Understanding the Protective Effect of Liquid Nitrogen Freezing on Crayfish Quality During Transportation and Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Crayfish
2.3. Extraction of Crayfish MP
2.4. Methods for Determining the Functional Properties of MP
2.4.1. Solubility
2.4.2. Turbidity
2.4.3. Foaming Properties and Foaming Stability Determination
2.4.4. Emulsibility and Emulsion Stability Determination
2.5. Observation of MP Microstructure
2.6. Methods for Determining the Protein Integrity of MP
2.6.1. SDS-PAGE Analysis
2.6.2. Ca2+-ATPase Activity
2.7. Determination of MP Conformation
2.7.1. Circular Dichroism Spectrum
2.7.2. Contents of Total Thiol and Disulfide Bonds
2.7.3. Determination of Surface Hydrophobicity
2.7.4. Determination of Fluorescence Intensity
2.8. Data Analysis
3. Results
3.1. Functional Properties of MP
3.1.1. Solubility
3.1.2. Foaming Properties
3.1.3. Emulsification Properties
3.2. Microstructural Analysis of MP
3.3. Molecular Weight of MP and Ca2+-ATPase Activity Analysis
3.3.1. SDS-PAGE
3.3.2. Ca2+-ATPase Activity
3.4. MP Conformation Analysis
3.4.1. Secondary Structure
3.4.2. Total Sulfhydryl Group and Disulfide Bond Contents
3.4.3. Surface Hydrophobicity
3.4.4. Fluorescence Intensity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Adebiyi, F.; Ore, O.; Ogunjimi, I. Evaluation of human health risk assessment of potential toxic metals in commonly consumed crayfish (Palaemon hastatus) in Nigeria. Heliyon 2020, 6, e03092. [Google Scholar] [CrossRef]
- Long, L.; Liu, H.; Cui, M.; Zhang, C.; Liu, C. Offshore aquaculture in China. Rev. Aquac. 2024, 16, 254–270. [Google Scholar] [CrossRef]
- Bonilla, F.; Reyes, V.; Chouljenko, A.; Dzandu, B.; Sathivel, S. Influence of energy removal rate on the quality of minced meat from undersized crawfish during frozen storage. Food Prod. Process. Nutr. 2020, 2, s43014–s43020. [Google Scholar] [CrossRef]
- You, Y.; Kang, T.; Jun, S. Control of ice nucleation for subzero food preservation. Food Eng. Rev. 2021, 13, 15–35. [Google Scholar] [CrossRef]
- Yang, K.; Bian, C.; Ma, X.; Mei, J.; Xie, J. Recent advances in emerging techniques for freezing and thawing on aquatic products’ quality. J. Food Process. Preserv. 2022, 46, e16609. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Z.; Li, G.; Xiang, Y.; Chen, J.; Hu, Y. Preservation mechanism of liquid nitrogen freezing on crayfish (Procambarus clarkia): Study on the modification effects in biochemical and structural properties. J. Food Process. Preserv. 2022, 46, e17116. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Wang, J.; Wang, Y.; Jin, G.; Teng, W.; Geng, F.; Cao, J. Myofibrillar protein denaturation/oxidation in freezing-thawing impair the heat-induced gelation: Mechanisms and control technologies. Trends Food Sci. Technol. 2023, 138, 655–670. [Google Scholar] [CrossRef]
- Shi, J.; Lei, Y.; Shen, H.; Hong, H.; Yu, X.; Zhu, B.; Luo, Y. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem. 2019, 272, 604–612. [Google Scholar] [CrossRef]
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of multiple freeze–thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018, 109, 65–71. [Google Scholar] [CrossRef]
