Germination and Heat Resistance of Parageobacillus and Geobacillus spp. Spores
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtention and Purification of Spore Suspensions
- -
- Protocol 1: four consecutive washes with distilled water [30];
- -
- Protocol 2: one wash with distilled water followed by ethanol treatment (50%, v/v; SAEQSA, Zaragoza, Spain) for 1 h at 25 °C and four washes with distilled water [31];
- -
- Protocol 3: four washes with distilled water followed by Tween 80 treatment (0.01%, v/v; Sigma-Aldrich) for 1 h at 25 °C and four washes with distilled water [32];
- -
- Protocol 4: one wash with distilled water followed by three washes with 0.1% (v/v) Tween 80, ethanol treatment (50%, v/v) for 1 h at 25 °C, three washes with 0.01% Tween 80, and four washes with distilled water [33].
2.2. Germination Assays
2.3. Thermal Treatments
2.4. Determination of Viability
2.5. Modeling of Heat Inactivation Curves
2.6. Statistical Analysis
3. Results and Discussion
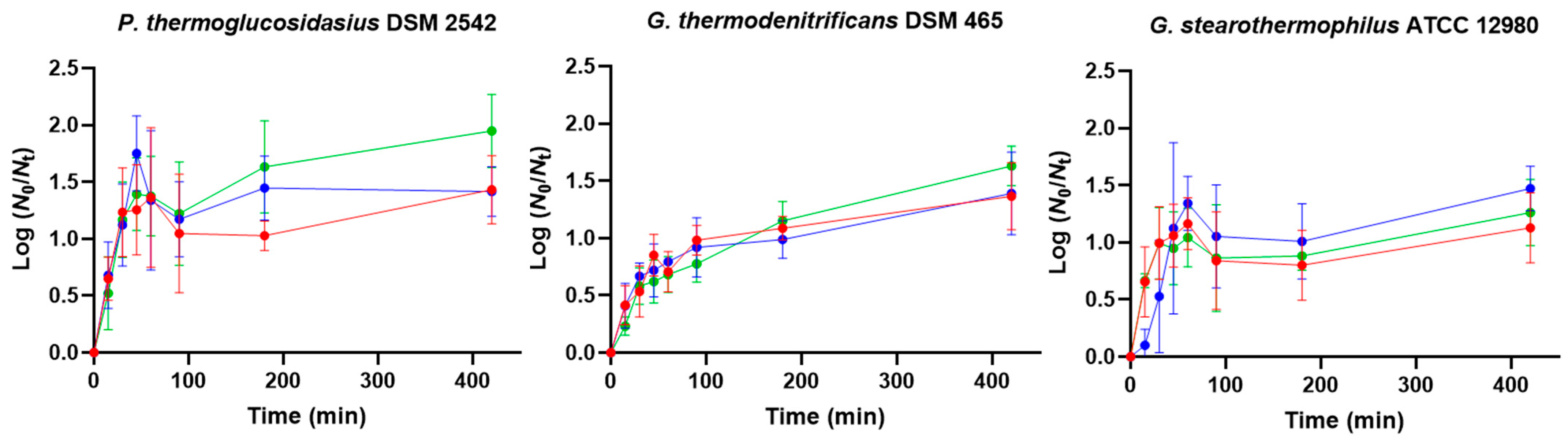
3.1. Germination in Milk Products
3.2. Effect of Purification Method on Germination and Heat Resistance
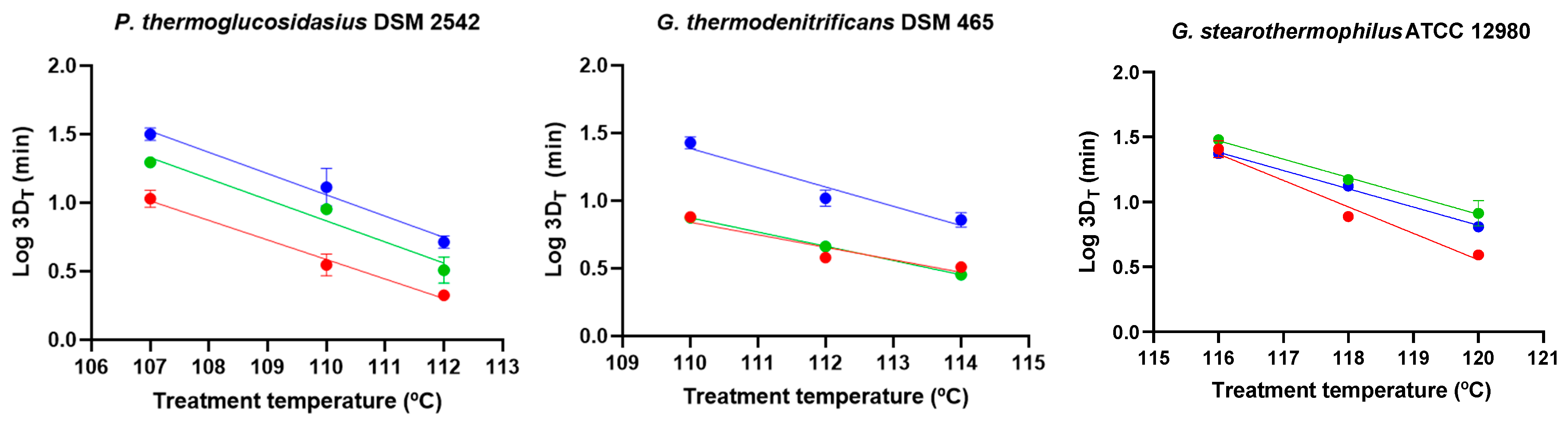
3.3. Effect of Maturation Time on Germination and Heat Resistance
3.4. Effect of Sporulation Temperature on Germination and Heat Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Najar, I.N.; Nagendra, T. A systematic review of the genera Geobacillus and Parageobacillus: Their evolution, current taxonomic status and major applications. Microbiology 2020, 166, 800–816. [Google Scholar] [CrossRef]
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Phylogenomic re-assessment of the thermophilic genus Geobacillus. Syst. Appl. Microbiol. 2016, 39, 527–533. [Google Scholar] [CrossRef]
- Zeigler, D.R. The Geobacillus paradox: Why is a thermophilic bacterial genus so prevalent on a mesophilic planet? Microbiology 2014, 160, 1–11. [Google Scholar] [CrossRef]
- Iacumin, L.; Pellegrini, M.; Colautti, A.; Orecchia, E.; Comi, G. Microbial Characterization of Retail Cocoa Powders and Chocolate Bars of Five Brands Sold in Italian Supermarkets. Foods 2022, 11, 2753. [Google Scholar] [CrossRef]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- André, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Li, Y.; Liu, T.; Flint, S.; Zhang, G.; He, G. A RAPD based study revealing a previously unreported wide range of mesophilic and thermophilic spore formers associated with milk powders in China. Int. J. Food Microbiol. 2016, 217, 200–208. [Google Scholar] [CrossRef]
- Yuan, D.-D.; Liu, G.-C.; Ren, D.-Y.; Zhang, D.; Zhao, L.; Kan, C.-P.; Yang, Y.-Z.; Ma, W.; Li, Y.; Zhang, L.-B. A survey on occurrence of thermophilic bacilli in commercial milk powders in China. Food Control 2012, 25, 752–757. [Google Scholar] [CrossRef]
- Delaunay, L.; Cozien, E.; Gehannin, P.; Mouhali, N.; Mace, S.; Postollec, F.; Leguerinel, I.; Mathot, A.-G. Occurrence and diversity of thermophilic sporeformers in French dairy powders. Int. Dairy J. 2021, 113, 104889. [Google Scholar] [CrossRef]
- Karaca, B.; Karakaya, A.B.; Ozcan, B.; Coleri Cihan, A. Rapid detection of Geobacillus and Anoxybacillus species by quantitative qPCR (qPCR) in commercial dairy products. J. Food Saf. 2022, 42, e12964. [Google Scholar] [CrossRef]
