Recent Advances in Functionalized Carbon Quantum Dots Integrated with Metal–Organic Frameworks: Emerging Platforms for Sensing and Food Safety Applications
Abstract
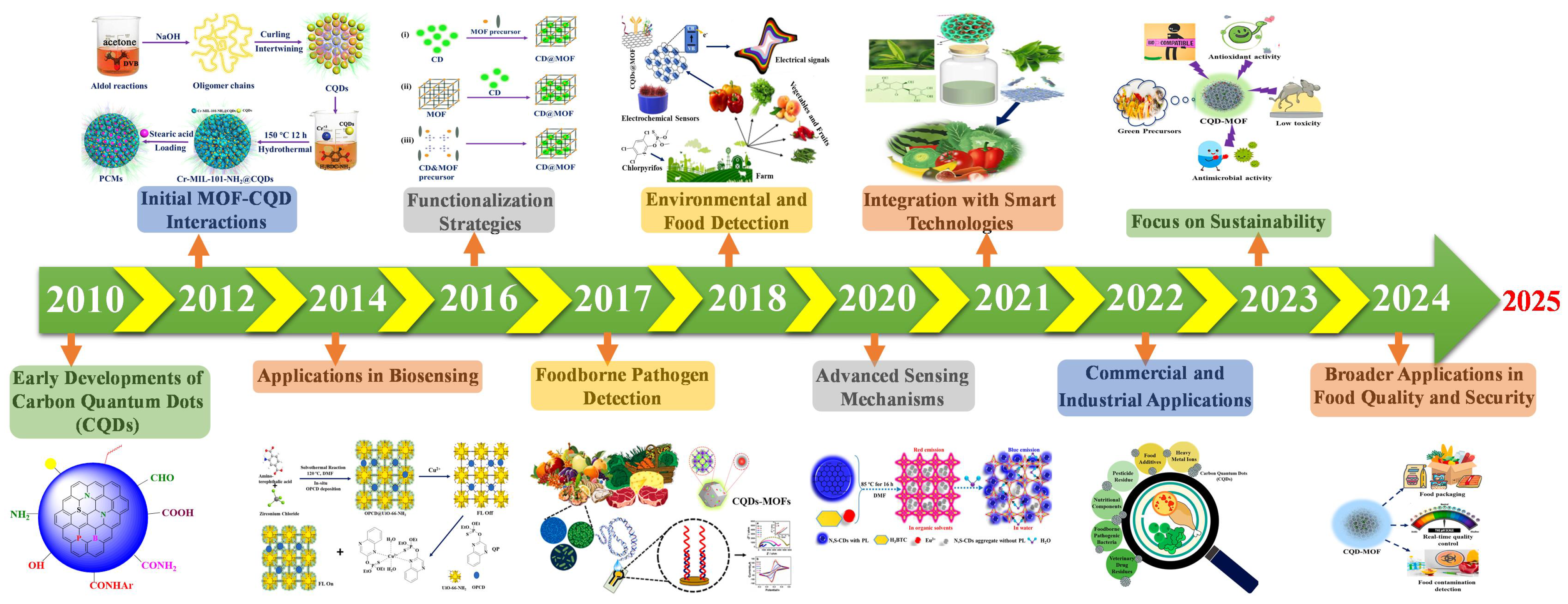
1. Introduction
2. Synthesis Methods and Optimal Conditions for CQD–MOF for Food Safety Applications
2.1. Top-Down Methods
Electrochemical Synthesis
| Method | Advantages | Limitations | Particle Size Control | Composite Stability | CQD@MOF Integration |
|---|---|---|---|---|---|
| Electrochemical |
|
| Moderate (~50–300 nm) | High (film-based) | CQDs co-deposited or anchored on MOF films |
| Hydrothermal/Solvothermal |
|
| Moderate (100–500 nm) | Moderate to high | CQDs embedded during MOF crystal growth |
| Mechanochemical |
|
| Nanocrystals to ~10 µm | Low (broad, irregular) | MOF crystals form around/with embedded CQDs |
| Microwave-Assisted Synthesis |
|
| Good (CQDs <10 nm; MOFs ~100 nm) | High | CQDs embedded or trapped in MOF matrix |
| Ultrasound-Assisted Synthesis |
|
| Good (<100 nm CQDs; MOF ~100–300 nm) | High | CQDs mixed with MOF under cavitation-assisted nucleation |
| Layer-by-Layer (LbL) Assembly |
|
| Excellent (nm-scale film thickness) | Very high | Alternating CQD/MOF layers or CQDs intercalated |
| Template-Assisted Synthesis |
|
| Variable (20 nm–µm range) | High | CQDs confined in templated cavities or structures |
2.2. Bottom-Up Methods
2.2.1. Hydrothermal/Solvothermal Synthesis
| S. No | CQDs@MOFs | Synthesis Methods | Emission Range (nm) | Size (nm) | Reference |
|---|---|---|---|---|---|
| 1 | BNCDs@Tb-MOF | Hydrothermal | 450, 490 and 544 | 3 | [38] |
| 2 | CQDs@ZIF-8 | Hydrothermal | - | - | [56] |
| 3 | CDs@Eu-MOFs | Hydrothermal | 365 | 3 | [57] |
| 4 | CuO/Cu2O-CdS/HgS | Hydrothermal | - | - | [5] |
| 5 | MOF/CdTe QDs | Hydrothermal | 425, 605 | - | [58] |
| 6 | CDs@ZIF-90 | Hydrothermal | 453 | - | [59] |
| 7 | E-CDs@ZIF-8 | Hydrothermal | 399 to 405 | - | [60] |
| 8 | PEG-ZnSQD@ZIF-67 | Green synthesis | 420 | - | [61] |
| 9 | CsPbBr3/HZIF-8 | Room temperature | 510 | 25 | [62] |
| 10 | CDs@ZIF-8@SMIP | Hydrothermal | 410–600 | 20 | [6] |
| 11 | CDs@Cu-MOFs | Room temperature | 430–600 | - | [13] |
| 12 | CdTe QDs@ZIF-8 | Room temperature | 524–650 | - | [63] |
| 13 | NH2-MIL-53 & N, P-CDs@MIP | Room temperature | 360–438 | - | [64] |
| 14 | MB@PApt-SP DNA@AZIS QDs@Ag-Pt NPs | Room temperature | - | - | [65] |
| 15 | M-TiO2-CdTe QDs/CdS QDs PEC | Room temperature | 390 | - | [66] |
| 16 | N-CDs@Eu-MOF@MIP | Room temperature | 430–616 | 3 | [67] |
| 17 | Fe-CDs/MOF-808 and Fe-CDs@MOF-808 | Room temperature | ~425 | - | [11] |
| 18 | CD@UIO-66-NH2 | Hydrothermal | 425 | 5 | [12] |
| 19 | N-CQDs@UiO-66-NH2 | heated at 90 °C for 24 h | - | - | [27] |
| 20 | CdS-Sm-BDC-g-C3N4-5 | Room temperature | - | - | [28] |
| 21 | CDs@Eu/UiO-67b | Hydrothermal | 442–612 | - | [20] |
| 22 | CdTe QDs@ZIF-8 | Room temperature | 521–672 | - | [29] |
| 23 | CDs@UiO-66-NH2 | Ultrasound | 328 | - | [68] |
| 24 | CD@MIP | Kettle Reflux | 450 | 5 | [69] |
| 25 | Ce, N-CDs@ZIF-67@MIP | Room temperature | 445 | - | [70] |
| 26 | His-GQDs-Ser@MOF | Room temperature | 460–618 | 5 | [71] |
| 27 | g-CDs@UiO-66 | Stirred for 12 h at 60 °C | 446–530 | - | [72] |
| 28 | Co-CD/PMOF | Hydrothermal | 350–450 | - | [30] |
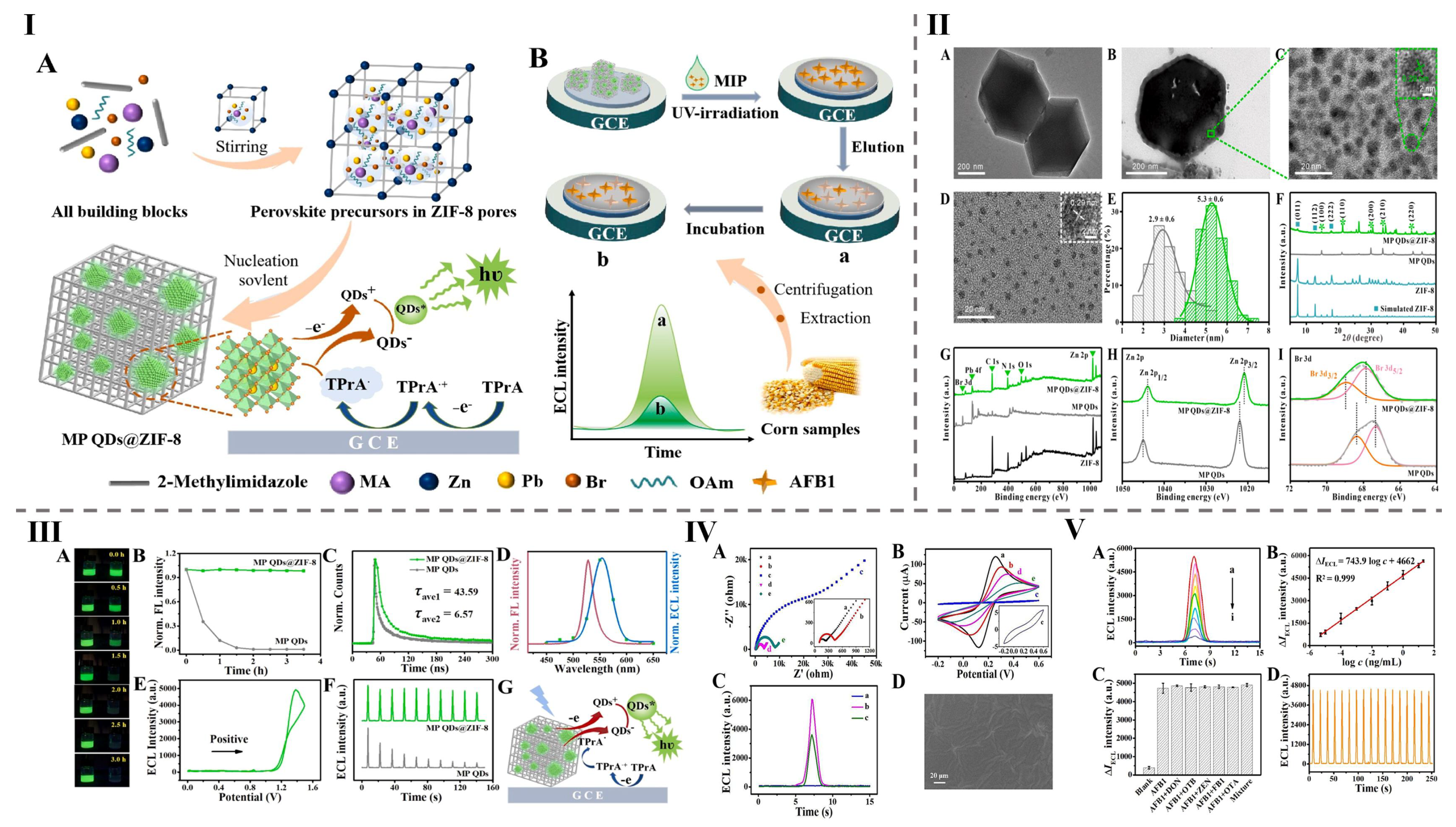
| 29 | MP QDs@ZIF-8 | Room temperature | 528 | 21 | [8] |
| 30 | Antibody/MoS2/UiO-66-NH2 | Microwave-assisted synthesis | - | - | [21] |
| 31 | NU66@QD-ICA | Room temperature | 400–670 | - | [73] |
| 32 | SQDs@MOF-5-NH2 | Solvothermal method | 645–755 | - | [74] |
| 33 | N-GQDs/Au@Cu-MOF | Hydrothermal | - | - | [75] |
| 34 | GQDs/Cu-MOF | Ultrasonication | - | - | [76] |
| 35 | rGO-MWCNT/CS/CQD | Room temperature | - | - | [77] |
| 36 | DP-CDs/TiO2 | Hydrothermal | 520–420 | - | [78] |
| 37 | [Zn(HCOO)3][C2H8N]/PEG and N-CQDs@[Zn(HCOO)3][C2H8N]/PEG | Hydrothermal and Room temperature | - | - | [31] |
| 38 | CD-Ab-COF | Room temperature | 365 | - | [9] |
| 39 | CDs@MIL-53(Fe)-NO2 | Microwave-assisted synthesis | 453 | - | [79] |
| 40 | CDs-MFMIPs | Room temperature | 400–600 | - | [80] |
| 41 | CDs@ZIF-7 | Room temperature | - | - | [81] |
| 42 | CDs@HKUST-1 | Hydrothermal | - | - | [82] |
| 43 | CDs@MOF-5@Rh-6G | Hydrothermal | 365, 435–560 | - | [10] |
| 44 | BYCDs@ZIF-8 | Room temperature | 365, 440–565 | - | [83] |
| 45 | CDs&ZIF-8@MIPs | Room temperature | - | - | [84] |
| 46 | N-GQDs@IRMOF-1@MIP | Room temperature | - | - | [85] |
| 47 | AgMOF@N-CD | Room temperature | - | - | [86] |
| 48 | B-CDs/P-CDs@ZIF-8 | Room temperature | 440–510 | - | [87] |
2.2.2. Mechanochemical Synthesis
2.2.3. Microwave-Assisted Synthesis
| Feature | Microwave-Assisted Synthesis | Ultrasound-Assisted Synthesis |
|---|---|---|
| Mechanism | Dielectric heating → rapid and uniform heating of reaction mixture | Acoustic cavitation → formation, growth, and implosion of bubbles that generate local hotspots |
| Reaction Time | Very short (minutes) | Short to moderate |
| Energy Input | Volumetric and uniform | Localized (at cavitation sites) |
| MOF Crystal Size Control | Good; can tune size by adjusting power/time | Moderate; harder to control due to stochastic cavitation |
| Quantum Dot (QD) Size Range | ~2–10 nm (depending on precursor and time) | ~3–15 nm, wider size distribution often observed |
| Product Homogeneity | Typically, high | Often lower (depends on sonication uniformity) |
| MOF Distribution on Substrate | More uniform coating possible | Can cause partial aggregation or uneven loading |
| Scalability | Moderate scalability (needs special equipment for large scale) | Easier to scale but uniformity issues persist |
| Advantages |
|
|
| Limitations |
|
|
2.2.4. Ultrasound Synthesis
2.2.5. Layer-by-Layer (LbL) Assembly
2.2.6. Template-Assisted Synthesis
3. The Use and Properties of CQDs@MOFs
3.1. Enhanced Sensitivity and Selectivity
3.2. Stability
3.3. Signal Amplification
4. Recent Progress in CQDs@MOFs-Based Sensing Applications
4.1. Enhanced Fluorescent Probes
| Contaminates | Food Samples | CQDs@MOFs | Sensors | Liner Range | LOD | Reference |
|---|---|---|---|---|---|---|
| Heavy Metals/ions | ||||||
| Pb2+ | Handpump water, Blue bird lake, Tap water, Chandigarh, NABI (Mohali), Manoli village water. | BNCDs@Tb-MOF | Fluorescent | 0–1000 nM | 5.97 nM | [38] |
| Pb2+, Cd2+ and Cu2+, | Tap water River water | CQDs@ZIF-8 | Electrochemical | 50 nM−1 μM | 0.04 nM | [56] |
| Hg2+ | Water | CDs@Eu-MOFs | Fluorescent | 0–300 μM | 0.12 nM | [57] |
| Hg2+ | Rice, Peanuts and Water | CuO/Cu2O-CdS/HgS | Photoelectrochemical | 0.5 pM to 2 μM | 0.00011 nM | [5] |
| Hg2+ and cu2+ | Lake water, Fruit juice and red wine | MOF/CdTe QDs | Fluorescence | - | 0.6996 nM and 0.8268 nM | [58] |
| Al3+ and Hg2+ | Yellow river water | CDs@ZIF-90 | Fluorescent | 1–200 μM for Al3+ and 0.05–240 μM for Hg2+ | 810 nM and 19.6 nM | [59] |
| Cu2+ | School lake, Xuanwu lake, and Yangtze River waters | E-CDs@ZIF-8 | Fluorescent | 3.48 nM | [60] | |
| Cu2+ | Tap water | PEG-ZnSQDs@ZIF-67 | Fluorescent | 3 to 500 nM | 0.96 nM | [61] |
| Cu2+ | Tap water | CsPbBr3/HZIF-8 | Fluorescent | 3–500 nM for Cu2+ and 30–1500 nM for melamine | 4.66 nM and 2.64 nM | [62] |
| Pesticides | ||||||
| Chloramphenicol | Milk, Honey, and Pork | CDs@ZIF-8@SMIP | Fluorescent | 0.323 μg L−1 (0.001 μM) to 8075.0 μg L−1 (25.0 μM), | 0.0022 nM | [6] |
| Pesticide thiophanate-methyl | Apple, Pear, and Tomato | CDs@Cu-MOFs | Fluorescence | 0.0307 to 0.769 μmol L−1 | ~ 3.67 nM | [13] |
| Chloramphenicol | Milk samples | M-TiO2-CdTe QDs/CdS QDs | Photoelectrochemical | 1 to 140 nmol L−1 | 0.14 nM | [66] |
| Malathion | Tap water, and Soil samples | N-CDs@Eu-MOF@MIP | Fluorescent | 1–10 μM | 50 nM | [67] |
| Organophosphorus pesticides | Pakchoi and Water sample | Fe-CDs/MOF-808 and Fe-CDs@MOF-808 | Fluorescent | 0.001–360 μM and 0.01–100 μM | 3.3 nM | [11] |
| Organophosphorus pesticide quinalphos | Tomato juice and Rice | OPCD@UiO-66-NH2 | Fluorescent | 0–16 μM | 0.3 nM | [12] |
| carbendazim | Vegetables and Environmental samples | N-CQDs@UiO-66-NH2 | Electrochemical | 0.02–126 µM | 20–126,000 nM and 5.8 nM | [27] |