- Tan, M.; Ding, Z.; Mei, J.; Xie, J. Effect of cellobiose on the myofibrillar protein denaturation induced by pH changes during freeze–thaw cycles. Food Chem. 2022, 373, 131511. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, Y.; Puolanne, E.; Ertbjerg, P. Role of freezing-induced myofibrillar protein denaturation in the generation of thaw loss: A review. Meat Sci. 2022, 190, 108841. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; He, X.; Wang, L.; Xu, L.; Jiao, C.; Chen, J. Effect of liquid nitrogen freezing on maintaining the quality of crayfish during freeze–thaw cycles: Muscle structure and myofibrillar proteins properties. Foods 2025, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Xiong, Y.L. Oxidative modification of amino acids in porcine myofibrillar protein isolates exposed to three oxidizing systems. Food Chem. 2007, 103, 607–616. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, X.; Yang, F.; Yu, L.; Li, X.; Huang, J.; Wang, S. Investigation of the cryoprotective mechanism and effect on quality characteristics of surimi during freezing storage by antifreeze peptides. Food Chem. 2022, 371, 131054. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Sharifian, A.; Soltanizadeh, N.; Abbaszadeh, R. Effects of dielectric barrier discharge plasma on the physicochemical and functional properties of myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2019, 54, 1–8. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Xiong, Y.; Ren, Y. Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen-thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Sci. 2010, 85, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Huang, L.; Han, D.; Li, C.; Bian, P.; Xie, P.; Yang, X. Octenyl succinylation of myofibrillar protein: Structural, physicochemical and emulsifying properties. LWT Food Sci. Technol. 2024, 201, 116279. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Xia, X.; Sun, F.; Kong, B. Effect of ultrasound-assisted immersion thawing on emulsifying and gelling properties of chicken myofibrillar protein. LWT-Food Sci. Technol. 2021, 142, 111016. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.; Nakai, S. Determination of SH- and SS-groups in some food proteins using Ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, H.; Mei, K.; Yang, W.; Wang, Z. Effects of high pressure on myofibrillar protein and moisture distribution of shrimp (Solenocera melantho) muscle. J. Aquat. Food Prod. Technol. 2020, 29, 220–228. [Google Scholar] [CrossRef]
- Chung, C.W.; Stephens, A.D.; Ward, E.; Feng, Y.; Davis, M.J.; Kaminski, C.F.; Kaminski Schierle, G.S. Label-free characterization of amyloids and alpha-synuclein polymorphs by exploiting their intrinsic fluorescence property. Anal. Chem. 2022, 94, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Huang, Y.; Zeng, X.; Brennan, M.A. Effect of soluble soybean polysaccharides on freeze-denaturation and structure of myofibrillar protein of bighead carp surimi with liquid nitrogen freezing. Int. J. Biol. Macromol. 2019, 135, 839–844. [Google Scholar] [CrossRef]
