Roasting Improves the Bioaccessibility and Bioactivity of Polyphenols from Highland Barley with a Protective Effect in Oxidatively Damaged HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Roasted HB
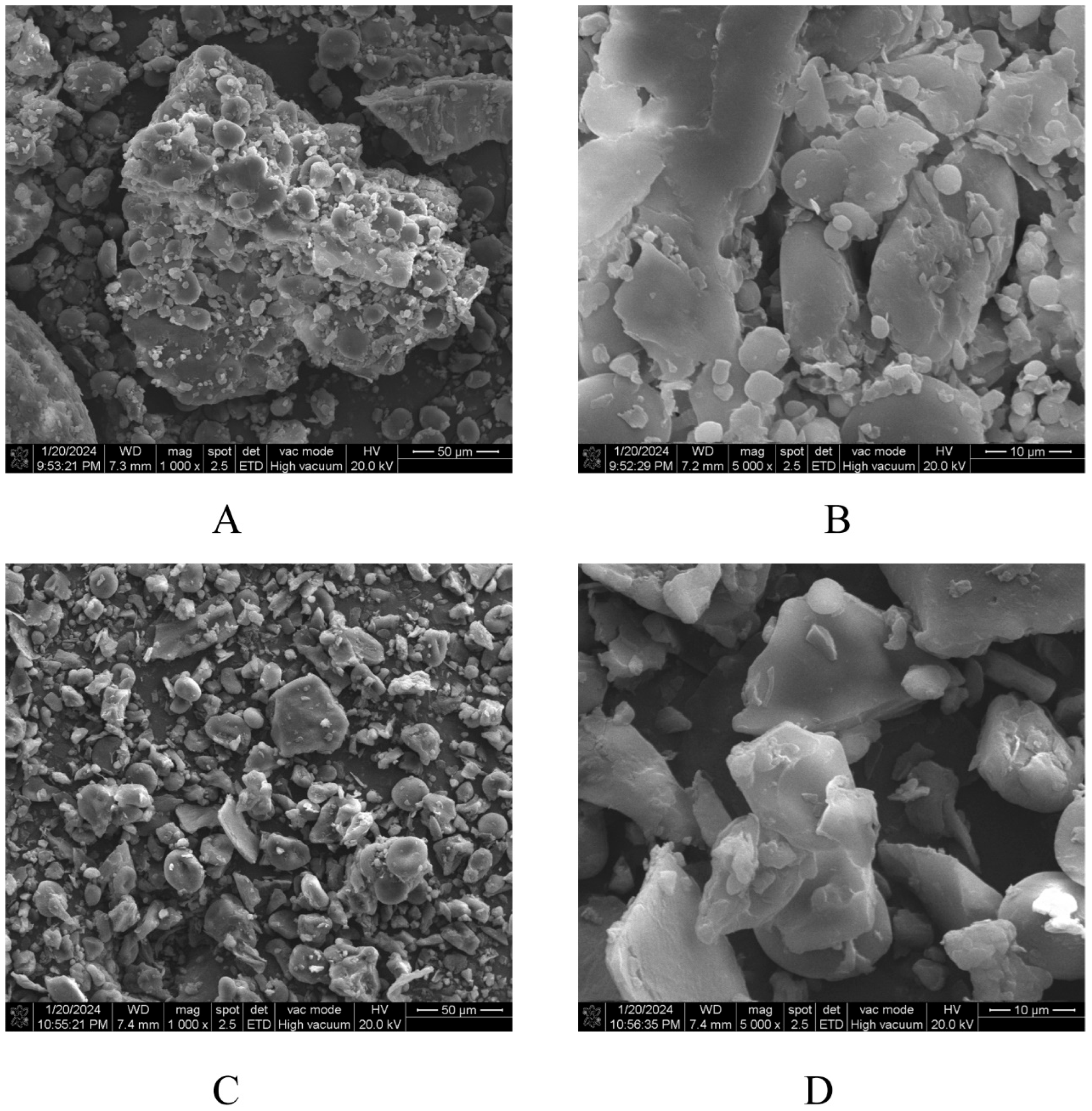
2.3. Morphological Property Analysis of HB
2.3.1. Scanning Electron Microscope (SEM)
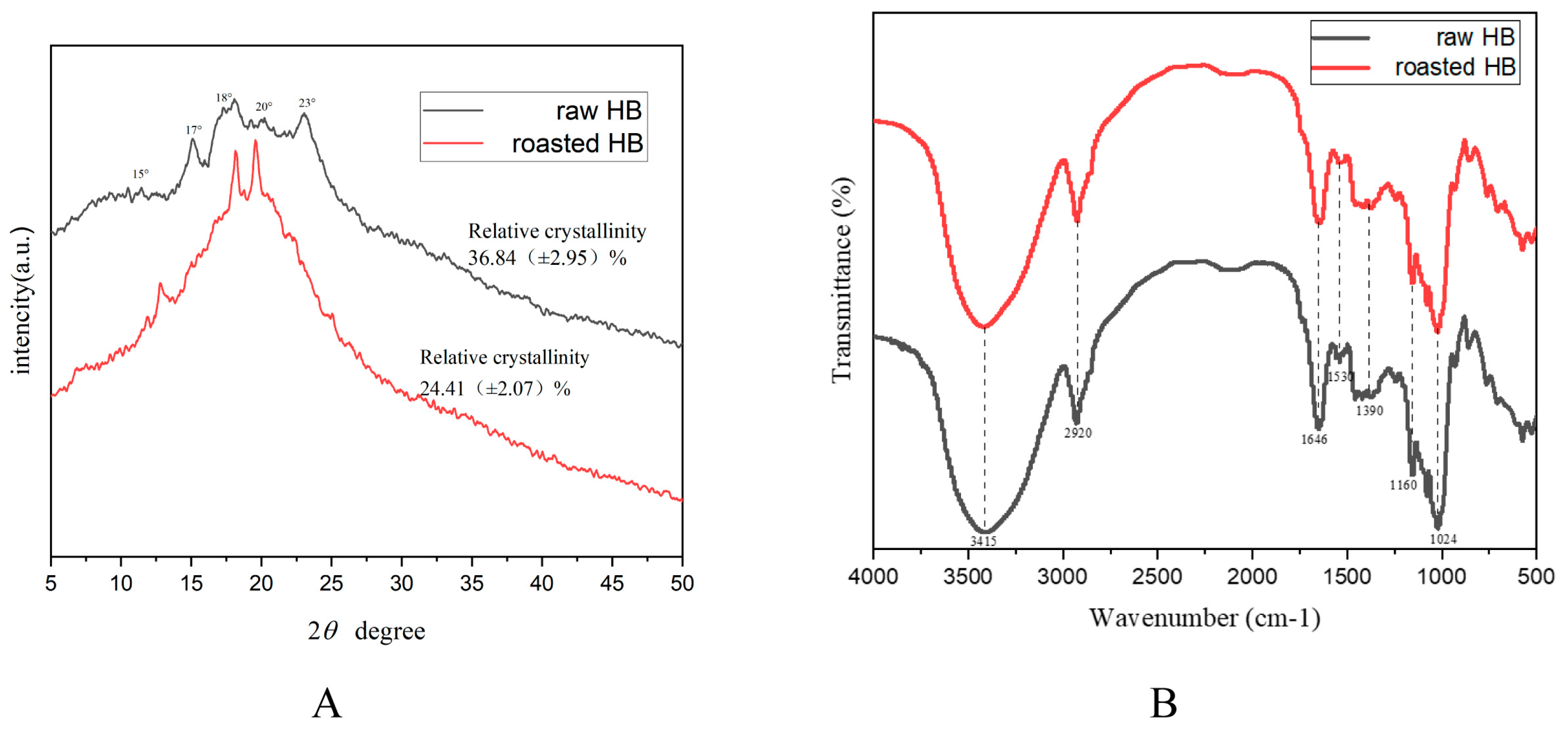
2.3.2. X-Ray Diffraction (XRD)
2.3.3. Fourier Transforms Infrared (FT-IR) Spectroscopy
2.4. Simulated In Vitro Digestion
2.4.1. In Vitro Gastrointestinal Digestion
2.4.2. In Vitro Colonic Fermentation
2.5. Extraction of Polyphenols
2.6. Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC) Measurement
2.6.1. Measurement of TPC
2.6.2. Measurement of TFC
2.7. Quantitative Analysis of Polyphenols
2.8. Assessment of Antioxidant Capacity
2.8.1. DPPH Radical Scavenging Capacity
2.8.2. Ferric-Reducing Antioxidant Power (FRAP)
2.8.3. ABTS Radical (ABTS•+) Scavenging Capacity
2.8.4. Hydrogen Peroxide (H2O2) Scavenging Capacity
2.8.5. Hydroxyl Radical (•OH) Scavenging Capacity
2.9. Inhibition of α-Amylase, α-Glucosidase, and Lipase Activity
2.9.1. α-Amylase Inhibition
2.9.2. α-Glucosidase (α-GLU) Inhibition
2.9.3. Lipase Inhibition
2.10. Cell Culture
2.11. MTT Assay
2.12. Assessment of Oxidative Stress Markers
2.13. Statistical Analysis
3. Results and Discussion
3.1. Morphological Property Analysis
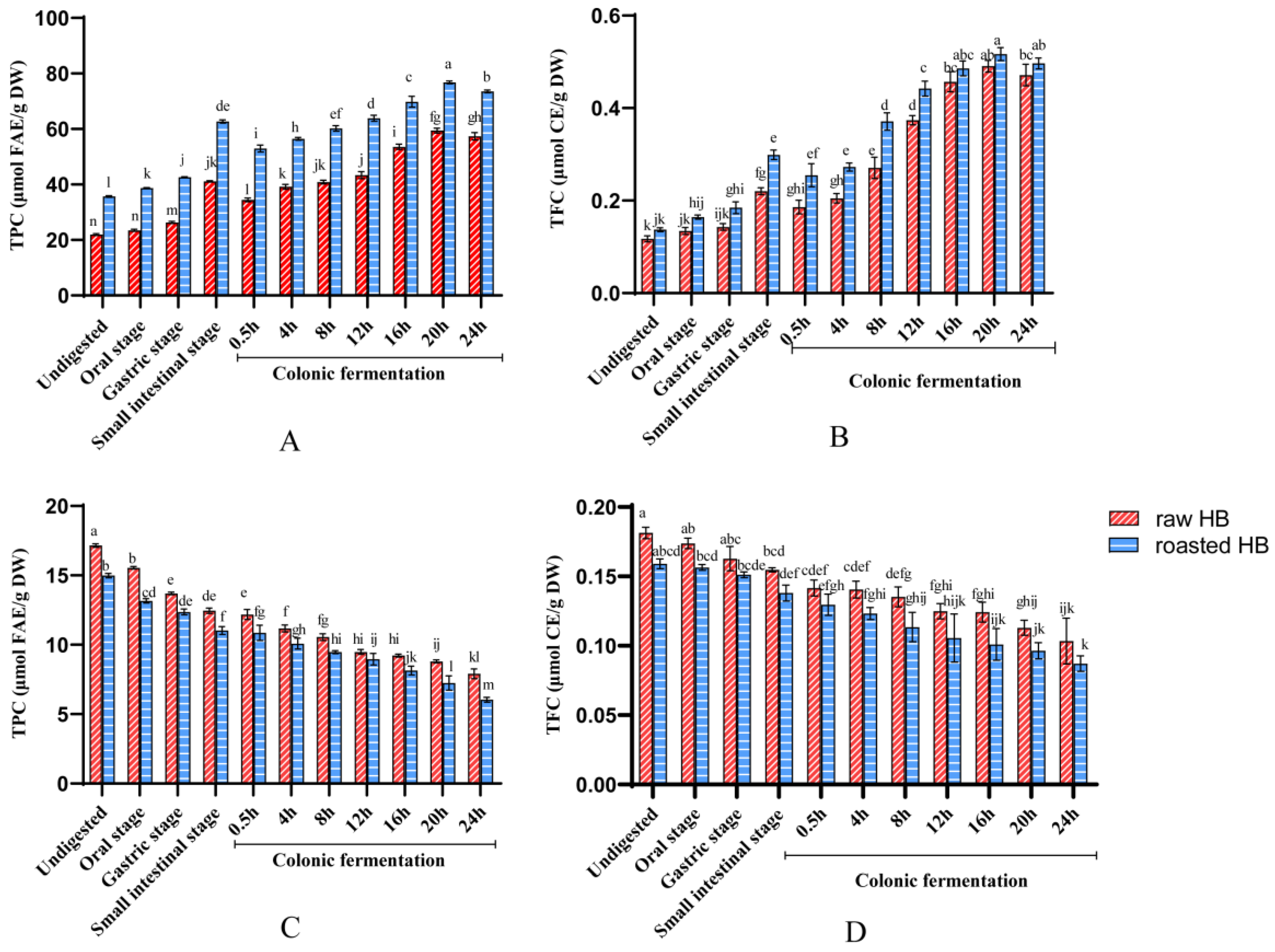
3.2. The Change in TPC and TFC Before and After In Vitro Digestion
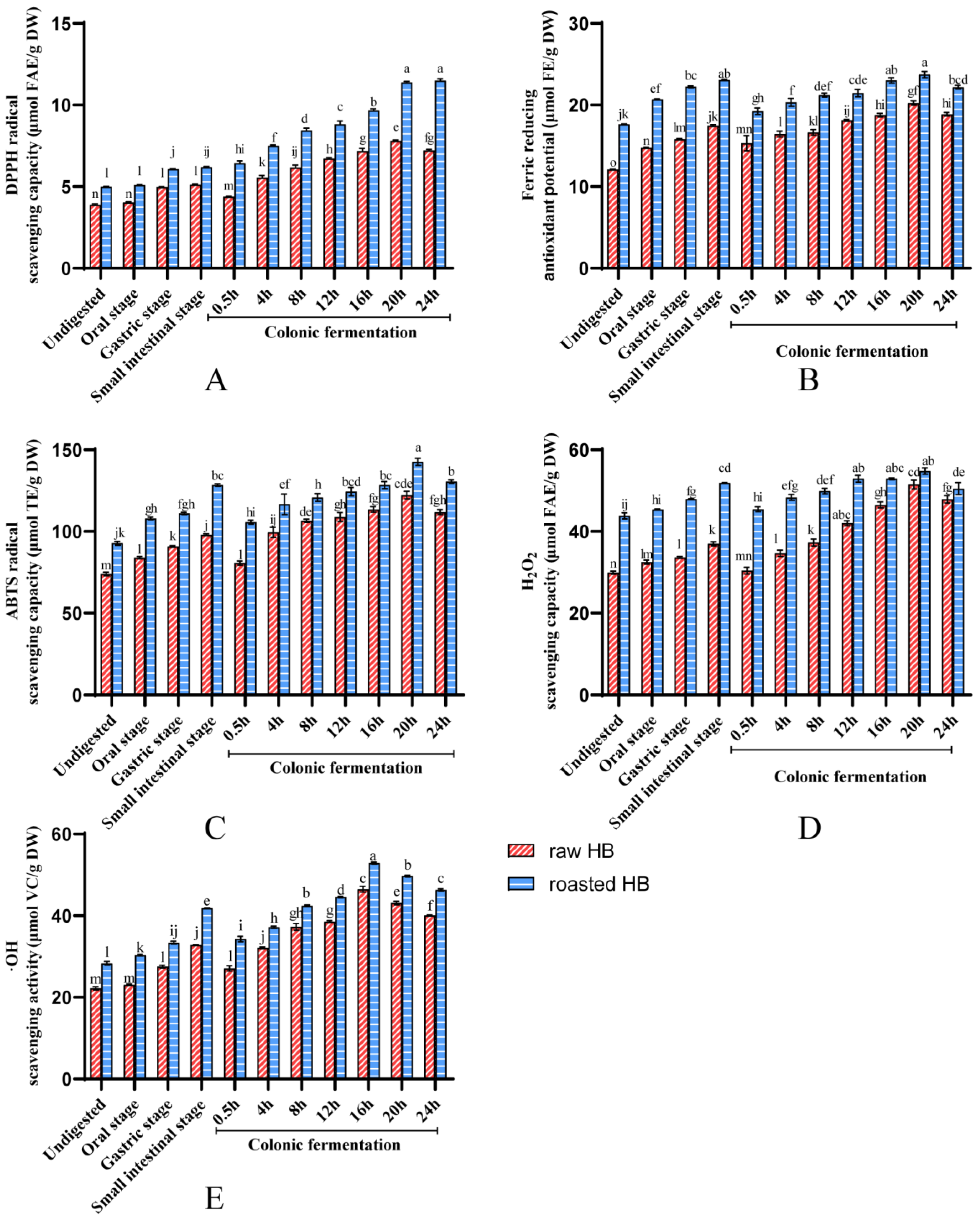
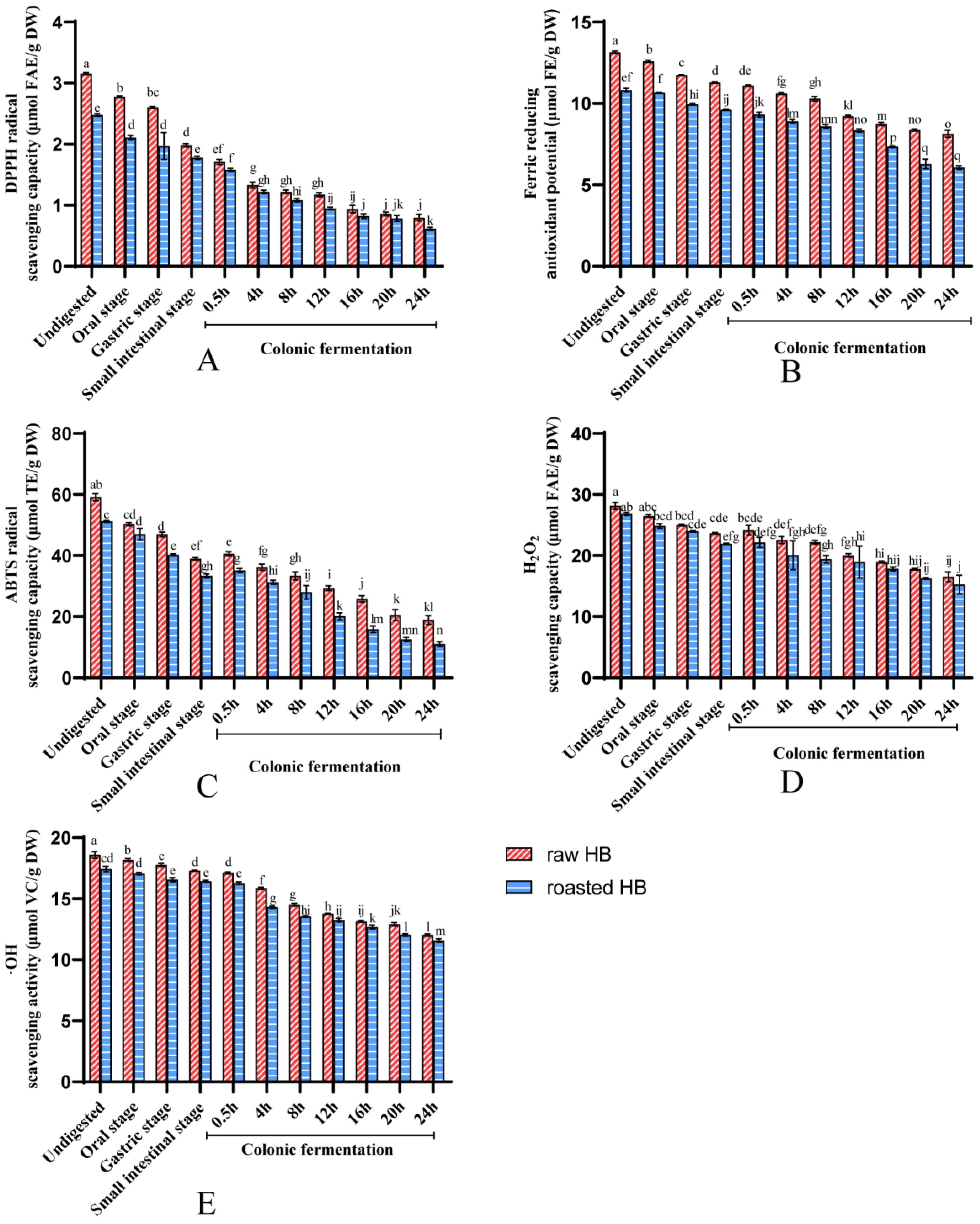
3.3. Antioxidant Capacity of Polyphenols
3.4. Bioaccessibility of Polyphenols
3.5. Quantification of Phenolic Compounds Using HPLC
3.6. Principal Component Analysis
3.7. Correlation Between TPC and Antioxidant Activity
3.8. Inhibition of Enzyme Activity
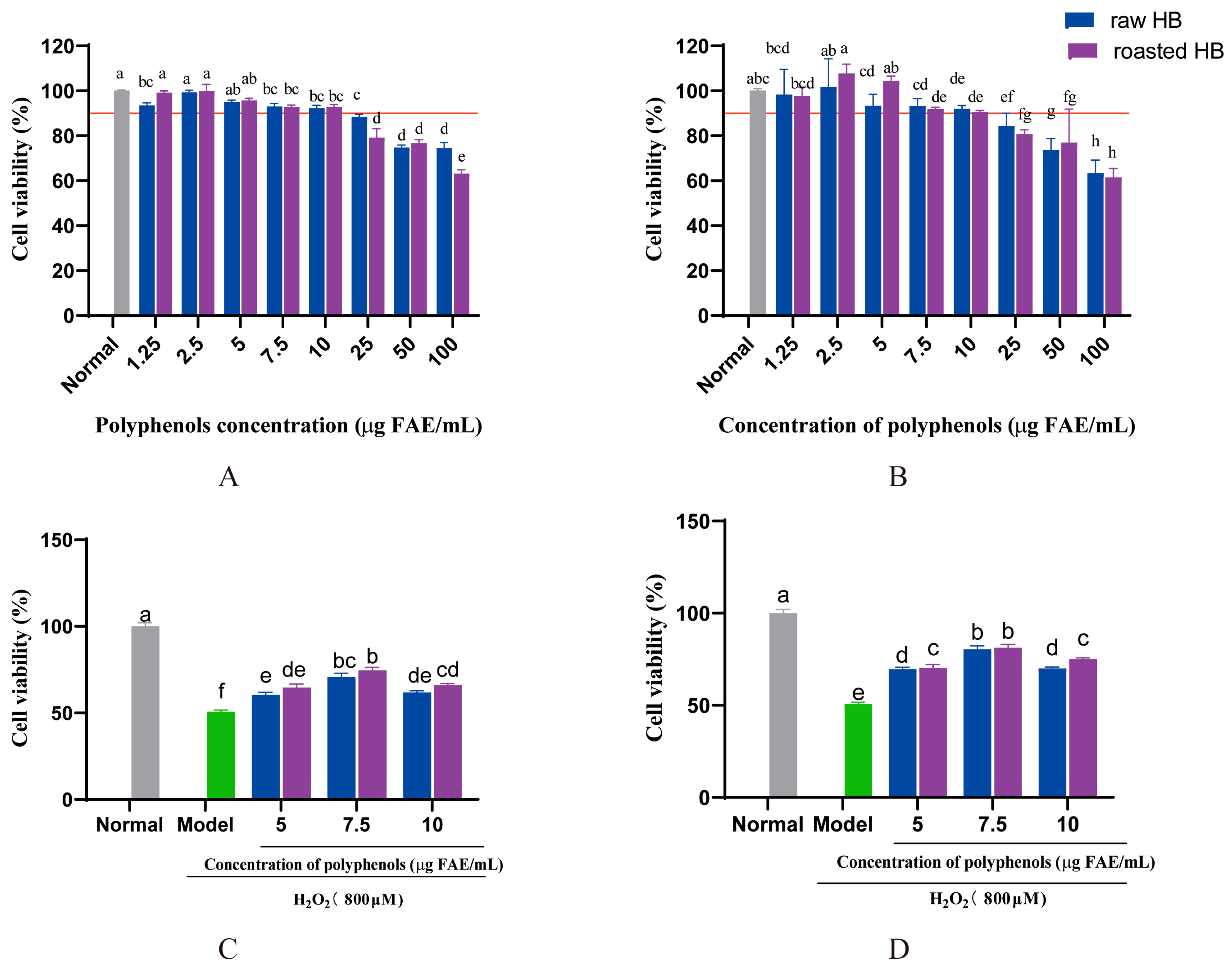
3.9. Cell Viability
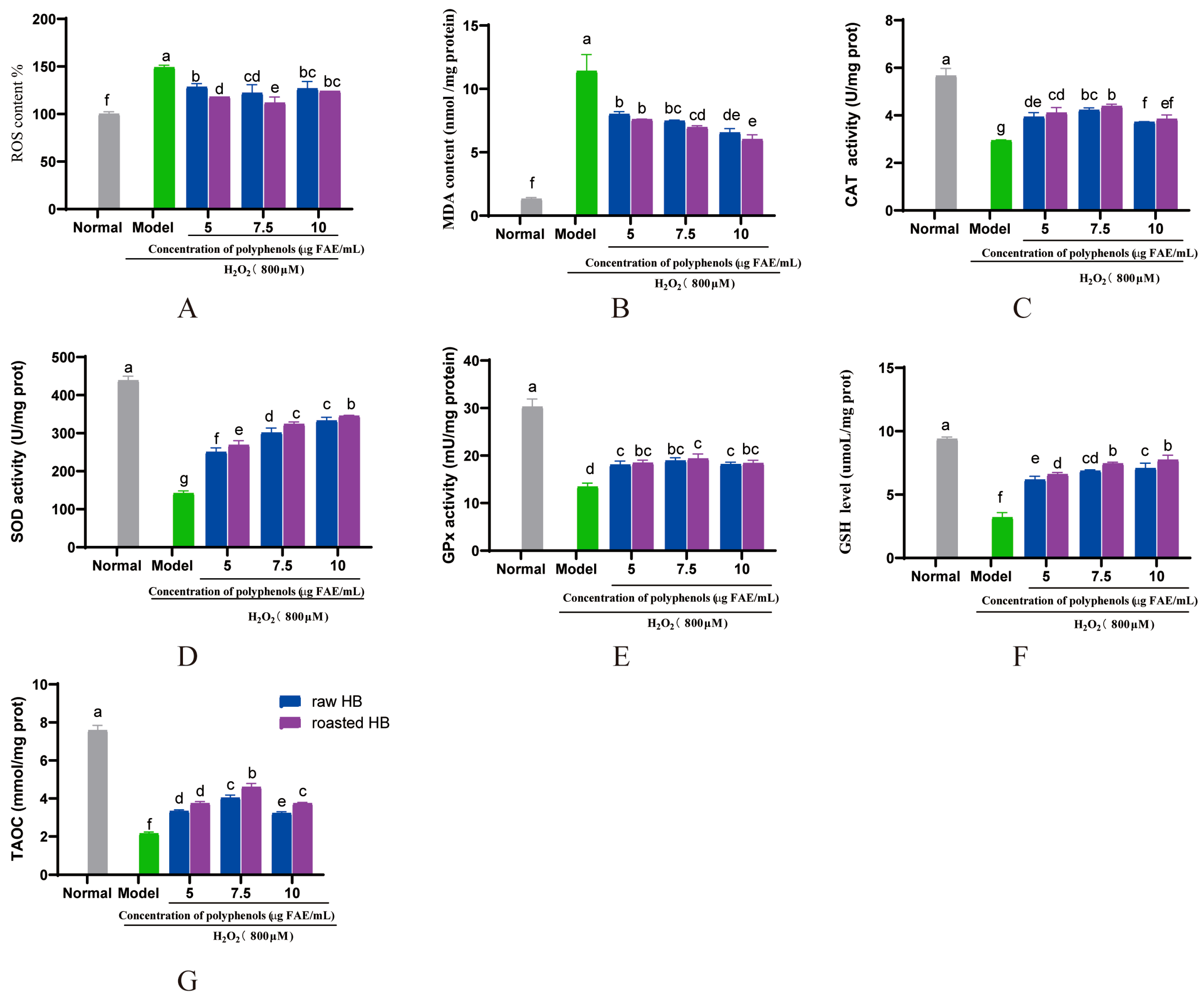
3.10. Impact of Polyphenol Extract on H2O2-Induced Oxidative Stress in HepG2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Assessment of the Phenolic Profiles, Hypoglycemic Activity, and Molecular Mechanism of Different Highland Barley (Hordeum vulgare L.) Varieties. Int. J. Mol. Sci. 2020, 21, 1175. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Dong, W.; Luo, Q.; Huang, Y.; Chen, B.; Wang, H.; Ren, N.; Luo, L.y.; Li, Y. The Bioaccessibility and Bioactivity of Polyphenols from Tsampa Prepared from Roasted Highland Barley Flour Solid-Fermented by Autochthonous Lactic Acid Bacteria. Food Res. Int. 2025, 203, 115817. [Google Scholar] [CrossRef]
- Wang, B.; Nie, C.; Li, T.; Zhao, J.; Fan, M.; Li, Y.; Qian, H.; Wang, L. Effect of Boiling and Roasting on Phenolic Components and Their Bioaccessibilities of Highland Barley. Food Res. Int. 2022, 162, 112137. [Google Scholar] [CrossRef]