| Malathion | Cabbage | CdS/g-C3N4/Sm-BDC MOF | Electrochemical (DPV) | 3.0 × 10−8 to 15.0 × 10−8 M | 7.4 nM | [28] |
| Antibiotics | ||||||
| Tetracycline | Animal feeds | CdTe QDs@ZIF-8 | Fluorescent/Colorimetry | 0–70 μM and 0–1000 μM | 15.5 nM 24.9 nM | [63] |
| Chlortetracycline | Milk | NH2-MIL-53 & N, P-CDs@MIP | Fluorescent and smartphone-integrated | 0.06–30 μg·mL−1 | 28,787.88 nM 50,000.00 nM | [64] |
| Penicillin | Milk samples | MB@PApt-SP DNA@AZIS QDs@Ag-Pt NPs | Photoelectrochemistry, Electrochemiluminescence, and Fluorescence signals. | 0.01 pg/mL−1 μg/mL (PEC), 1 pg/mL−1 μg/mL (ECL), and 1 pg/mL−1 μg/mL (FL), | 0.0000034 nM, 0.00029 nM and 0.00047 nM | [65] |
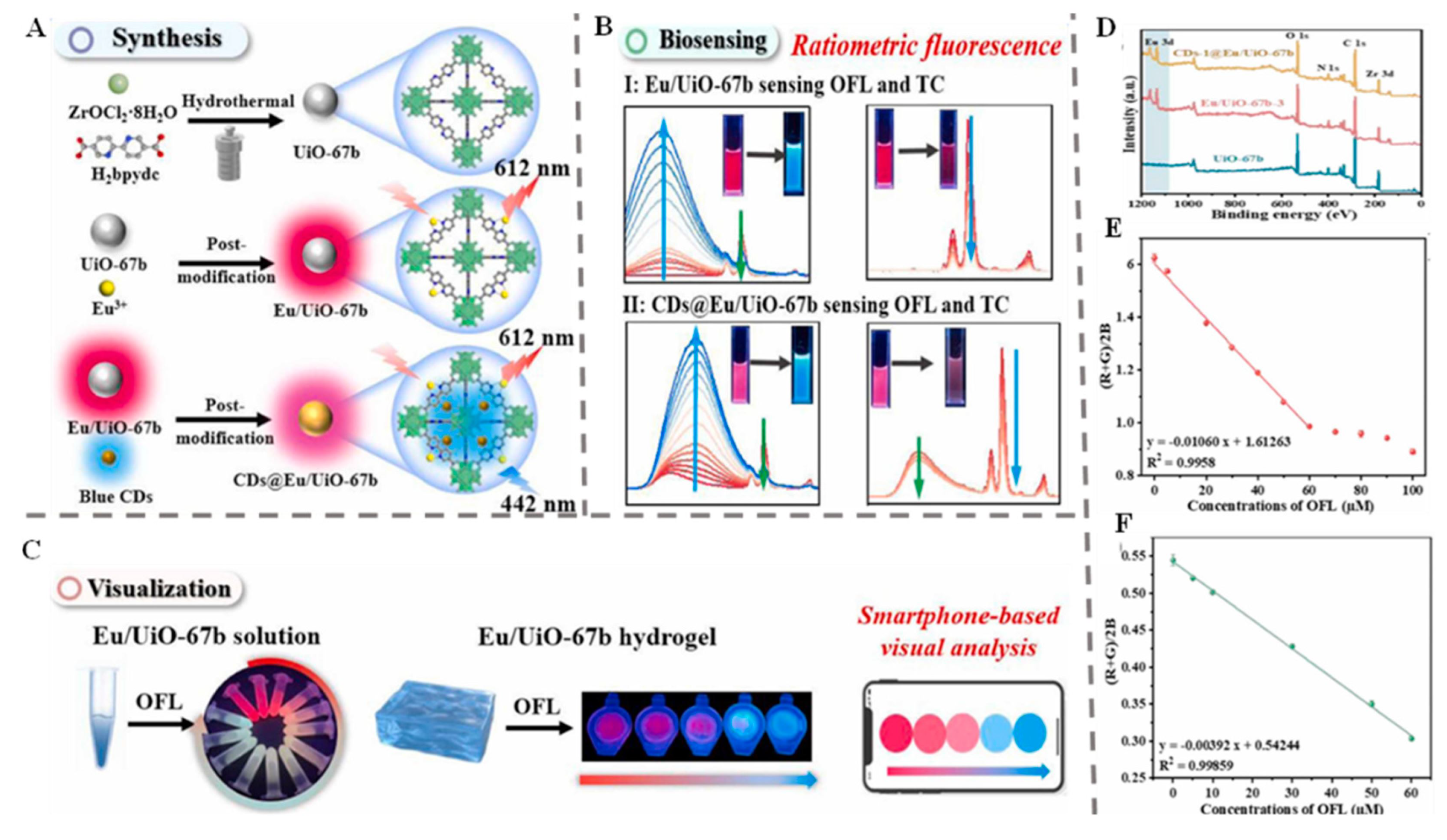
| Ofloxacin and Tetracycline | Tap water and Chicken | Eu3+/CDs-modified UiO-67b | Fluorescent | 0–60 µM and 0–10 µM | 22/27 nM | [20] |
| Chlortetracycline | Basa fish and Pure milk | CdTe QDs@ZIF-8 | Fluorescent | - | 37 nM | [29] |
| Tetracycline and norfloxacin | Water, Milk and Soil samples | CDs@UiO-66-NH2 | Fluorescent | - | 150 nM and 870 nM, | [68] |
| Tetracycline | Milk samples | CD@MIP | Fluorescence | 0–400 μmol L−1 | 590 nM | [69] |
| oxytetracycline | Milk | Ce, N-CDs@ZIF-67@MIP | Fluorescent | 0.05–20 μg mL−1 | 15.13 nM | [70] |
| doxycycline | Milk | His-GQDs-Ser@MOF | Fluorescent | 0.003–6.25 μM and 6.25–25 μM | 1.8 nM | [71] |
| norfloxacin | Milk and Pork | g-CDs@UiO-66 | fluorescent | 1–8 μM | 82 nM | [72] |
4.2. Dual-Mode Sensing
4.3. Enzyme Mimicry
| Contaminates | Food Samples | CQDs@MOFs | Sensors | Liner Range | LOD | Reference |
|---|---|---|---|---|---|---|
| Mycotoxins | ||||||
| Aflatoxin B1 (AFB1) | Canal water and liquid milk samples | Co-CD/PMOF | Chemiluminescence/ Fluorescence | 0.63–69.36 ng/mL | 0.217 ng/mL and 0.027 ng/mL | [30] |
| Aflatoxin B1 | Corn | MP QDs@ZIF-8 | Electrochemiluminescence | 11.55 fg/mL to 20 ng/mL | 0.0000035 nM | [8] |
| Aflatoxin M1 | Milk samples | Antibody/MoS2/UiO-66-NH2 | Electrochemical | 0.2–10 ng/mL | 0.06 ng/mL | [21] |
| Aflatoxin B1, Fumonisin B1, Deoxynivalenol, T-2 toxins, and Zearalenone | Cereals and Feed | NU66@QD-ICA | Fluorescent | - | 0.04, 0.28, 0.25, 0.09, and 0.08 μg/kg. | [73] |
| Patulin (PAT) | Apple juice samples | SQDs@MOF-5-NH2 | Fluorescent | - | 0.000753 ng/mL | [74] |
| Patulin (PAT) | Apple juices | N-GQDs/Au@Cu-MOF | Electrochemical | 0.001 to 70.0 ng/mL | 0.0007 ng/mL | [75] |
| Bacteria | ||||||
| Staphylococcus aureus | Tap water, Milk, Lonicera japonica, Urine, and Zhangjiang River. | GQDs/Cu-MOF | Electrochemical aptasensor | 5.0 × 100 to 5.0 × 108 CFU·mL−1 | 0.97 CFU/mL | [76] |
| Acinetobacter baumannii | Skim milk powder | rGO-MWCNT/CS/CQD | Electrochemical aptasensor | 10 to 1 × 107 CFU/mL | 1 CFU/mL | [77] |
| Vibrio harveyi | Shrimps | DP-CDs/TiO2 | Fluorescent | - | - | [78] |
| Escherichia coli | - | [Zn(HCOO)3][C2H8N]/PEG and N-CQDs@[Zn(HCOO)3][C2H8N]/PEG | Fluorescent | - | - | [31] |
| E. coli O157:H7 | Milk | CD-Ab-COF | Fluorescent | 0 to 106 CFU/mL | 7 CFU/mL | [9] |
| Aromatic compounds | ||||||
| Gallic acid (GA) | Green tea drink samples | CDs@MIL-53(Fe)-NO2 | Colorimetric/Fluorescent | 17, 16 and 27 nM | [79] | |
| 4-nitrophenol | Tap water, Fish and Shrimp meat | CDs-MFMIPs | Fluorescent | 0.05–50 μM | 17.44 nM. | [80] |
| Allura Red AC (AR) | Candy, Jelly, Strawberry flavored syrup, Pomegranate flavored drink, Energy drinks, Drink water, Commercial food colorant solution, and Carbonated beverages were determined. | CDs@ZIF-7 | Fluorescent | 0.30–7.00 nM | 0.60 nM | [81] |
| Catechol | Tea samples | CDs@HKUST-1 | Electrochemiluminescence | 5.0 × 10−9 to 2.5 × 10−5 mol/L | 3.8 nM | [82] |
| Curcumin | Cur in mustard, Curry, and red pepper powders. | CDs@MOF-5@Rh-6G | Fluorescent | 0.1–5 μmol/L | 15 nM | [10] |
| Glutathione | Grape and Cucumber | BYCDs@ZIF-8 | Fluorescent | 3–25 nM | 0.90 nM | [83] |
| Malachite green (MG) | River water, Tap water, Deionized water and Aquaculture water | CDs&ZIF-8@MIPs | Fluorescent | 20–180 nM | 2.93 nM | [84] |
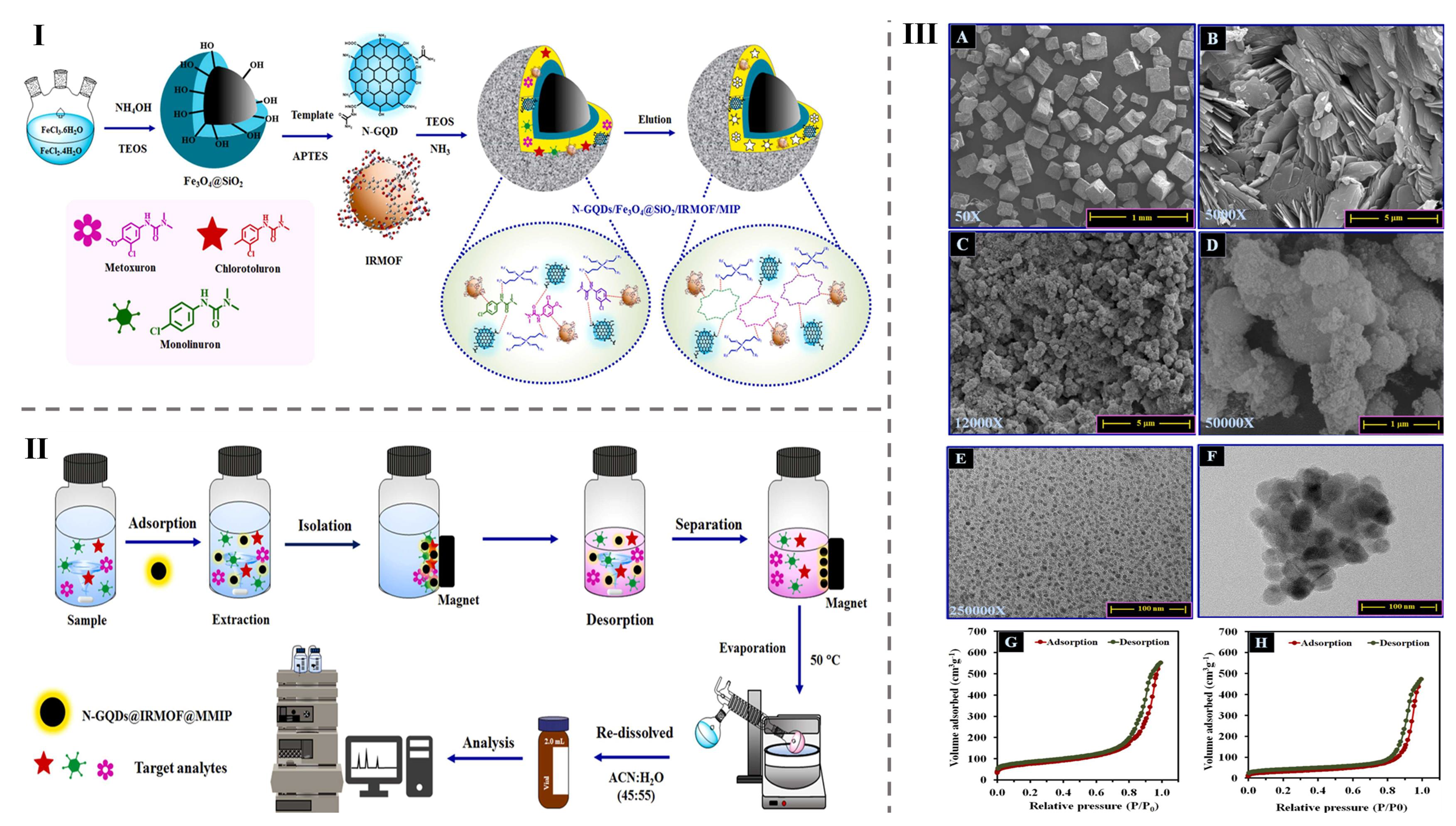
| Phenylureas | Tomato, Cucumber, Radish and Soybean milk | N-GQDs@IRMOF-1@MIP | Adsorbent | 1.0–150 µg L−1 | 1.0 µg L−1 | [85] |
| Trilobatin | Lithocarpus polystachyus Rehd | AgMOF@N-CD | Electrochemiluminescence | 1.0 × 10−7 M to 1.0 × 10−3 M | 5.99 nM | [86] |
| Triticonazole | Water and fruit juice samples | B-CDs/P-CDs@ZIF-8 | Fluorescence | 10–400 nM | 4.0 nM | [87] |
5. Recent Advances in CQDs@MOFs for Detection Applications of Food Contaminates
5.1. Metal Ions
5.2. Pesticides
5.3. Antibiotic
5.4. Mycotoxins
5.5. Pathogens
5.6. Aromatic Compounds
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delnavaz, E.; Amjadi, M.; Farajzadeh, M.A. Metal-organic framework with dual-loading of nickel/nitrogen-doped carbon dots and magnetic nanoparticles for fluorescence detection of fenitrothion in food samples. J. Food Compos. Anal. 2023, 115, 104873. [Google Scholar] [CrossRef]
- Pan, M.; Xie, X.; Liu, K.; Yang, J.; Hong, L.; Wang, S. Fluorescent carbon quantum dots—Synthesis, functionalization and sensing application in food analysis. Nanomaterials 2020, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Elugoke, S.E.; Uwaya, G.E.; Quadri, T.W.; Ebenso, E.E. Carbon quantum dots: Basics, properties, and fundamentals. In Carbon Dots: Recent Developments and Future Perspectives; ACS Publications: Washington, DC, USA, 2024; pp. 3–42. [Google Scholar]
- Li, B.; Suo, T.; Xie, S.; Xia, A.; Ma, Y.-j.; Huang, H.; Zhang, X.; Hu, Q. Rational design, synthesis, and applications of carbon dots@ metal–organic frameworks (CD@ MOF) based sensors. TrAC Trends Anal. Chem. 2021, 135, 116163. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wang, J.; Zhou, B.; Shi, J.; Zhang, H. Metal organic framework-derived CuO/Cu2O polyhedron-CdS quantum dots double Z-scheme heterostructure for cathodic photoelectrochemical detection of Hg2+ in food and environment. Food Chem. 2024, 450, 139261. [Google Scholar] [CrossRef]
- Pan, M.; Sun, J.; Wang, Y.; Yang, J.; Wang, Z.; Li, L.; Wang, S. Carbon-dots encapsulated luminescent metal–organic frameworks@ surface molecularly imprinted polymer: A facile fluorescent probe for the determination of chloramphenicol. Food Chem. 2024, 442, 138461. [Google Scholar] [CrossRef]
- Hu, F.; Fu, Q.; Li, Y.; Yan, C.; Xiao, D.; Ju, P.; Hu, Z.; Li, H.; Ai, S. Zinc-doped carbon quantum dots-based ratiometric fluorescence probe for rapid, specific, and visual determination of tetracycline hydrochloride. Food Chem. 2024, 431, 137097. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, C.; Li, J.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.-M. High-performance electrochemiluminescence sensors based on ultra-stable perovskite quantum dots@ ZIF-8 composites for aflatoxin B1 monitoring in corn samples. Food Chem. 2023, 410, 135325. [Google Scholar] [CrossRef]
- Wang, S.; Liang, N.; Hu, X.; Li, W.; Guo, Z.; Zhang, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J. Carbon dots and covalent organic frameworks based FRET immunosensor for sensitive detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.; Chen, J.; Li, R.; Chen, Q.; Chen, X. Ratiometric fluorescence method comprising carbon dots and rhodamine 6G encapsulated in metal–organic framework microcubes for curcumin detection. Microchim. Acta 2024, 191, 337. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ou, Q.; Yuan, J.; Yang, J.; Xu, S.; Zhang, X. Multifunctional Fe-doped carbon dots and metal-organic frameworks nanoreactor for cascade degradation and detection of organophosphorus pesticides. Chem. Eng. J. 2023, 464, 142480. [Google Scholar] [CrossRef]
