Abstract
Metabolic syndrome (MetS) is a complex condition defined by central obesity, insulin resistance, dyslipidemia, and systemic inflammation. Kefir, a fermented beverage rich in probiotics and beneficial compounds, has emerged as a functional food that may offer metabolic advantages. Nevertheless, preclinical results have been variable. This systematic review and meta-analysis aimed to assess the influence of kefir and its derived compositions on parameters associated with MetS, inflammation, and oxidative stress in rodent studies. A comprehensive literature search was conducted in PubMed, Scopus, AMED, and LILACS through June 2024. Eligible studies involving kefir interventions in rodent MetS models were included. Data extraction followed PRISMA guidelines, with the risk of bias assessed using the CAMARADES and SYRCLE tools. Meta-analyses were performed with a random effects model. Thirty-eight studies involving 1462 rodents (mice and rats) were analyzed. Kefir significantly reduced body weight gain in both mice (MD = –3.33; 95% CI: –4.89 to –1.77) and rats (MD = –41.53; 95% CI: –54.33 to –28.72). In mice, triglycerides and LDL-C levels decreased significantly; in rats, kefir lowered total cholesterol and triglycerides. Insulin levels were reduced (MD = –0.69; 95% CI: –1.16 to –0.22), suggesting improved insulin sensitivity. Several studies also reported reductions in TNF-α, IL-1β, and IL-6. Despite promising results, the high heterogeneity and methodological variability emphasize the need for standardized preclinical protocols and clinical validation. These findings support the role of kefir as a functional food for metabolic health promotion.
1. Introduction
Metabolic syndrome (MetS) consists of interconnected metabolic abnormalities such as central obesity, insulin resistance, dyslipidemia, and hypertension, leading to a heightened risk of type 2 diabetes, cardiovascular diseases (CVD), and some cancers. It significantly contributes to the ongoing epidemics of diabetes and CVD, with expected increases in these conditions [1,2]. Individuals with MetS are at a considerably greater risk for cardiovascular disease (CVD), experiencing a 32% increased likelihood and a 69.5% higher risk of heart failure [3]. Combining impaired glucose tolerance with MetS further elevates cardiovascular mortality risk, as indicated by a hazard ratio of 2.96 compared to those without MetS [4]. In chronic kidney disease patients, MetS is linked to a 26% higher all-cause mortality risk and a 48% increased risk of cardiovascular events [5]. Globally, the prevalence of MetS ranges from 10% to 50%, with a marked increase in South and Southeast Asia over the last three decades due to urbanization and economic development, shifting the disease burden from communicable to non-communicable diseases [6,7,8]. Chronic low-grade inflammation and oxidative stress are crucial in the pathogenesis of MetS [9]. Obesity, particularly visceral adiposity, heightens pro-inflammatory macrophage polarization in adipose tissue. This leads to increased production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), alongside a dysregulated secretion of adipokines, which includes elevated levels of leptin and decreased levels of adiponectin. These changes contribute to systemic inflammation, insulin resistance, and metabolic dysregulation in individuals with MetS [10]. Additionally, oxidative stress causes mitochondrial dysfunction and lipid peroxidation, impairing cellular functions and contributing to MetS components. The interaction between inflammation and oxidative stress creates a vicious cycle that exacerbates metabolic dysfunction in MetS [11].
Fermented foods and beverages containing probiotics or synbiotics, including kefir, yogurt, synbiotic yogurt (fortified with prebiotics), kimchi, kombucha, and sauerkraut, have demonstrated effectiveness in alleviating metabolic syndromes by reducing chronic inflammation and oxidative stress [12]. Key components of metabolic syndrome, including glycemic indices and lipid profiles, show improvement with the consumption of probiotics [12,13,14]. Probiotics enhance gut health by altering the microbiota and producing bioactive metabolites, particularly short-chain fatty acids (SCFAs), which further mitigate oxidative stress. Specific strains, such as Bifidobacterium lactis, Enterococcus faecium, and Lactobacillus paracasei reduce inflammatory markers in both healthy and metabolic-syndrome-afflicted models [15,16]. At present, probiotic beverages, including kefir [17], kombucha [18], drinkable yogurt [19], and kvass [20], have garnered significant attention from consumers. These beverages are regarded as alternative interventions that facilitate the stability of the gut microbiome and demonstrate advantageous effects on MetS, reducing systemic inflammation and oxidative stress. Thus, incorporating these functional beverages into the diet might represent a practical approach for managing MetS alongside conventional medical treatments.
Kefir is a probiotic-rich beverage made from the fermentation of milk or plant-based substrates by a symbiotic mix of bacteria and yeasts known as kefir grains. It includes beneficial microorganisms such as Lactobacillus, Leuconostoc, Lactococcus, and Acetobacter, as well as yeasts like Saccharomyces and Kluyveromyces [17,21]. Kefir also contains bioactive metabolites, including peptides, exopolysaccharides, and organic acids, which enhance its antioxidant, anti-inflammatory, antimicrobial, and hypocholesterolemic effects [17,21,22,23]. Preclinical studies conducted in rodent models have shown their potential to improve lipid profiles, reduce body weight gain, enhance glucose tolerance, and suppress inflammatory cytokines such as TNF-α and IL-6 [24,25,26]. These anti-inflammatory effects are thought to involve multiple pathways of action across various cell types, including the modulation of immune cell signaling, inhibition of NF-κB activation, and regulation of the JAK2 signaling pathway, all of which collectively contribute to the reduction of systemic inflammation. A systematic review highlighted the role of kefir in modulating immune responses and reducing inflammation through the alteration of pro-inflammatory cytokines and enhancement of anti-inflammatory responses, alongside changes in intestinal microbiota composition [17,27,28]. However, the evidence across studies remains fragmented due to methodological variations, including differences in kefir type (e.g., dairy vs. non-dairy, live vs. heat-treated), animal species used, duration of intervention, and measurement of outcomes.
Despite the increasing number of experimental studies, a thorough synthesis of findings from animal research and an assessment of the overall effectiveness of kefir and its components in relation to MetS remains scarce. Systematic reviews and meta-analyses of preclinical data play a crucial role in guiding future investigations, improving experimental designs, and contributing to the translational potential for clinical applications. Therefore, this study aimed to systematically evaluate and quantitatively assess the impacts of kefir and its derivatives, which include kefir grains, kefir-fermented products, isolated probiotics, peptides, exopolysaccharides, and other kefir-derived bioactive compounds, on factors associated with metabolic syndrome, including body weight, lipid levels, glucose and insulin concentrations, and inflammatory and oxidative stress markers in rodent models. The findings from this study are expected to offer significant insights into the therapeutic potential of kefir as a functional food for managing metabolic health.
2. Methods
The design and reporting of our study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. This systematic review was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY®) register (INPLASY2024100010; DOI: 10.37766/inplasy2024.10.0010).
2.1. Data Sources and Searches
A comprehensive review of studies was conducted using the online databases PubMed, Scopus, AMED, and LILACS, covering publications up to June 2024 with common keywords related to kefir and metabolic syndromes without language restrictions. All search terms can be found in the Supplementary Materials (Table S1). Furthermore, we conducted a manual examination of the bibliographies of all selected articles to identify additional pertinent papers that were not included in the initial database search.
2.2. Study Selection and Eligibility
All abstracts and titles were screened by three authors (ZNQ, WPL, and BBL) to include papers that addressed kefir consumption in rodent studies related to metabolic syndromes. Duplicate articles across all databases were removed. Additionally, studies involving humans, in vitro studies, letters to the editor, dissertations, theses, and papers not relevant to the main issue were excluded from the study. We included studies that met the following inclusion criteria: (1) use of kefir or isolated bacterial strains and metabolites as monotherapy or in combination with other drugs, chemicals, or types of interventions; (2) utilization of a rodent model for metabolic syndromes; (3) a control group receiving alternative treatments or compared with a placebo; (4) outcomes reported concerning metabolic-syndrome-related parameters (lipid profile, glucose levels, blood pressure, blood glucose, and insulin resistance), inflammation markers (interleukin (IL)-6, IL-1, tumor necrosis factor (TNF)), or oxidative stress markers (MDA, ROS, and GSH levels).
2.3. Data Collection
Data related to experimental design were extracted, and for each comparison, the mean, median, and percentage reduction of the specified parameters in both the treated and control groups were recorded. General characteristics (year of publication, author, date of publication, and country), as well as characteristics of the experimental model (animal lineage, number of animals, sex, age, and initial weight) and experimental techniques (number of experimental groups, number of animals per group, metabolic syndrome (MetS) induction, presence of a control group, treatment and dosage, and duration of the intervention), were among the parameters of interest in the included studies. The primary outcome measures for further analysis included direct metabolic parameters (e.g., body weight, glucose, insulin, lipid profiles), inflammatory markers, and oxidative stress status, which were based on the availability of data from the included studies. While cortisol levels and parameters related to the hypothalamic–pituitary–adrenal (HPA) axis are recognized as key factors in regulating metabolic syndrome, a comprehensive review of the included preclinical studies showed no findings regarding these hormonal outcomes. Consequently, cortisol and HPA axis parameters were excluded from this current review. It is important to emphasize that a comprehensive understanding of the role of kefir in modulating stress-related metabolic dysfunction is urgently needed.
In instances where the data were not clearly or comprehensively described, the authors were contacted seeking the information; studies from which no response was received after a two-week period were subsequently excluded. Furthermore, a quality assessment of the studies was conducted utilizing the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist items [30] (Supplementary Materials, Table S2).
2.4. Quality Assessment of the Included Articles
All articles selected for this review underwent an analysis of the risk of bias utilizing SYRCLE’s Risk of Bias (RoB) tool, specifically designed for animal intervention studies [31], which comprises ten components (Supplementary Materials, Table S3). These components pertain to six distinct types of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Terminology signifying low, high, or unclear was employed to delineate each domain. Three researchers (WPL, KYP, and MMS) conducted independent assessments of the quality of the included articles.