- Zhao, Z.; Ming, J.; Zhao, G.; Lei, L. Color, Starch Digestibility, and In Vitro Fermentation of Roasted Highland Barley Flour with Different Fractions. Foods 2022, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Baima, C.; Jiang, J.; Liu, Z.; Wang, J.; Chen, X.D.; Wu, P. In Vitro Gastric Digestion and Emptying of Tsampa under Simulated Elderly and Young Adult Digestive Conditions Using a Dynamic Stomach System. J. Food Eng. 2022, 327, 11105. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Xu, F.; Zhang, Y.; Li, Z.; Ju, X.; Wang, L. Insoluble-Bound Polyphenols of Adlay Seed Ameliorate H2O2-Induced Oxidative Stress in HepG2 Cells via Nrf2 Signalling. Food Chem. 2020, 325, 126865. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Wang, H. Physicochemical Properties, Bioaccessibility and Antioxidant Activity of the Polyphenols from Pine Cones of Pinus Koraiensis. Int. J. Biol. Macromol. 2019, 126, 385–391. [Google Scholar] [CrossRef]
- Hithamani, G.; Srinivasan, K. Effect of Domestic Processing on the Polyphenol Content and Bioaccessibility in Finger Millet (Eleusine coracana) and Pearl Millet (Pennisetum glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef]
- Yi, L.; Wang, Q.; Luo, H.; Lei, D.; Tang, Z.; Lei, S.; Xiao, H. Inhibitory Effects of Polyphenols-Rich Components From Three Edible Seaweeds on Inflammation and Colon Cancer in Vitro. Front. Nutr. 2022, 9, 856273. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Mendoza, S.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A.; Villegas-Ochoa, M.A.; Quintero-Vargas, J.T.J.; Wall-Medrano, A. First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach. Antioxidants 2020, 9, 311. [Google Scholar] [CrossRef]
- Gallego, M.; Arnal, M.; Barat, J.M.; Talens, P. Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes. Foods 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.D.; Ruiz del Castillo, M.L.; Blanch, G.P.; de Pascual-Teresa, S. Black Bean (Phaseolus vulgaris L. Cv. “Tolosa”) Polyphenolic Composition through Cooking and in Vitro Digestion. Food Funct. 2024, 15, 6395–6407. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, M.; Zhang, Y.; Zhang, J.; Wang, C.; Zhang, Y. Effect of Steam, Microwave, and Hot-air Drying on Antioxidant Capacity and in Vitro Digestion Properties of Polyphenols in Oat Bran. J. Food Process Preserv. 2021, 45, e16013. [Google Scholar] [CrossRef]
- Obayiuwana, O.A.; Behrends, V.; Calle-Patino, Y.; Barone, M.; Turroni, S.; Brigidi, P.; Costabile, A.; Corona, G. Cooking, Digestion, and In Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition. Int. J. Mol. Sci. 2023, 24, 14111. [Google Scholar] [CrossRef] [PubMed]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A. The Bioavailability of Polyphenols Following Acute Consumption of Pigmented Barley and Wheat. Food Funct. 2024, 15, 9330–9934. [Google Scholar] [CrossRef]