- Bera, M.K.; Behera, L.; Mohapatra, S. A fluorescence turn-down-up detection of Cu2+ and pesticide quinalphos using carbon quantum dot integrated UiO-66-NH2. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126792. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, G.; Luo, X.; Lin, W.; Han, Y.; Huang, J.; Li, Z. Carbon dots@ Cu metal–organic frameworks hybrids for ratiometric fluorescent determination of pesticide thiophanate-methyl. Microchim. Acta 2022, 189, 325. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Lin, H.; Jiang, H.; Yao-Say Solomon Adade, S.; Xue, Z.; Chen, Q. Advanced applications of chemo-responsive dyes based odor imaging technology for fast sensing food quality and safety: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5145–5172. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, S.; Cheng, L.; Ma, H.; Gao, X.; Brennan, C.S.; Yan, J.-K. Micro-nano-bubble technology and its applications in food industry: A critical review. Food Rev. Int. 2023, 39, 4213–4235. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Rajoka, M.S.R.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Ren, J.; Meijerink, A.; Zhou, X.; Wu, J.; Zhang, G.; Wang, Y. In situ embedding synthesis of CsPbBr3@ Ce-MOF@ SiO2 nanocomposites for high efficiency light-emitting diodes: Suppressing reabsorption losses through the waveguiding effect. ACS Appl. Mater. Interfaces 2022, 14, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- Dassouki, K.; Dasgupta, S.; Dumas, E.; Steunou, N. Interfacing metal organic frameworks with polymers or carbon-based materials: From simple to hierarchical porous and nanostructured composites. Chem. Sci. 2023, 14, 12898–12925. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Li, J.; Li, J. Hydrogel-involved portable ratiometric fluorescence sensor based on Eu3+/CDs-modified metal-organic framework for detection of ofloxacin and tetracycline. Sens. Actuators B Chem. 2025, 422, 136573. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, S.; Singh, S.; Bhardwaj, N.; Deep, A. Selective and sensitive electrochemical sensor for aflatoxin M1 with a molybdenum disulfide quantum dot/metal–organic framework nanocomposite. ACS Omega 2022, 7, 17600–17608. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Song, Y.; Pei, H.; Mo, Z. Synthesis strategies and applications of metal–organic framework-quantum dot (MOF@ QD) functional composites. J. Ind. Eng. Chem. 2023, 128, 17–54. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.; Chen, Q. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Y.; Li, L.; Zhou, J. Carbon quantum dots activated metal organic frameworks for selective detection of Cu (II) and Fe (III). Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124378. [Google Scholar] [CrossRef]
- Bazazi, S.; Hashemi, E.; Mohammadjavadi, M.; Saeb, M.R.; Liu, Y.; Huang, Y.; Xiao, H.; Seidi, F. Metal-organic framework (MOF)/C-dots and covalent organic framework (COF)/C-dots hybrid nanocomposites: Fabrications and applications in sensing, medical, environmental, and energy sectors. Adv. Colloid Interface Sci. 2024, 328, 103178. [Google Scholar] [CrossRef] [PubMed]
- Giri, L.; Rout, S.R.; Varma, R.S.; Otyepka, M.; Jayaramulu, K.; Dandela, R. Recent advancements in metal–organic frameworks integrating quantum dots (QDs@ MOF) and their potential applications. Nanotechnol. Rev. 2022, 11, 1947–1976. [Google Scholar] [CrossRef]
- Velmurugan, S.; Traiwatcharanon, P.; Jiwanti, P.K.; Cheng, S.-H.; Wongchoosuk, C. Highly selective carbendazim fungicide sensing performance of nitrogen-doped carbon quantum dots encapsulated aminated-UiO-66 zirconium metal-organic framework electrocatalyst. Electrochim. Acta 2024, 479, 143911. [Google Scholar] [CrossRef]
- Yassin, J.M.; Taddesse, A.M.; Tsegaye, A.A.; Sánchez-Sánchez, M. CdS/g-C3N4/Sm-BDC MOF nanocomposite modified glassy carbon electrodes as a highly sensitive electrochemical sensor for malathion. Appl. Surf. Sci. 2024, 648, 158973. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, X.; Gao, F.; Li, H.; Fu, B.; Guo, D.-Y.; Wang, F.; Pan, Q. CdTe QDs@ ZIF-8 composite-based recyclable ratiometric fluorescent sensor for rapid and sensitive detection of chlortetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120785. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xiao, S.; Kang, X.; Long, F.; Zhu, A. Bifunctional MOF-encapsulated cobalt-doped carbon dots nanozyme-powered chemiluminescence/fluorescence dual-mode detection of aflatoxin B1. ACS Appl. Mater. Interfaces 2024, 16, 16494–16504. [Google Scholar] [CrossRef]
- Tabatabaeian, K.; Simayee, M.; Fallah-Shojaie, A.; Mashayekhi, F.; Hadavi, M. Novel MOF-based mixed-matrix membranes, N-CQDs@[Zn (HCOO)3][C2H8 N]/PEG, as the effective antimicrobials. J. Iran. Chem. Soc. 2020, 17, 2987–2995. [Google Scholar] [CrossRef]
- Xu, L.; Pan, M.; Fang, G.; Wang, S. Carbon dots embedded metal-organic framework@ molecularly imprinted nanoparticles for highly sensitive and selective detection of quercetin. Sens. Actuators B Chem. 2019, 286, 321–327. [Google Scholar] [CrossRef]
- Lin, X.; Gao, G.; Zheng, L.; Chi, Y.; Chen, G. Encapsulation of strongly fluorescent carbon quantum dots in metal–organic frameworks for enhancing chemical sensing. Anal. Chem. 2014, 86, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Kurniawan, D.; Tsai, M.-D.; Chang, J.-W.; Chang, Y.-N.; Yang, S.-C.; Chiang, W.-H.; Kung, C.-W. Two-dimensional metal–organic framework for post-synthetic immobilization of graphene quantum dots for photoluminescent sensing. Commun. Chem. 2024, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.V.; Rhim, J.-W. Fluorescent carbon quantum dots for food contaminants detection applications. J. Environ. Chem. Eng. 2024, 12, 111999. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and applications of metal-organic framework–quantum dot (QD@ MOF) composites. Coord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Kung, C.-W.; Venkatesana, S.; Teng, H.; Lee, Y.-L. Synthesis of the composites of carbon quantum dots and metal organic framework and their application in hydrogen evolution. Int. J. Hydrogen Energy 2024, 68, 321–330. [Google Scholar] [CrossRef]
- Jain, S.; Dilbaghi, N.; Singhal, N.K.; Kaushik, A.; Kim, K.-H.; Kumar, S. Carbon quantum dots@ metal–organic framework based catalytic nucleic acid fluorescent system for highly sensitive and selective detection of Pb2+ in aqueous solutions. Chem. Eng. J. 2023, 457, 141375. [Google Scholar] [CrossRef]
- Varadharajan, S.; Vasanthan, K.S.; Mathur, V.; Hariperumal, N.; Mazumder, N. Green synthesis and multifaceted applications: Challenges and innovations in carbon dot nanocomposites. Discov. Nano 2024, 19, 205. [Google Scholar] [CrossRef]
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the methods for the synthesis of carbon dots and their emerging applications. Polymers 2021, 13, 3190. [Google Scholar] [CrossRef] [PubMed]
- Sher, F.; Hayward, A.; El Guerraf, A.; Ziani, I.; Hrnjić, H.; Boškailo, E.; Wang, B.; Chupin, A.; Nemțanu, M.R. Advanced metal-organic frameworks for superior carbon capture, high-performance energy storage and environmental photocatalysis—A critical review. J. Mater. Chem. A 2024, 12, 27932–27973. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fang, G.; Liu, J.; Pan, M.; Wang, R.; Wang, S. One-pot synthesis of nanoscale carbon dots-embedded metal–organic frameworks at room temperature for enhanced chemical sensing. J. Mater. Chem. A 2016, 4, 15880–15887. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Tang, T.; Liao, S. One-pot preparation and the electrochemical properties of the composite of the NiCo bimetallic organic framework and carbon quantum dots. CrystEngComm 2024, 26, 4448–4457. [Google Scholar] [CrossRef]
- Rocco, D.; Moldoveanu, V.G.; Feroci, M.; Bortolami, M.; Vetica, F. Electrochemical synthesis of carbon quantum dots. ChemElectroChem 2023, 10, e202201104. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A review of MOFs and their composites-based photocatalysts: Synthesis and applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Yan, X.; Huang, S.; Wang, Y.; Tang, Y.; Tian, Y. Bottom-up self-assembly based on DNA nanotechnology. Nanomaterials 2020, 10, 2047. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Chai, Z.; Childress, A.; Busnaina, A.A. Directed assembly of nanomaterials for making nanoscale devices and structures: Mechanisms and applications. ACS Nano 2022, 16, 17641–17686. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on metal–organic framework classification, synthetic approaches, and influencing factors: Applications in energy, drug delivery, and wastewater treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef]