- Karaca, B.; Buzrul, S.; Coleri Cihan, A. Anoxybacillus and Geobacillus biofilms in the dairy industry: Effects of surface material, incubation temperature and milk type. Biofouling 2019, 35, 551–560. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Flint, S.; He, G. Microbiota of milk powders and the heat resistance and spoilage potential of aerobic spore-forming bacteria. Int. Dairy J. 2018, 85, 159–168. [Google Scholar] [CrossRef]
- Manachini, P.L.; Mora, D.; Nicastro, G.; Parini, C.; Stackebrandt, E.; Pukall, R.; Fortina, M.G. Bacillus thermodenitrificans sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 3, 1331–1337. [Google Scholar] [CrossRef]
- Murphy, S.C.; Martin, N.H.; Barbano, D.M.; Wiedmann, M. Influence of raw milk quality on processed dairy products: How do raw milk quality test results relate to product quality and yield? J. Dairy Sci. 2016, 99, 10128–10149. [Google Scholar] [CrossRef]
- Pujol, L.; Albert, I.; Magras, C.; Johnson, N.B.; Membré, J.M. Probabilistic exposure assessment model to estimate aseptic-UHT product failure rate. Int. J. Food Microbiol. 2015, 192, 124–141. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Misiou, O.D.; Kakagianni, M.N. Climate change threatens the microbiological stability of non-refrigerated foods. Food Res. Int. 2022, 162, 111990. [Google Scholar] [CrossRef]
- Peñalver-Soto, J.L.; Garre, A.; Aznar, A.; Fernández, P.S.; Egea, J.A. Dynamics of Microbial Inactivation and Acrylamide Production in High-Temperature Heat Treatments. Foods 2021, 10, 2535. [Google Scholar] [CrossRef]
- Kakagianni, M.; Koutsoumanis, K.P. Mapping the risk of evaporated milk spoilage in the Mediterranean region based on the effect of temperature conditions on Geobacillus stearothermophilus growth. Food Res. Int. 2018, 111, 104–110. [Google Scholar] [CrossRef]
- Kakagianni, M.; Gougouli, M.; Koutsoumanis, K.P. Development and application of Geobacillus stearothermophilus growth model for predicting spoilage of evaporated milk. Food Microbiol. 2016, 57, 28–35. [Google Scholar] [CrossRef]
- Jha, S.; Singh, N.; Anand, S. Occurrence of aerobic bacterial endospores in dried dairy ingredients. Int. J. Dairy Technol. 2023, 76, 1025–1029. [Google Scholar] [CrossRef]
- Champidou, C.; Ellouze, M.; Haddad, N.; Membré, J.-M. Modeling Geobacillus stearothermophilus spores inactivation in plant-based drinks to design UHT processing. Food Res. Int. 2025, 201, 115518. [Google Scholar] [CrossRef]
- Feurhuber, M.; Neuschwander, R.; Taupitz, T.; Frank, C.; Hochenauer, C.; Schwarz, V. Mathematically modelling the inactivation kinetics of Geobacillus stearothermophilus spores: Effects of sterilization environments and temperature profiles. Phys. Med. 2022, 13, 100046. [Google Scholar] [CrossRef]
- Bressuire-Isoard, C.; Broussolle, V.; Carlin, F. Sporulation environment influences spore properties in Bacillus: Evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol. Rev. 2018, 42, 614–626. [Google Scholar] [CrossRef]
- Setlow, P. Observations on Research with Spores of Bacillales and Clostridiales Species. J. Appl. Microbiol. 2019, 126, 348–358. [Google Scholar] [CrossRef]
