Tea and Its Active Ingredients in Preventing and Alleviating Depression: A Comprehensive Review
Abstract
1. Introduction
2. The Etiological Mechanism of Depression and the Antidepressant Effects of Tea Components
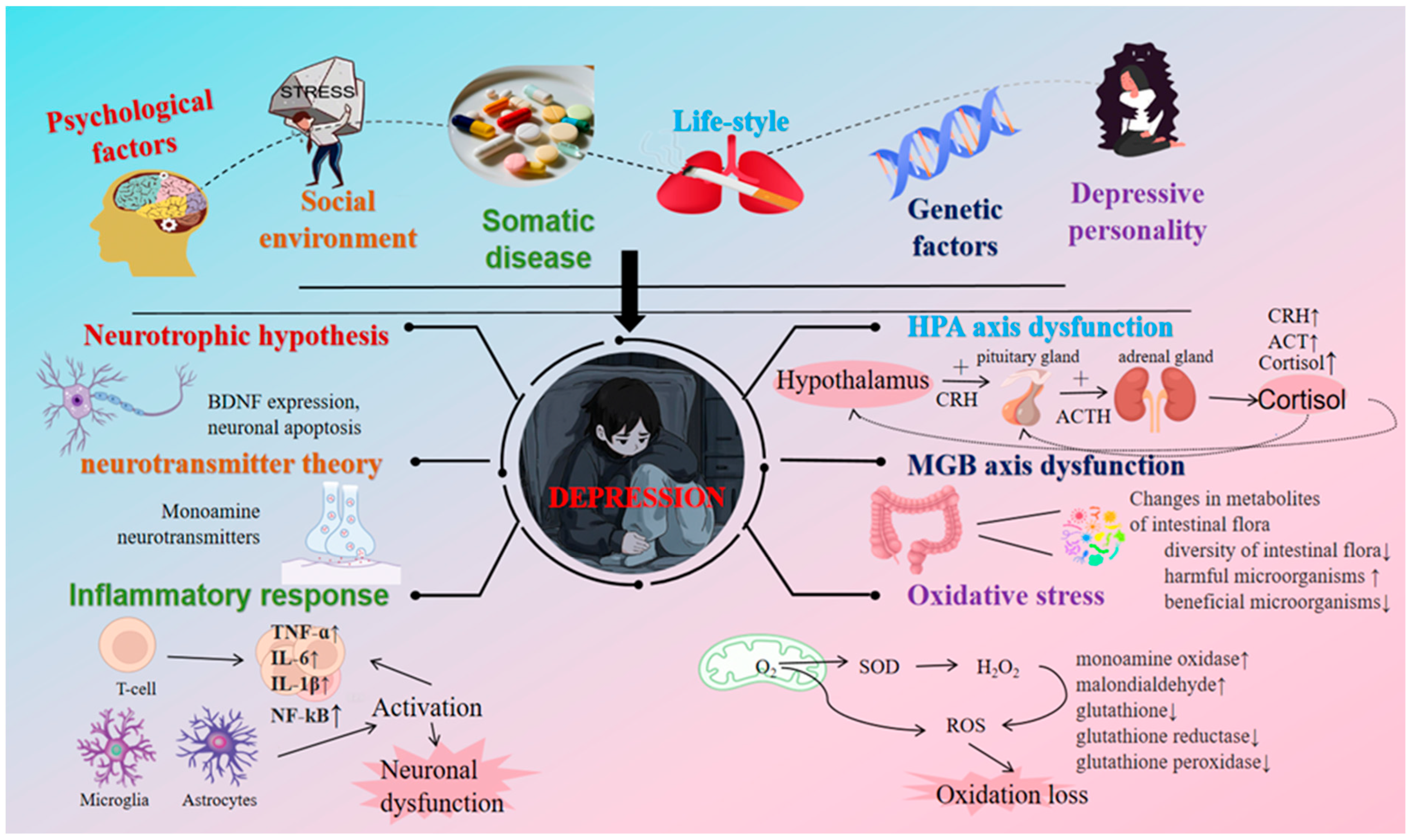
2.1. Etiological Mechanism of Depression
2.2. Research Approaches
2.2.1. Observational Studies
2.2.2. Intervention Studies
2.3. Mechanism of Tea’s Action on Depression
2.3.1. L-Theanine
L-Theanine Regulates the HPA Axis
Neuroregulatory Mechanism of L-Theanine
L-Theanine and the Immune System
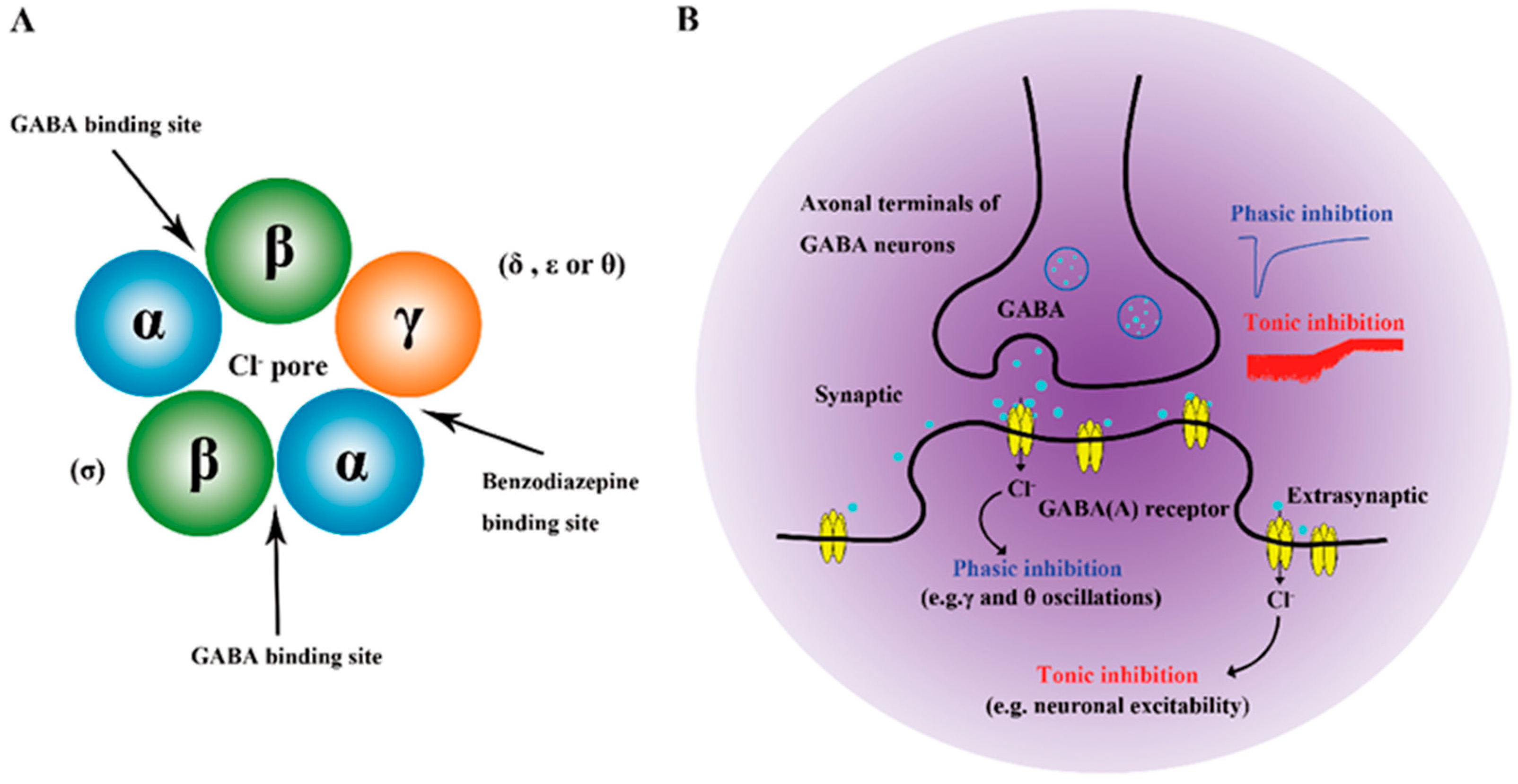
2.3.2. GABA
2.3.3. Tea Polyphenols
Flavonoids
EGCG
- (1)
- EGCG Regulating the HPA Axis
- (2)
- EGCG Regulating Nervous System
- (3)
- EGCG Regulates the Intestinal System
- (4)
- EGCG Regulates Oxidative Stress
- (5)
- Tea Polyphenol Mixture Antidepressant
2.3.4. Tea Pigments
2.3.5. Tea Saponin
3. Discussion
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothenberg, D.; Zhang, L. Mechanisms underlying the anti-depressive effects of regular tea consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, D.; Rive, B.; Denee, T. The humanistic and economic burden of treatment-resistant depression in Europe: A cross-sectional study. BMC Psychiatry 2019, 19, 247. [Google Scholar] [CrossRef]
- Santomauro, D.; Herrera, A.; Shadid, J. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Egbert, A.; Karpiak, S.; Havlik, R.; Cankurtaran, S. Global occurrence of depressive symptoms during the COVID-19 pandemic. J. Psychosom. Res. 2023, 166, 111145. [Google Scholar] [CrossRef]
- Olfson, M.; Blanco, C.; Marcus, S.C. Treatment of adult depression in the United States. JAMA Intern. Med. 2016, 176, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Alang, S.; McAlpine, D. Treatment modalities and perceived effectiveness of treatment among adults with depression. Health Serv. Insights 2020, 13, 1178632920918288. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, H.G.; Kessler, R.C.; Kelley, D.; Ben-Hamadi, R.; Joish, V.N.; Greenberg, P.E. Employer burden of mild, moderate, and severe major depressive disorder: Mental health services utilization and costs, and work performance. Depress. Anxiety 2010, 27, 78–89. [Google Scholar] [CrossRef]
- Rozental, A.; Castonguay, L.; Dimidjian, S.; Lambert, M.; Shafran, R.; Andersson, G.; Carlbring, P. Negative effects in psychotherapy: Commentary and recommendations for future research and clinical practice. BJPsych Open 2018, 4, 307–312. [Google Scholar] [CrossRef]
- Cuijpers, P.; Miguel, C.; Harrer, M.; Plessen, C.Y.; Ciharova, M.; Ebert, D.; Karyotaki, E. Cognitive behavior therapy vs. control conditions, other psychotherapies, pharmacotherapies and combined treatment for depression: A comprehensive meta-analysis including 409 trials with 52,702 patients. World Psychiatry 2023, 22, 105–115. [Google Scholar] [CrossRef]
- Lai, C.; Boag, S. The association between gut-health promoting diet and depression: A mediation analysis. J. Affect. Disord. 2023, 324, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Sánchez-Pedreño, F.O.; Van, W.; Martínez-González, M.A. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Gutiérrez-Rojas, L.; Molina, R.; Rodríguez-Jimenez, R.; et al. Biological role of nutrients, food and dietary patterns in the prevention and clinical management of major depressive disorder. Nutrients 2022, 14, 3099. [Google Scholar] [CrossRef]
- Liu, Q.; Leng, P.; Gu, Y.; Shang, X.; Zhou, Y.; Zhang, H.; Zuo, L.; Mei, G.; Xiong, C.; Wu, T.; et al. The dose-effect relationships of cigarette and alcohol consumption with depressive symptoms: A multiple-center, cross-sectional study in 5965 Chinese middle-aged and elderly men. BMC Psychiatry 2022, 22, 657. [Google Scholar] [CrossRef]
- Fazelian, S.; Sadeghi, E.; Firouzi, S.; Haghighatdoost, F. Adherence to the vegetarian diet may increase the risk of depression: A systematic review and meta-analysis of observational studies. Nutr. Rev. 2022, 80, 242–254. [Google Scholar] [CrossRef]
- Hu, D.; Cheng, L.; Jiang, W. Sugar-sweetened beverages consumption and the risk of depression: A meta-analysis of observational studies. J. Affect. Disord. 2019, 245, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [PubMed]
- Samanta, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Dassanayake, T.L.; Wijesundara, D.; Kahathuduwa, C.N.; Weerasinghe, V.S. Dose-response effect of L-theanine on psychomotor speed, sustained attention, and inhibitory control: A double-blind, placebo-controlled, crossover study. Nutr. Neurosci. 2023, 26, 1138–1146. [Google Scholar] [CrossRef]