2.5. Statistical Analysis
A meta-analysis of extracted data, specifically concentrating on parameters associated with metabolic syndrome—such as body weight gain; lipid profiles including total cholesterol, HDL cholesterol, and LDL cholesterol; triglycerides; plasma glucose; and insulin levels—was performed utilizing MetaAnalysisOnline.com (https://metaanalysisonline.com; accessed on 10 January 2025) [32]. The mean values, standard deviations (SD), and sample sizes from both the intervention and control groups were directly entered into the tool. When studies presented standard errors (SE), these were converted into SD prior to data input. Continuous outcomes were analyzed employing a random effects model, due to the anticipated methodological and biological heterogeneity across studies. The mean difference (MD) with a 95% confidence interval (CI) was utilized to ascertain effect sizes, taking into account the various measurement scales applied in the studies. The between-study variance was estimated using the method of moments (DerSimonian and Laird), with the heterogeneity among studies assessed through Cochran’s Q-test and quantified using the I2 statistic. An I2 value exceeding 50% signified substantial heterogeneity. Publication bias was visually examined through funnel plots generated by MetaAnalysisOnline.com.
3. Results
3.1. Study Inclusion
The article selection process adhered to the PRISMA guidelines [29], and a PRISMA flow diagram (Figure 1) was created to illustrate the number of articles included or excluded at each stage of the protocol. A total of 120 records were located in electronic databases. The following studies were excluded: 27 due to repetitive content, 49 for failing to meet exclusion criteria based on an evaluation of titles and abstracts, and 6 after a thorough review of full-text articles for reasons such as being review articles, clinical studies, or in vitro experiments, or lacking sufficient outcome information. Finally, 38 studies were included in the final analysis of this article.
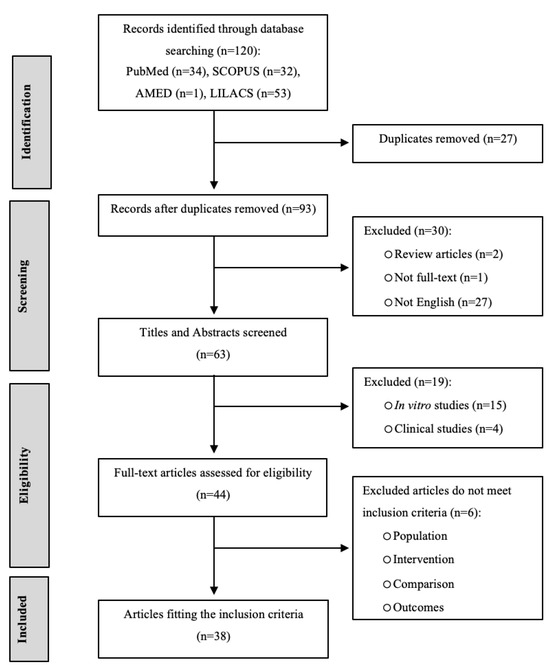
Figure 1.
PRISMA flow diagram of the systematic review process.
3.2. Characteristics of the Included Studies
A total of 38 studies involving 1462 animals were included: 457 rats and 1005 mice. The details of the experimental studies are provided in the Supplementary Materials (Table S4). The studies selected from 2006 [33] to 2024 [34] were conducted across 11 distinct countries. Notably, South Korea emerged as the leading nation, accounting for 29% (n = 11) of the studies [25,33,34,35,36,37,38,39,40,41,42], followed by Taiwan with 21% (n = 8) [24,43,44,45,46,47,48,49]. Nineteen additional studies were conducted in Turkey (n = 5; [26,50,51,52,53]), Canada (n = 4; [22,54,55,56]), China (n = 2; [57,58]), Indonesia (n = 2; [59,60]), Brazil (n = 2; [61,62]), Malaysia [63], Egypt [64], Argentina [65], and Tunisia [66], as illustrated in Figure 2.
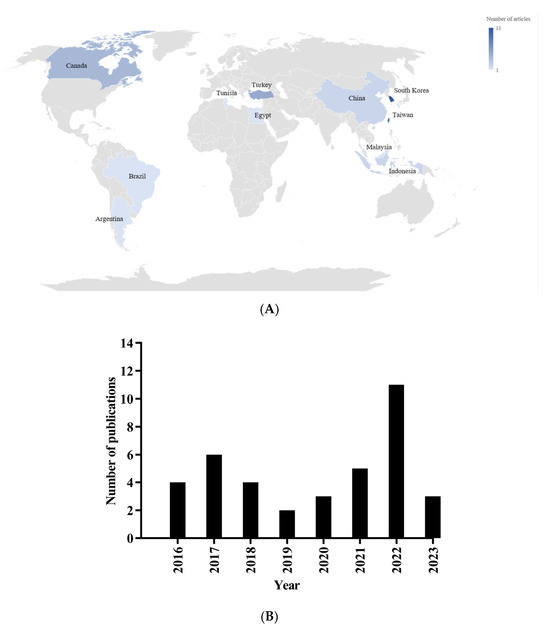
Figure 2.
Geographical distribution (A) and publication year (B) of included studies.
3.3. Experimental Models
In studies using animal models, mice were the experimental animals in 67% of cases (n = 24) [22,24,25,34,35,36,37,38,39,40,41,42,43,45,46,47,49,54,55,56,60,62,63,65], while rats were used in 33% (n = 14) [26,33,44,48,50,51,52,53,57,58,59,61,64,66]. Most of the animals were male, accounting for 82% (n = 32), with female rodents only representing 12% (n = 5) [22,55,57,58,66]. Only two studies, or 5% (n = 2) [54,63], involved both male and female rodents (Figure 3 and Supplementary Materials, Table S4). The age of the animals utilized in these studies varied from 3 [26,50] to 22 weeks [35]. The age distribution among the rat models indicates that approximately 50% of the studies employed rats younger than 8 weeks, while 35.71% involved rats aged under 8 weeks, and 14.29% did not specify the age of the rats. The majority (79.17%) of studies involving mice utilized animals younger than 8 weeks, whereas a smaller proportion (20.83%) included mice that were 8 weeks or older.
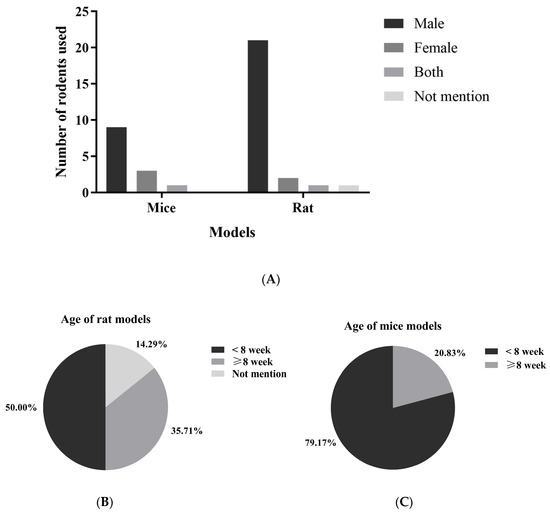
Figure 3.
The gender (A) and age (B,C) distribution of rodents used in kefir evaluation studies represent the demographics of rodents used to assess the beneficial effects of kefir in studies involving metabolic syndrome.
All 38 studies employed dietary strategies to induce metabolic syndrome, primarily using high-fat diets (HFD), which accounted for 36.84% (14 out of 38 studies). In particular, supplying around 60% of calories from fats was the predominant strategy utilized in six studies [34,40,43,46,47,53]. Other approaches included fructose-rich diets (FRD), high-fructose corn syrup solutions (HFCS), hypercholesterolemic diets, and Western diets (WD). The dietary interventions ranged from straightforward high-fat diets that provided 40% to 60% of calories from fats to more complex combinations, such as high-fat high-fructose (HFHF), atherogenic diets, and high-fat high-sucrose (HFHS) diets (Supplementary Materials, Table S5 and Figure 4).
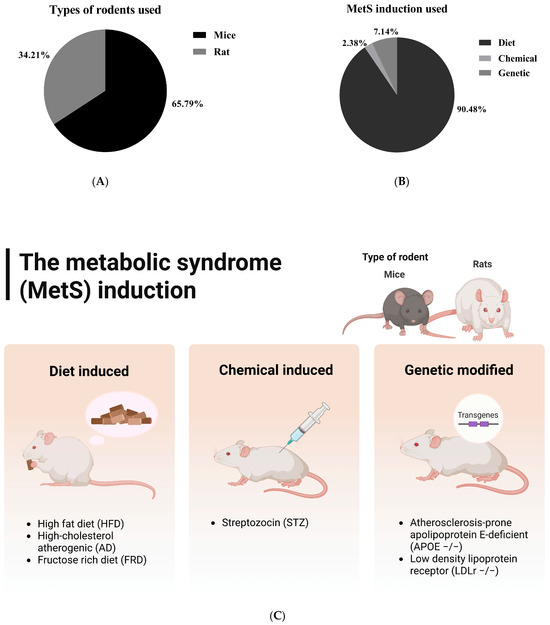
Figure 4.
Types of rodents used in the included studies (A) and the detailed methods (B,C) for inducing metabolic syndrome in these rodents. The first group represents diet-induced metabolic syndrome, while the second and third models combine dietary and chemical induction (e.g., high-fat diet plus streptozotocin) and dietary and genetic induction (e.g., ApoE knockout mice), respectively. This classification reflects the experimental strategies utilized in the included studies.
The dietary induction and treatment duration varied significantly in duration across studies, ranging from a brief 6 days [26] to an extensive 15 weeks [50]. However, most studies typically employed induction methods lasting between 6 and 12 weeks, which reflects a standard approach to effectively induce metabolic syndrome (Supplementary Materials, Table S5 and Figure 4). Interestingly, there are only a few studies that combine dietary methods with chemical or genetic approaches. Two studies used a genetic induction model with ApoE −/− mice [45,49], making them susceptible to severe hypercholesterolemia and atherosclerosis, while one study employed chemical induction using streptozotocin (STZ) [63].