- Juneja, V.K.; Osoria, M.; Altuntas, E.G.; Taneja, N.K.; Thakur, S.; Kumar, G.D.; Setlow, P. Effects of spore purity on the wet heat resistance of Clostridium perfringens, Bacillus cereus and Bacillus subtilis spores. Food Res. Int. 2024, 177, 113904. [Google Scholar] [CrossRef]
- Swarge, B.; Nafid, C.; Vischer, N.; Kramer, G.; Setlow, P.; Brul, S. Investigating Synthesis of the MalS Malic Enzyme during Bacillus subtilis Spore Germination and Outgrowth and the Influence of Spore Maturation and Sporulation Conditions. mSphere 2020, 5, e00464-20. [Google Scholar] [CrossRef]
- Ramirez-Peralta, A.; Zhang, P.; Li, Y.-Q.; Setlow, P. Effects of Sporulation Conditions on the Germination and Germination Protein Levels of Bacillus subtilis Spores. Appl. Environ. Microbiol. 2012, 78, 2689–2697. [Google Scholar] [CrossRef]
- Li, L.; Jin, J.; Hu, H.; Deveau, I.F.; Foley, S.L.; Chen, H. Optimization of sporulation and purification methods for sporicidal efficacy assessment on Bacillus spores. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac014. [Google Scholar] [CrossRef]
- Leguérinel, I.; Couvert, O.; Mafart, P. Modelling the influence of the incubation temperature upon the estimated heat resistance of heated Bacillus spores. Lett. Appl. Microbiol. 2006, 43, 17–21. [Google Scholar] [CrossRef]
- Georget, E.; Kushman, A.; Callanan, M.; Ananta, E.; Heinz, V.; Mathys, A. Geobacillus stearothermophilus ATCC 7953 spore chemical germination mechanisms in model systems. Food Control 2015, 50, 141–149. [Google Scholar] [CrossRef]
- Koransky, J.R.; Allen, S.D.; Dowell, V.R., Jr. Use of ethanol for selective isolation of sporeforming microorganisms. Appl. Environ. Microbiol. 1978, 35, 762–765. [Google Scholar] [CrossRef]
- Abhyankar, W.; Beek, A.T.; Dekker, H.; Kort, R.; Brul, S.; de Koster, C.G. Gel-free proteomic identification of the Bacillus subtilis insoluble spore coat protein fraction. Proteomics 2011, 11, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Begyn, K.; Kim, T.D.; Heyndrickx, M.; Michiels, C.; Aertsen, A.; Rajkovic, A.; Devlieghere, F. Directed evolution by UV-C treatment of Bacillus cereus spores. Int. J. Food Microbiol. 2020, 317, 108424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Dong, Z.; Setlow, P.; Li, Y.-Q. Kinetics of Germination of Individual Spores of Geobacillus stearothermophilus as Measured by Raman Spectroscopy and Differential Interference Contrast Microscopy. PLoS ONE 2013, 8, e74987. [Google Scholar] [CrossRef]
- Condón, S.; Arrizubieta, M.J.; Sala, F.J. Microbial heat determinations by the multipoint system with the thermorresistometer TR-SC Improvement of this methodology. J. Microbiol. Methods 1993, 18, 357–366. [Google Scholar] [CrossRef]
- Dawson, R.M.C.; Elliot, D.C.; Elliot, W.H.; Jones, K.M. (Eds.) pH, buffers and physiological media. In Data for Biochemical Research, 2nd ed.; Clarendon Press: Oxford, UK, 1974. [Google Scholar]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Valdramidis, V.; Bernaerts, K.; Van Impe, J.; Geeraerd, A. An Alternative Approach to Non-Log-Linear Thermal Microbial Inactivation: Modelling the Number of Log Cycles Reduction with Respect to Temperature. Food Technol. Biotechnol. 2005, 43, 321–327. [Google Scholar]