- Raja, D.S.; Tsai, D.-H. Recent Advances in Continuous Flow Synthesis of Metal-Organic Frameworks and Their Composites. Chem. Commun. 2024, 60, 8497–8515. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-organic frameworks: Synthetic methods and potential applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Dzinnik, M.J.; Akmaz, N.E.; Hannebauer, A.; Schaate, A.; Behrens, P.; Haug, R.J. Locally controlled MOF growth on functionalized carbon nanotubes. Commun. Mater. 2024, 5, 38. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oloyede, S.O. Synthesis of Metal–Organic Frameworks Quantum Dots Composites as Sensors for Endocrine-Disrupting Chemicals. Int. J. Mol. Sci. 2022, 23, 7980. [Google Scholar] [CrossRef]
- Gu, Z.G.; Li, D.J.; Zheng, C.; Kang, Y.; Wöll, C.; Zhang, J. MOF-templated synthesis of ultrasmall photoluminescent carbon-nanodot arrays for optical applications. Angew. Chem. 2017, 129, 6957–6962. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, X.; Liu, Z.; Gai, L.; Yue, Y.; Ma, H. Sensitive and selective electrochemical detection of lead (II) based on waste-biomass-derived carbon quantum dots@ zeolitic imidazolate framework-8. Materials 2023, 16, 3378. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Wu, N.; Xu, M.; Wang, M.; Zhang, L.; Yang, W. In-situ synthesis of carbon dots-embedded europium metal-organic frameworks for ratiometric fluorescence detection of Hg2+ in aqueous environment. Anal. Chim. Acta 2021, 1141, 13–20. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Cao, J.; Wu, Y. On-site, rapid and visual determination of Hg2+ and Cu2+ in red wine by ratiometric fluorescence sensor of metal-organic frameworks and CdTe QDs. Food Chem. 2020, 328, 127119. [Google Scholar] [CrossRef]
- Peng, L.; Guo, H.; Wu, N.; Wang, M.; Hao, Y.; Ren, B.; Hui, Y.; Ren, H.; Yang, W. A dual-functional fluorescence probe CDs@ ZIF-90 for highly specific detection of Al3+ and Hg2+ in environmental water samples. Anal. Chim. Acta 2024, 1288, 342171. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Wang, X.; Feng, X.; Deng, Y.; Cheng, W.; Xiong, R.; Huang, C. Xylan derived carbon dots composite ZIF-8 and its immobilized carbon fibers membrane for fluorescence selective detection Cu2+ in real samples. Chem. Eng. J. 2023, 474, 145804. [Google Scholar] [CrossRef]
- Asadi, F.; Azizi, S.N.; Chaichi, M.J. Green synthesis of fluorescent PEG-ZnS QDs encapsulated into Co-MOFs as an effective sensor for ultrasensitive detection of copper ions in tap water. Mater. Sci. Eng. C 2019, 105, 110058. [Google Scholar] [CrossRef]
- Ahmed, S.; Lahkar, S.; Doley, S.; Mohanta, D.; Dolui, S.K. A hierarchically porous MOF confined CsPbBr3 quantum dots: Fluorescence switching probe for detecting Cu (II) and melamine in food samples. J. Photochem. Photobiol. A Chem. 2023, 443, 114821. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, M.; Liu, Y.; Peng, L.; Tian, J.; Ren, B.; Guo, H.; Yang, W. Dual response signal CdTe QDs@ ZIF-8 with butterfly spectrum for dual-mode fluorescence/colorimetric detection of tetracycline in animal feeds. Anal. Bioanal. Chem. 2024, 416, 5841–5851. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Li, B.; Li, X.; Xu, Y. Hydrogen bonding-mediated assembly of carbon dot@ Zr-based metal organic framework as a multifunctional fluorescence sensor for chlortetracycline, pH and temperature detection. New J. Chem. 2022, 46, 13021–13029. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Dong, Y.; Jie, G. A multi-modal biosensing platform based on Ag-ZnIn2S4@ Ag-Pt nanosignal probe-sensitized UiO-66 for ultra-sensitive detection of penicillin. Food Chem. 2024, 444, 138665. [Google Scholar] [CrossRef]
- Ou, P.; Wu, J.; Lin, Y.; Tan, X.; Wu, Y.; Chen, Z.; Wei, F.; Huang, K. Flexible photoelectrochemical sensor for highly sensitive chloramphenicol detection based on M-TiO 2-CdTe QDs/CdS QDs composite. Anal. Bioanal. Chem. 2022, 414, 2065–2078. [Google Scholar] [CrossRef]
- Yang, L.; Hu, W.; Pei, F.; Liu, Z.; Wang, J.; Tong, Z.; Mu, X.; Du, B.; Xia, M.; Wang, F. A ratiometric fluorescence imprinted sensor based on N-CDs and metal–organic frameworks for visual smart detection of malathion. Food Chem. 2024, 438, 138068. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hong, D.; Zhang, D.; Jiang, Y. Carbon dots-functionalized metal-organic framework for the dual detection of tetracycline and norfloxacin. J. Mol. Struct. 2024, 1312, 138567. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, H.; Xu, Y.; Wang, H.; Guan, K.; Gong, B. Dual-functional molecularly imprinted doped carbon dot based on metal-organic frameworks for tetracycline adsorption and determination. Microchim. Acta 2023, 190, 463. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Hu, X.; Cai, L.; Wang, H.; Fang, G.; Wang, S. A novel fluorescent biomimetic sensor based on cerium, nitrogen co-doped carbon quantum dots embedded in cobalt-based metal organic framework@ molecularly imprinted polymer for selective and sensitive detection of oxytetracycline. Microchem. J. 2023, 190, 108606. [Google Scholar] [CrossRef]
- Han, Y.; Li, P.; Du, Y. Encapsulating functionalized graphene quantum dot into metal-organic framework as a ratiometric fluorescent nanoprobe for doxycycline sensing. Microchim. Acta 2023, 190, 234. [Google Scholar] [CrossRef]
- Wu, S.; Chen, C.; Chen, J.; Li, W.; Sun, M.; Zhuang, J.; Lin, J.; Liu, Y.; Xu, H.; Zheng, M. Construction of carbon dots/metal–organic framework composite for ratiometric sensing of norfloxacin. J. Mater. Chem. C 2022, 10, 15508–15515. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.; Zhang, J.; Shen, X.; Xu, Z.; Li, X.; Lei, H. High bioaffinity controllable assembly nanocarrier UiO-66-NH2@ quantum dot-based immunochromatographic assay for simultaneous detection of five mycotoxins in cereals and feed. J. Agric. Food Chem. 2023, 71, 16797–16806. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, Y.; Du, G.; Guo, Q.; Chen, H.; He, Q.; Zhao, Q.; Ye, H.; Wang, J.; Yuan, Y. Magnetic capture of sulfur quantum dots encapsulated in MOF-5-NH2 via a target-driven self-cycling catalyzed hairpin assembly for the sensitive detection of patulin. Chem. Eng. J. 2022, 433, 133624. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Beheshti, H.R.; Boroushaki, M.T. Ultra-sensitive molecularly imprinted electrochemical sensor for patulin detection based on a novel assembling strategy using Au@ Cu-MOF/N-GQDs. Sens. Actuators B Chem. 2020, 318, 128219. [Google Scholar] [CrossRef]
- Lin, X.; Liu, C.; Lei, Q.; Nan, X.; Zhu, Y.; Liao, J.; Du, Z.; Ye, C.; Xiong, Y.; Yang, M. A novel ratiometric electrochemical aptasensor based on graphene quantum dots/Cu-MOF nanocomposite for the on-site determination of Staphylococcus aureus. J. Hazard. Mater. 2025, 485, 136845. [Google Scholar] [CrossRef]
- Abedi, R.; Raoof, J.B.; Mohseni, M.; Hashkavayi, A.B. Sandwich-type electrochemical aptasensor based on hemin-graphite oxide as a signal label and rGO/MWCNTs/chitosan/carbon quantum dot modified electrode for sensitive detection of Acinetobacter baumannii bacteria. Anal. Chim. Acta 2024, 1303, 342491. [Google Scholar] [CrossRef]
- Alexpandi, R.; Gopi, C.V.M.; Durgadevi, R.; Kim, H.-J.; Pandian, S.K.; Ravi, A.V. Metal sensing-carbon dots loaded TiO2-nanocomposite for photocatalytic bacterial deactivation and application in aquaculture. Sci. Rep. 2020, 10, 12883. [Google Scholar] [CrossRef]
- Liu, J.; Ma, W.; Wang, Y.; Gu, Q.; Pan, Q.; Zong, S.; Qin, M.; Li, J. Enhanced oxidase-mimic constructed by luminescent carbon dots loaded on MIL-53 (Fe)-NO2 for dual-mode detection of gallic acid and biothiols in food and humans. Food Chem. 2024, 433, 137241. [Google Scholar] [CrossRef]
- Yan, C.; Han, S.; Sun, R.; Zhao, L.; Zhang, X.; Zhang, X.; Wang, Y.; Zhai, M. A smart imprinted fluorescence sensor of biomass carbon dots based on magnetic covalent organic frameworks for real-time and sensitive detection of 4-nitrophenol in food. Microchem. J. 2024, 207, 111949. [Google Scholar] [CrossRef]
- Esmail, L.A.; Jabbar, H.S. Encapsulation of amaranth CDs at ZIF-7 MOFs as a novel adsorbent for ultrasonic-assisted dispersive nano-solid-phase microextraction and ultrasensitive determination of allura red in food samples. Microchem. J. 2023, 195, 109474. [Google Scholar] [CrossRef]