- Gilbert, N. The science of tea’s mood-altering magic. Nature 2019, 566, S8. [Google Scholar] [CrossRef]
- Zhu, W.L.; Shi, H.S.; Wei, Y.M.; Wang, S.J.; Sun, C.Y.; Ding, Z.B.; Lu, L. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol. Res. 2012, 65, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Gan, R.Y.; Li, B.Y.; Mao, Q.; Shang, A.; Xu, X.; Li, H.; Li, H. Effects and mechanisms of tea on Parkinson′s disease, Alzheimer′s disease and depression. Food Rev. Int. 2021, 39, 278–306. [Google Scholar] [CrossRef]
- Sohtorik, Y.; McWilliams, N. Hugging, drinking tea, and listening: Mental health needs of Turkish immigrants. J. Multicult. Couns. D 2011, 39, 66–77. [Google Scholar] [CrossRef]
- Bzdok, D.; Ioannidis, J.P.A. Exploration, inference, and prediction in neuroscience and biomedicine. Trends Neurosci. 2019, 42, 251–262. [Google Scholar] [CrossRef]
- Racagni, G.; Popoli, M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin. Neuro. 2008, 10, 385–400. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-López-Alberca, C.; González-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The role of BDNF on neural plasticity in depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Wang, J.Q.; Mao, L. The ERK pathway: Molecular mechanisms and treatment of depression. Mol. Neurobiol. 2019, 56, 6197–6205. [Google Scholar] [CrossRef]
- Jeon, S.W.; Kim, Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J. Psychiatr. 2016, 6, 283. [Google Scholar] [CrossRef]
- Palucha, A.; Pilc, A. The involvement of glutamate in the pathophysiology of depression. Drug News Perspect. 2005, 18, 262–268. [Google Scholar]
- Sartorius, A.; Mahlstedt, M.M.; Vollmayr, B.; Henn, F.A.; Ende, G. Elevated spectroscopic glutamate/γ-amino butyric acid in rats bred for learned helplessness. Neuroreport 2007, 18, 1469–1473. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.W. Social roles, context and evolution in the origins of depression. J. Health Soc. Behav. 2002, 43, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Alshaya, D.S. Genetic and epigenetic factors associated with depression: An updated overview. Saudi J. Biol. Sci. 2022, 29, 103311. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing depression from the microbiota-gut-brain axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Gonda, X.; Dome, P.; Neill, J.C.; Tarazi, F. Novel antidepressant drugs: Beyond monoamine targets. CNS Spectrums 2023, 28, 6–15. [Google Scholar] [CrossRef]
- Török, B.; Sipos, E.; Pivac, N.; Zelena, D. Modelling posttraumatic stress disorders in animals. Prog. Neuro-Psychopharmacol. Bio Psychiatry 2019, 90, 117–133. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Dong, X.; Gu, Y.; Rayamajhi, S.; Thapa, A.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Zhang, S.; Zhang, T.; et al. Green tea consumption and risk of depressive symptoms: Results from the TCLSIH Cohort Study. J. Affect. Disord. 2022, 310, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Nanri, A.; Kurotani, K.; Kuwahara, K.; Kume, A.; Sato, M.; Hayabuchi, H.; Mizoue, T. Green tea and coffee consumption is inversely associated with depressive symptoms in a Japanese working population. Public Health Nutr. 2014, 17, 625–633. [Google Scholar] [CrossRef]
- Ng, T.P.; Gao, Q.; Gwee, X.; Chua, D.Q.L. Tea consumption and depression from follow up in the Singapore Longitudinal Ageing Study. J. Nutr. Health Aging 2021, 25, 295–301. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J. Green tea, coffee, and caffeine consumption are inversely associated with self-report lifetime depression in the Korean population. Nutrients 2018, 10, 1201. [Google Scholar] [CrossRef]
- Guo, X.; Park, Y.; Freedman, N.D.; Sinha, R.; Hollenbeck, A.; Blair, A.; Chen, H. Sweetened beverages, coffee, and tea and depression risk among older US adults. PLoS ONE 2014, 9, e94715. [Google Scholar] [CrossRef]
- Ruusunen, A.; Lehto, S.M.; Tolmunen, T.; Mursu, J.; Kaplan, G.A.; Voutilainen, S. Coffee, tea and caffeine intake and the risk of severe depression in middle-aged finnish men: The kuopio ischaemic heart disease risk factor study. Public Health Nutr. 2010, 13, 1215–1220. [Google Scholar] [CrossRef]
- Esmaeilpour-Bandboni, M.; Seyedpourchafi, Z.; Kahneh, E. The effect of green tea drinking on the depression of elderly people. J. Nurse Pract. 2021, 17, 983–987. [Google Scholar] [CrossRef]
- Asil, E.; Yılmaz, M.V.; Yardimci, H. Effects of black tea consumption and caffeine intake on depression risk in black tea consumers. Afr. Health Sci. 2021, 21, 858–865. [Google Scholar] [CrossRef]