- Paredes-Sabja, D.; Setlow, P.; Sarker, M.R. Germination of spores of Bacillales and Clostridiales species: Mechanisms and proteins involved. Trends Microbiol. 2011, 19, 85–94. [Google Scholar] [CrossRef]
- Setlow, P. Summer Meeting 2013—When the Sleepers Wake: The Germination of Spores of Bacillus Species. J. Appl. Microbiol. 2013, 115, 1251–1268. [Google Scholar] [CrossRef]
- Burgess, S.A.; Flint, S.H.; Lindsay, D.; Cox, M.P.; Biggs, P.J. Insights into the Geobacillus stearothermophilus species based on phylogenomic principles. BMC Microbiol. 2017, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Caspers, M.P.; Abee, T.; Siezen, R.J.; Kort, R. Complete genome sequence of Geobacillus thermoglucosidans TNO-09.020, a thermophilic sporeformer associated with a dairy-processing environment. J. Bacteriol. 2012, 194, 4118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kumar, M.; Caspers, M.P.M.; Nierop Groot, M.N.; Van Der Vossen, J.M.B.M.; Abee, T. Short communication: Growth of dairy isolates of Geobacillus thermoglucosidans in skim milk depends on lactose degradation products supplied by Anoxybacillus flavithermus as secondary species. J. Dairy Sci. 2018, 101, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Flint, S.; Palmer, J.; Chanapha, S.; Hall, C. Influence of Incubation Temperature and Total Dissolved Solids on Biofilm and Spore Formation by Dairy Isolates of Geobacillus stearothermophilus. Appl. Environ. Microbiol. 2021, 87, e02311-20. [Google Scholar] [CrossRef]
- Wang, T.; Flint, S.; Palmer, J. Magnesium and calcium ions: Roles in bacterial cell attachment and biofilm structure maturation. Biofouling 2019, 35, 959–974. [Google Scholar] [CrossRef]
- Wang, T.; Flint, S.; Palmer, J. Heterogeneous response of Geobacillus stearothermophilus biofilms to calcium. Int. Dairy J. 2021, 116, 104961. [Google Scholar] [CrossRef]
- Hyatt, M.T.; Levinson, H.S. Effect of sugars and other carbon compounds on germination and postgerminative development of Bacillus megaterium spores. J. Bacteriol. 1964, 88, 1403–1415. [Google Scholar] [CrossRef]
- Craven, S.E.; Blankenship, L.C. Activation and injury of Clostridium perfringens spores by alcohols. Appl. Environ. Microbiol. 1985, 50, 249–256. [Google Scholar] [CrossRef]
- Kim, J.; Foegeding, P.M. Effects of heat-, CaCl2- and ethanol-treatments on activation of Bacillus spores*. J. Appl. Bacteriol. 1990, 69, 414–420. [Google Scholar] [CrossRef]
- Luu, S.; Cruz-Mora, J.; Setlow, B.; Feeherry, F.E.; Doona, C.J.; Setlow, P. The Effects of Heat Activation on Bacillus Spore Germination, with Nutrients or under High Pressure, with or without Various Germination Proteins. Appl. Environ. Microbiol. 2015, 81, 2927–2938. [Google Scholar] [CrossRef]
- Berg, R.W.; Sandine, W.E. Activation of bacterial spores. A Review. J. Food Prot. 1970, 33, 435–441. [Google Scholar]
- Setlow, B.; Loshon, C.A.; Genest, P.C.; Cowan, A.E.; Setlow, C.; Setlow, P. Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol. J. Appl. Microbiol. 2002, 92, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Cronin, U.P.; Wilkinson, M.G. Monitoring changes in germination and permeability of bacillus cereus endospores following chemical, heat and enzymatic treatments using flow cytometry. J. Rapid Methods Autom. Microbiol. 2008, 16, 164–184. [Google Scholar] [CrossRef]
