (mm) | Appearance Deformity | Number/Rate of Appearance Abnormalities (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Encephalocele | Edema | No toe | Total | Deformity Rate (%) | ||||||
| CG-T | 212 | 81.0 ± 22.8 | 3.95 ± 0.25 | 62.59 ± 1.62 | 0 | 0 | 0 | 0 | 0.0 | 0/0.0 |
| LDG-T | 232 | 86.6 ± 12.7 | 3.75 ± 0.32 | 61.42 ± 1.71 | 0 | 0 | 0 | 0 | 0.0 | 0/0.0 |
| MDG-T | 252 | 92.8 ± 20.3 | 4.14 ± 0.65 | 62.18 ± 3.16 | 0 | 0 | 0 | 0 | 0.0 | 0/0.0 |
| HDG-T | 220 | 83.9 ± 13.3 | 3.94 ± 0.27 | 61.97 ± 1.36 | 0 | 0 | 0 | 0 | 0.0 | 0/0.0 |
| Groups | Number of Fetal Rats | Internal Organs Deformity and Abnormal Cases | Number/Rate of Viscera Abnormalities (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Ventricular Abnormalities/Hypoplasia | Eyes Were Absent or Varied in Size | Cleft Palate | Hydronephrosis | Total | Deformity Rate (%) | |||
| CG-T | 101 | 0 | 0 | 0 | 1 | 1 | 1.0 | 1/6.3 |
| LDG-T | 113 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0/0.0 |
| MDG-T | 122 | 0 | 0 | 0 | 3 | 3 | 2.5 | 2/11.8 |
| HDG-T | 105 | 0 | 0 | 0 | 5 | 5 | 4.8 | 4/25.0 |
| Groups | Number of Fetal Rats | Number of Skeletal Deformities and Abnormalities | Number/Rate of Skeleton Abnormalities (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Interparietal Bone and Posterior Skull Were Missing | Sternum Was Absent and Incompletely Calcified | Rib Abnormalities (Multiple Ribs, Missing Ribs, Wavy Ribs) | Number of Spinal Bones Was Abnormal | Total | Deformity Rate (%) | |||
| CG-T | 111 | 0 | 6 | 0 | 0 | 6 | 5.4 | 4/25.0 |
| LDG-T | 119 | 0 | 6 | 0 | 0 | 6 | 5 | 3/18.8 |
| MDG-T | 130 | 0 | 8 | 0 | 0 | 8 | 6.2 | 4/23.5 |
| HDG-T | 115 | 0 | 5 | 0 | 0 | 5 | 4.3 | 5/31.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Peng, T.; Zeng, Z.; Wang, P.; Gao, Y.; Wen, X.; Xia, J.; Gong, D.; Yu, P. Edible Safety Evaluation of Cinnamomum camphora Seed Kernel Oil: Sub-Chronic Toxicity and Teratogenicity Assessments. Foods 2025, 14, 2116. https://doi.org/10.3390/foods14122116
Yan X, Peng T, Zeng Z, Wang P, Gao Y, Wen X, Xia J, Gong D, Yu P. Edible Safety Evaluation of Cinnamomum camphora Seed Kernel Oil: Sub-Chronic Toxicity and Teratogenicity Assessments. Foods. 2025; 14(12):2116. https://doi.org/10.3390/foods14122116
Chicago/Turabian StyleYan, Xianghui, Ting Peng, Zheling Zeng, Pengbo Wang, Yifang Gao, Xuefang Wen, Jiaheng Xia, Deming Gong, and Ping Yu. 2025. "Edible Safety Evaluation of Cinnamomum camphora Seed Kernel Oil: Sub-Chronic Toxicity and Teratogenicity Assessments" Foods 14, no. 12: 2116. https://doi.org/10.3390/foods14122116
APA StyleYan, X., Peng, T., Zeng, Z., Wang, P., Gao, Y., Wen, X., Xia, J., Gong, D., & Yu, P. (2025). Edible Safety Evaluation of Cinnamomum camphora Seed Kernel Oil: Sub-Chronic Toxicity and Teratogenicity Assessments. Foods, 14(12), 2116. https://doi.org/10.3390/foods14122116