- Li, F.; Du, X.; Wang, B.; Pan, N.; Xia, X.; Bao, Y. Inhibiting effect of ice structuring protein on the decreased gelling properties of protein from quick-frozen pork patty subjected to frozen storage. Food Chem. 2021, 353, 129104. [Google Scholar] [CrossRef]
- Han, J.; Sun, Y.; Zhang, T.; Wang, C.; Xiong, L.; Ma, Y.; Zhu, Y.; Gao, R.; Wang, L.; Jiang, N. The preservable effects of ultrasound-assisted alginate oligosaccharide soaking on cooked crayfish subjected to freeze–thaw cycles. Ultrason. Sonochem. 2023, 92, 106259. [Google Scholar] [CrossRef]
- Gao, W.; Hou, R.; Zeng, X. Synergistic effects of ultrasound and soluble soybean polysaccharide on frozen surimi from grass carp. J. Food Eng. 2019, 240, 1–8. [Google Scholar] [CrossRef]
- Gharbi, N.; Labbafi, M. Influence of treatment-induced modification of egg white proteins on foaming properties. Food Hydrocoll. 2019, 90, 72–81. [Google Scholar] [CrossRef]
- Xu, J.; Yang, L.; Nie, Y.; Yang, M.; Wu, W.; Wang, Z.; Wang, X.; Zhong, J. Effect of transglutaminase crosslinking on the structural, physicochemical, functional, and emulsion stabilization properties of three types of gelatins. LWT-Food Sci. Technol. 2022, 163, 113543. [Google Scholar] [CrossRef]
- Kieserling, H.; Pankow, A.; Keppler, J.K.; Wagemans, A.M.; Drusch, S. Conformational state and charge determine the interfacial film formation and film stability of β-lactoglobulin. Food Hydrocoll. 2021, 114, 106561. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, C.; Li, Q.; Xia, X.; Kong, B. Changes in functional properties of common carp (Cyprinus carpio) myofibrillar protein as affected by ultrasound-assisted freezing. J. Food Sci. 2020, 85, 2879–2888. [Google Scholar] [CrossRef]
- Chen, A.; Tanidjaja, I.; Damodaran, S. Nanostructure and functionality of enzymatically repolymerized whey protein hydrolysate. Food Chem. 2018, 256, 405–412. [Google Scholar] [CrossRef]
- Pan, N.; Wan, W.; Du, X.; Kong, B.; Liu, Q.; Lv, H.; Xia, X.; Li, F. Mechanisms of change in emulsifying capacity induced by protein denaturation and aggregation in quick-frozen pork patties with different fat levels and freeze–thaw cycles. Foods 2021, 11, 44. [Google Scholar] [CrossRef]
- Anese, M.; Manzocco, L.; Panozzo, A.; Beraldo, P.; Foschia, M.; Nicoli, M. Effect of radiofrequency assisted freezing on meat microstructure and quality. Food Res. Int. 2012, 46, 50–54. [Google Scholar] [CrossRef]
- Chen, H.; Cao, M.; Cai, Q.; Su, W.; Mao, H.; Liu, G. Purification and characterisation of sarcoplasmic calcium-binding protein, a novel allergen of red swamp crayfish (Procambarus clarkii). Food Chem. 2013, 139, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Jiang, X.; Zhong, H.; Zhang, Z.; Su, W. Degradation of myofibrillar proteins by a myofibril-bound serine proteinase in the skeletal muscle of crucian carp (Carasius auratus). Food Chem. 2006, 94, 7–13. [Google Scholar] [CrossRef]
- Lan, W.; Hu, X.; Sun, X.; Zhang, X.; Xie, J. Effect of the number of freeze–thaw cycles number on the quality of Pacific white shrimp (Litopenaeus vannamei): An emphasis on moisture migration and microstructure by LF-NMR and SEM. Aquac. Fish. 2020, 5, 193–200. [Google Scholar] [CrossRef]