- Loison, P.; Gervais, P.; Perrier-Cornet, J.M.; Kuimova, M.K. Effect of ethanol perturbation on viscosity and permeability of an inner membrane in Bacillus subtilis spores. Biochim. Biophys. Acta 2016, 1858, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, H.; Wang, X.; Peng, L.; Peng, Y.; Li, Y.Q. Probing the germination kinetics of ethanol-treated Bacillus thuringiensis spores. Appl. Opt. 2017, 56, 3263–3269. [Google Scholar] [CrossRef]
- Zhou, K.X.; Li, N.; Christie, G.; Wilson, D.I. Assessing the Impact of Germination and Sporulation Conditions on the Adhesion of Bacillus Spores to Glass and Stainless Steel by Fluid Dynamic Gauging. J. Food Sci. 2017, 82, 2614–2625. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 2007, 153, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Petersen, M.A.; Risbo, J.; Hümmer, M.; Clausen, A. Implications of modifying membrane fatty acid composition on membrane oxidation, integrity, and storage viability of freeze-dried probiotic, Lactobacillus acidophilus La-5. Biotechnol. Prog. 2015, 31, 799–807. [Google Scholar] [CrossRef]
- Reitermayer, D.; Kafka, T.A.; Lenz, C.A.; Vogel, R.F. Interrelation between Tween and the membrane properties and high pressure tolerance of Lactobacillus plantarum. BMC Microbiol. 2018, 18, 72. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Kiosseoglou, V. The Role of Tween in Inhibiting Heat-Induced Destabilization of Yolk-Based Emulsions. Food Hydrocoll. 2007, 21, 1310–1318. [Google Scholar] [CrossRef]
- Porter, W.R.; Staack, H.; Brandt, K.; Manning, M.C. Thermal stability of low molecular weight urokinase during heat treatment. I. Effects of protein concentration, pH and ionic strength. Thromb. Res. 1993, 71, 265–279. [Google Scholar] [CrossRef]
- Kanaan, J.; Murray, J.; Higgins, R.; Nana, M.; DeMarco, A.M.; Korza, G.; Setlow, P. Resistance properties and the role of the inner membrane and coat of Bacillus subtilis spores with extreme wet heat resistance. J. Appl. Microbiol. 2022, 132, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Korza, G.; DePratti, S.; Fairchild, D.; Wicander, J.; Kanaan, J.; Shames, H.; Nichols, F.C.; Cowan, A.; Brul, S.; Setlow, P. Expression of the 2Duf protein in wild-type Bacillus subtilis spores stabilizes inner membrane proteins and increases spore resistance to wet heat and hydrogen peroxide. J. Appl. Microbiol. 2023, 134, lxad040. [Google Scholar] [CrossRef]
- Yu, B.; Kanaan, J.; Shames, H.; Wicander, J.; Aryal, M.; Li, Y.; Korza, G.; Stanley, B.; Kramer, G.; Li, Y.-Q.; et al. Identification and characterization of new proteins crucial for bacterial spore resistance and germination. Front. Microbiol. 2023, 14, 1161604. [Google Scholar] [CrossRef]
- Salvador, M.; Yruela, I.; Lau, M.S.H.; Minton, N.P.; Condón, S.; Gayán, E. Identification and Role of Germinant Receptors in the Revival of Geobacillus and Parageobacillus Spores. In Proceedings of the 11th European Spores Conference, Cambridge, UK, 8–10 April 2025. [Google Scholar]