3.4. Interventions
A comprehensive overview of the experimental designs and kefir treatment protocols utilized in the 38 eligible preclinical studies that evaluate the effects of kefir on metabolic syndrome in rodent models is presented in Table 1. The research explored various types of kefir and its bioactive ingredients. This includes milk-based kefir grains [26,50,62], commercial kefir products [22,51,52,58], lactic acid bacteria derived from kefir [35], isolated probiotics like Lactobacillus kefiri and Lactobacillus mali APS1 [37,44], kefir peptides [45,48,49], and various formulations of symbiotic and postbiotic kefir [42,59]. Dosages varied considerably across studies. Liquid kefir administration ranged from low volumes of 0.001 mL/g body weight daily [26] to higher doses of 22 mL/kg body weight daily [62]. Probiotic dosages commonly ranged between 106 to 1010 CFU/mouse/day [44,63], while powdered forms of kefir and its active components were generally provided in the range of 0.05 mg/g to 400 mg/kg body weight daily [24,48,49]. Dietary incorporation varied from 0.1% to 10% w/w [33,40]. The duration of kefir administration across studies was variable, ranging from a minimum of 3 weeks [60,61] to a maximum of 16 weeks [53]. Most studies used treatments of 6 to 12 weeks, indicating a sufficient period to observe metabolic and physiological effects in animal models of metabolic syndrome. These studies mainly utilized control groups such as water [26], saline [38,43], phosphate-buffered saline (PBS) [40,46], microcrystalline cellulose [39,40], milk [25,37,62], or standard chow diet (SCD) [36,55].

Table 1.
Experimental designs and treatment protocols for animal models of metabolic syndromes used in the eligibility studies.
3.5. Quality Assessment
The methodological quality of the 38 studies was assessed using the 10-item CAMARADES checklist (Supplementary Materials Table S2), which evaluates the internal validity of animal research. Most studies showed moderate to high quality. Key aspects such as peer-reviewed publication, random group allocation, and compliance with animal welfare regulations were noted in nearly all studies, indicating strong adherence to experimental standards. However, blinded outcome assessments and sample size calculations were rarely reported, suggesting a lack of rigor and potential observer bias. Less than half disclosed blinded induction of metabolic syndrome or conflicts of interest, indicating transparency issues. Only a few satisfied all 10 criteria, with most scoring between 6 and 8, suggesting good practices but neglect of reproducibility and blinding elements.
The SYRCLE risk of bias tool was used to assess six domains of bias, including selection, performance, detection, attrition, reporting, and other potential sources. Most studies were rated as low risk for random sequence generation, but allocation concealment was often rated high risk or not reported, indicating inadequate control over group assignment processes. Similarly, few studies employed random housing or blinded care of animals, leading to a high or unclear risk for this domain across the dataset. There was consistently high risk due to the lack of random outcome assessment and blinded outcome assessment, which suggests that the results could be influenced by observer expectations. These were often rated as unclear because of insufficient information regarding incomplete data handling and outcome reporting (Supplementary Materials, Table S3 and Figure 5).
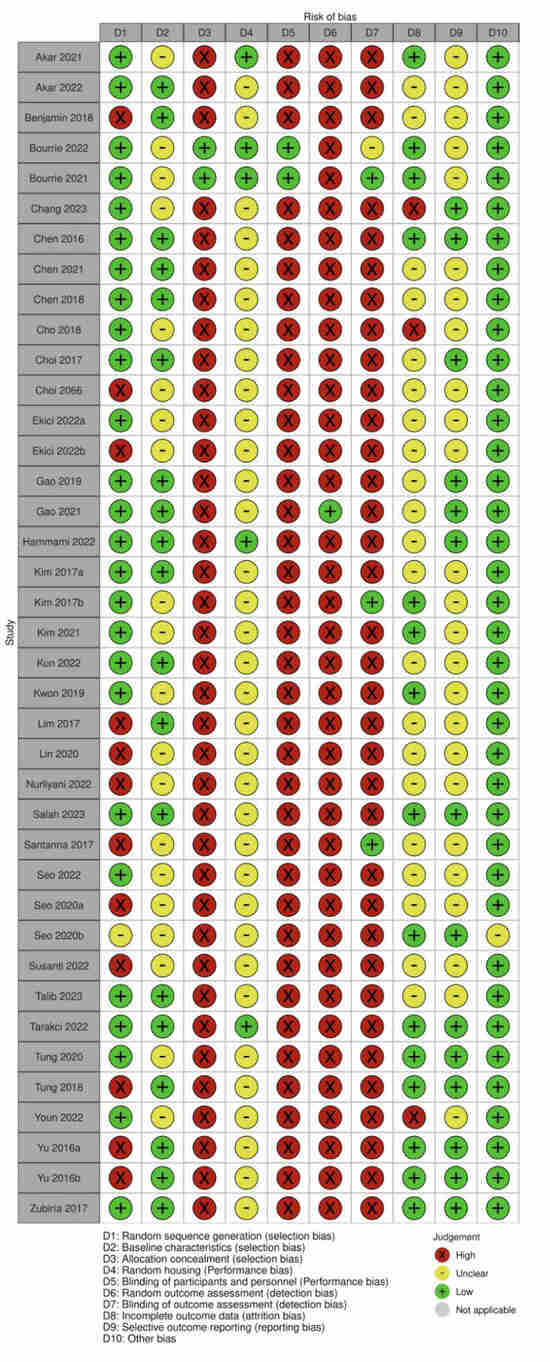
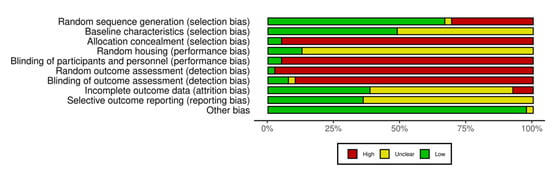
Figure 5.
Quality of reporting and bias evaluation conducted with SYRCLE’s risk of bias tool. The upper panel illustrates the quality of reporting and bias risk in the included studies [22,24,25,26,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66], while the lower panel evaluates biases related to selection, performance, detection, attrition, and other factors.
3.6. Qualitative Synthesis
A comprehensive summary of the outcomes related to metabolic syndrome, inflammation, and oxidative stress parameters influenced by kefir consumption or its active components in rodent models across 38 studies is provided in the Supplementary Materials (Table S6) and summarized in Figure 6. Kefir administration resulted in a reduction of body weight gain, indicating a beneficial effect of kefir on managing the weight increase commonly associated with metabolic syndrome (e.g., [26,43,54,56]. Additionally, the majority of studies demonstrated improvements in lipid profiles, including total cholesterol (TC), triglycerides (TG), LDL-C, and HDL-C. The consumption of kefir consistently reduced total cholesterol (e.g., [22,34,58]), triglycerides (e.g., [24,26,33]), and LDL cholesterol (e.g., [25,35,37,46]). HDL cholesterol levels were commonly elevated by kefir administration (e.g., [33,39,66]), though some studies reported reductions (e.g., [45,56,58]). Many studies found kefir administration decreased plasma glucose and insulin levels, suggesting a potential role in improving glucose homeostasis and insulin sensitivity (e.g., [26,46,51,52]). Few studies, however, showed mixed or elevated glucose outcomes [56].
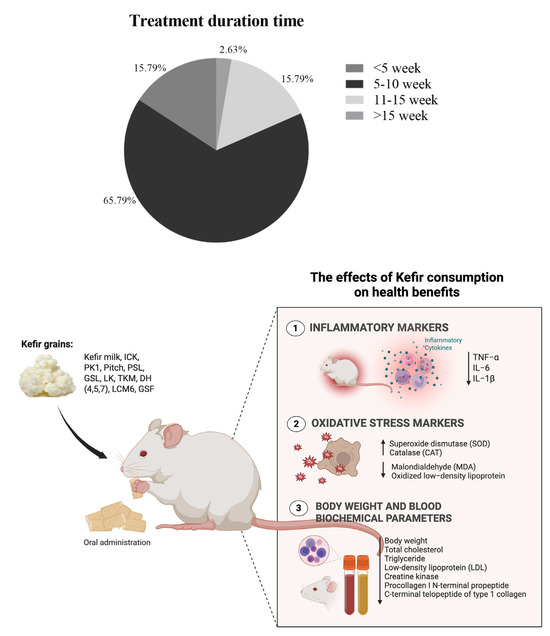
Figure 6.
Overview of study characteristics and outcome measures. The upper panel summarizes the distribution of treatment durations across the included studies, while the lower panel presents the inflammatory and oxidative stress markers evaluated in those studies.
Several studies have reported significant anti-inflammatory effects of kefir, particularly in reducing inflammatory cytokines such as TNF-α, IL-1β, and IL-6. A total of 38.58% (n = 12) of the studies reported that dietary interventions such as fructose, HFD, HFHF, and HFCS led to significant increases in TNF-α levels, which were subsequently attenuated by kefir treatment. Five studies (13.16%) focused on the impact of kefir on IL-1β, and the results indicated that kefir consumption greatly reduced elevated levels of IL-1β caused by various diets, including the AD diet, HFD, and HFCS. Similarly, three studies reported a reduction of IL-6, all noting the effectiveness of kefir in this regard. Specifically, Akar et al., (2021) and Chang et al., (2023) [26,45] highlighted the ability of kefir to significantly reduce TNF-α and IL-1β. Kim et al., (2017) reported mixed outcomes, showing increased TNF-α and IL-1β but decreased IL-6 levels [25,37]. Santanna et al., (2017) and Tung et al., (2020) [49,62] also noted decreases in TNF-α and IL-6 levels. It should be noted that only one study [45] explicitly evaluated oxidative stress markers, reporting that kefir intake significantly reduced levels of MDA and oxidized LDL, suggesting potential antioxidant properties of kefir components.
3.7. Quantitative Synthesis
The meta-analysis was performed on synthesized data from multiple preclinical studies examining the effects of kefir interventions on metabolic syndrome (MetS)-related parameters, including weight gain, lipid profiles, plasma glucose, and insulin levels in both mouse and rat models. The included studies for meta-analysis contain similar diets and a consistent number of animals per group (i.e., studies with varying numbers of animals in different groups were excluded) (Supplementary Materials, Table S7).
The meta-analysis for inflammatory and oxidative stress parameters was excluded due to the limited number of comparable studies. Specifically, a predefined threshold was applied, necessitating the inclusion of at least three independent studies reporting on the same outcome for the meta-analysis. This criterion was established to ensure sufficient statistical power and to avoid the instability of effect estimates that may result from extremely small data sets (Supplementary Materials, Table S8). The results of the meta-analysis are summarized as follows.