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-theanine administration on stress-related symptoms and cognitive functions in healthy adults: A randomized controlled trial. Nutrients 2019, 11, 2362. [Google Scholar] [CrossRef]
- Yılmaz, C.; Özdemir, F.; Gökmen, V. Investigation of free amino acids, bioactive and neuroactive compounds in different types of tea and effect of black tea processing. Lwt-Food Sci. Technol. 2020, 117, 108655. [Google Scholar] [CrossRef]
- Das, C.; Banerjee, A.; Saha, M.; Chatterjee, S. A review of the health benefits of tea: Implications of the biochemical properties of the bioactive constituents. Curr. Res. Nutr. Food Sci. J. 2022, 10, 458–475. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Zhao, G.; Kan, Z.; Wang, X.; Wang, H.; Huang, J.; Wang, T.; Qian, F.; Ho, C.T.; et al. Protective effect and mechanism of theanine on lipopolysaccharide-induced inflammation and acute liver injury in mice. J. Agric. Food Chem. 2018, 66, 7674–7683. [Google Scholar] [CrossRef]
- Yamada, T.; Terashima, T.; Okubo, T.; Juneja, L.R.; Yokogoshi, H. Effects of theanine, r-glutamylethylamide, on neurotransmitter release and its relationship with glutamic acid neurotransmission. Nutr. Neurosci. 2013, 8, 219–226. [Google Scholar] [CrossRef]
- Yamada, T.; Terashima, T.; Kawano, S.; Furuno, R.; Okubo, T.; Juneja, L.R.; Yokogoshi, H. Theanine, γ-glutamylethylamide, a unique amino acid in tea leaves, modulates neurotransmitter concentrations in the brain striatum interstitium in conscious rats. Amino Acids 2009, 36, 21–27. [Google Scholar] [CrossRef]
- Shen, M.; Yang, Y.; Wu, Y.; Zhang, B.B.; Wu, H.; Wang, L.; Tang, H.; Chen, J. L-theanine ameliorate depressive-like behavior in a chronic unpredictable mild stress rat model via modulating the monoamine levels in limbic-cortical-striatal-pallidal-thalamic-circuit related brain regions. Phytother. Res. 2019, 33, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, S.; Xie, Z.; Wan, X. Synaptic modification by L-theanine, a natural constituent in green tea, rescues the impairment of hippocampal long-term potentiation and memory in AD mice. Neuropharmacol. 2018, 138, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Taguchi, K.; Konishi, T.; Ozeki, M.; Nakamura, Y. Theanine, a tea-leaf-specific amino acid, alleviates stress through modulation of npas4 expression in group-housed older mice. Int. J. Mol. Sci. 2023, 24, 3983. [Google Scholar] [CrossRef]
- Rao, T.; Ozeki, M.; Juneja, L. In search of a safe natural sleep aid. J. Am. Coll. Nutr. 2015, 34, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lin, L.; Chen, L.; Xiao, W.; Gong, Z. L-theanine ameliorates d-galactose-induced brain damage in rats via inhibiting AGE formation and regulating sirtuin1 and BDNF signaling pathways. Oxid. Med. Cell. Longev. 2021, 2021, 8850112. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jin, S.W.; Choi, J.H.; Choi, C.Y.; Kim, H.G.; Kim, S.J.; Kim, Y.; Lee, K.J.; Chung, Y.C.; Jeong, H.G. Inhibitory effects of l-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem. Toxicol. 2017, 99, 162–169. [Google Scholar] [CrossRef]
- Zeng, W.J.; Tan, Z.; Lai, X.F.; Xu, Y.N.; Mai, C.L.; Zhang, J.; Lin, Z.J.; Liu, X.G.; Sun, S.L.; Zhou, L.J. Topical delivery of L-theanine ameliorates TPA-induced acute skin inflammation via downregulating endothelial PECAM-1 and neutrophil infiltration and activation. Chem. Biol. Interact. 2018, 284, 69–79. [Google Scholar] [CrossRef]
- Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. L-Theanine and Immunity: A Review. Molecules 2023, 28, 3846. [Google Scholar] [CrossRef]
- Wang, D.; Cai, M.; Wang, T.; Liu, T.; Huang, J.; Wang, Y.; Granato, D. Ameliorative effects of L-theanine on dextran sulfate sodium induced colitis in C57BL/6J mice are associated with the inhibition of inflammatory responses and attenuation of intestinal barrier disruption. Food Res. Int. 2020, 137, 109409. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed moods: A review of the interactions between inflammation and mood disorders. Prog. Neuro-Psychoph. 2014, 53, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, W.; Peng, Y.; Deng, Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab. Brain Dis. 2019, 34, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Strosznajder, J.B. Glutamate and GABA in microglia-neuron cross-talk in Alzheimer’s disease. Int. J. Mol. Sci. 2011, 22, 11677. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Kim, S.R. Beneficial effects of flavonoids against Parkinson’s disease. J. Med. Food 2018, 21, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.H. Effects of epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic-pituitary-adrenal axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress. J. Med. Food 2018, 21, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, F.; Jin, H.; Li, R.; Wang, Y.; Zhang, W.; Wang, H.; Chen, W. Involvement of PKCα and ERK1/2 signaling pathways in EGCG′s protection against stress-induced neural injuries in Wistar rats. Neuroscience 2017, 346, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhang, J.; Liu, G.; Chen, F.; Lin, Y. Neuroprotection by (-)-epigallocatechin-3-gallate in a rat model of stroke is mediated through inhibition of endoplasmic reticulum stress. Mol. Med. Rep. 2014, 9, 69–72. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, C.S.; Kim, D.J.; Chao, M.H.; Jin, B.K.; Pie, J.E.; Chung, W.G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002, 23, 367–374. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, M.; Liang, Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.; Kumari, A.; Kulurkar, P.M.; Raj, R.; Gulati, A.; Padwad, Y.S. Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male swiss albino mice. Biogerontology 2017, 18, 367–382. [Google Scholar] [CrossRef]
- Zhou, J.; Mao, L.; Xu, P.; Wang, Y. Effects of (−)-epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients 2018, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lin, J.K. (−)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-κB. Mol. Pharmacol. 1997, 52, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, F.; Sha, L.; Wang, S.; Tao, L.; Yao, L.; He, M.; Yao, Z.; Liu, H.; Zhu, Z.; et al. (−)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75 NTR signaling in APP/PS1 transgenic mice. Mol. Neurobiol. 2014, 49, 1350–1363. [Google Scholar] [CrossRef]
- Johnston, G.A.R. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem. Int. 2015, 89, 120–125. [Google Scholar] [CrossRef]
- Payne, A.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Molecular mechanisms of the anti-inflammatory effects of epigallocatechin 3-gallate (EGCG) in LPS-activated BV-2 microglia cells. Brain Sci. 2023, 13, 632. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, J.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Ski. Pharmacol. Phys. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef]
- Liang, Y.; Ip, M.S.M.; Mak, J.C.W. (-)-Epigallocatechin-3-gallate suppresses cigarette smoke-induced inflammation in human cardiomyocytes via ROS-mediated MAPK and NF-κB pathways. Phytomedcine 2019, 58, 152768. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Xu, Q.; Chen, Y.; Lv, L.; Zheng, B.; Wang, Q.; Wang, S.; Li, L. Epigallocatechin-3-gallate improves intestinal gut microbiota homeostasis and ameliorates clostridioides difficile infection. Nutrients 2022, 14, 3756. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Elias, R.J.; Vijay-Kumar, M.; Lambert, J. (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol. Nutr. Food Res. 2016, 60, 2267–2274. [Google Scholar] [CrossRef]
- Luo, Y.P.; Tang, X.F.; Zhang, Y.C.; Chen, S.M.; Wu, Q.; Li, W.J. Epigallocatechin-3-gallate alleviates galactose-induced aging impairment via gut-brain communication. Food Funct. 2022, 13, 11200–11209. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, M.; Xue, J.; Xiang, L.; Li, Y.; Xiao, J.; Xiao, G.; Wang, H. EGCG ameliorates neuronal and behavioral defects by remodeling gut microbiota and TotM expression in Drosophila models of Parkinson’s disease. FASEB J. 2020, 34, 5931–5950. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, J.; Wang, X.; Zhou, C.; Zheng, X.; Lin, W. Effects of EGCG on depression-related behavior and serotonin concentration in a rat model of chronic unpredictable mild stress. Food Funct. 2020, 11, 8780–8787. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Ye, L.; Xu, X.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. BMC Complement. Altern. Med. 2012, 12, 82. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, S.; Doo, M.; Kim, Y. Green Tea (-)-Epigallotocatechin-3-gallate induces PGC-1α gene expression in HepG2 cells and 3T3-L1 adipocytes. Prev. Nutr. Food Sci. 2016, 21, 62–67. [Google Scholar] [CrossRef]
- Rothenberg, D.O.N.; Zhou, C.; Zhang, L. A review on the weight-loss effects of oxidized tea polyphenols. Molecules 2018, 23, 1176. [Google Scholar] [CrossRef] [PubMed]