- Abhyankar, W.; Pandey, R.; Ter Beek, A.; Brul, S.; De Koning, L.J.; De Koster, C.G. Reinforcement of Bacillus subtilis spores by cross-linking of outer coat proteins during maturation. Food Microbiol. 2015, 45, 54–62. [Google Scholar] [CrossRef]
- Ghosh, S.; Korza, G.; Maciejewski, M.; Setlow, P. Analysis of Metabolism in Dormant Spores of Bacillus Species by 31P Nuclear Magnetic Resonance Analysis of Low-Molecular-Weight Compounds. J. Bacteriol. 2015, 197, 992–1001. [Google Scholar] [CrossRef]
- Setlow, P.; Christie, G. New Thoughts on an Old Topic: Secrets of Bacterial Spore Resistance Slowly Being Revealed. Microbiol. Mol. Biol. Rev. 2023, 87, e00080-22. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salas, J.-L.; Setlow, B.; Zhang, P.; Li, Y.-Q.; Setlow, P. Maturation of Released Spores Is Necessary for Acquisition of Full Spore Heat Resistance during Bacillus subtilis Sporulation. Appl. Environ. Microbiol. 2011, 77, 6746–6754. [Google Scholar] [CrossRef]
- Camilleri, E.; Korza, G.; Green, J.; Yuan, J.; Li, Y.-Q.; Caimano, M.J.; Setlow, P. Properties of Aged Spores of Bacillus subtilis. J. Bacteriol. 2019, 201, e00231-19. [Google Scholar] [CrossRef] [PubMed]
- Ursem, R.; Swarge, B.; Abhyankar, W.R.; Buncherd, H.; De Koning, L.J.; Setlow, P.; Brul, S.; Kramer, G. Identification of Native Cross-Links in Bacillus subtilis Spore Coat Proteins. J. Proteome Res. 2021, 20, 1809–1816. [Google Scholar] [CrossRef]
- Isticato, R.; Lanzilli, M.; Petrillo, C.; Donadio, G.; Baccigalupi, L.; Ricca, E. Bacillus subtilis builds structurally and functionally different spores in response to the temperature of growth. Environ. Microbiol. 2020, 22, 170–182. [Google Scholar] [CrossRef]
- Ghosh, S.; Setlow, B.; Wahome, P.G.; Cowan, A.E.; Plomp, M.; Malkin, A.J.; Setlow, P. Characterization of spores of Bacillus subtilis that lack most coat layers. J. Bacteriol. 2008, 190, 6741–6748. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.E.; Olivastro, E.M.; Koppel, D.E.; Loshon, C.A.; Setlow, B.; Setlow, P. Lipids in the inner membrane of dormant spores of Bacillus species are largely immobile. Proc. Natl. Acad. Sci. USA 2004, 101, 7733–7738. [Google Scholar] [CrossRef]
- Garcia, D.; Der Voort, M.V.; Abee, T. Comparative analysis of Bacillus weihenstephanensis KBAB4 spores obtained at different temperatures. Int. J. Food Microbiol. 2010, 140, 146–153. [Google Scholar] [CrossRef]
- Freire, V.; Condón, S.; Gayán, E. Impact of sporulation temperature on germination of Bacillus subtilis spores under optimal and adverse environmental conditions. Food Res. Int. 2024, 182, 114064. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, W.R.; Kamphorst, K.; Swarge, B.N.; Van Veen, H.; Van Der Wel, N.N.; Brul, S.; de Koster, C.G.; de Koning, L.J. The Influence of Sporulation Conditions on the Spore Coat Protein Composition of Bacillus subtilis Spores. Front. Microbiol. 2016, 7, 1636. [Google Scholar] [CrossRef]