A total of 14 studies were analyzed, encompassing a cumulative total of 129 mice across both experimental and control groups. The analysis was conducted utilizing a random effects model in conjunction with the inverse variance method to compare the mean difference (MD). The results indicate a statistically significant difference between the two groups, with the summarized MD reported as −3.33 and a 95% confidence interval ranging from −4.89 to −1.77. The overall effect test reveals significance at p < 0.01. Additionally, substantial heterogeneity was identified (p < 0.01), implying inconsistent effects in either magnitude or direction. The I2 value denotes that 99% percent of the variability among studies is attributed to heterogeneity rather than random chance. Similarly, weight gain in rats across six studies showed that the summarized MD was −41.53 with a 95% confidence interval ranging from −54.33 to −28.72, showing a significant reduction in weight gain in the experimental group compared to controls (p < 0.01). These findings emphasize the potential of kefir in managing diet-induced obesity (Figure 7 and Supplementary Materials, Figure S1).
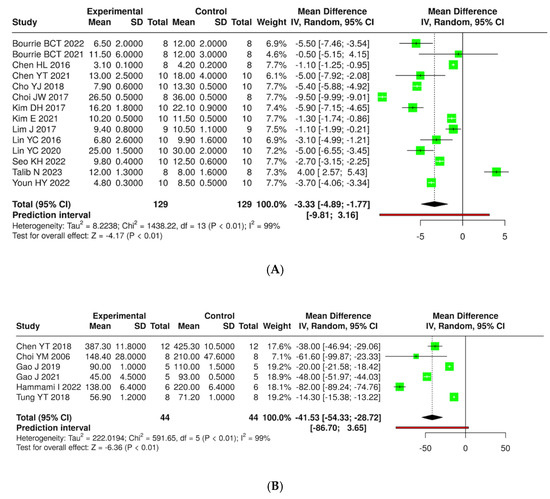
Figure 7.
Forest plot analysis presenting the effects of consuming different types of kefirs, their isolated bacteria, or active components, compared to the control group, on weight gain in mouse (A) [24,25,35,36,38,40,41,42,43,46,47,54,55,63] and rat (B) [36,44,48,57,58,66] models. Each green square shows the mean difference (MD) point estimate for individual studies, while the red line denotes the 95% prediction interval for the overall meta-analysis.
Figure 8 illustrates that the consumption of kefir enhances glycemic control in murine models, as evidenced by a reduction in fasting plasma glucose and insulin levels. A total of eight studies involving 79 mice (both experimental and control groups) were analyzed for plasma glucose; however, no statistically significant difference between the groups was observed (pooled mean difference −29.62, 95% CI: −86.17 to 26.93), with a heterogeneity of 100% (p < 0.01). In a subsequent analysis of five studies for insulin level, each encompassing 49 mice, a significant difference was found (pooled MD −0.69, 95% CI: −1.16 to −0.22, p < 0.05); nonetheless, substantial heterogeneity continued to exist (p < 0.01, I2 = 100%) (Supplementary Materials, Figure S2).
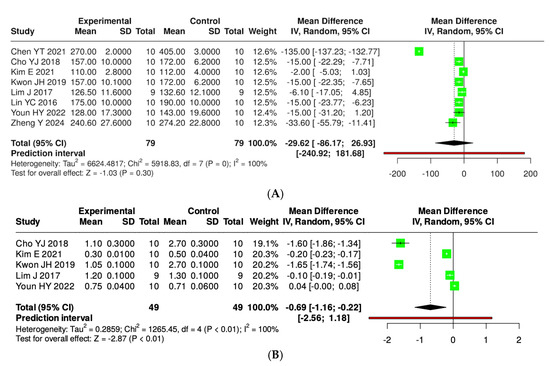
Figure 8.
Forest plot analysis showing the effects of consuming various types of kefirs, their isolated bacteria, or active components, compared to the control group, on plasma glucose (A) [34,35,38,39,40,42,43] and insulin (B) [35,38,39,40,42] levels in mouse models. Each green square shows the mean difference (MD) point estimate for individual studies, while the red line denotes the 95% prediction interval for the overall meta-analysis.
The effects of kefir and its derivatives on lipid profiles—including total cholesterol, triglycerides, low-density lipoprotein (LDL), and high-density lipoprotein (HDL)—were evaluated using random effects meta-analyses based on data from studies conducted exclusively in mice (Figure 9 and Supplementary Materials, Figure S3) and rats (Figure 10 and Supplementary Materials, Figure S4). In a mouse model comprising 14 studies with 123 mice, a meta-analysis assessed total cholesterol levels and found no significant difference between kefir-treated and control groups (MD = −37.84, 95% CI: −99.94 to 24.26). High heterogeneity was noted (p < 0.01, I2 = 100%). For triglycerides, 11 studies with 96 mice per group indicated a significant reduction in levels with kefir consumption (MD = −13.29, 95% CI: −18.39 to −8.19, p < 0.05), although high heterogeneity persisted (p < 0.01, I2 = 97%). The analysis of low-density lipoprotein (LDL) from ten studies involving 85 mice per group revealed a significant reduction in LDL levels (MD = −34.60, 95% CI: −59.26 to −9.93, p < 0.05), accompanied by high heterogeneity (p < 0.01, I2 = 100%). Conversely, no significant difference was found for high-density lipoprotein (HDL) across 11 studies (MD = −0.38, 95% CI: −6.64 to 5.87), with substantial heterogeneity (p < 0.01, I2 = 98%).
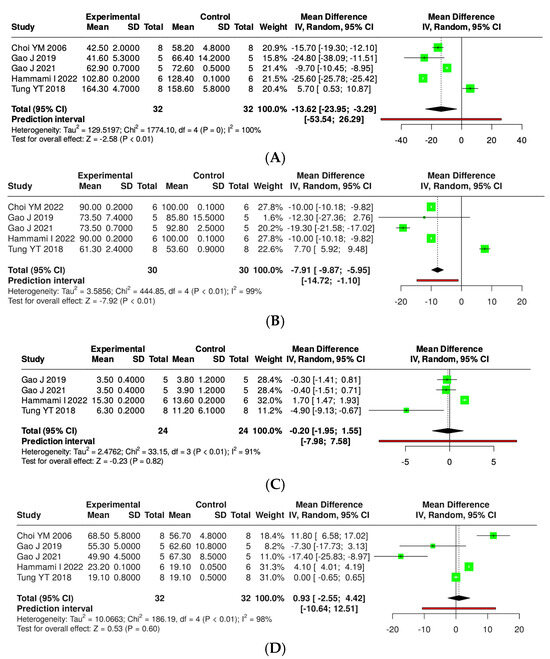
Figure 9.
Forest plot analysis illustrating the effects of consuming various types of kefirs, their isolated bacteria, or active components, in comparison to the control group, on lipid profiles, including levels of total cholesterol (A) [36,48,57,58,66], triglycerides (B) [36,48,57,58,66], low-density lipoprotein (C) [48,57,58,66], and high-density lipoprotein (D) [36,48,57,58,66] in rat models. Each green square shows the mean difference (MD) point estimate for individual studies, while the red line denotes the 95% prediction interval for the overall meta-analysis.
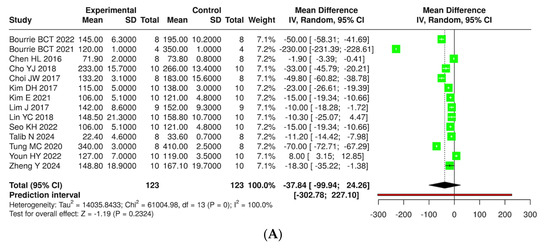
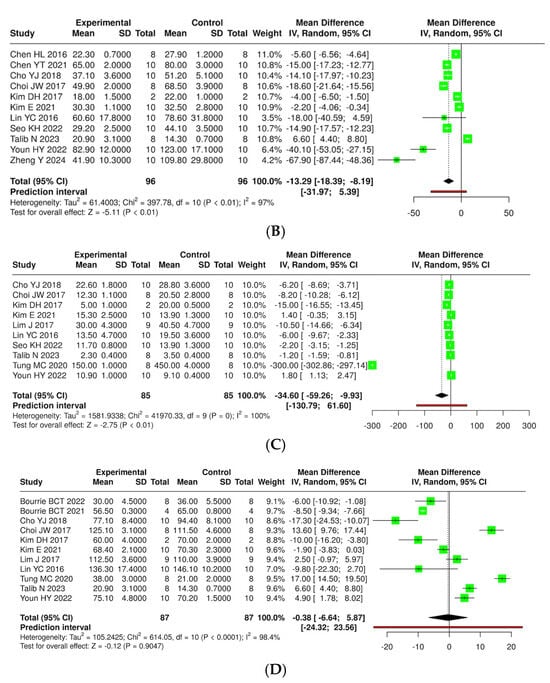
Figure 10.
Forest plot analysis illustrating the effects of consuming various types of kefirs, their isolated bacteria, or active components, in comparison to the control group, on lipid profiles, including levels of total cholesterol (A) [24,25,34,35,36,38,40,41,42,46,49,54,55,63], triglycerides (B) [24,25,34,35,36,38,41,42,43,46,63], low-density lipoprotein (C) [25,35,36,38,40,41,42,46,49,63], and high-density lipoprotein (D) [25,35,36,38,40,42,46,49,54,55,63] in mouse models. Each green square shows the mean difference (MD) point estimate for individual studies, while the red line denotes the 95% prediction interval for the overall meta-analysis.