- Odaira, T.; Nakagawasai, O.; Takahashi, K.; Nemoto, W.; Sakuma, W.; Lin, J.R.; Tan-No, K. Mechanisms underpinning AMP-activated protein kinase-related effects on behavior and hippocampal neurogenesis in an animal model of depression. Neuropharmacology 2019, 150, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Nosheen, F.; Arshad, M.U.; Saeed, F.; Afzaal, M.; Islam, F.; Imran, A.; Noreen, R.; Amer Ali, Y.; Shah, M.A. Isolation and antioxidant characterization of theaflavin for neuroprotective effect in mice model. Food Sci. Nutr. 2023, 11, 3485–3496. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Wu, H.; Yang, Z.; Fei, L.; Zhu, J. Theaflavin attenuates cerebral ischemia/reperfusion injury by abolishing miRNA-128-3p-mediated Nrf2 inhibition and reducing oxidative stress. Mol. Med. Rep. 2019, 20, 4893–4904. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Kita, M.; Taniguchi, Y.; Kondo, K. Theaflavins improve memory impairment and depression-like behavior by regulating microglial activation. Molecules 2019, 24, 467. [Google Scholar] [CrossRef]
- Lagha, A.B.; Grenier, D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci. Rep. 2016, 6, 34520. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, F.; Wang, Y.; Li, F.; Wang, L.; Wang, Q.; Ren, Z.; Wang, Y. In vitro and in vivo anti-inflammatory effects of theaflavin-3, 3′-digallate on lipopolysaccharide-induced inflammation. Eur. J. Pharmacol. 2017, 794, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Lo, C.Y.; Wang, B.J.; Chiou, R.Y.; Lin, S.M. Theaflavin-3, 3′-digallate, a black tea polyphenol, attenuates adipocyte-activated inflammatory response of macrophage associated with the switch of M1/M2-like phenotype. J. Funct. Foods 2014, 11, 36–48. [Google Scholar] [CrossRef]
- Kita, M.; Uchida, S.; Yamada, K.; Ano, Y. Anxiolytic effects of theaflavins via dopaminergic activation in the frontal cortex. Biosci. Biotechnol. Biochem. 2019, 83, 1157–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Quaisie, J.; Gu, C.; Guo, L.; Liu, D.; Chen, Y.; Zhang, T. Tea saponin extracted from seed pomace of Camellia oleifera Abel ameliorates DNCB-induced atopic dermatitis-like symptoms in BALB/c mice. J. Funct. Foods 2022, 91, 105001. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Szabo, A.; Wu, Z.; Wang, H.; Li, D.; Huang, X.F. Teasaponin reduces inflammation and central leptin resistance in diet-induced obese male mice. Endocrinology 2013, 154, 3130–3140. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.F.; Zhang, P.; Newell, K.A.; Wang, H.; Zheng, K.; Yu, Y. Dietary tea saponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci. Rep. 2017, 7, 12203. [Google Scholar]
- Herman, J.P.; Mueller, N.K.; Figueiredo, H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann. N. Y. Acad. Sci. 2004, 1018, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Evanson, N.K.; Herman, J.P. Role of paraventricular nucleus glutamate signaling in regulation of HPA axis stress responses. Interdiscip. Inf. Sci. 2015, 21, 253–260. [Google Scholar] [CrossRef]
- Boonstra, E.; De Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef]
- Gundersen, R.Y.; Vaagenes, P.; Breivik, T.; Fonnum, F.; Opstad, P.K. Glycine-an important neurotransmitter and cytoprotective agent. Acta Anaesthesiol. Scand. 2005, 49, 1108–1116. [Google Scholar] [CrossRef]
- Yadid, G.; Pacak, K.; Golomb, E.; Harvey-White, J.D.; Lieberman, D.M.; Kopin, I.J.; Goldstein, D.S. Glycine stimulates striatal dopamine release in conscious rats. Br. J. Pharmacol. 1993, 110, 50–53. [Google Scholar] [CrossRef]
- Schuster, J.; Mitchell, E.S. More than just caffeine: Psychopharmacology of methylxanthine interactions with plant-derived phytochemicals. Prog. Neuo-Psychopharmcol. Biol. Psychiatry 2019, 89, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Calabrese, F.; Auletta, F.; Olivier, J.; Racagni, G.; Homberg, J.; Riva, M.A. Developmental influence of the serotonin transporter on the expression of npas4 and GABAergic markers: Modulation by antidepressant treatment. Neuropsychoph 2012, 37, 746–758. [Google Scholar] [CrossRef]
- Drouet, J.B.; Peinnequin, A.; Faure, P.; Denis, J.; Fidier, N.; Maury, R.; Buguet, A.; Cespuglio, R.; Canini, F. Stress-induced hippocampus Npas4 mRNA expression relates to specific psychophysiological patterns of stress response. Brain Res. 2018, 1679, 75–83. [Google Scholar] [CrossRef]