- Atchan Nwakiban, A.P.; Cicolari, S.; Piazza, S.; Gelmini, F.; Sangiovanni, E.; Martinelli, G.; Bossi, L.; Carpentier-Maguire, E.; Deutou Tchamgoue, A.; Agbor, G.A.; et al. Oxidative Stress Modulation by Cameroonian Spice Extracts in HepG2 Cells: Involvement of Nrf2 and Improvement of Glucose Uptake. Metabolites 2020, 10, 182. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhao, W.; Ren, T.; Wang, X.; Hu, X. Extracts from Tartary Buckwheat Sprouts Restricts Oxidative Injury Induced by Hydrogen Peroxide in HepG2 by Upregulating the Redox System. Foods 2024, 13, 3726. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated Gastrointestinal Digestion and in Vitro Colonic Fermentation of Carob Polyphenols: Bioaccessibility and Bioactivity. LWT 2020, 117, 10862. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat. Foods 2022, 11, 3697. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; Yu, Q. The Recovery, Catabolism and Potential Bioactivity of Polyphenols from Carrot Subjected to in Vitro Simulated Digestion and Colonic Fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Gallo, A.; Bernardi, J.; Marocco, A.; Lucini, L. Effect of Dietary Polyphenols on the in Vitro Starch Digestibility of Pigmented Maize Varieties under Cooking Conditions. Food Res. Int. 2018, 108, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Cabero, A.-I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.-L.; Carrière, F.; et al. A Standardised Semi-Dynamic in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in Vitro Batch Fermentation Protocol for Studying the Contribution of Food to Gut Microbiota Composition and Functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Fuhrman, B.; Volkova, N.; Suraski, A.; Aviram, M. White Wine with Red Wine-like Properties: Increased Extraction of Grape Skin Polyphenols Improves the Antioxidant Capacity of the Derived White Wine. J. Agric. Food Chem. 2001, 49, 3164–3168. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yin, J.; Nie, S.; Zhou, C.; Wan, Y.; Xie, M. Chemical Characteristics and Antioxidant Activities of Polysaccharide Purified from the Seeds of Plantago asiatica L. J. Sci. Food Agric. 2010, 90, 210–217. [Google Scholar] [CrossRef]
- Guo, X.; Long, P.; Meng, Q.; Ho, C.-T.; Zhang, L. An Emerging Strategy for Evaluating the Grades of Keemun Black Tea by Combinatory Liquid Chromatography-Orbitrap Mass Spectrometry-Based Untargeted Metabolomics and Inhibition Effects on α-Glucosidase and α-Amylase. Food Chem. 2018, 246, 74–81. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular Antioxidant Activity and in Vitro Inhibition of α-Glucosidase, α-Amylase and Pancreatic Lipase of Oregano Polyphenols under Simulated Gastrointestinal Digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Y.; Yao, Y.; Xu, Q.; Wang, L. Antioxidant Mechanism of a Newly Found Phenolic Compound from Adlay (NDPS) in HepG2 Cells via Nrf2 Signalling. Food Chem. 2022, 378, 132034. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, Y.; Ham, H.; Jeong, H.-S.; Lee, J. Protective Effects of Oligomeric and Polymeric Procyanidin Fractions from Defatted Grape Seeds on Tert-Butyl Hydroperoxide-Induced Oxidative Damage in HepG2 Cells. Food Chem. 2013, 137, 136–141. [Google Scholar]