- Zhou, L.; Shan, X.; Jiang, D.; Wang, W.; Chen, Z. Electrochemical luminescence sensor based on CDs@ HKUST-1 composite for detection of catechol. J. Electroanal. Chem. 2020, 871, 114215. [Google Scholar] [CrossRef]
- Jalili, R.; Khataee, A.; Rashidi, M.-R.; Luque, R. Dual-colored carbon dot encapsulated metal-organic framework for ratiometric detection of glutathione. Sens. Actuators B Chem. 2019, 297, 126775. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Wang, T.; Deng, P.; Yan, Y. Carbon dots incorporated metal–organic framework for enhancing fluorescence detection performance. J. Mater. Sci. 2020, 55, 14153–14165. [Google Scholar] [CrossRef]
- Sa-Nguanprang, S.; Phuruangrat, A.; Bunkoed, O. A magnetic adsorbent of nitrogen-doped graphene quantum dots, zinc metal-organic framework and molecularly imprinted polymer to extract phenylureas. J. Food Compos. Anal. 2024, 126, 105911. [Google Scholar] [CrossRef]
- Yao, L.; Mei, X.; Zhi, J.; Wang, W.; Li, Q.; Jiang, D.; Chen, X.; Chen, Z. A novel electrochemiluminescent sensor based on AgMOF@ N-CD composites for sensitive detection of trilobatin. Analyst 2024, 149, 5265–5276. [Google Scholar] [CrossRef]
- Shokri, R.; Amjadi, M. A ratiometric fluorescence sensor for triticonazole based on the encapsulated boron-doped and phosphorous-doped carbon dots in the metal organic framework. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118951. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Ng, M.; Milner, P.J. Rapid mechanochemical synthesis of metal–organic frameworks using exogenous organic base. Dalton Trans. 2020, 49, 16238–16244. [Google Scholar] [CrossRef]
- Hasan, H.; Kulbir; Kumar, P.; Sk, M.P. Efficient Mechanochemical Conversion of Elemental Sulfur to Circularly Polarized Luminescent Sulfur Quantum Dots. J. Phys. Chem. C 2024, 128, 8114–8122. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la O Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A green approach in the preparation of pharmaceutical cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Wenger, L.E.; Hanusa, T.P. Synthesis without solvent: Consequences for mechanochemical reactivity. Chem. Commun. 2023, 59, 14210–14222. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Mahmoud, N.E.; Mahdi, A.A.; Emam, H.E.; Abdelhameed, R.M. Affinity of carbon quantum dots anchored within metal organic framework matrix as enhancer of plant nourishment. Heliyon 2022, 8, e12396. [Google Scholar] [CrossRef]
- Kayani, K.F.; Shatery, O.B.; Mohammed, S.J.; Ahmed, H.R.; Hamarawf, R.F.; Mustafa, M.S. Synthesis and applications of luminescent metal organic frameworks (MOFs) for sensing dipicolinic acid in biological and water samples: A review. Nanoscale Adv. 2024, 7, 13–41. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, C.; Ma, W.; Liu, Y.; Liu, C.; Zhang, T.; Xiao, H. Microwave-assisted synthesis of N, S Co-doped carbon quantum dots for fluorescent sensing of Fe (III) and hydroquinone in water and cell imaging. Nanomaterials 2024, 14, 1827. [Google Scholar] [CrossRef]
- Chen, X.; Ding, J.; Ji, D.; He, S.; Ma, H. Optimization of ultrasonic-assisted extraction conditions for bioactive components from coffee leaves using the Taguchi design and response surface methodology. J. Food Sci. 2020, 85, 1742–1751. [Google Scholar] [CrossRef]
- Liu, W.; Xu, S.; Li, Z.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Layer-by-layer assembly of carbon dots-based ultrathin films with enhanced quantum yield and temperature sensing performance. Chem. Mater. 2016, 28, 5426–5431. [Google Scholar] [CrossRef]
- Xiang, F.; Popczun, E.J.; Hopkinson, D.P. Layer-by-layer assembly of metal-organic framework nanosheets with polymer. Nanotechnology 2019, 30, 345602. [Google Scholar] [CrossRef]
- Chen, D.-H.; Gliemann, H.; Wöll, C. Layer-by-layer assembly of metal-organic framework thin films: Fabrication and advanced applications. Chem. Phys. Rev. 2023, 4, 011305. [Google Scholar] [CrossRef]
- Choi, S.; Jin, H.; Bang, J.; Kim, S. Layer-by-layer quantum dot assemblies for the enhanced energy transfers and their applications toward efficient solar cells. J. Phys. Chem. Lett. 2012, 3, 3442–3447. [Google Scholar] [CrossRef]
- Xu, J.; Guo, H.; Hao, Y.; Tian, J.; Liu, Y.; Ren, H.; Yang, W. NiFe2O4 quantum dots anchored on flower-like Ni-MOF with enhanced electrochemical performance for supercapacitors. J. Mater. Chem. C 2023, 11, 15624–15637. [Google Scholar] [CrossRef]
- Altaf, A.; Khan, I.; Khan, A.; Sadiq, S.; Humayun, M.; Khan, S.; Zaman, S.; Khan, A.; Abumousa, R.A.; Bououdina, M. Metal/covalent organic framework encapsulated lead-free halide perovskite hybrid nanocatalysts: Multifunctional applications, design, recent trends, challenges, and prospects. ACS Omega 2024, 9, 34220–34242. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, X.; He, H.; Fan, X.; Peng, W.; Li, Y. Template-Assisted in situ synthesis of superaerophobic bimetallic MOF composites with tunable morphology for boosted oxygen evolution reaction. J. Colloid Interface Sci. 2024, 676, 238–248. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A review of polymeric micelles and their applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Bjørnestad, V.A.; Li, X.; Tribet, C.; Lund, R.; Cascella, M. Micelle kinetics of photoswitchable surfactants: Self-assembly pathways and relaxation mechanisms. J. Colloid Interface Sci. 2023, 646, 883–899. [Google Scholar] [CrossRef]
- Ghaedi, H.; Zhao, M. Review on template removal techniques for synthesis of mesoporous silica materials. Energy Fuels 2022, 36, 2424–2446. [Google Scholar] [CrossRef]
- Hao, P.; Shi, R.; Wang, X.; Zhang, J.; Li, B.; Wang, J.; Liu, B.; Liu, Y.; Qiao, X.; Wang, Z. Efficient tetracycline degradation using carbon quantum dot modified TiO2@LaFeO3 hollow core shell photocatalysts. Sci. Rep. 2024, 14, 27057. [Google Scholar] [CrossRef]
- Shi, F.; Xing, B.; Zeng, H.; Guo, H.; Qu, X.; Huang, G.; Cao, Y.; Li, P.; Feng, L.; Zhang, C. Facile synthesis of ultrathin carbon nanosheets through NaCl-KCl templates coupled with ice-induced assembly strategy from carbon quantum dots as lithium-ion batteries anodes. J. Alloys Compd. 2024, 976, 173325. [Google Scholar] [CrossRef]
- Kaur, A.; Bajaj, B.; Kaushik, A.; Saini, A.; Sud, D. A review on template assisted synthesis of multi-functional metal oxide nanostructures: Status and prospects. Mater. Sci. Eng. B 2022, 286, 116005. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, X.; Bi, J.; Fu, H.; Liu, N.; Zhang, X.; Wang, Z.; Han, Y. Template-assisted synthesis of 3D ordered mesoporous graphitic carbon nitride decorated with gold nanoparticles for dopamine sensing. New J. Chem. 2024, 48, 17928–17934. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Han, Y.; Lv, X.; Hou, H.; Fan, Y. Template-assisted synthesis of Co, Mn-MOFs with magnetic properties based on pyridinedicarboxylic acid. Cryst. Growth Des. 2012, 12, 3505–3513. [Google Scholar] [CrossRef]
- Hou, X.; Wang, W.; Gao, X.; Ran, K.; Huang, Y.; Zhang, Z.; Fang, Y.; Wang, S.; He, D.; Ye, W. Salt template assisted synthesis of Fe@ graphene for high-performance electromagnetic wave absorption. Carbon 2022, 199, 268–278. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Li, Y.; Shi, J.; Huang, X.; Li, Z.; Zhang, J.; Li, W.; Xu, Y.; Zou, X. Easy-to-Use visual sensing system for milk freshness, sensitized with acidity-responsive N-doped carbon quantum dots. Foods 2022, 11, 1855. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef]
- Yang, W.; Cao, L.; Lu, H.; Huang, Y.; Yang, W.; Cai, Y.; Li, S.; Li, S.; Zhao, J.; Xu, W. Custom-printed microfluidic chips using simultaneous ratiometric fluorescence with “Green” carbon dots for detection of multiple antibiotic residues in pork and water samples. J. Food Sci. 2024, 89, 5980–5992. [Google Scholar] [CrossRef]
- Song, W.; Zhai, X.; Shi, J.; Zou, X.; Xue, Y.; Sun, Y.; Sun, W.; Zhang, J.; Huang, X.; Li, Z. A ratiometric fluorescence amine sensor based on carbon quantum dot-loaded electrospun polyvinylidene fluoride film for visual monitoring of food freshness. Food Chem. 2024, 434, 137423. [Google Scholar] [CrossRef]