- Cai, L.; Feng, J.; Cao, A.; Tian, H.; Wang, J.; Liu, Y.; Gong, L.; Li, J. Effect of partial substitutes of NaCl on the cold-set gelation of grass carp myofibrillar protein mediated by microbial transglutaminase. Food Bioprocess Technol. 2018, 11, 1876–1886. [Google Scholar] [CrossRef]
- Zhang, Y.; Ertbjerg, P. Effects of frozen-then-chilled storage on proteolytic enzyme activity and water-holding capacity of pork loin. Meat Sci. 2018, 145, 375–382. [Google Scholar] [CrossRef]
- Mao, W.; Li, X.; Fukuoka, M.; Liu, S.; Ji, H.; Sakai, N. Study of Ca2+-ATPase activity and solubility in the whole kuruma prawn (marsupenaeus japonicus) meat during heating: Based on the kinetics analysis of myofibril protein thermal denaturation. Food Bioprocess Technol. 2016, 9, 1511–1520. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C. Ultrasound treatment of frozen crayfish with chitosan Nano-composite water-retaining agent: Influence on cryopreservation and storage qualities. Food Res. Int. 2019, 126, 108670. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, G.; Que, F.; Xia, Y.; Li, X.; Yang, H.; Shi, L.; Wu, W.; Ding, A.; Li, X. Effect of microstructure and chemical proximate composition on mechanical properties of Procambarus clarkii shell. LWT-Food Sci. Technol. 2022, 165, 113731. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, F.; Zhang, L.; Wang, H.; Wang, X. Effect of different extent of protein oxidation on the frozen storage stability of muscle protein in obscure pufferfish (Takifugu obscurus). LWT-Food Sci. Technol. 2021, 137, 110416. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef]
- Cui, X.; Xiong, Y.; Kong, B.; Zhao, X.; Liu, N. Hydroxyl radical-stressed whey protein isolate: Chemical and structural properties. Food Bioprocess Technol. 2012, 5, 2454–2461. [Google Scholar] [CrossRef]
- Shen, H.; Elmore, J.S.; Zhao, M.; Sun, W. Effect of oxidation on the gel properties of porcine myofibrillar proteins and their binding abilities with selected flavour compounds. Food Chem. 2020, 329, 127032. [Google Scholar] [CrossRef]
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative studies on the effect of the freeze–thawing process on the physicochemical properties and microstructures of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) muscle. Food Chem. 2007, 104, 113–121. [Google Scholar] [CrossRef]
- Mutilangi, W.; Panyam, D.; Kilara, A. Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J. Food Sci. 1996, 61, 270–275. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Kong, B.; Han, J.; He, X. The influence of superchilling and cryoprotectants on protein oxidation and structural changes in the myofibrillar proteins of common carp (Cyprinus carpio) surimi. LWT-Food Sci. Technol. 2014, 57, 603–611. [Google Scholar] [CrossRef]
- Mi, J.; Zhao, X.; Huang, P.; Hong, J.; Jia, R.; Deng, S.; Yu, X.; Wei, H.; Yang, W. Effect of hydroxypropyl distarch phosphate on the physicochemical characteristics and structure of shrimp myofibrillar protein. Food Hydrocoll. 2022, 125, 107417. [Google Scholar] [CrossRef]
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.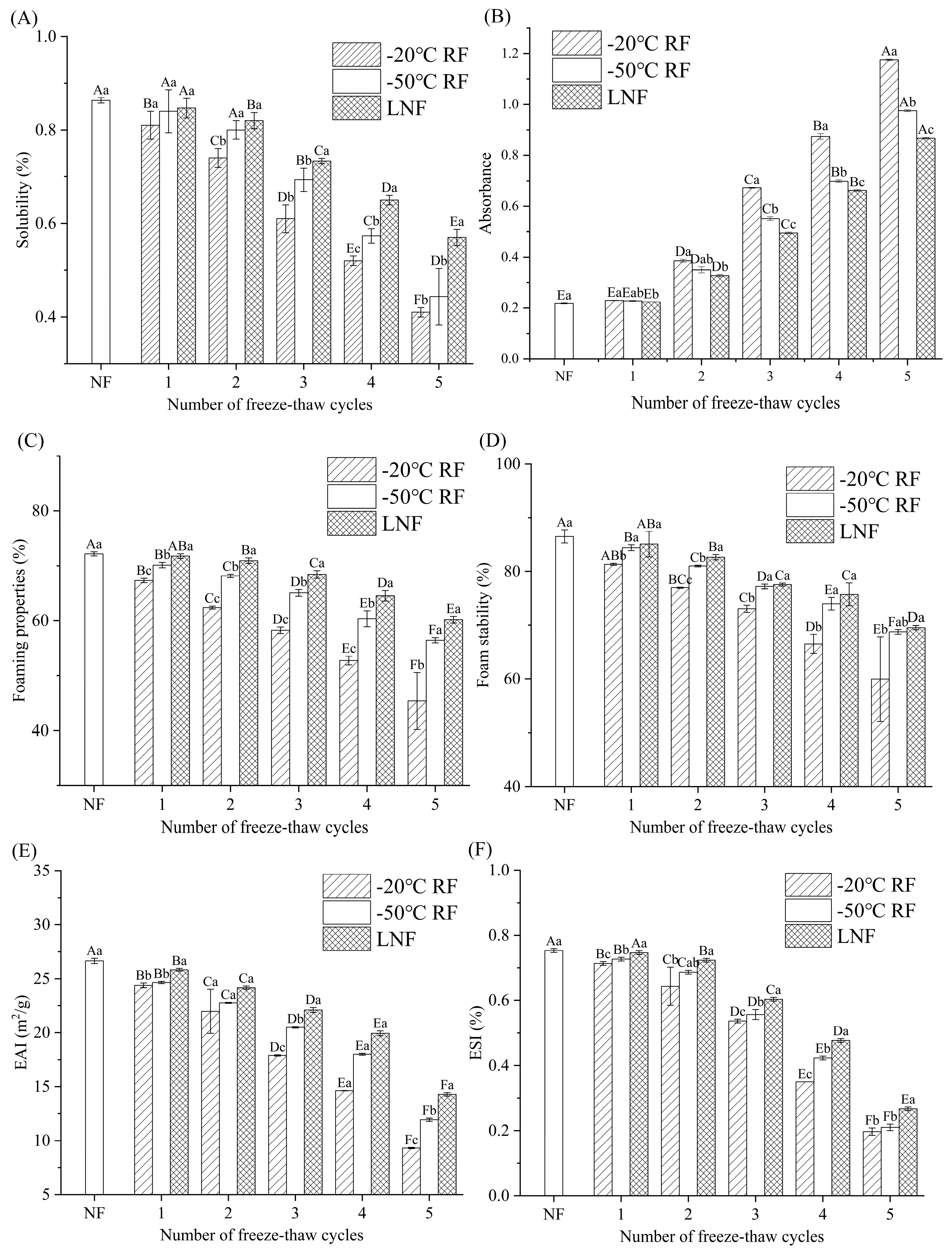
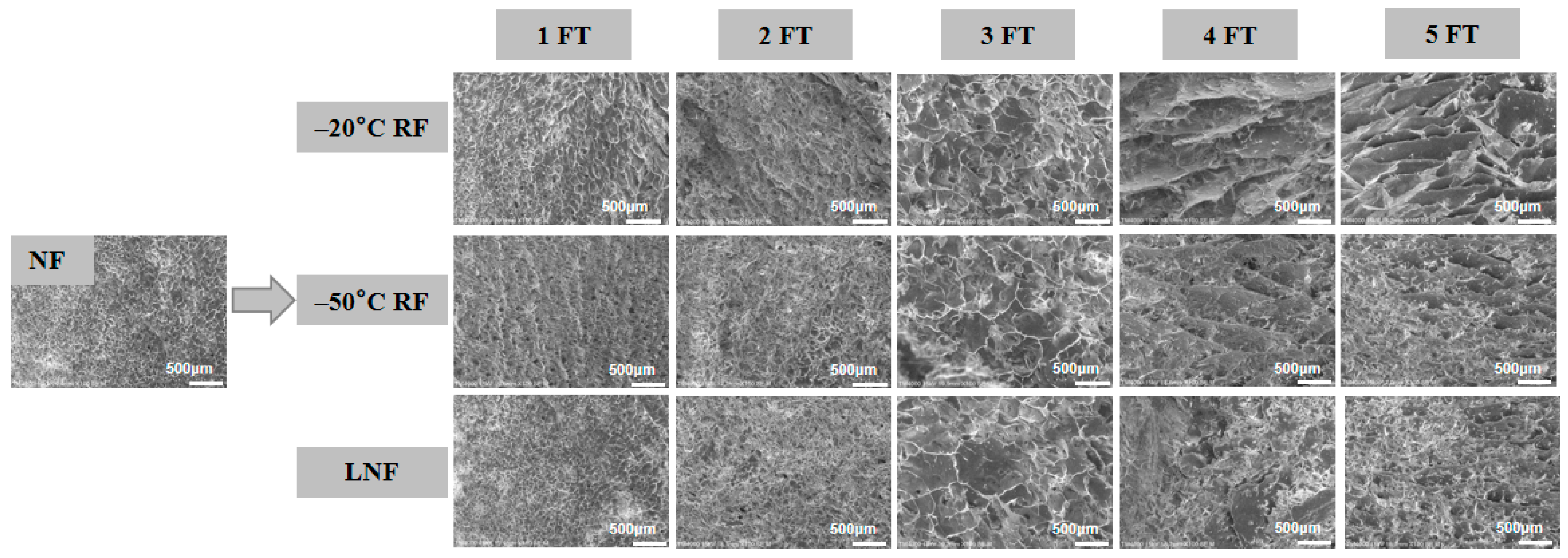
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.