- Huang, W.; Tian, F.; Wang, H.; Wu, S.; Jin, W.; Shen, W.; Hu, Z.; Cai, Q.; Liu, G. Comparative Assessment of Extraction, Composition, and in Vitro Antioxidative Properties of Wheat Bran Polyphenols. LWT 2023, 180, 114706. [Google Scholar] [CrossRef]
- Hong, Q.; Chen, G.; Wang, Z.; Chen, X.; Kan, J. Effects of Different Thermal Processing Methods on Bioactive Components, Phenolic Compounds, and Antioxidant Activities of Qingke (Highland Hull-Less Barley). Food Sci. Human. Wellness 2023, 12, 119–129. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, Z.; Huang, G.; Liu, J.; Ye, F.; Chen, J.; Ming, J.; Zhao, G.; Lei, L. The Contribution of Cell Wall Integrity to Gastric Emptying and in Vitro Starch Digestibility and Fermentation Performance of Highland Barley Foods. Food Res. Int. 2023, 169, 112912. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Xia, R.; Wu, D.; Cheng, W.; Meng, L.; Wang, Z.; Tang, X. Toward a Comprehensive Understanding of Various Milling Methods on the Physicochemical Properties of Highland Barley Flours and Eating Quality of Corresponding Sugar-Free Cookies. Food Chem. 2023, 413, 135657. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Q.; Liu, H.; Fan, Z.; Shi, J.; Liu, X. Effect of Various Thermal Processing on the Structural and in Vitro Prebiotic Characteristics of β-Glucan from Hulless Barley. Food Hydrocoll. 2023, 142, 108818. [Google Scholar] [CrossRef]
- Si, J.; Xie, J.; Zheng, B.; Xie, J.; Chen, Y.; Yang, C.; Sun, N.; Wang, Y.; Hu, X.; Yu, Q. Release Characteristic of Bound Polyphenols from Tea Residues Insoluble Dietary Fiber by Mixed Solid-State Fermentation with Cellulose Degrading Strains CZ-6 and CZ-7. Food Res. Int. 2023, 173, 113319. [Google Scholar] [CrossRef]
- Wen, J.; Sui, Y.; Shi, J.; Cai, S.; Xiong, T.; Cai, F.; Zhou, L.; Li, S.; Mei, X. In Vitro Gastrointestinal Digestion of Various Sweet Potato Leaves: Polyphenol Profiles, Bioaccessibility and Bioavailability Elucidation. Antioxidants 2024, 13, 520. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Zhang, Y.; Chen, Y.; Ge, X.; Sui, W.; Zhu, Q.; Geng, J.; Zhang, M. Release of Bound Polyphenols from Wheat Bran Soluble Dietary Fiber during Simulated Gastrointestinal Digestion and Colonic Fermentation in Vitro. Food Chem. 2023, 402, 134111. [Google Scholar] [CrossRef] [PubMed]
- Sirisena, S.; Ajlouni, S.; Ng, K. Simulated Gastrointestinal Digestion and in Vitro Colonic Fermentation of Date (Phoenix dactylifera L.) Seed Polyphenols. Int. J. Food Sci. Technol. 2018, 53, 412–422. [Google Scholar] [CrossRef]