A subgroup meta-analysis was carried out to assess the impact of kefir and its bioactive compounds on serum lipid levels in rat models of metabolic syndrome. Five studies involving 30 rats from both experimental and control groups indicated a statistically significant reduction in total cholesterol levels following kefir treatment. The pooled mean difference (MD) was −7.91, with a 95% confidence interval ranging from −9.87 to −5.95 (p < 0.05). However, there was significant heterogeneity (I2 = 99%, p < 0.01), suggesting considerable variability across the studies. In a separate analysis of five studies with 32 rats per group for triglycerides, kefir supplementation led to a significant reduction in triglyceride levels, with an averaged MD of −13.62 (95% CI: −23.95 to −3.29, p < 0.05). Nevertheless, this analysis also revealed high heterogeneity (I2 = 100%, p < 0.01), indicating variability in the magnitude and directions of the effects. For LDL, four studies involving 24 rats per group showed that kefir treatment did not significantly change LDL levels, resulting in a pooled MD of −0.20 (95% CI: −1.95 to 1.55), with the overall effect test yielding no significance. Yet, significant heterogeneity was noted (I2 = 91%, p < 0.01), highlighting inconsistencies among the findings. Lastly, five studies with 32 rats per group investigated the effects of kefir on HDL levels, revealing no statistically significant difference between the experimental and control groups (MD = 0.93; 95% CI: −2.55 to 4.42). Similar to other lipid parameters, significant heterogeneity was observed (I2 = 98%, p < 0.01).
The pooled analyses strongly support the metabolic regulatory potential of kefir and its constituents in rodent models of metabolic syndrome. Significant improvements were observed in body weight management, glycemic control, and lipid profile modulation, particularly in reducing total cholesterol and triglycerides. However, effects on LDL-C and HDL-C levels were inconsistent, highlighting a need for further mechanistic exploration and standardized kefir formulations in future research.
4. Discussion
This systematic review and meta-analysis present a comprehensive synthesis of preclinical evidence on the impacts of kefir and its bioactive components on MetS-related outcomes in rodent models. The findings strongly support the metabolic regulatory capabilities of kefir, especially its effectiveness in reducing body weight gain, enhancing lipid parameters, and adjusting glycemic and inflammatory markers. These findings underscore the significance of kefir as a promising functional food with potential applications in the dietary management of metabolic syndrome.
The pooled analyses indicated that kefir interventions significantly diminished weight gain in both mice and rats, thereby suggesting its potential as an anti-obesity agent in the context of diet-induced metabolic dysfunction. This aligns with previous studies indicating that probiotics in fermented foods may reduce adiposity by influencing lipid metabolism and energy balance through interactions involving the microbiota–gut–brain axis, mediated by neural, hormonal, and immune pathways shaped by gut microbiota composition [67,68,69]. Probiotics can enhance gut microbiota and stimulate the production of SCFAs such as acetate, propionate, and butyrate, which is critical for regulating metabolism, glucose, and energy homeostasis [70]. For example, studies indicate that specific probiotic strains, such as Faecalibacterium prausnitzii, may aid in weight management and lipid metabolism, especially in high-fat diet models [71].
This review highlights studies that have demonstrated the influence of probiotics and peptides derived from kefir on weight gain, which may involve the modulation of metabolic pathways, such as PPARγ and AMPK. Additionally, kefir, kefir-derived probiotics, and peptides possibly exert these effects by promoting beneficial shifts in gut microbiota and enhancing the production of SCFAs, particularly butyrate [22,26,36,54]. While direct evidence from included animal studies remains limited, previous in vitro studies suggest that SCFAs, which are metabolites enhanced by probiotic fermentation, can activate peroxisome proliferator-activated receptor gamma (PPARγ) [72] and AMP-activated protein kinase (AMPK) pathways [73]. Kefir-derived bioactive peptides have been shown to activate AMPK signaling and upregulate PPARγ expression in cultured hepatocytes and adipocytes, contributing to enhanced lipid oxidation and decreased lipogenesis [24,36,37]. The consumption of kefir peptides reduces body weight and fat accumulation in obesity models induced by high-fat diets. It could possibly enhance lipid metabolism by inhibiting lipogenesis and promoting fatty acid oxidation via increased liver phosphorylated AMPK and PPARα expression [36,48]. Moreover, PPAR agonists that kefir activates can influence AMPK activity, indicating a synergistic effect for metabolic regulation [74]. Therefore, its anti-obesity effects are fundamentally linked to these metabolic pathways.
Furthermore, notable reductions in triglyceride levels [24,34,35,36,37,38,41,42,43,46,63] and LDL cholesterol levels [35,36,37,38,40,41,42,46,49,63] have been observed due to kefir consumption, particularly in mouse models. However, mixed results were found regarding total cholesterol levels. A significant reduction was noted in rats [33,48,57,58,66], while no statistically significant effect was observed in mice [22,24,34,35,36,37,38,40,42,46,49,63]. Moreover, no consistent or significant effects concerning HDL levels were detected across models. Clinical trials have also indicated that kefir consumption can lead to reductions in LDL cholesterol and triglycerides, particularly in individuals with dyslipidemic conditions, although the effects may vary depending on the specific microbial composition of the kefir consumed [75]. The presence of Lactobacillus plantarum in kefir plays a significant role in cholesterol reduction by exhibiting bile salt hydrolase (BSH) activity, which hydrolyzes bile salts, leading to the precipitation of cholesterol and its subsequent removal from the body [76,77]. Kluyveromyces strains, in particular Kluyveromyces marxianus and Kluyveromyces lactis, have demonstrated high cholesterol-reducing capabilities due to their efficient BSH activity, which facilitates the breakdown of bile salts and cholesterol [78].
In murine models, significantly decreased insulin levels were also noted, suggesting improved insulin sensitivity. Some studies reported reductions in glucose levels, but the overall impact was insignificant. Clinical studies indicate that kefir supplementation significantly lowers fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) levels while increasing C-peptide levels, which suggests enhanced insulin secretion and sensitivity [79,80]. In contrast, other research found that kefir can notably reduce fasting insulin levels and insulin resistance (HOMA-IR) without impacting FBS or HbA1c levels [81]. Kefir influences insulin and glucose regulation through the insulin signaling pathway, primarily via its probiotic components, such as lactic acid bacteria and Bifidobacterium spp [82]. Kefir has been shown to enhance glucose uptake in insulin-responsive muscle cells by activating the phosphatidylinositol 3-kinase (PI 3-kinase) pathway [83], which is a critical component of the insulin signaling cascade. This activation leads to glucose transporter 4 (GLUT4) translocation to the cell membrane, thereby increasing glucose uptake.
The probiotic bacteria present in kefir not only contribute to its hypolipidemic and hypoglycemic properties but also serve to reduce oxidative stress and inflammatory markers [84,85]. Indeed, a principal strength of this analysis resides in the evidence substantiating the anti-inflammatory properties of kefir. Multiple preclinical studies demonstrate that kefir administration reduces pro-inflammatory cytokine levels, including TNF-α, IL-1β, and IL-6, in rodent models of metabolic syndrome. For example, Akar et al., (2021) [26] and Chang et al., (2023) [45] reported significant decreases in TNF-α and IL-1β levels in high-fructose-fed and atherogenic-diet-fed rats and ApoE knockout mice, respectively, after kefir or kefir peptide supplementation. Kim et al., (2017) [25] and Kim et al., (2021) [37] observed mixed outcomes, with reductions in IL-6 but varying effects on TNF-α and IL-1β in high-fat-diet-induced obese mice treated with kefir-derived probiotics. Similarly, Santanna et al., (2017) [62] and Tung et al., (2020) [49] found significant decreases in TNF-α and IL-6 levels following kefir intervention in LDL-receptor-deficient mice and diet-induced obesity models. These studies involved various kefir formulations, including whole kefir, isolated peptides, and kefir-derived probiotics, administered over treatment periods ranging from 3 to 16 weeks at doses between 0.05 mg/g and 22 mL/kg body weight. This anti-inflammatory profile aligns with proposed mechanisms in which kefir-derived metabolites interact with toll-like receptors and nuclear factor-kappa B (NF-κB) signaling pathways to suppress inflammatory responses [86]. However, despite the presence of promising findings, the quantitative synthesis of inflammatory and oxidative stress parameters remains constrained due to the limited number of studies reporting comparable outcomes. Kefir exhibits radical-scavenging effects, chelates ferrous ions, and boosts the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase. These functions play a crucial role in neutralizing reactive oxygen species (ROS), thereby enhancing their protective properties against oxidative stress [28,63,84,87,88].
Kefir consumption demonstrates considerable anti-inflammatory effects, especially in regulating the immune response [17,28,49]. It decreases pro-inflammatory cytokines while elevating anti-inflammatory cytokines, thus achieving a balance between Th1 and Th2 responses. It additionally promotes the abundance of beneficial bacteria, including Lachnospiraceae and Roseburia, which are recognized for their capacity to generate SCFAs with anti-inflammatory properties [28,82]. These SCFAs regulate intestinal pH, improve barrier integrity, and affect immune responses by activating signaling pathways that are involved in the synthesis of host defense peptides [89]. The polysaccharide extract from kefir, known as kefiran, was found to possess anti-inflammatory properties by inhibiting granuloma formation and reducing paw edema in animal models [90]. Kefir peptides, Kef-1, have demonstrated an ability to inhibit pathways such as NF-κB and MAPK, which play a role in inflammation [91].
The heterogeneity (I2) value observed across the meta-analyses of preclinical studies was consistently high, ranging from 91% to 100%. Differences in study design, animal species, sex, age, induction methods for MetS, kefir strain composition, dosage, and treatment duration all contributed to the variability observed in the outcomes. Furthermore, the quality assessment revealed that several studies were deficient in adequate blinding, randomization, and transparency regarding sample size calculations, which may have introduced bias and compromised the reliability of the results. Additionally, the elevated heterogeneity observed in the present meta-analysis may be partially influenced by publication bias, particularly the inclination for studies yielding positive or statistically significant results to be more frequently published.
Another major methodological limitation identified in this review was the significant inconsistency in how outcomes were reported in preclinical studies. Firstly, various outcomes related to inflammatory and oxidative stress markers could not be quantitatively analyzed due to an insufficient number of eligible studies (i.e., less than three studies reporting the same outcome). Secondly, an imbalance in sex and age was noted among the included studies. The majority of experiments were conducted on male rodents (82%), with relatively few studies involving female animals. Many studies exhibited considerable variation in the age of animals at the beginning of the study. Sex-specific hormonal and metabolic differences can significantly affect responses to interventions for metabolic syndrome, including changes in lipid metabolism, insulin sensitivity, and inflammatory pathways. Similarly, the stage of development or aging may influence metabolic outcomes and the effectiveness of kefir interventions. Consequently, these factors may limit the generalizability of the findings across both sexes and various life stages. Future preclinical studies should include balanced sex representation and standardized age groups to enhance the translational relevance of the results for broader populations.