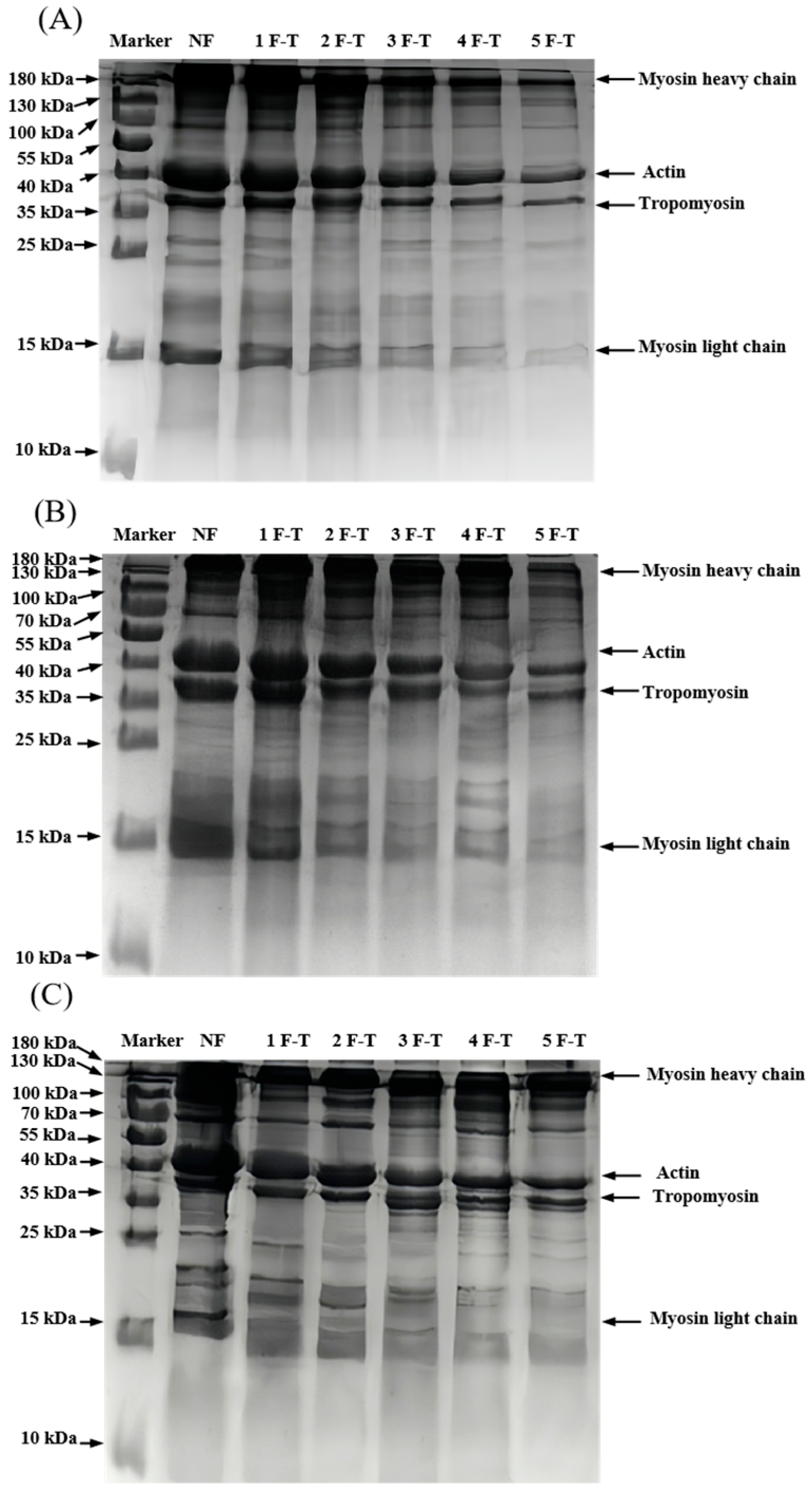
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.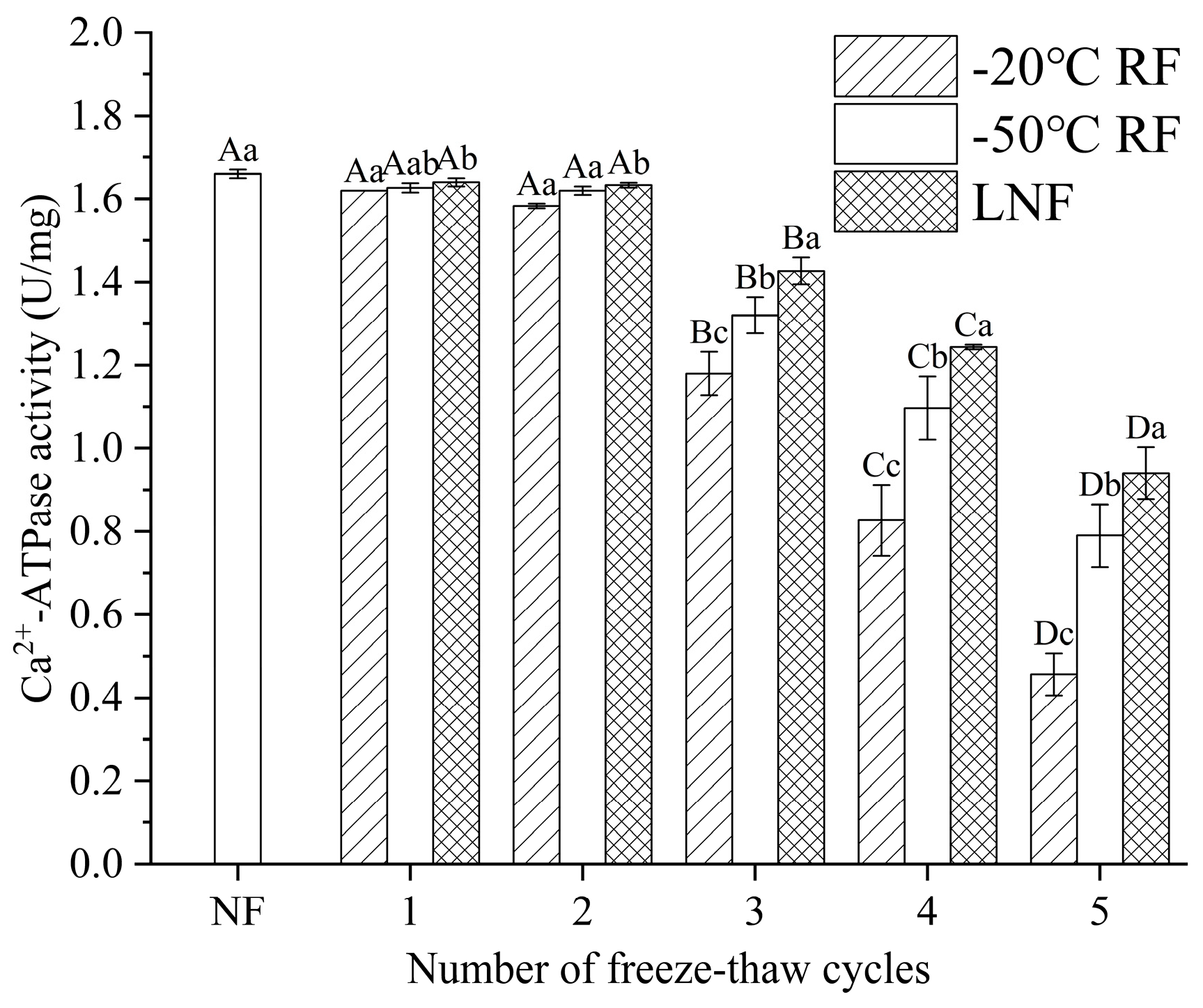
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish subjected to 1, 2, 3, 4 and 5 freeze–thaw cycles.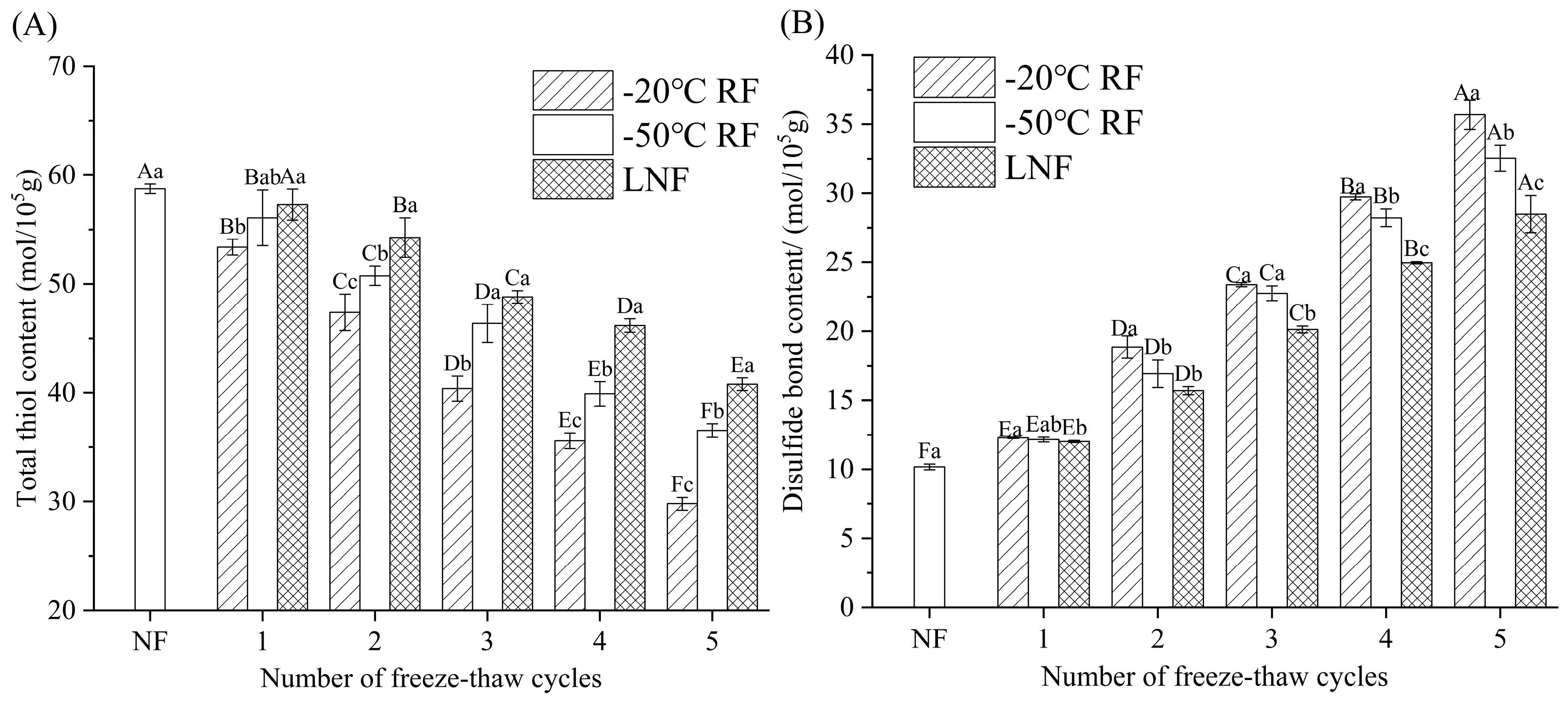
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.
 ); RF indicates refrigerator freezing, −20 °C RF (
); RF indicates refrigerator freezing, −20 °C RF ( ), −50 °C RF (
), −50 °C RF ( ); LNF indicates −80 °C liquid nitrogen freezing (
); LNF indicates −80 °C liquid nitrogen freezing ( ). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.
). The numbers 1, 2, 3, 4 and 5 indicate frozen crayfish after 1, 2, 3, 4 and 5 freeze–thaw cycles.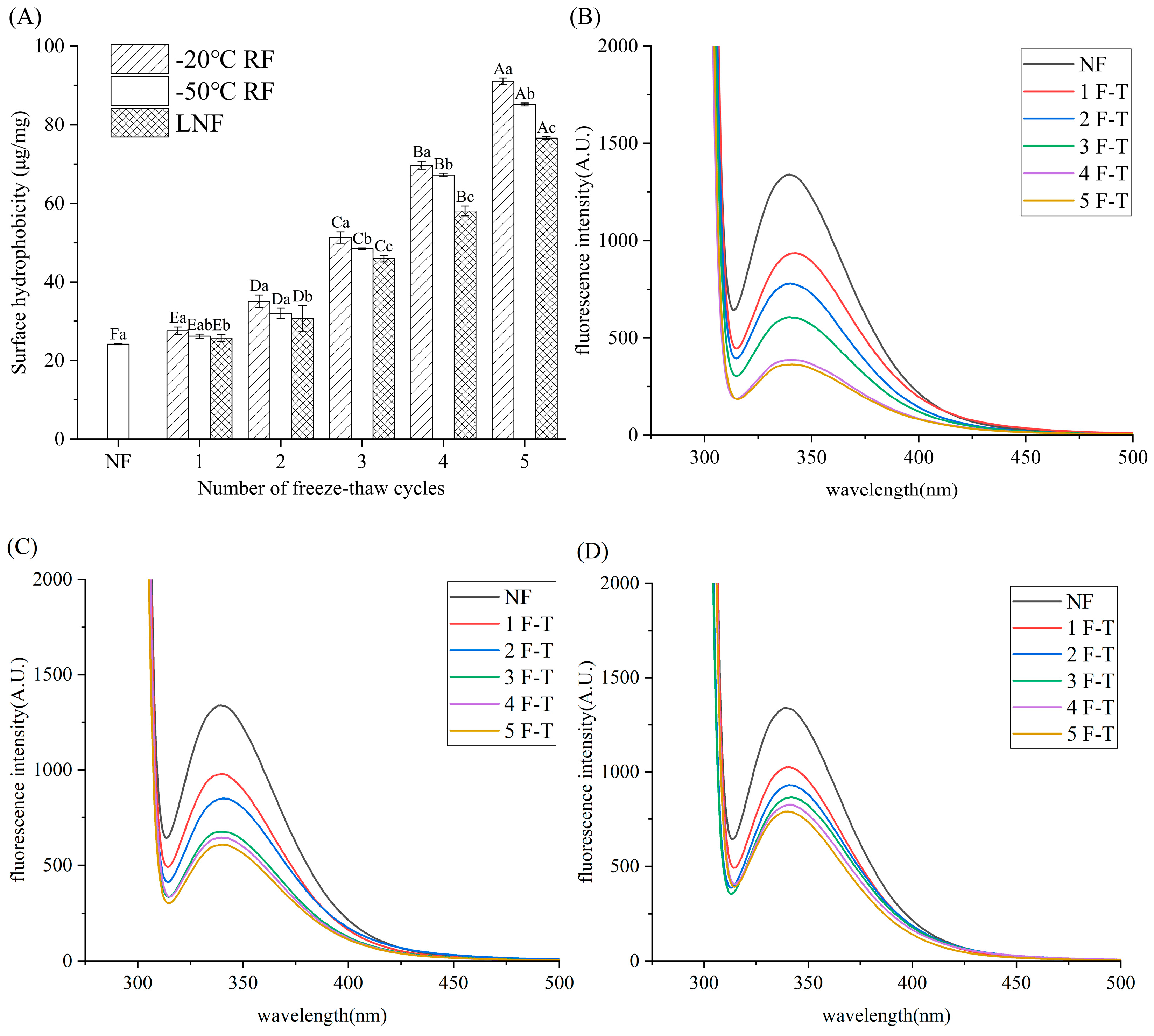
| Crayfish | Secondary Structure | ||||