- Ozkan, G.; Sakarya, F.B.; Tas, D.; Yurt, B.; Ercisli, S.; Capanoglu, E. Effect of In Vitro Digestion on the Phenolic Content of Herbs Collected from Eastern Anatolia. ACS Omega 2023, 8, 12730–12738. [Google Scholar] [CrossRef]
- Xie, Y.; Gong, T.; Liu, H.; Fan, Z.; Zhaojun, C.; Liu, X. In Vitro and In Vivo Digestive Fate and Antioxidant Activities of Polyphenols from Hulless Barley: Impact of Various Thermal Processing Methods and β-Glucan. J. Agric. Food Chem. 2022, 70, 7683–7694. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fan, S.; Shangguan, C.; Zhang, J. Evaluation of the Effects of Simulated in Vitro Digestion by Gastrodia Elata on Biological Activity and Gut Microflora Regulation. Food Biosci. 2022, 50, 10214. [Google Scholar] [CrossRef]
- Xie, J.; Sun, N.; Huang, H.; Xie, J.; Chen, Y.; Hu, X.; Hu, X.; Dong, R.; Yu, Q. Catabolism of Polyphenols Released from Mung Bean Coat and Its Effects on Gut Microbiota during in Vitro Simulated Digestion and Colonic Fermentation. Food Chem. 2022, 396, 133719. [Google Scholar] [CrossRef]
- Collins, A.; Francis, N.; Chinkwo, K.; Santhakumar, A.B.; Blanchard, C. Effect of In Vitro Gastrointestinal Digestion on the Polyphenol Bioaccessibility and Bioavailability of Processed Sorghum (Sorghum bicolor L. Moench). Molecules 2024, 29, 5229. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Villasante, J.; Fogliano, V.; Pastore, G.M. In Vitro Gastrointestinal Digestion and Gut Microbiota Fermentation of Phenolic Compounds from Uvaia. Food Chem. 2025, 477, 143462. [Google Scholar] [CrossRef]
- Del Burgo-Gutiérrez, C.; Ludwig, I.A.; De Peña, M.-P.; Cid, C. Industrial and Culinary Treatments Applied to Piquillo Pepper (Capsicum Annuum Cv. Piquillo) Impact Positively on (Poly)Phenols’ Bioaccessibility and Gut Microbiota Catabolism. Food Funct. 2024, 15, 2443–2458. [Google Scholar] [CrossRef]
- Attri, S.; Sharma, K.; Raigond, P.; Goel, G. Colonic Fermentation of Polyphenolics from Sea Buckthorn (Hippophae rhamnoides) Berries: Assessment of Effects on Microbial Diversity by Principal Component Analysis. Food Res. Int. 2018, 105, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhao, C.; Shang, X.; Li, B.; Guo, J.; Wang, J.; Wu, B.; Fu, Y. Ameliorative Effects of Raisin Polyphenol Extract on Oxidative Stress and Aging In Vitro and In Vivo via Regulation of Sirt1–Nrf2 Signaling Pathway. Foods 2024, 14, 71. [Google Scholar] [CrossRef]
- Cañas, S.; Rebollo-Hernanz, M.; Martín-Trueba, M.; Braojos, C.; Gil-Ramírez, A.; Benítez, V.; Martín-Cabrejas, M.A.; Aguilera, Y. Exploring the Potential of Phenolic Compounds from the Coffee Pulp in Preventing Cellular Oxidative Stress after in Vitro Digestion. Food Res. Int. 2023, 172, 113116. [Google Scholar] [CrossRef]