- Yang, W.; Liu, C.; Zhang, B.; Wu, C.; Cao, Y.; Huang, W.; Xu, W. Construction of a Nitrogen-Doped Carbon Quantum Dot Fluorescent Molecularly Imprinted Sensor for Ultra-Sensitive Detection of Sulfadiazine in Pork Samples. Food Anal. Methods 2024, 17, 1689–1701. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum dots applied to methodology on detection of pesticide and veterinary drug residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; Shi, X.; Han, Y.; Guo, Z.; Liu, Y. Development of carbon quantum dot–labeled antibody fluorescence immunoassays for the detection of morphine in hot pot soup base. Food Anal. Methods 2020, 13, 1042–1049. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Wang, C.; Tian, X.; Chang, X.; Ren, Y.; Yu, S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef]
- Liu, P.; Dong, Y.; Li, X.; Zhang, Y.; Liu, Z.; Lu, Y.; Peng, X.; Zhai, R.; Chen, Y. Multilayered Fe3O4@(ZIF-8) 3 combined with a computer-vision-enhanced immunosensor for chloramphenicol enrichment and detection. J. Hazard. Mater. 2024, 470, 134150. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Jayan, H.; Gao, S.; Zhou, R.; Yosri, N.; Zou, X.; Guo, Z. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef]
- Li, H.; Murugesan, A.; Shoaib, M.; Sheng, W.; Chen, Q. Functionalized metal-organic frameworks with biomolecules for sensing and detection applications of food contaminants. Crit. Rev. Food Sci. Nutr. 2024, 1–33. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Xu, X.; Zhang, W.; Zou, X.; Shi, J.; Zheng, K.; Arslan, M. A portable test strip based on fluorescent europium-based metal–organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Zhai, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J. A simple and sensitive electrochemical sensing based on amine-functionalized metal–organic framework and polypyrrole composite for detection of lead ions in meat samples. J. Food Meas. Charact. 2024, 18, 5813–5825. [Google Scholar] [CrossRef]
- Dai, Y.; Peng, W.; Ji, Y.; Wei, J.; Che, J.; Huang, Y.; Huang, W.; Yang, W.; Xu, W. A self-powered photoelectrochemical aptasensor using 3D-carbon nitride and carbon-based metal-organic frameworks for high-sensitivity detection of tetracycline in milk and water. J. Food Sci. 2024, 89, 8022–8035. [Google Scholar] [CrossRef]
- Yosri, N.; Gao, S.; Zhou, R.; Wang, C.; Zou, X.; El-Seedi, H.R.; Guo, Z. Innovative quantum dots-based SERS for ultrasensitive reporting of contaminants in food: Fundamental concepts and practical implementations. Food Chem. 2024, 467, 142395. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Zhai, X.; Shen, T.; Shi, J.; He, Y. A novel sustainable biomass-based fluorescent probe for sensitive detection of salicylic acid in rice. Food Chem. 2024, 434, 137260. [Google Scholar] [CrossRef]
- Zhihua, L.; Xue, Z.; Xiaowei, H.; Xiaobo, Z.; Jiyong, S.; Yiwei, X.; Xuetao, H.; Yue, S.; Xiaodong, Z. Hypha-templated synthesis of carbon/ZnO microfiber for dopamine sensing in pork. Food Chem. 2021, 335, 127646. [Google Scholar] [CrossRef]
- Meng, S.; Liu, D.; Li, Y.; Dong, N.; Chen, T.; You, T. Engineering the signal transduction between CdTe and CdSe quantum dots for in situ ratiometric photoelectrochemical immunoassay of Cry1Ab protein. J. Agric. Food Chem. 2022, 70, 13583–13591. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Li, Z. A ratiometric fluorescent sensor based on silicon quantum dots and silver nanoclusters for beef freshness monitoring. Foods 2023, 12, 1464. [Google Scholar] [CrossRef]
- Wang, C.; Gu, C.; Zhao, X.; Yu, S.; Zhang, X.; Xu, F.; Ding, L.; Huang, X.; Qian, J. Self-designed portable dual-mode fluorescence device with custom python-based analysis software for rapid detection via dual-color FRET aptasensor with IoT capabilities. Food Chem. 2024, 457, 140190. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Huang, X.; Zhai, X.; Li, Z.; Shi, J.; Sobhy, R.; Khalifa, I.; Zou, X. Lemon-derived carbon quantum dots incorporated guar gum/sodium alginate films with enhanced the preservability for blanched asparagus active packaging. Food Res. Int. 2025, 202, 115736. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Adade, S.Y.-S.S.; Nunekpeku, X.; Chen, Q. Investigation of heat-induced pork batter quality detection and change mechanisms using Raman spectroscopy coupled with deep learning algorithms. Food Chem. 2024, 461, 140798. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Mwakosya, A.W.; Guo, Z.; Xu, Y.; Huang, X.; Li, Z.; Zhang, X.; Zou, X. Fluorescence and colorimetric dual-mode sensor for visual detection of malathion in cabbage based on carbon quantum dots and gold nanoparticles. Food Chem. 2021, 343, 128494. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, X.; Li, W.; Liang, N.; Hu, X.; Xiao, J.; Wang, D.; Zou, X.; Shi, J. An intrinsic dual-emitting fluorescence sensing toward tetracycline with self-calibration model based on luminescent lanthanide-functionalized metal-organic frameworks. Food Chem. 2023, 400, 133995. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Shi, Y.; Zou, X.; Tahir, H.E.; Holmes, M.; Zhang, W.; Huang, X.; Li, Z.; Xu, Y. A dual-mode sensor for colorimetric and fluorescent detection of nitrite in hams based on carbon dots-neutral red system. Meat Sci. 2019, 147, 127–134. [Google Scholar] [CrossRef]
- Zeng, K.; Chen, B.; Li, Y.; Meng, H.; Wu, Q.; Yang, J.; Liang, H. Gold nanoparticle-carbon nanotube nanohybrids with peroxidase-like activity for the highly-sensitive immunoassay of kanamycin in milk. Int. J. Food Sci. Technol. 2022, 57, 6028–6037. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of heavy metals in food and agricultural products by surface-enhanced Raman spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Chen, P.; Yin, L.; El-Seedi, H.R.; Zou, X.; Guo, Z. Green reduction of silver nanoparticles for cadmium detection in food using surface-enhanced Raman spectroscopy coupled multivariate calibration. Food Chem. 2022, 394, 133481. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, J.; Yao, K.; Xu, M.; Tang, N.; Zhou, X. Nondestructive detection of lead content in oilseed rape leaves based on MRF-HHO-SVR and hyperspectral technology. J. Food Process Eng. 2021, 44, e13793. [Google Scholar] [CrossRef]
- Zhang, K.; Kwadzokpui, B.A.; Adade, S.Y.-S.S.; Lin, H.; Chen, Q. Quantitative and qualitative detection of target heavy metals using anti-interference colorimetric sensor Array combined with near-infrared spectroscopy. Food Chem. 2024, 459, 140305. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Feng, X.; Lin, L.; Nie, P.; Shi, J.; Zou, X.; He, Y. Sensing of mercury ions in Porphyra by Copper@ Gold nanoclusters based ratiometric fluorescent aptasensor. Food Chem. 2021, 344, 128694. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Haruna, S.A.; Hassan, M.M.; Chen, Q. Recent advances in rare earth ion-doped upconversion nanomaterials: From design to their applications in food safety analysis. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3732–3764. [Google Scholar] [CrossRef] [PubMed]