Despite these limitations, the conclusions drawn from this investigation carry significant implications. Primarily, they advocate for incorporating kefir and its derived components into functional food frameworks for managing MetS. Furthermore, the findings underscore the necessity for enhanced standardization in the production of kefir products, particularly regarding microbial content and bioactive metabolites. This standardization is crucial for ensuring both reproducibility and efficacy. Lastly, the results provide a scientific basis for transitioning these findings into clinical trials involving human participants. Conducting randomized controlled trials among individuals diagnosed with MetS or its related components is essential to authenticate the preclinical findings regarding the efficacy of kefir and to elucidate its safety and long-term health implications.
5. Conclusions
In conclusion, this systematic review and meta-analysis provide robust preclinical evidence supporting the beneficial effects of kefir on weight management, lipid modulation, and insulin regulation, as well as the reduction of oxidative stress and inflammation in rodent models of metabolic syndrome. Although the findings are promising, notable heterogeneity and methodological limitations prevalent across the studies highlight the necessity of more standardized investigations. Furthermore, due to the complex composition of kefir, which contains multiple strains of probiotics, bioactive peptides, exopolysaccharides, and organic acids, additional mechanistic studies are warranted to clarify the specific pathways and molecular targets responsible for its beneficial effects. Kefir continues to be a compelling candidate within the functional food domain for the prevention and management of metabolic syndrome, thereby warranting further exploration in both translational and clinical contexts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14122077/s1, Table S1: Database search strategy for in vivo experiments investigating the beneficial effects of kefir consumption on metabolic syndrome; Table S2: Assessment of methodological quality in the included studies using the 10-item CAMARADES checklist; Table S3: Risk of bias assessment of the included studies using the SYRCLE tool; Table S4: General characteristics of included studies; Table S5: Strategies for inducing metabolic syndrome in animal models for included studies; Table S6: The summary of outcomes related to parameters associated with metabolic syndrome, inflammation, and oxidative stress highlights the effects of kefir and its active components in rodent models; Table S7: Evaluating outcomes associated with metabolic syndrome parameters highlights the effects of kefir and its active components in rodent models; Table S8: Assessing outcomes related to inflammatory and oxidative stress markers highlights the effects of kefir and its active components in rodent models of metabolic syndrome; Figure S1: A tunnel plot illustrating the distribution of publication biases associated with the consumption of various types of kefirs, their isolated bacteria, or active components, compared to the control group, regarding weight gain in mouse (A) and rat (B) models; Figure S2: A tunnel plot illustrating the distribution of publication biases associated with the consumption of various types of kefirs, their isolated bacteria, or active components, compared to the control group, on plasma glucose (A) and insulin (B) levels in mouse models; Figure S3: A tunnel plot illustrating the distribution of publication biases associated with the consumption of various types of kefirs, their isolated bacteria, or active components, in comparison to the control group, on lipid profiles, including levels of total cholesterol (A), triglycerides (B), low-density lipoprotein (C), and high-density lipoprotein (D) in rat models; Figure S4: A tunnel plot illustrating the distribution of publication biases associated with the consumption of various types of kefirs, their isolated bacteria, or active components, in comparison to the control group, on lipid profiles, including levels of total cholesterol (A), triglycerides (B), low-density lipoprotein (C), and high-density lipoprotein (D) in mouse models.
Author Contributions
S.C.: funding acquisition, conceptualization, supervision, writing—review and editing. Z.N.Q.: writing the original draft, data curation, validation, and formal analysis. W.P.L., B.B.L., K.Y.P., and M.M.S.: article searching, screening, and data curation. N.A. and S.A.: validation and visualization. R.P., S.P.N.A., C.H., and N.B.: co-funding acquisition, conceptualization, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by “Innovative functional beverage with cholesterol-lowering effects: In vivo assessment and a randomized controlled trial of Coconut water Kefir (CWK)” from the funding agency “Program Management Unit for Competitiveness (PMUC)” (grant no. C02F660307) and the postdoctoral fellowship fund from “Mae Fah Luang University, Thailand (Contract No. 10/2024)”.
Institutional Review Board Statement
This manuscript is a review and does not present any negative impact issues. All operations adhere to ethical standards.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that might appear to influence the work reported in this paper.
References
- Zhang, H.; Zhou, X.D.; Shapiro, M.D.; Lip, G.Y.H.; Tilg, H.; Valenti, L.; Somers, V.K.; Byrne, C.D.; Targher, G.; Yang, W.; et al. Global burden of metabolic diseases, 1990–2021. Metabolism 2024, 160, 155999. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, L.; Han, Z.; Xiong, P. The global burden of disease attributable to high body mass index in 204 countries and territories: Findings from 1990 to 2019 and predictions to 2035. Diabetes Obes. Metab. 2024, 26, 3998–4010. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zuo, P.; Ma, G. Association of weight-adjusted waist index with cardiovascular disease and mortality among metabolic syndrome population. Sci. Rep. 2024, 14, 18684. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; He, Y.; Wang, J.; Yu, L.; Gong, Q.; Chen, Y.; An, Y.; He, S.; Li, G.; Zhang, B. Influence of impaired glucose tolerance alone and combined with metabolic syndrome on long-term risk of cardiovascular events and mortality. J. Diabetes 2024, 16, e13598. [Google Scholar] [CrossRef]
- Pammer, L.M.; Lamina, C.; Schultheiss, U.T.; Kotsis, F.; Kollerits, B.; Stockmann, H.; Lipovsek, J.; Meiselbach, H.; Busch, M.; Eckardt, K.U.; et al. Association of the metabolic syndrome with mortality and major adverse cardiac events: A large chronic kidney disease cohort. J. Intern. Med. 2021, 290, 1219–1232. [Google Scholar] [CrossRef]
- Sahoo, J.P.; Mukherjee, J.J.; Lee, K.O.; Khoo, C.M. Chapter 7—Epidemiology of metabolic syndrome in South and South-East Asia. In Metabolic Syndrome; Mukhopadhyay, S., Mondal, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 73–83. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, F.; Wang, W.; Zhang, K.; Fan, M.; Lin, R. The global burden of disease attributable to high body mass index in 204 countries and territories from 1990 to 2021 with projections to 2050: An analysis of the Global Burden of Disease Study 2021. Eur. J. Heart Fail. 2025, 27, 354–365. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.E.; Caruntu, C.; Benea, S.N. The Interplay between obesity and inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Li, A.; Zheng, N.; Ding, X. Mitochondrial abnormalities: A hub in metabolic syndrome-related cardiac dysfunction caused by oxidative stress. Heart Fail. Rev. 2022, 27, 1387–1394. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Majeed, O.S.; Gaaz, T.S.; Akpoghelie, P.O.; Isoje, E.F.; Igbuku, U.A.; Owheruo, J.O.; Opiti, R.A.; Garba, Y.; et al. A review on probiotics and dietary bioactives: Insights on metabolic well-being, gut microbiota, and inflammatory responses. Food Chem. Adv. 2025, 6, 100919. [Google Scholar] [CrossRef]
- Peng, X.; Xian, H.; Ge, N.; Hou, L.; Tang, T.; Xie, D.; Gao, L.; Yue, J. Effect of probiotics on glycemic control and lipid profiles in patients with type 2 diabetes mellitus: A randomized, double blind, controlled trial. Front. Endocrinol. 2024, 15, 1440286. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ma, C.; Yang, Y.; Liu, X.; Wang, B.; Wang, Y.; Zhang, G.; Bian, X.; Zhang, N. The role and mechanism of probiotics supplementation in blood glucose regulation: A Review. Foods 2024, 13, 2719. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Li, Y.; Wu, L.; Fan, C.; Liang, T.; Xi, Y.; Yang, S.; Li, H.; Zhang, J.; et al. Evaluation of the cholesterol-lowering mechanism of Enterococcus faecium strain 132 and Lactobacillus paracasei strain 201 in hypercholesterolemia rats. Nutrients 2021, 13, 1982. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simão, A.N.C.; de Souza, C.H.B.; Alfieri, D.F.; Segura, L.G.; Costa, G.N.; Dichi, I. Effect of Bifidobacterium lactis HN019 on inflammatory markers and oxidative stress in subjects with and without the metabolic syndrome. Br. J. Nutr. 2018, 120, 645–652. [Google Scholar] [CrossRef]