|---|---|---|---|---|---|
| Temperature | Freeze–Thaw | α-Helix | β-Turn | β-Sheet | Random Coil |
| −20 °C | 1 | 24.32 ± 2.20 Aa | 36.27 ± 2.36 Aa | 28.51 ± 2.13 Cb | 10.91 ± 2.10 Ca |
| 2 | 21.05 ± 1.05 Aa | 28.80 ± 0.43 Bb | 29.62 ± 2.00 Cc | 20.54 ± 0.94 Ba | |
| 3 | 9.32 ± 0.89 Bb | 18.86 ± 0.69 Cb | 47.08 ± 1.18 Bb | 24.74 ± 0.38 Aa | |
| 4 | 9.47 ± 0.49 Ba | 17.97 ± 1.55 Ca | 61.09 ± 1.70 Aa | 11.47 ± 0.55 Cc | |
| 5 | 6.00 ± 0.51 Cb | 10.58 ± 1.13 Db | 63.05 ± 1.08 Aa | 20.37 ± 1.16 Bb | |
| −50 °C | 1 | 22.19 ± 2.59 Aa | 28.34 ± 1.74 Ab | 37.84 ± 1.22 Da | 11.64 ± 0.92 Ca |
| 2 | 21.19 ± 2.24 Aa | 25.53 ± 1.82 Bb | 41.52 ± 1.37 Ca | 11.44 ± 0.86 Cb | |
| 3 | 10.18 ± 1.41 Bb | 21.17 ± 1.80 Cb | 54.42 ± 1.99 Ba | 14.24 ± 0.58 Bb | |