- Selva Sharma, A.; Marimuthu, M.; Varghese, A.W.; Wu, J.; Xu, J.; Xiaofeng, L.; Devaraj, S.; Lan, Y.; Li, H.; Chen, Q. A review of biomolecules conjugated lanthanide up-conversion nanoparticles-based fluorescence probes in food safety and quality monitoring applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 6129–6159. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, X.; Xu, Y.; Liu, C.; Zou, X.; Li, Z.; Shi, J.; Huang, X. Facile fabrication of three-dimensional gold nanodendrites decorated by silver nanoparticles as hybrid SERS-active substrate for the detection of food contaminants. Food Control 2021, 122, 107772. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Ouyang, Q.; Chen, Q. Lanthanide ion (Ln3+)-based upconversion sensor for quantification of food contaminants: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3531–3578. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Li, Q.; Shi, Y.; Zhai, X.; Xu, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J. Copper nanoclusters@ nitrogen-doped carbon quantum dots-based ratiometric fluorescence probe for lead (II) ions detection in porphyra. Food Chem. 2020, 320, 126623. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Liu, F.; Zou, X.; Xu, Y.; Xu, X. A smart-phone-based electrochemical platform with programmable solid-state-microwave flow digestion for determination of heavy metals in liquid food. Food Chem. 2020, 303, 125378. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, X.; Luo, W.; Bai, X.; Xu, L.; Jiang, H. Microwave detection technique combined with deep learning algorithm facilitates quantitative analysis of heavy metal Pb residues in edible oils. J. Food Sci. 2024, 89, 6005–6015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Z.; Deng, J.; Ding, Z.; Chen, Q. Quantitative detection of heavy metal Cd in vegetable oils: A nondestructive method based on Raman spectroscopy combined with chemometrics. J. Food Sci. 2024, 89, 8054–8065. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Rajarathinam, T.; Jayaraman, S.; Jang, H.-G.; Yoon, J.-H.; Lee, J.; Paik, H.-j.; Chang, S.-C. Recent advances in miniaturized electrochemical analyzers for hazardous heavy metal sensing in environmental samples. Coord. Chem. Rev. 2024, 499, 215487. [Google Scholar] [CrossRef]
- Chokkiah, B.; Eswaran, M.; Annamalai, P.; Ramalingam, M.; Mijakovic, I.; Sivakumar, B.; Ponnusamy, V.K.; Ramakrishnan, K. Trace level detection of heavy metal ions using electrochemical sensors: Mercury, lead, cadmium, and chromium. In Materials Technology for the Energy and Environmental Nexus, Volume 2; IOP Publishing: Bristol, UK, 2023; pp. 13-11–13-33. [Google Scholar]
- Zhao, Y.; Ma, Y.; Zhou, R.; He, Y.; Wu, Y.; Yi, Y.; Zhu, G. Highly sensitive electrochemical detection of paraoxon ethyl in water and fruit samples based on defect-engineered graphene nanoribbons modified electrode. J. Food Meas. Charact. 2022, 16, 2596–2603. [Google Scholar] [CrossRef]
- Azam, S.R.; Ma, H.; Xu, B.; Devi, S.; Siddique, M.A.B.; Stanley, S.L.; Bhandari, B.; Zhu, J. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: A review. Trends Food Sci. Technol. 2020, 97, 417–432. [Google Scholar] [CrossRef]
- Huang, A.-J.; Dong, X.-X.; Tan, S.; Chen, K.; Zhang, M.; Li, B.; Deng, H.; He, F.; Ni, H.; Wang, H. A covalent organic framework-derived pretreatment for pesticides in vegetables and fruits. Front. Sustain. Food Syst. 2024, 8, 1472174. [Google Scholar] [CrossRef]
- Marimuthu, M.; Xu, K.; Song, W.; Chen, Q.; Wen, H. Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 2024, 463, 141288. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem. 2024, 459, 140379. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; Swanson, C.S.; He, Q.; Mintah, B.K.; Gao, E.; Zheng, X.; He, M. Optimization of medium composition and culture conditions for cell multiplication of a high quality milk beer fermentation yeast (Kluyveromyces marxianus). Food Sci. Technol. Res. 2020, 26, 351–361. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Shi, Y.; Zou, X.; Arslan, M.; Zhang, W.; Huang, X.; Li, Z.; Xu, Y. Use of a smartphone for visual detection of melamine in milk based on Au@ Carbon quantum dots nanocomposites. Food Chem. 2019, 272, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Lu, J.; Wu, Y.; Meng, M.; Yu, C.; Sun, C.; Chen, M.; Da, Z.; Yan, Y. Antifouling molecularly imprinted membranes for pretreatment of milk samples: Selective separation and detection of lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef]
- Han, F.; Huang, X.; Aheto, J.H.; Zhang, D.; Feng, F. Detection of beef adulterated with pork using a low-cost electronic nose based on colorimetric sensors. Foods 2020, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zou, B.; Liu, F.; Wang, P.; Yan, Y. Sensitive glucose biosensor based on cyclodextrin modified carbon nanotubes for detecting glucose in honey. J. Food Compos. Anal. 2022, 105, 104221. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Wang, Y.; Guo, Z.; Huang, X.; Li, Z.; Zhang, X.; Zhang, J.; Xiao, J. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Li, Y.; Zhang, L.; Bi, N.; Gou, J.; Zhu, T.; Jia, L. A novel intelligently integrated MOF-based ratio fluorescence sensor for ultra-sensitive monitoring of TC in water and food samples. Food Chem. 2023, 405, 134899. [Google Scholar] [CrossRef]
- Li, H.; Geng, W.; Zheng, Z.; Haruna, S.A.; Chen, Q. Flexible SERS sensor using AuNTs-assembled PDMS film coupled chemometric algorithms for rapid detection of chloramphenicol in food. Food Chem. 2023, 418, 135998. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, X.; Jayan, H.; Yin, L.; Xue, S.; El-Seedi, H.R.; Zou, X. Recent developments and applications of surface enhanced Raman scattering spectroscopy in safety detection of fruits and vegetables. Food Chem. 2024, 434, 137469. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, W.; Li, N.; Salah, M.; Guan, S.; Li, X.; Wang, Y. Development of a Sensitive Monoclonal Antibody-Based Colloidal Gold Immunochromatographic Strip for Lomefloxacin Detection in Meat Products. Foods 2024, 13, 2550. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Wang, S.; Yan, Y. Mesoporous silica-based molecularly imprinted fluorescence sensor for the ultrafast and sensitive recognition of oxytetracycline. J. Food Compos. Anal. 2022, 108, 104427. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, R.; Zhang, D.; Zheng, X.; El-Seedi, H.R.; Chen, S.; Niu, L.; Li, X.; Guo, Z.; Zou, X. Magnetic nanoparticle-based immunosensors and aptasensors for mycotoxin detection in foodstuffs: An update. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13266. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent trends in detecting, controlling, and detoxifying of patulin mycotoxin using biotechnology methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472. [Google Scholar] [CrossRef]
- Zhai, W.; You, T.; Ouyang, X.; Wang, M. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1887–1909. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; He, Y.; Yuan, B.; Li, L.; Luo, L.; You, T. Simultaneous detection of multiple mycotoxins in agricultural products: Recent advances in optical and electrochemical sensing methods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70062. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Niu, R.; Yang, Y.; Wang, Y. Development and evaluation of a rapid immunomagnetic extraction for effective detection of zearalenone in agricultural products. Food Control 2020, 110, 106973. [Google Scholar] [CrossRef]
- You, F.; Wen, Z.; Yuan, R.; Qian, J.; Long, L.; Wang, K. Sensitive and stable detection of deoxynivalenol based on electrochemiluminescence aptasensor enhanced by 0D/2D homojunction effect in food analysis. Food Chem. 2023, 403, 134397. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Ding, L.; Liu, Y.; Fan, C.; You, F.; Wei, J.; Qian, J.; Wang, K. A miniaturized organic photoelectrochemical transistor aptasensor based on nanorod arrays toward high-sensitive T-2 toxin detection in milk samples. Food Chem. 2023, 423, 136285. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, S.; Li, L.; Liu, X.; Zhang, J.; Li, X.; Liu, D.; You, T. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@ SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef]
- Gao, S.; Wei, Z.; Zheng, X.; Wang, T.; Huang, X.; Shen, T.; Zhang, D.; Guo, Z.; Zhang, Y.; Zou, X. Multiplexed lateral-flow immunoassays for the simultaneous detection of several mycotoxins in foodstuffs. Trends Food Sci. Technol. 2024, 156, 104858. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Chen, T.; You, T. Label-free ratiometric homogeneous electrochemical aptasensor based on hybridization chain reaction for facile and rapid detection of aflatoxin B1 in cereal crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef]
- Hassan, M.M.; Yi, X.; Zareef, M.; Li, H.; Chen, Q. Recent advancements of optical, electrochemical, and photoelectrochemical transducer-based microfluidic devices for pesticide and mycotoxins in food and water. Trends Food Sci. Technol. 2023, 142, 104230. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Sun, Q.; Guo, Z.; Zhang, D.; Zou, X. Enzyme-assisted patulin detoxification: Recent applications and perspectives. Trends Food Sci. Technol. 2024, 146, 104383. [Google Scholar] [CrossRef]