- He, S.; Cui, X.; Khan, A.; Liu, Y.; Wang, Y.; Cui, Q.; Zhao, T.; Cao, J.; Cheng, G. Activity Guided Isolation of Phenolic Compositions from Anneslea Fragrans Wall. and Their Cytoprotective Effect against Hydrogen Peroxide Induced Oxidative Stress in HepG2 Cells. Molecules 2021, 26, 3690. [Google Scholar] [CrossRef]
- Jiao, X.; Li, B.; Zhang, Q.; Gao, N.; Zhang, X.; Meng, X. Effect of in Vitro-simulated Gastrointestinal Digestion on the Stability and Antioxidant Activity of Blueberry Polyphenols and Their Cellular Antioxidant Activity towards HepG2 Cells. Int. J. Food Sci. Technol. 2018, 53, 61–71. [Google Scholar] [CrossRef]
- González-Burgos, E.; Ureña-Vacas, I.; Sánchez, M.; Gómez-Serranillos, M.P. Nutritional Value of Moringa Oleifera Lam. Leaf Powder Extracts and Their Neuroprotective Effects via Antioxidative and Mitochondrial Regulation. Nutrients 2021, 13, 2203. [Google Scholar] [CrossRef]
- Zheng, Q.; Feng, K.; Zhong, W.; Tan, W.; Rengaowa, S.; Hu, W. Investigating the Hepatoprotective Properties of Mulberry Leaf Flavonoids against Oxidative Stress in HepG2 Cells. Molecules 2024, 29, 2597. [Google Scholar] [CrossRef]



| Indigestion | Gastrointestinal Digestion | Bioaccessibility(%) | ||||
|---|---|---|---|---|---|---|
| Raw HB | Roasted HB | Raw HB | Roasted HB | Raw HB | Roasted HB | |
| TPC (µmol FAE/g DW) | 21.95 ± 0.32 b | 35.67 ± 0.32 a | 41.24 ± 0.27 b | 62.78 ± 0.56 a | 187.28 b | 285.65 a |
| TFC (µmol CE/g DW) | 0.12 ± 0.01 b | 0.14 ± 0.00 a | 0.22 ± 0.01 b | 0.30 ± 0.01 a | 188.13 b | 255.36 a |
| DPPH (μmol FAE/g DW) | 3.88 ± 0.05 b | 4.99 ± 0.01 a | 5.14 ± 0.06 b | 6.20 ± 0.04 a | 131.65 b | 159.43 a |
| FRAP (μmol FE/g DW) | 12.09 ± 0.08 b | 17.64 ± 0.04 a | 17.53 ± 0.12 b | 23.07 ± 0.06 a | 144.50 b | 190.83 a |
| ABTS (μmol TE/g DW) | 29.90 ± 0.37 b | 43.83 ± 0.74 a | 37.17 ± 0.52 b | 51.81 ± 0.11 a | 123.63 b | 173.45 a |
| H2O2 (μmol FAE/g DW) | 22.22 ± 0.38 b | 28.35 ± 0.41 a | 32.89 ± 0.1 b | 41.82 ± 0.08 a | 147.76 b | 188.26 a |
| •OH (μmol VC/g DW) | 73.95 ± 1.13 b | 92.76 ± 1.16 a | 97.76 ± 0.56 b | 128.35 ± 0.71 a | 132.48 b | 173.69 a |
| Colonic Fermentation for 0.5 h | Colonic Fermentation for 24 h | Bioaccessibility(%) | ||||
|---|---|---|---|---|---|---|
| Raw HB | Roasted HB | Raw HB | Roasted HB | Raw HB | Roasted HB | |
| TPC (µmol FAE/g DW) | 34.46 ± 0.7 b | 52.95 ± 1.25 a | 57.38 ± 1.33 b | 73.55 ± 0.52 a | 166.51 b | 213.41 a |
| TFC (µmol CE/g DW) | 0.19 ± 0.01 a | 0.25 ± 0.03 a | 0.47 ± 0.02 b | 0.50 ± 0.01 a | 253.53 a | 267.09 a |
| DPPH (μmol FAE/g DW) | 4.38 ± 0.03 b | 6.43 ± 0.15 a | 7.22 ± 0.06 b | 11.50 ± 0.10 a | 164.97 b | 262.77 a |
| FRAP (μmol FE/g DW) | 15.32 ± 0.93 b | 19.24 ± 0.4 a | 18.87 ± 0.21 b | 22.18 ± 0.22 a | 123.16 b | 144.75 a |
| ABTS (μmol TE/g DW) | 30.39 ± 0.83 b | 45.42 ± 0.61 a | 47.89 ± 0.89 b | 50.48 ± 1.47 a | 157.56 b | 166.10 a |
| H2O2 (μmol FAE/g DW) | 27.08 ± 0.67 b | 34.27 ± 0.66 a | 40.06 ± 0.13 b | 46.31 ± 0.28 a | 147.94 b | 171.03 a |