- Lovato, N.; Gradisar, M. A meta-analysis and model of the relationship between sleep and depression in adolescents: Recommendations for future research and clinical practice. Sleep Med. Rev. 2014, 18, 521–529. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.; Han, S.; Suh, H.J. GABA and L-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 64–72. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Khanjani, S.; Moghaddam, A.H.; Braidy, N.; Nabavi, S. Improvement of antioxidant defences and mood status by oral GABA tea administration in a mouse model of post-stroke depression. Nutrients 2017, 9, 446. [Google Scholar] [CrossRef]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Konishi, T.; Nakamura, Y. L-arginine exerts excellent anti-stress effects on stress-induced shortened lifespan, cognitive decline and depression. Int. J. Mol. Sci. 2021, 22, 508. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Brain-derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF-a key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Lotrich, F.E. Inflammatory cytokine-associated depression. Brain Res. 2015, 1617, 113–125. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Anders, S.; Tanaka, M.; Kinney, D.K. Depression as an evolutionary strategy for defense against infection. Brain Behav. Immune. 2013, 31, 9–22. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, W.J. Anti-inflammatory cytokines and depression. Prog. Biochem. Biophys. 2014, 41, 1099–1108. [Google Scholar]
- Ghose, S.; Winter, M.K.; McCarson, K.E.; Tamminga, C.A.; Enna, S.J. The GABAβ receptor as a target for antidepressant drug action. Bri. J. Pharmacol. 2011, 162, 1–17. [Google Scholar] [CrossRef]
- Xu, M.-y.; Wong, A. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol. Sin. 2018, 39, 733–753. [Google Scholar] [CrossRef]
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.A.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Liu, X.; Cao, Y. gadA gene locus in Lactobacillus brevis NCL912 and its expression during fed-batch fermentation. FEMS Microbiol. Lett. 2013, 349, 108–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. Front. Psychiatry 2021, 12, 637863. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Zhou, W.; Zeng, Z.; Zhao, W.; Huang, Y.; Zhang, X. Quality components and antidepressant-like effects of GABA green tea. Food Funct. 2017, 8, 3311–3318. [Google Scholar] [CrossRef]
- Tanaka, T.; Kouno, I. Oxidation of tea catechins: Chemical structures and reaction mechanism. Food Sci. Technol. Res. 2003, 9, 128–133. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Tounekti, T.; Joubert, E.; Hernández, I.; Munné-Bosch, S. Improving the polyphenol content of tea. Crit Rew. Plant Sci. 2013, 32, 192–215. [Google Scholar] [CrossRef]
- Zhao, K.; Yao, M.; Zhang, X.; Xu, F.; Shao, X.; Wei, Y.; Wang, H. Flavonoids and intestinal microbes interact to alleviate depression. J. Sci. Food Agric. 2022, 102, 1311–1318. [Google Scholar] [CrossRef]
- Mossine, V.V.; Waters, J.K.; Gu, Z.; Sun, G.Y.; Mawhinney, T.P. Bidirectional responses of eight neuroinflammation-related transcriptional factors to 64 flavonoids in astrocytes with transposable insulated signaling pathway reporters. CS Chem. Neurosci. 2022, 13, 613–623. [Google Scholar] [CrossRef]
- Park, E.; Chun, H.S. Green tea polyphenol Epigallocatechine gallate (EGCG) prevented LPS-induced BV-2 micoglial cell activation. J. Life Sci. 2016, 26, 640–645. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Park, S.Y.; Hwang, J.W.; Park, P.J.; Lim, J.H.; Choi, D.K. Attenuation of inflammatory-mediated neurotoxicity by Saururus chinensis extract in LPS-induced BV-2 microglia cells via regulation of NF-κB signaling and anti-oxidant properties. BMC Complement. Altern. Med. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection against oxidative stress: Phytochemicals targeting TrkB signaling and the Nrf2-ARE antioxidant system. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- McKernan, D.P.; Dinan, T.G.; Cryan, J.F. “Killing the Blues”: A role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog. Neurobiol. 2009, 88, 246–263. [Google Scholar] [CrossRef]
- Alho, H.; Costa, E.; Ferrero, P.; Fujimoto, M.; Cosenza-Murphy, D.; Guidott, A. Diazepam-binding inhibitor: A neuropeptide located in selected neuronal populations of rat brain. Science 1985, 229, 179–182. [Google Scholar] [CrossRef]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The role of the microbiome-brain-gut axis in the pathogenesis of depressive disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, G.; Tang, M.; Fang, J.; Jiang, H. Epigallocatechin gallate can protect mice from acute stress induced by LPS while stabilizing gut microbes and serum metabolites levels. Front. Immunol. 2021, 12, 640305. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, and elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Valenti, D.; de Bari, L.; de Rasmo, D.; Signorile, A.; Henrion-Caude, A.; Contestabile, A.; Vacca, R.A. The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. BBA-Mol. Basis Dis. 2016, 1862, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Vacca, R.A.; Valenti, D.; Caccamese, S.; Daglia, M.; Braidy, N.; Nabavi, S.M. Plant polyphenols as natural drugs for the management of Down syndrome and related disorders. Neurosci. Biobehav. R. 2016, 71, 865–877. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Mood by microbe: Towards clinical translation. Genome Med. 2016, 8, 1–3. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X. Fermentation in vitro of EGCG, GCG and EGCG3" Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Samad, N.; Jabeen, S.; Imran, I.; Zulfiqar, I.; Bilal, K. Protective effect of gallic acid against arsenic-induced anxiety-/depression-like behaviors and memory impairment in male rats. Metab. Brain Dis. 2019, 34, 1091–1102. [Google Scholar] [CrossRef]
- Can, Ö.D.; Turan, N.; Özkay, Ü.D.; Öztürk, Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. [Google Scholar] [CrossRef]
- Cabrera-Suárez, B.M.; Hernández-Fleta, J.L.; Ana González-Pinto, P.M.; Claudio Cabrera, F.L.; Chiclana-Actis, C.; Sánchez-Villegas, A. Mediterranean diet-based intervention to improve depressive symptoms: Analysis of the PREDIDEP randomized trial. Nutr. Neurosci. 2024, 27, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, H.; Zheng, Y. Editorial: Exploration of major depressive disorder among children and adolescents: From pathogenesis to intervention. Front. Psychiatry. 2024, 14, 1350201. [Google Scholar] [CrossRef] [PubMed]
- Menardo, E.; Balboni, G.; Cubelli, R. Environmental factors and teenagers’ personalities: The role of personal and familial Socio-Cultural Level. Behav. Brain Res. 2017, 325, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Cusin, C.; Yang, H.; Yeung, A.; Fava, M. Rating scales for depression. In Handbook of Clinical Rating Scales and Assessment in Psychiatry and Mental Health; Springer: Berlin/Heidelberg, Germany, 2010; pp. 7–35. [Google Scholar]
- Moncrieff, J.; Kirsch, I. Empirically derived criteria cast doubt on the clinical significance of antidepressant-placebo differences. Contemp. Clin. Trials 2015, 43, 60–62. [Google Scholar] [CrossRef]
| Tea Ingredients | Theoretical Approach | Action Mechanism | Ref. |
|---|---|---|---|
| L -theanine | Improved HPA axis | 1. Decreases ACTH, increases cortisol, and inhibits NF-κB phosphorylation | [53] |
| 2. Inhibits the release of glutamate and decarboxylates glutamate | [54] | ||
| Neuromodulation | 1. Increases glycine concentration in striatum | [55] | |
| 2. The concentration of PFC, NAc and AN and the 5-HT, norepinephrine and dopamine in HIP are increased | [56] | ||
| 3. Activation of the dopamine D1/5 receptor-PKA pathway improves monoamine transmission in the HIP | [57] | ||
| 4. The expression of holo-GR and DNMT3a is inhibited, and the expression of NPAS4 is indirectly promoted | [58] | ||
| 5. Indirectly reduces the intermittent arousal and improves sleep quality | [59] | ||
| 6. Inhibits the formation of AGE and regulate Sirtuin1 and BDNF signaling pathways | [60] | ||
| Immunomodulation | 1. Reduces inflammation in many types of cells | [61,62] | |
| 2. Inhibit of NF-κB pathway to reduce inflammatory cytokine levels | [63] | ||
| 3. Ameliorate intestinal pathological injury and inhibit various inflammatory cytokines | [64] | ||
| GABA | Improved HPA axis | 1. GABA inhibits glutamate release and reduces the activation of CRF neurons | [65] |
| 2. GABA promotes recurrent GABAergic synaptic connections between PVN CRFR1 and CRF neurons | [66] | ||
| Neuromodulation | 1. GABA system activation promotes neuronal signal transmission | [67] | |
| Intestinal regulation | 1. Long-term administration of Lactobacillus rhamnosus JB-1 improves GABA receptor expression in the cortico-hippocampus, thereby reducing CORT and depressive behaviors | [68] | |
| Flavonoids | 1. Protects neurons from oxidative stress, inhibits neuroinflammation and improves signaling pathways | [69] | |
| EGCG | Improved HPA axis | 1. Reduces CORT, CRH, and ACTH levels | [70] |
| 2. Activation of ERK pathway inhibits HPA activity and improves neural status in mice | [71,72] | ||
| 3. Protects the level of DA and its metabolites and prevents monoaminergic disorders in the nervous system | [73] | ||
| 4. Prevents the decrease in dopaminergic neurons and inhibits the increase in serum concentrations of TNF-α and IL-6 | [74] | ||
| 5. Regulates DHEA hormone levels in the HPA axis | [75] | ||
| Neuromodulation | 1. Inhibits the phosphorylation of NF-κB and STAT3 in the hypothalamus and inhibits the production and release of TNF-α, IL-6 and IL-1β | [76] | |