| 4 | 6.72 ± 1.36 Cb | 15.67 ± 0.64 Cb | 56.04 ± 1.21 Bb | 21.57 ± 1.31 Ab | |
| 5 | 5.43 ± 0.68 Cb | 20.88 ± 0.25 Da | 59.40 ± 0.61 Ab | 14.28 ± 0.74 Bc | |
| −80 °C | 1 | 23.36 ± 2.03 Aa | 36.15 ± 1.13 Aa | 28.44 ± 1.55 Db | 11.05 ± 1.16 Ca |
| 2 | 22.75 ± 0.71 Aa | 34.97 ± 2.67 Aa | 33.20 ± 1.65 Cb | 9.08 ± 1.24 Cc | |
| 3 | 15.00 ± 1.85 Ba | 35.00 ± 2.32 Aa | 35.00 ± 2.15 Cc | 15.00 ± 1.24 Bb | |
| 4 | 9.00 ± 0.74 Ca | 19.00 ± 0.41 Ba | 47.00 ± 1.28 Bc | 25.00 ± 1.13 Aa | |
| 5 | 7.93 ± 0.10 Ca | 11.55 ± 0.49 Cb | 54.63 ± 0.57 Ac | 26.06 ± 2.51 Aa | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, G.; Zhang, P.; Xu, L.; Wang, L.; He, X.; Chen, J. Understanding the Protective Effect of Liquid Nitrogen Freezing on Crayfish Quality During Transportation and Storage. Foods 2025, 14, 2078. https://doi.org/10.3390/foods14122078
Lei G, Zhang P, Xu L, Wang L, He X, Chen J. Understanding the Protective Effect of Liquid Nitrogen Freezing on Crayfish Quality During Transportation and Storage. Foods. 2025; 14(12):2078. https://doi.org/10.3390/foods14122078
Chicago/Turabian StyleLei, Gehao, Peng Zhang, Limin Xu, Liuqing Wang, Xiaoyue He, and Jiwang Chen. 2025. "Understanding the Protective Effect of Liquid Nitrogen Freezing on Crayfish Quality During Transportation and Storage" Foods 14, no. 12: 2078. https://doi.org/10.3390/foods14122078
APA StyleLei, G., Zhang, P., Xu, L., Wang, L., He, X., & Chen, J. (2025). Understanding the Protective Effect of Liquid Nitrogen Freezing on Crayfish Quality During Transportation and Storage. Foods, 14(12), 2078. https://doi.org/10.3390/foods14122078