- Yin, L.; Cai, J.; Ma, L.; You, T.; Arslan, M.; Jayan, H.; Zou, X.; Gong, Y. Dual function of magnetic nanocomposites-based SERS lateral flow strip for simultaneous detection of aflatoxin B1 and zearalenone. Food Chem. 2024, 446, 138817. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Liu, X.; Luo, L.; Cheng, Z.; Sun, J.; Cai, Z.; Liu, J.; You, T. Inner filter effect-modulated ratiometric fluorescence aptasensor based on competition strategy for zearalenone detection in cereal crops: Using mitoxantrone as quencher of CdTe QDs@ SiO2. Food Chem. 2021, 349, 129171. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, Y.; Xu, Y.; Gan, Z.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Li, Y. Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021, 339, 127775. [Google Scholar] [CrossRef]
- Han, J.; Ullah, M.; Andoh, V.; Khan, M.N.; Feng, Y.; Guo, Z.; Chen, H. Engineering Bacterial Chitinases for Industrial Application: From Protein Engineering to Bacterial Strains Mutation! A Comprehensive Review of Physical, Molecular, and Computational Approaches. J. Agric. Food Chem. 2024, 72, 23082–23096. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, J.; Wen, J.; Wu, Z.; Dong, Y.; Chen, Y. Unsupervised Learning-Assisted Acoustic-Driven Nano-Lens Holography for the Ultrasensitive and Amplification-Free Detection of Viable Bacteria. Adv. Sci. 2025, 12, 2406912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, X.; Ma, A.; Jiang, F.; Chen, Y. Multiplexed food-borne pathogen detection using an argonaute-mediated digital sensor based on a magnetic-bead-assisted imaging transcoding system. Nat. Food 2025, 6, 170–181. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Wahia, H.; Ji, Q.; Fakayode, O.A.; Zhang, L.; Zhou, C. Multiple-frequency ultrasound for the inactivation of microorganisms on food: A review. J. Food Process Eng. 2024, 47, e14587. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Okoye, C.O.; Gao, L.; Chen, X.; Wang, Y.; Jiang, J. Fermentation profile and bioactive component retention in honeysuckle residue silages inoculated with lactic acid bacteria: A promising feed additive for sustainable agriculture. Ind. Crops Prod. 2025, 224, 120315. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol. 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.; Chen, Q. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Huang, A.; He, L.; Cai, C.; You, T. Recent advances in foodborne pathogen detection using photoelectrochemical biosensors: From photoactive material to sensing strategy. Front. Sustain. Food Syst. 2024, 8, 1432555. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of hyperspectral imaging as a nondestructive technique for foodborne pathogen detection and characterization. Foodborne Pathog. Dis. 2019, 16, 712–722. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Sobhy, M.; Abdelkarim, E.A.; Hussein, M.A.; Aziz, T.; Al-Asmari, F.; Alabbosh, K.F.; Cui, H.; Lin, L. Essential Oils as Antibacterials Against Multidrug-Resistant Foodborne pathogens: Mechanisms, Recent Advances, and Legal Considerations. Food Biosci. 2025, 64, 105937. [Google Scholar] [CrossRef]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-based detection methodology studies in food safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Yixuan, L.; Qaria, M.A.; Sivasamy, S.; Jianzhong, S.; Daochen, Z. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind. Crops Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Osae, R.; Zhou, C.; Tchabo, W.; Xu, B.; Bonah, E.; Alenyorege, E.A.; Ma, H. Optimization of osmosonication pretreatment of ginger (Zingiber officinale Roscoe) using response surface methodology: Effect on antioxidant activity, enzyme inactivation, phenolic compounds, and physical properties. J. Food Process Eng. 2019, 42, e13218. [Google Scholar] [CrossRef]
- Hassan, M.M.; Xu, Y.; Zareef, M.; Li, H.; Rong, Y.; Chen, Q. Recent advances of nanomaterial-based optical sensor for the detection of benzimidazole fungicides in food: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 2851–2872. [Google Scholar] [CrossRef] [PubMed]
- Fayek, N.M.; Xiao, J.; Farag, M.A. A multifunctional study of naturally occurring pyrazines in biological systems; formation mechanisms, metabolism, food applications and functional properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 5322–5338. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ezeorba, T.P.C.; Okoye, C.O.; Chen, Y.; Mao, G.; Feng, W.; Wu, X. Analytical detection methods for azo dyes: A focus on comparative limitations and prospects of bio-sensing and electrochemical nano-detection. J. Food Compos. Anal. 2022, 114, 104778. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Tang, Q.; Guo, Y.; Zhang, Z.; Zhang, W.; Zou, X.; Sun, Z. Molecularly imprinted electrochemical sensor for ethyl carbamate detection in Baijiu based on “on-off” nanozyme-catalyzing process. Food Chem. 2024, 453, 139626. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, W.; Arslan, M.; Hu, X.; Zhang, X.; Li, Z.; Shi, J.; Zou, X. Ratiometric fluorescent metal–organic framework biosensor for ultrasensitive detection of acrylamide. J. Agric. Food Chem. 2022, 70, 10065–10074. [Google Scholar] [CrossRef]
- Khan, I.A.; Luo, J.; Shi, H.; Zou, Y.; Khan, A.; Zhu, Z.; Xu, W.; Wang, D.; Huang, M. Mitigation of heterocyclic amines by phenolic compounds in allspice and perilla frutescens seed extract: The correlation between antioxidant capacities and mitigating activities. Food Chem. 2022, 368, 130845. [Google Scholar] [CrossRef]
- Guo, L.; Hong, C.; Wang, W.; Zhang, X.; Chen, J.; Chen, Z.; Ashokkumar, M.; Ma, H. Evaluation of low-temperature ultrasonic marination of pork meat at various frequencies on physicochemical properties, myoglobin levels, and volatile compounds. Meat Sci. 2024, 217, 109606. [Google Scholar] [CrossRef]
- Khan, I.A.; Khan, A.; Zou, Y.; Zongshuai, Z.; Xu, W.; Wang, D.; Huang, M. Heterocyclic amines in cooked meat products, shortcomings during evaluation, factors influencing formation, risk assessment and mitigation strategies. Meat Sci. 2022, 184, 108693. [Google Scholar] [CrossRef]
- Ji, D.; Wang, Q.; Lu, T.; Ma, H.; Chen, X. The effects of ultrasonication on the phytochemicals, antioxidant, and polyphenol oxidase and peroxidase activities in coffee leaves. Food Chem. 2022, 373, 131480. [Google Scholar] [CrossRef]
- Manikandan, R.; Jang, H.-G.; Kim, C.-S.; Yoon, J.-H.; Lee, J.; Paik, H.-j.; Chang, S.-C. Electrodes Coated with Polyaniline/Cerium Tungstate Nanostructures for Detection of Hydrazine in Food and Environmental Samples. ACS Appl. Nano Mater. 2024, 7, 24112–24122. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Manikandan, R.; Sekar, S.; Lee, D.J.; Jeon, H.C.; Lee, S.; Chang, S.-C.; Kim, D.Y. Two dimensional FeVO4 nanoflakes decorated on Ti3C2 MXene hybrid nanocomposites as a novel effective electrochemical biosensor for ultrasensitive and selective detection of serotonin (5-HT). Appl. Surf. Sci. 2025, 680, 161411. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugesan, A.; Li, H.; Shoaib, M. Recent Advances in Functionalized Carbon Quantum Dots Integrated with Metal–Organic Frameworks: Emerging Platforms for Sensing and Food Safety Applications. Foods 2025, 14, 2060. https://doi.org/10.3390/foods14122060
Murugesan A, Li H, Shoaib M. Recent Advances in Functionalized Carbon Quantum Dots Integrated with Metal–Organic Frameworks: Emerging Platforms for Sensing and Food Safety Applications. Foods. 2025; 14(12):2060. https://doi.org/10.3390/foods14122060
Chicago/Turabian StyleMurugesan, Arul, Huanhuan Li, and Muhammad Shoaib. 2025. "Recent Advances in Functionalized Carbon Quantum Dots Integrated with Metal–Organic Frameworks: Emerging Platforms for Sensing and Food Safety Applications" Foods 14, no. 12: 2060. https://doi.org/10.3390/foods14122060
APA StyleMurugesan, A., Li, H., & Shoaib, M. (2025). Recent Advances in Functionalized Carbon Quantum Dots Integrated with Metal–Organic Frameworks: Emerging Platforms for Sensing and Food Safety Applications. Foods, 14(12), 2060. https://doi.org/10.3390/foods14122060