- Culpepper, T. The Effects of kefir and kefir components on immune and metabolic physiology in pre-clinical studies: A narrative review. Cureus 2022, 14, e27768. [Google Scholar] [CrossRef]
- Júnior, J.; Meireles Mafaldo, Í.; de Lima Brito, I.; Tribuzy de Magalhães Cordeiro, A.M. Kombucha: Formulation, chemical composition, and therapeutic potentialities. Curr. Res. Food Sci. 2022, 5, 360–365. [Google Scholar] [CrossRef]
- Mousavi, S.N.; Saboori, S.; Asbaghi, O. Effect of daily probiotic yogurt consumption on inflammation: A systematic review and meta-analysis of randomized Controlled Clinical trials. Obes. Med. 2020, 18, 100221. [Google Scholar] [CrossRef]
- Khanturgaev, A.G.; Khamagaeva, I.S.; Shiretorova, V.G. Production of probiotic kvass beverages enriched with pine nut shell extract and propionic acid bacteria. Food Sci. Technol. 2023, 43. [Google Scholar] [CrossRef]
- Apalowo, O.E.; Adegoye, G.A.; Mbogori, T.; Kandiah, J.; Obuotor, T.M. Nutritional characteristics, health impact, and applications of kefir. Foods 2024, 13, 1026. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Cotter, P.D.; Willing, B.P. Traditional kefir reduces weight gain and improves plasma and liver lipid profiles more successfully than a commercial equivalent in a mouse model of obesity. J. Funct. Foods 2018, 46, 29–37. [Google Scholar] [CrossRef]
- Saleem, K.; Ali, I.; Farhan, S.; Muhammad, A.; Huda, A.; Muzammal, H.; Awais, R.; Amara, R.; Aasma, A.; and Asif Shah, M. Nutritional and functional properties of kefir: Review. Int. J. Food Prop. 2023, 26, 3261–3274. [Google Scholar] [CrossRef]
- Chen, H.L.; Tsai, T.C.; Tsai, Y.C.; Liao, J.W.; Yen, C.C.; Chen, C.M. Kefir peptides prevent high-fructose corn syrup-induced non-alcoholic fatty liver disease in a murine model by modulation of inflammation and the JAK2 signaling pathway. Nutr. Diabetes 2016, 6, e237. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, H.; Jeong, D.; Kang, I.-B.; Chon, J.-W.; Kim, H.-S.; Song, K.-Y.; Seo, K.-H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef]
- Akar, F.; Sumlu, E.; Alçığır, M.E.; Bostancı, A.; Sadi, G. Potential mechanistic pathways underlying intestinal and hepatic effects of kefir in high-fructose-fed rats. Food Res. Int. 2021, 143, 110287. [Google Scholar] [CrossRef]
- Vieira, C.P.; Rosario, A.; Lelis, C.A.; Rekowsky, B.S.S.; Carvalho, A.P.A.; Rosário, D.K.A.; Elias, T.A.; Costa, M.P.; Foguel, D.; Conte-Junior, C.A. Bioactive compounds from kefir and their potential benefits on health: A systematic review and meta-analysis. Oxid. Med. Cell Longev. 2021, 2021, 9081738. [Google Scholar] [CrossRef]
- Albuquerque Pereira, M.d.F.; Matias Albuini, F.; Gouveia Peluzio, M.d.C. Anti-inflammatory pathways of kefir in murine model: A systematic review. Nutr. Rev. 2023, 82, 210–227. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Macleod, M.R.; O’Collins, T.; Howells, D.W.; Donnan, G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004, 35, 1203–1208. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. MetaAnalysisOnline.com: Web-Based tool for the rapid meta-analysis of clinical and epidemiological studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Bae, S.H.; Kang, D.H.; Suh, H.J. Hypolipidemic effect of lactobacillus ferment as a functional food supplement. Phytother. Res. 2006, 20, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lee, Y.; Kweon, M.; Kim, S.; Kang, I.-J. Evaluate and compare the anti-obesity effects of two probiotic preparations in high-fat diet-induced obese mice. J. Funct. Foods 2024, 116, 106199. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, H.G.; Seo, K.H.; Yokoyama, W.; Kim, H. Antiobesity effect of prebiotic polyphenol-rich grape seed flour supplemented with probiotic kefir-derived lactic acid bacteria. J. Agric. Food Chem. 2018, 66, 12498–12511. [Google Scholar] [CrossRef]
- Choi, J.W.; Kang, H.W.; Lim, W.C.; Kim, M.K.; Lee, I.Y.; Cho, H.Y. Kefir prevented excess fat accumulation in diet-induced obese mice. Biosci. Biotechnol. Biochem. 2017, 81, 958–965. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kang, I.B.; Kim, H.; Song, K.Y.; Seo, K.H. Dual function of Lactobacillus kefiri DH5 in preventing high-fat-diet-induced obesity: Direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue. Mol. Nutr. Food Res. 2017, 61, 1700252. [Google Scholar] [CrossRef]
- Kim, E.; Lee, H.G.; Han, S.; Seo, K.H.; Kim, H. Effect of surface layer proteins derived from paraprobiotic kefir lactic acid bacteria on inflammation and high-fat diet-induced obesity. J. Agric. Food Chem. 2021, 69, 15157–15164. [Google Scholar] [CrossRef]
- Kwon, J.H.; Lee, H.G.; Seo, K.H.; Kim, H. Combination of whole grapeseed flour and newly isolated kefir lactic acid bacteria reduces high-fat-induced hepatic steatosis. Mol. Nutr. Food Res. 2019, 63, e1801040. [Google Scholar] [CrossRef]
- Lim, J.; Kale, M.; Kim, D.H.; Kim, H.S.; Chon, J.W.; Seo, K.H.; Lee, H.G.; Yokoyama, W.; Kim, H. Antiobesity effect of exopolysaccharides isolated from kefir grains. J. Agric. Food Chem. 2017, 65, 10011–10019. [Google Scholar] [CrossRef]
- Seo, K.-H.; Gyu Lee, H.; Young Eor, J.; Jin Jeon, H.; Yokoyama, W.; Kim, H. Effects of kefir lactic acid bacteria-derived postbiotic components on high fat diet-induced gut microbiota and obesity. Food Res. Int. 2022, 157, 111445. [Google Scholar] [CrossRef]
- Youn, H.-Y.; Seo, K.-H.; Kim, H.-J.; Kim, Y.-S.; Kim, H. Effect of postbiotics derived from kefir lactic acid bacteria-mediated bioconversion of citrus pomace extract and whey on high-fat diet-induced obesity and gut dysbiosis. Food Res. Int. 2022, 162, 111930. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Hsu, A.H.; Chiou, S.Y.; Lin, Y.C.; Lin, J.S. AB-Kefir reduced body weight and ameliorated inflammation in adipose tissue of obese mice fed a high-fat diet, but not a high-sucrose diet. Nutrients 2021, 13, 2182. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Yang, N.S.; Lin, Y.C.; Ho, S.T.; Li, K.Y.; Lin, J.S.; Liu, J.R.; Chen, M.J. A combination of Lactobacillus mali APS1 and dieting improved the efficacy of obesity treatment via manipulating gut microbiome in mice. Sci. Rep. 2018, 8, 6153. [Google Scholar] [CrossRef]
- Chang, G.R.; Cheng, W.Y.; Fan, H.C.; Chen, H.L.; Lan, Y.W.; Chen, M.S.; Yen, C.C.; Chen, C.M. Kefir peptides attenuate atherosclerotic vascular calcification and osteoporosis in atherogenic diet-fed ApoE (−/−) knockout mice. Front. Cell Dev. Biol. 2023, 11, 1158812. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, Y.-T.; Hsieh, H.-H.; Chen, M.-J. Effect of Lactobacillus mali APS1 and L. kefiranofaciens M1 on obesity and glucose homeostasis in diet-induced obese mice. J. Funct. Foods 2016, 23, 580–589. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, Y.T.; Li, K.Y.; Chen, M.J. Investigating the Mechanistic Differences of obesity-inducing Lactobacillus kefiranofaciens M1 and anti-obesity Lactobacillus mali APS1 by microbolomics and metabolomics. Front. Microbiol. 2020, 11, 1454. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, H.L.; Wu, H.S.; Ho, M.H.; Chong, K.Y.; Chen, C.M. Kefir peptides prevent hyperlipidemia and obesity in high-fat-diet-induced obese rats via lipid metabolism modulation. Mol. Nutr. Food Res. 2018, 62, 1700505. [Google Scholar] [CrossRef]
- Tung, M.-C.; Lan, Y.-W.; Li, H.-H.; Chen, H.-L.; Chen, S.-Y.; Chen, Y.-H.; Lin, C.-C.; Tu, M.-Y.; Chen, C.-M. Kefir peptides alleviate high-fat diet-induced atherosclerosis by attenuating macrophage accumulation and oxidative stress in ApoE knockout mice. Sci. Rep. 2020, 10, 8802. [Google Scholar] [CrossRef]
- Akar, F.; Yildirim, O.G.; Yucel Tenekeci, G.; Tunc, A.S.; Demirel, M.A.; Sadi, G. Dietary high-fructose reduces barrier proteins and activates mitogenic signalling in the testis of a rat model: Regulatory effects of kefir supplementation. Andrologia 2022, 54, e14342. [Google Scholar] [CrossRef]
- Ekici, O.; Aslan, E.; Guzel, H.; Korkmaz, O.A.; Sadi, G.; Gurol, A.M.; Boyaci, M.G.; Pektas, M.B. Kefir alters craniomandibular bone development in rats fed excess dose of high fructose corn syrup. J. Bone Miner. Metab. 2022, 40, 56–65. [Google Scholar] [CrossRef]
- Ekici, Ö.; Aslan, E.; Aladağ, T.; Güzel, H.; Korkmaz, Ö.A.; Bostancı, A.; Sadi, G.; Pektaş, M.B. Masseter muscle and gingival tissue inflammatory response following treatment with high-fructose corn syrup in rats: Anti-inflammatory and antioxidant effects of kefir. J. Food Biochem. 2022, 46, e13732. [Google Scholar] [CrossRef] [PubMed]
- Tarakci, N.G.; Erdem, N.Z.; Dumen, E. Probiotic Foods Are Effective on Weight Loss, Biochemical Parameters, and Intestinal Microbiota in Wistar Albino Rats with Obese Microbiota. Int. J. Clin. Pract. 2022, 2022, 4569100. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.C.T.; Forgie, A.J.; Ju, T.; Richard, C.; Cotter, P.D.; Willing, B.P. Consumption of the cell-free or heat-treated fractions of a pitched kefir confers some but not all positive impacts of the corresponding whole kefir. Front. Microbiol. 2022, 13, 1056526. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Ju, T.; Fouhse, J.M.; Forgie, A.J.; Sergi, C.; Cotter, P.D.; Willing, B.P. Kefir microbial composition is a deciding factor in the physiological impact of kefir in a mouse model of obesity. Br. J. Nutr. 2021, 125, 129–138. [Google Scholar] [CrossRef]