| •OH (μmol VC/g DW) | 80.65 ± 1.28 b | 105.70 ± 1.29 a | 111.87 ± 1.62 b | 130.54 ± 1.05 a | 138.72 b | 161.86 a |
| Identification | Indigestion | Gastrointestinal Digestion | Colonic Fermentation | |||
|---|---|---|---|---|---|---|
| Raw HB (μg/g DW) | Roasted HB (μg/g DW) | Raw HB (μg/g DW) | Roasted HB (μg/g DW) | Raw HB (μg/g DW) | Roasted HB (μg/g DW) | |
| Hydroxybenzoic acids | ||||||
| Gallic acid | 6.92 ± 0.23 c | 32.54 ± 0.66 b | ND | ND | 220.10 ± 1.84 a | ND |
| Protocatechuic acid | ND | ND | ND | ND | 37.30 ± 1.21 | 53.75 ± 0.46 |
| p-Hydroxybenzoic acid | ND | ND | ND | 25.21 ± 0.81 | ND | ND |
| Vanillin | ND | ND | ND | ND | ND | 3.87 ± 0.32 |
| Vanillic acid | ND | ND | ND | 29.72 ± 0.96 | ND | ND |
| Total | 6.92 ± 0.23 e | 32.54 ± 0.66 d | ND | 54.92 ± 1.36 c | 257.40 ± 1.53 a | 57.62 ± 0.44 b |
| Hydroxycinnamic acid | ||||||
| Chlorogenic acid | ND | 114.58 ± 1.40 b | ND | 153.86 ± 1.37 a | 14.31 ± 0.22 c | ND |
| Ferulic acid | 6.66 ± 0.48 d | 4.19 ± 0.66 e | 6.45 ± 0.04 d | 9.25 ± 0.15 c | 25.52 ± 0.30 a | 18.21 ± 0.60 b |
| Sinapic acid | 5.66 ± 0.28 c | ND | 17.13 ± 0.23 b | 17.59 ± 0.52 b | 41.46 ± 1.26 a | 40.34 ± 1.07 a |
| trans-Cinnamic acid | 1.90 ± 0.02 e | 1.48 ± 0.07 f | 3.03 ± 0.00 d | 5.24 ± 0.11 b | 11.97 ± 0.15 a | 3.80 ± 0.25 c |
| o-Coumaric acid | 3.32 ± 0.13 e | 3.06 ± 0.13 e | 8.64 ± 0.07 d | 17.60 ± 0.11 b | 23.80 ± 0.37 a | 14.04 ± 0.21 c |
| Total | 17.55 ± 0.53 f | 123.32 ± 1.98 b | 35.26 ± 0.19 e | 203.54 ± 1.54 a | 117.06 ± 1.76 c | 76.38 ± 1.98 d |
| Flavonoids | ||||||
| (−)-Epigallocatechin | 128.89 ± 2.36 b | 64.10 ± 2.19 d | ND | 80.40 ± 2.34 c | ND | 310.34 ± 1.82 a |
| Epicatechin | 33.27 ± 0.99 | 22.73 ± 1.20 | ND | ND | ND | ND |
| vitexin | ND | 8.95 ± 0.32 e | 15.37 ± 0.38 d | 26.21 ± 0.76 c | 56.45 ± 1.26 a | 42.65 ± 0.46 b |
| Ellagic acid | 9.81 ± 0.77 e | 8.12 ± 0.38 f | 21.77 ± 0.15 d | 36.90 ± 0.55 c | 144.51 ± 0.50 a | 105.78 ± 0.07 b |
| Myricetin | 18.34 ± 0.63 e | 16.24 ± 0.45 e | 21.65 ± 0.73 d | 83.49 ± 1.03 c | 145.93 ± 1.31 b | 163.23 ± 0.67 a |
| Quercetin | 14.94 ± 1.11 e | 9.60 ± 0.42 f | 41.93 ± 0.26 d | 55.50 ± 1.73 c | 140.77 ± 1.99 a | 85.61 ± 0.87 b |
| Kaempferol | 7.59 ± 0.39 e | 5.25 ± 1.09 f | 13.51 ± 0.11 c | 47.61 ± 0.93 a | 35.01 ± 0.81 b | 13.32 ± 0.56 c |
| Apigenin | 11.19 ± 0.22 c | 5.55 ± 0.40 d | ND | 33.17 ± 0.06 b | 45.99 ± 0.54 a | 5.85 ± 0.07 d |
| Total | 224.03 ± 2.17 d | 139.90 ± 0.36 e | 114.22 ± 1.02 f | 363.727 ± 4.30 c | 568.65 ± 4.67 b | 726.79 ± 3.19 a |
| TPC | 490.08 ± 5.47 e | 558.97 ± 2.81 d | 298.96 ± 2.41 f | 1243.48 ± 9.19 c | 1886.23 ± 11.20 a | 1721.57 ± 6.75 b |
| Identification | Indigestion | Gastrointestinal Digestion | Colonic Fermentation | |||
|---|---|---|---|---|---|---|