- Saggese, A.; Barletta, G.D.G.; Vittoria, M.; Donadio, G.; Isticato, R.; Baccigalupi, L.; Ricca, E. CotG Mediates Spore Surface Permeability in Bacillus subtilis. mBio 2022, 13, e02760-22. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Korza, G.; DeMarco, A.M.; Kuipers, O.P.; Li, Y.Q.; Setlow, P. Properties of spores of Bacillus subtilis with or without a transposon that decreases spore germination and increases spore wet heat resistance. J. Appl. Microbiol. 2021, 131, 2918–2928. [Google Scholar] [CrossRef]
- Planchon, S.; Dargaignaratz, C.; Levy, C.; Ginies, C.; Broussolle, V.; Carlin, F. Spores of Bacillus cereus strain KBAB4 produced at 10 °C and 30 °C display variations in their properties. Food Microbiol. 2011, 28, 291–297. [Google Scholar] [CrossRef]
- Trunet, C.; Mtimet, N.; Mathot, A.G.; Postollec, F.; Leguerinel, I.; Couvert, O.; Broussolle, V.; Carlin, F.; Coroller, L. Suboptimal Bacillus licheniformis and Bacillus weihenstephanensis Spore Incubation Conditions Increase Heterogeneity of Spore Outgrowth Time. Appl. Environ. Microbiol. 2020, 86, e02061-19. [Google Scholar] [CrossRef]
- Mtimet, N.; Trunet, C.; Mathot, A.-G.; Venaille, L.; Leguérinel, I.; Coroller, L.; Couvert, O. Modeling the behavior of Geobacillus stearothermophilus ATCC 12980 throughout its life cycle as vegetative cells or spores using growth boundaries. Food Microbiol. 2015, 48, 153–162. [Google Scholar] [CrossRef]
- Baril, E.; Coroller, L.; Couvert, O.; Leguérinel, I.; Postollec, F.; Boulais, C.; Carlin, F.; Mafart, P. Modeling heat resistance of Bacillus weihenstephanensis and Bacillus licheniformis spores as function of sporulation temperature and pH. Food Microbiol. 2012, 30, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Palop, A.; Mañas, P.; Condón, S. Sporulation temperature and heat resistance of Bacillus spores: A review. J. Food Saf. 1999, 19, 57–72. [Google Scholar] [CrossRef]
- Wells-Bennik, M.H.J.; Janssen, P.W.M.; Klaus, V.; Yang, C.; Zwietering, M.H.; Den Besten, H.M.W. Heat resistance of spores of 18 strains of Geobacillus stearothermophilus and impact of culturing conditions. Int. J. Food Microbiol. 2019, 291, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, V.; Alonso, R.; Mañas, P.; Condón, S.; Condón-Abanto, S. Geobacillus stearothermophilus STCC4517 spore suspensions showed survival curves with shoulder phenomena independent of sporulation temperature and pH, whose duration was an exponential function of treatment temperature. Food Microbiol. 2022, 104, 103969. [Google Scholar] [CrossRef]
- Sala, F.J.; Ibarz, P.; Palop, A.; Raso, J.; Condón, S. Sporulation Temperature and Heat Resistance of Bacillus subtilis at Different pH Values. J. Food Prot. 1995, 58, 239–243. [Google Scholar] [CrossRef]
- Kumar, M. A Study of the Abiotic Factors Influencing the Biofilm and Spore Formation of Dairy Isolates of Geobacillus stearothermophilus and Characterisation of Spores Based on Their Heat Resistance. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2021. [Google Scholar]
- Beaman, T.C.; Gerhardt, P. Heat resistance of bacterial spores correlated with protoplast dehydration, mineralization, and thermal adaptation. Appl. Environ. Microbiol. 1986, 52, 1242–1246. [Google Scholar] [CrossRef]
- Melly, E.; Setlow, P. Heat Shock Proteins Do Not Influence Wet Heat Resistance of Bacillus subtilis Spores. J. Bacteriol. 2001, 183, 779–784. [Google Scholar] [CrossRef]