- Seo, K.H.; Jeong, J.; Kim, H. Synergistic Effects of Heat-Killed Kefir Paraprobiotics and Flavonoid-Rich Prebiotics on Western Diet-Induced Obesity. Nutrients 2020, 12, 2465. [Google Scholar] [CrossRef]
- Gao, J.; Mao, K.; Wang, X.; Mi, S.; Fu, M.; Li, X.; Xiao, J.; Simal-Gandara, J.; Sang, Y. Tibet Kefir Milk Regulated Metabolic Changes Induced by High-Fat Diet via Amino Acids, Bile Acids, and Equol Metabolism in Human-Microbiota-Associated Rats. J. Agric. Food Chem. 2021, 69, 6720–6732. [Google Scholar] [CrossRef]
- Gao, J.; Ding, G.; Li, Q.; Gong, L.; Huang, J.; Sang, Y. Tibet kefir milk decreases fat deposition by regulating the gut microbiota and gene expression of Lpl and Angptl4 in high fat diet-fed rats. Food Res. Int. 2019, 121, 278–287. [Google Scholar] [CrossRef]
- Nurliyani, N.; Harmayani, E.; Sunarti, S. Synbiotic goat milk kefir improves health status in rats fed a high-fat and high-fructose diet. Vet. World 2022, 15, 173–181. [Google Scholar] [CrossRef]
- Susanti, S.; Nurwantoro, N.; Elto, J.; Nugroho, T.; Erma Suryani, A.; Rizqiati, H. Preclinical study of goat milk kefir as an antihyperglycemic food. Funct. Food Health Dis. 2022, 12, 705. [Google Scholar] [CrossRef]
- Angelis-Pereira, M.C.; Barcelos Mde, F.; Sousa, M.S.; Pereira Jde, A. Effects of the kefir and banana pulp and skin flours on hypercholesterolemic rats. Acta Cir. Bras. 2013, 28, 481–486. [Google Scholar] [CrossRef]
- Santanna, A.F.; Filete, P.F.; Lima, E.M.; Porto, M.L.; Meyrelles, S.S.; Vasquez, E.C.; Endringer, D.C.; Lenz, D.; Abdalla, D.S.P.; Pereira, T.M.C.; et al. Chronic administration of the soluble, nonbacterial fraction of kefir attenuates lipid deposition in LDLr(−/−) mice. Nutrition 2017, 35, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Talib, N.; Mohamad, N.E.; Yeap, S.K.; Ho, C.L.; Masarudin, M.J.; Abd-Aziz, S.; Izham, M.N.M.; Kumar, M.R.; Hussin, Y.; Alitheen, N.B. Anti-diabetic effect of Lactobacillus paracasei isolated from Malaysian water kefir grains. Probiotics Antimicrob. Proteins 2024, 16, 2161–2180. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Eissa, S.; Mansour, A.; El Magd, N.M.A.; Hasanin, A.H.; El Mahdy, M.M.; Hassan, M.K.; Matboli, M. Evaluation of the role of kefir in management of non-alcoholic steatohepatitis rat model via modulation of NASH linked mRNA-miRNA panel. Sci. Rep. 2023, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Zubiría, M.G.; Gambaro, S.E.; Rey, M.A.; Carasi, P.; Serradell, M.; Giovambattista, A. Deleterious metabolic effects of high fructose intake: The preventive effect of Lactobacillus kefiri administration. Nutrients 2017, 9, 470. [Google Scholar] [CrossRef]
- Hammami, I.; Ben Ali, R.; Nahdi, A.; Boussada, M.; Mahjoub, R.; Bibi, A.; El May, M.V. Kefir milk consumption decreases sperm alterations due to the high-fat diet in adult male rats. Andrologia 2022, 54, 1631–1642. [Google Scholar] [CrossRef]
- Jalili, M.; Nazari, M.; Magkos, F. Fermented foods in the management of obesity: Mechanisms of action and future challenges. Int. J. Mol. Sci. 2023, 24, 2665. [Google Scholar] [CrossRef]
- Tian, Y.; Li, F.; Du, L.; Peng, D.; Yang, Z.; Li, J.; Zhang, J. Fermented fruits ameliorate obesity by controlling food intake and regulating lipid metabolism in high-fat dietary mice. J. Funct. Foods 2024, 114, 106072. [Google Scholar] [CrossRef]
- Xiao, X.; Li, S.; Zhou, X.; Li, M.; Zhang, Y.; Ye, H. The anti-obesogenic effects and underpinning mechanisms of fermented plant-based foods: A review. Trends Food Sci. Technol. 2023, 136, 1–10. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.H.; Shin, J.H.; Lee, D.; Lee, S.N.; Seo, J.G.; Shin, J.H.; Nam, Y.D.; Kim, H.; Sun, X. Pharmaceutical efficacy of novel human-origin Faecalibacterium prausnitzii strains on high-fat-diet-induced obesity and associated metabolic disorders in mice. Front. Endocrinol. 2023, 14, 1220044. [Google Scholar] [CrossRef]
- Alex, S.; Lange, K.; Amolo, T.; Grinstead, J.S.; Haakonsson, A.K.; Szalowska, E.; Koppen, A.; Mudde, K.; Haenen, D.; Al-Lahham, S.; et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol. Cell. Biol. 2013, 33, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Elamin Elhaseen, E.; Masclee Ad, A.; Jan, D.; Harm-Jan, P.; Jonkers Daisy, M. Short-chain fatty acids activate amp-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, S.G. AMPK-Dependent metabolic regulation by PPAR Agonists. PPAR Res. 2010, 2010, 549101. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Forgie, A.J.; Makarowski, A.; Cotter, P.D.; Richard, C.; Willing, B.P. Consumption of kefir made with traditional microorganisms resulted in greater improvements in LDL cholesterol and plasma markers of inflammation in males when compared to a commercial kefir: A randomized pilot study. Appl. Physiol. Nutr. Metab. 2023, 48, 668–677. [Google Scholar] [CrossRef]
- Arslan, B.; Yilmaz, I. The effect of kefir consumption on the lipid profile for individuals with normal and dyslipidemic properties: A randomized controlled trial. Rev. Nutr. 2022, 35, e210098. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, F.; Wang, X.; Sui, Y.; Yang, L.; Wang, J. Characterization of Lactobacillus plantarum Lp27 isolated from Tibetan kefir grains: A potential probiotic bacterium with cholesterol-lowering effects. J. Dairy Sci. 2013, 96, 2816–2825. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; Xiong, L.; Dong, R.; Pan, C.; Teng, G.; Zhang, H. Effect and Mechanism of cholesterol-lowering by Kluyveromyces from Tibetan kefir. Adv. Mater. Res. 2011, 343–344, 1290–1298. [Google Scholar] [CrossRef]
- Salari, A.; Ghodrat, S.; Gheflati, A.; Jarahi, L.; Hashemi, M.; Afshari, A. Effect of kefir beverage consumption on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. Complement. Ther. Clin. Pract. 2021, 44, 101443. [Google Scholar] [CrossRef]
- Hadisaputro, S.; Ks, I.; Cahyono, B.; Suzery, M.; Widiastuti, Y.; Purnawan, A. Effects of clear kefir on biomolecular aspects of glycemic status of Type 2 diabetes mellitus (T2DM) patients in bandung, West Java [Study on Human Blood Glucose, c Peptide, and Insulin]. Funct. Foods Health Dis. 2014, 4, 340–348. [Google Scholar] [CrossRef]
- Yahyapoor, F.; Haghighat, N.; Sohrabi, Z.; Asbaghi, O.; Bagherniya, M.; Jamialahmadi, T.; Sahebkar, A. Effects of kefir consumption on cardiometabolic risk factors: A systematic review and meta-analysis of randomized controlled trials. Curr. Drug Targets 2023, 24, 599–612. [Google Scholar] [CrossRef]
- Peluzio, M.; Dias, M.M.E.; Martinez, J.A.; Milagro, F.I. Kefir and intestinal microbiota modulation: Implications in human health. Front. Nutr. 2021, 8, 638740. [Google Scholar] [CrossRef]
- Teruya, K.; Yamashita, M.; Tominaga, R.; Nagira, T.; Shim, S.Y.; Katakura, Y.; Tokumaru, S.; Tokumaru, K.; Barnes, D.; Shirahata, S. Fermented milk, Kefram-kefir enhances glucose uptake into insulin-responsive muscle cells. Cytotechnology 2002, 40, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, D.; Chrysikopoulou, V.; Rampaouni, A.; Tsoupras, A. Antioxidant and anti-inflammatory properties of water kefir microbiota and its bioactive metabolites for health promoting bio-functional products and applications. AIMS Microbiol. 2024, 10, 756–811. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Sharma, H.; Melekoglu, E.; Ozogul, F. Recent developments in dairy kefir-derived lactic acid bacteria and their health benefits. Food Biosci. 2022, 46, 101592. [Google Scholar] [CrossRef]
- Aligita, W.; Singgih, M.; Sutrisno, E.; Adnyana, I.K. Hepatoprotective study of Indonesian water kefir against CCl4-induced liver injury in rats. J. Pharm. Pharmacogn. Res. 2023, 11, 1002–1016. [Google Scholar] [CrossRef]
- Kumar, M.R.; Yeap, S.K.; Lee, H.C.; Mohamad, N.E.; Nazirul Mubin Aziz, M.; Khalid, M.; Masarudin, M.J.; Leow, A.T.C.; Abdullah, J.O.; Alitheen, N.B. Selected kefir water from Malaysia attenuates hydrogen peroxide-induced oxidative stress by upregulating endogenous antioxidant levels in SH-SY5Y neuroblastoma cells. Antioxidants 2021, 10, 940. [Google Scholar] [CrossRef]
- Pugliero, S.; Lima, D.Y.; Rodrigues, A.M.; Bogsan, C.S.B.; Rogero, M.M.; Punaro, G.R.; Higa, E.M.S. Kefir reduces nitrosative stress and upregulates Nrf2 in the kidney of diabetic rats. Int. Dairy J. 2021, 114, 104909. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Z.; Yang, Z.; Qiao, X. Microbiota-derived short-chain fatty acids and modulation of host-derived peptides formation: Focused on host defense peptides. Biomed. Pharmacother. 2023, 162, 114586. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Carvalho, J.C.; Schneedorf, J.M. Anti-inflammatory properties of kefir and its polysaccharide extract. Inflammopharmacology 2005, 13, 485–492. [Google Scholar] [CrossRef]
- Aires, R.; Amorim, F.; Côco, L.; Conceição, A.; Zanardo, T.; Taufner, G.; Nogueira, B.; Vasquez, E.; Pereira, T.; Campagnaro, B.; et al. Kefir peptide (Kef-1) as an emerging approach for the treatment of oxidative stress and inflammation in 2K1C mice. Food Funct 2022, 13, 1965–1974. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).