| 2. The binding of NF-kB to iNOS promoter is prevented, thus inhibiting the transcription of iNOS to produce NO and reducing the activity and protein level of iNOS in microglia | [77] | ||
| 3. Decreases the expression of Caspase 3 in HIP and apoptotic neurons | [78] | ||
| 4. Increasing the ratio of NGF/proNGF can improve the relative expression level of NGF, inhibit Aβ deposition and neuronal apoptosis in HIP | [78] | ||
| 5. The full function of GABA receptors may be restored by substituting DBI type antianxiety ligands | [79] | ||
| Immunomodulation | 1. Neuroimmunological coordination is achieved through NF-κB, PI3k-Akt-mTOR, and NO pathways | [80] | |
| 2. Decreases levels of IL-1β and TNF-α in the HIP | [70] | ||
| 3. The levels of TNF-α, IL-1β, IL-6 and IL-8 are inhibited | [81] | ||
| 4. Inhibition of NFκB/ activator protein 1 (AP-1) pathway shows anti-inflammatory effects | [82] | ||
| 5. Inhibits the activation of ERK1/2, P38MAPK, and NF-κB and reduces the production of inflammatory chemokine IL-8 in the supernatant of AC16 cardiomyocytes | [83] | ||
| Protect intestinal health | 1. Protects intestinal mucosal barrier from damage and improves intestinal microbial homeostasis | [84] | |
| 2. Decreases plasma lfructose–rhamnose–ratio and plasma sucralose-to-erythrolitol ratio, decreases colon protein levels of IL-1β, IL-6, and TNF-β, and colon lipid peroxides. | [85] | ||
| 3. Up-regulates SCFA level in cecal contents of mice | [84] | ||
| 4. The levels of cortisol, ACTH, and CRF are significantly decreased, and the production of intestinal SCFAs is induced | [86] | ||
| 5. Enriches SCFAs producing bacteria | [87] | ||
| 6. TotM connects the gut microbiota formed after ingesting EGCG with host neurons and motor function | [88] | ||
| 7. Enhances the protective effect of intestinal structure and decreases the level of 5-HT in the colon; increases the level of 5-HT in the HIP or inhibits the apoptosis of hippocampal neurons | [89] | ||
| Reduce oxidative stress | 1. Combined administration of EGCG and green tea polyphenols can up-regulate PGC-1α | [90,91] | |
| 2. EGCG activates PGC-1α by promoting AMPK phosphorylation | [92,93] | ||
| 3. EGCG can regulate oxidative stress and neuroimmunity through the NO pathway, can regulate the expression levels of genes including iNOS, COX-2 and TNF-α, and can prevent the binding of NF-kB to iNOS promoters | [77,80] | ||
| Tea pigments | 1. TFs increases overall antioxidant capacity and decreases OS levels | [94] | |
| 2. Theaflavin administration significantly increases Nrf2 protein and Nrf2 mRNA levels, and TF treatment can reduce the apoptosis rate of CI/RI rats | [95] | ||
| 3. Inhibiting the production of inflammatory cytokines significantly reduces depressive behavior in mice | [96] | ||
| 4. Inhibition of NF-κB activation significantly reduce IL-1β, TNF-α, and IL-6 secretion in a dose-dependent manner | [97] | ||
| 5. TFDG inhibits the expression of TNF-α, IL-1β and IL-6 | [98,99] | ||
| 6. The circulating level of DA in the frontal cortex of mice is increased | [100] | ||
| Tea saponin | 1. BALB/c mice can significantly improve the symptoms of skin inflammation and significantly reduce the level of inflammatory factors in serum and skin tissue | [101] | |
| 2. The levels of inflammatory signaling molecules, proinflammatory cytokines, and inflammatory signaling molecules in adipose tissue and liver of obese mice are decreased | [102] | ||
| 3. The changes in the intestinal microbiota of mice are reduced, the recognition and memory disorders are effectively prevented, and the neuroinflammation and BDNF defects in the HIP of the experimental group of mice are improved | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Li, Y.; Jiang, H.; Hou, S.; Wang, Y.; Wang, D.; Teng, J. Tea and Its Active Ingredients in Preventing and Alleviating Depression: A Comprehensive Review. Foods 2025, 14, 2054. https://doi.org/10.3390/foods14122054
Xiao S, Li Y, Jiang H, Hou S, Wang Y, Wang D, Teng J. Tea and Its Active Ingredients in Preventing and Alleviating Depression: A Comprehensive Review. Foods. 2025; 14(12):2054. https://doi.org/10.3390/foods14122054
Chicago/Turabian StyleXiao, Shuangling, Yi Li, Haiyan Jiang, Sitong Hou, Yaoyao Wang, Di Wang, and Jie Teng. 2025. "Tea and Its Active Ingredients in Preventing and Alleviating Depression: A Comprehensive Review" Foods 14, no. 12: 2054. https://doi.org/10.3390/foods14122054
APA StyleXiao, S., Li, Y., Jiang, H., Hou, S., Wang, Y., Wang, D., & Teng, J. (2025). Tea and Its Active Ingredients in Preventing and Alleviating Depression: A Comprehensive Review. Foods, 14(12), 2054. https://doi.org/10.3390/foods14122054