- Bressuire-Isoard, C.; Bornard, I.; Henriques, A.O.; Carlin, F.; Broussolle, V. Sporulation Temperature Reveals a Requirement for CotE in the Assembly of both the Coat and Exosporium Layers of Bacillus cereus Spores. Appl. Environ. Microbiol. 2016, 82, 232–243. [Google Scholar] [CrossRef]



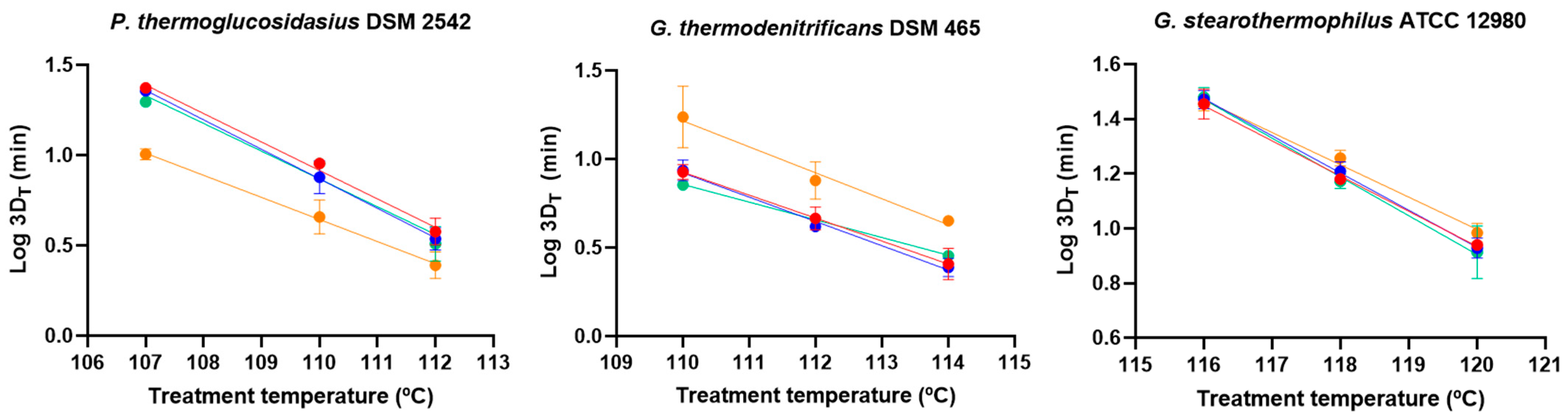

| Strain | Treatment Temperature (°C) | Purification Method | Sl (min) | Kmax (min−1) | 3DT (min) | R2 | RMSE |
|---|---|---|---|---|---|---|---|
| P. thermoglucosidasius DSM 2542 | 112 | Water | 0.57 a (0.08) | 2.47 a (0.58) | 3.35 a (0.40) | 0.990 | 0.125 |
| Ethanol | 0.12 b (0.07) | 2.29 ab (0.64) | 3.23 a (0.75) | 0.988 | 0.151 | ||
| Tween | 0.27 ab (0.53) | 1.61 b (0.21) | 4.57 b (0.57) | 0.970 | 0.414 | ||
| Tween + ethanol | 1.13 c (0.23) | 1.88 b (0.09) | 4.81 b (0.07) | 0.973 | 0.351 | ||
| G. thermodenitrificans DSM 465 | 114 | Water | 1.21 a (0.10) | 4.61 a (1.09) | 2.72 a (0.28) | 0.988 | 0.133 |
| Ethanol | 1.25 a (0.16) | 4.33 a (0.20) | 2.85 a (0.11) | 0.981 | 0.280 | ||
| Tween | 0.51 a (0.99) | 2.87 b (0.88) | 3.41 b (0.38) | 0.989 | 0.136 | ||
| Tween + ethanol | 0.32 a (0.75) | 1.97 b (0.43) | 4.47 b (1.23) | 0.982 | 0.148 | ||
| G. stearothermophilus ATCC 12980 | 120 | Water | 2.32 a (0.32) | 1.90 a (0.35) | 6.12 a (0.82) | 0.984 | 0.145 |
| Ethanol | 2.15 a (0.86) | 1.16 a (0.02) | 8.18 a (1.77) | 0.957 | 0.248 | ||
| Tween | 2.05 a (0.36) | 1.27 a (0.35) | 7.57 a (1.03) | 0.965 | 0.333 | ||
| Tween + ethanol | 2.23 a (0.56) | 1.22 a (0.07) | 7.91 a (0.83) | 0.983 | 0.145 |
| Strain | Maturation Time (d) | Sporulation Temperatura (°C) | z (°C) | R2 |
|---|---|---|---|---|
| P. thermoglucosidasius DSM 2542 | 1 | 55 | 6.22 a (0.69) | 0.962 |
| 2 | 55 | 6.12 a (0.33) | 0.979 | |
| 4 | 55 | 6.54 a (0.92) | 0.964 | |
| 7 | 55 | 8.22 a (1.23) | 0.990 | |
| 4 | 50 | 7.03 a (0.59) | 0.974 | |
| 4 | 60 | 6.48 a (0.83) | 0.958 | |
| G. thermodenitrificans DSM 465 | 1 | 55 | 7.86 ab (1.73) | 0.990 |
| 2 | 55 | 7.36 a (1.23) | 0.986 | |
| 4 | 55 | 9.78 bc (0.88) | 0.996 | |
| 7 | 55 | 6.98 a (1.70) | 0.948 | |
| 4 | 50 | 10.83 c (0.63) | 0.877 | |
| 4 | 60 | 7.03 a (0.54) | 0.914 | |
| G. stearothermophilus ATCC 12980 | 1 | 55 | 8.21 a (0.41) | 0.993 |
| 2 | 55 | 7.38 a (0.67) | 0.990 | |
| 4 | 55 | 7.08 a (1.10) | 0.958 | |
| 7 | 55 | 8.47 a (0.49) | 0.992 | |
| 4 | 50 | 5.07 b (0.49) | 0.925 | |
| 4 | 60 | 7.11 a (0.21) | 0.992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, M.; Condón, S.; Gayán, E. Germination and Heat Resistance of Parageobacillus and Geobacillus spp. Spores. Foods 2025, 14, 2061. https://doi.org/10.3390/foods14122061
Salvador M, Condón S, Gayán E. Germination and Heat Resistance of Parageobacillus and Geobacillus spp. Spores. Foods. 2025; 14(12):2061. https://doi.org/10.3390/foods14122061
Chicago/Turabian StyleSalvador, Maika, Santiago Condón, and Elisa Gayán. 2025. "Germination and Heat Resistance of Parageobacillus and Geobacillus spp. Spores" Foods 14, no. 12: 2061. https://doi.org/10.3390/foods14122061
APA StyleSalvador, M., Condón, S., & Gayán, E. (2025). Germination and Heat Resistance of Parageobacillus and Geobacillus spp. Spores. Foods, 14(12), 2061. https://doi.org/10.3390/foods14122061