| Raw HB (μg/g DW) | Roasted HB (μg/g DW) | Raw HB (μg/g DW) | Roasted HB (μg/g DW) | Raw HB (μg/g DW) | Roasted HB (μg/g DW) | |
| Hydroxycinnamic acid | ||||||
| Sinapic acid | ND | ND | ND | ND | 21.95±0.80 | ND |
| o-Coumaric acid | ND | ND | ND | ND | ND | 0.61 ± 0.05 |
| trans-Cinnamic acid | 16.11 ± 0.38 b | 1.18 ± 0.06 d | 2.87 ± 0.11 c | ND | 39.97 ± 0.78 a | ND |
| Total | 16.11 ± 0.38 b | 1.18 ± 0.06 d | 2.87 ± 0.11 c | ND | 24.81 ± 0.71 a | 0.61 ± 0.05 e |
| Flavonoids | ||||||
| Myricetin | ND | 6.29 ± 0.44 | ND | ND | 39.97 ± 0.78 | ND |
| Quercetin | ND | 3.71 ± 0.35 | ND | ND | 65.56 ± 0.68 | ND |
| Kaempferol | 3.62 ± 0.27 d | 5.71 ± 0.24 c | 6.44 ± 0.27 b | 4.20 ± 0.11 d | 14.53 ± 0.34 a | 4.04 ± 0.23 d |
| Apigenin | 2.18 ± 0.15 c | 3.28 ± 0.11 b | 1.98 ± 0.19 c | 0.63 ± 0.01 d | 8.16 ± 0.17 a | 3.02 ± 0.18 b |
| Total | 5.80 ± 0.30 e | 18.99 ± 0.58 b | 8.43 ± 0.34 c | 4.83 ± 0.12 f | 128.22 ± 0.37 a | 7.06 ± 0.32 d |
| TPC | 38.02 ± 0.66 b | 21.36 ± 0.61 c | 8.43 ± 0.34 d | 4.83 ± 0.12 e | 177.86 ± 1.72 a | 8.29 ± 0.42 d |
| IC50 (Mean ± SD in μg FAE/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Indigestion | Gastrointestinal Digestion | Colonic Fermentation | Acarbose/Orlistat | ||||
| Raw HB | Roasted HB | Raw HB | Roasted HB | Raw HB | Roasted HB | ||
| α-Amylase | 107.24 ± 0.51 c | 82.13 ± 0.61 e | 89.31 ± 0.78 c | 71.31 ± 1.35 f | 120.65 ± 2.35 b | 87.51 ± 1.26 d | 133.25 ± 4.47 a |
| α-GLU | 98.17 ± 0.24 c | 81.35 ± 0.49 e | 82.20 ± 0.87 d | 60.44 ± 0.14 f | 108.94 ± 1.08 b | 95.31 ± 0.37 d | 133.25 ± 4.47 a |
| Lipase | 105.24 ± 2.49 b | 96.40 ± 1.19 c | 68.16 ± 1.87 c | 52.94 ± 2.51 d | 133.22 ± 0.46 a | 102.28 ± 2.20 b | 138.38 ± 2.39 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Pang, S.; Zao, X.; Luo, Q.; Luo, L.; Dong, W.; Li, Y. Roasting Improves the Bioaccessibility and Bioactivity of Polyphenols from Highland Barley with a Protective Effect in Oxidatively Damaged HepG2 Cells. Foods 2025, 14, 2095. https://doi.org/10.3390/foods14122095
Chen N, Pang S, Zao X, Luo Q, Luo L, Dong W, Li Y. Roasting Improves the Bioaccessibility and Bioactivity of Polyphenols from Highland Barley with a Protective Effect in Oxidatively Damaged HepG2 Cells. Foods. 2025; 14(12):2095. https://doi.org/10.3390/foods14122095
Chicago/Turabian StyleChen, Nuo, Shuyu Pang, Xingru Zao, Qin Luo, Lingyuan Luo, Wenming Dong, and Yongqiang Li. 2025. "Roasting Improves the Bioaccessibility and Bioactivity of Polyphenols from Highland Barley with a Protective Effect in Oxidatively Damaged HepG2 Cells" Foods 14, no. 12: 2095. https://doi.org/10.3390/foods14122095
APA StyleChen, N., Pang, S., Zao, X., Luo, Q., Luo, L., Dong, W., & Li, Y. (2025). Roasting Improves the Bioaccessibility and Bioactivity of Polyphenols from Highland Barley with a Protective Effect in Oxidatively Damaged HepG2 Cells. Foods, 14(12), 2095. https://doi.org/10.3390/foods14122095





