Purslane-Fortified Yogurt: In-Line Process Control by FT-NIR Spectroscopy and Storage Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Yogurt Ingredients
2.2. Yogurt Production and Storage
2.3. Monitoring of Fermentation
2.4. Microbiological Analyses
2.5. Texture Analysis
2.6. Data Processing
3. Results and Discussion
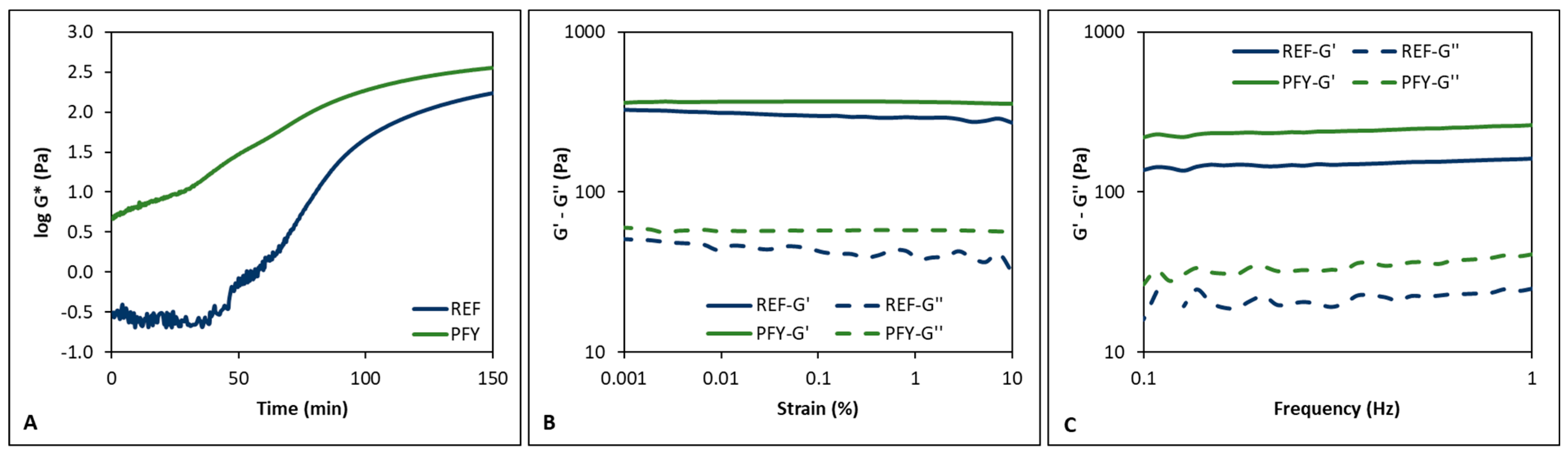
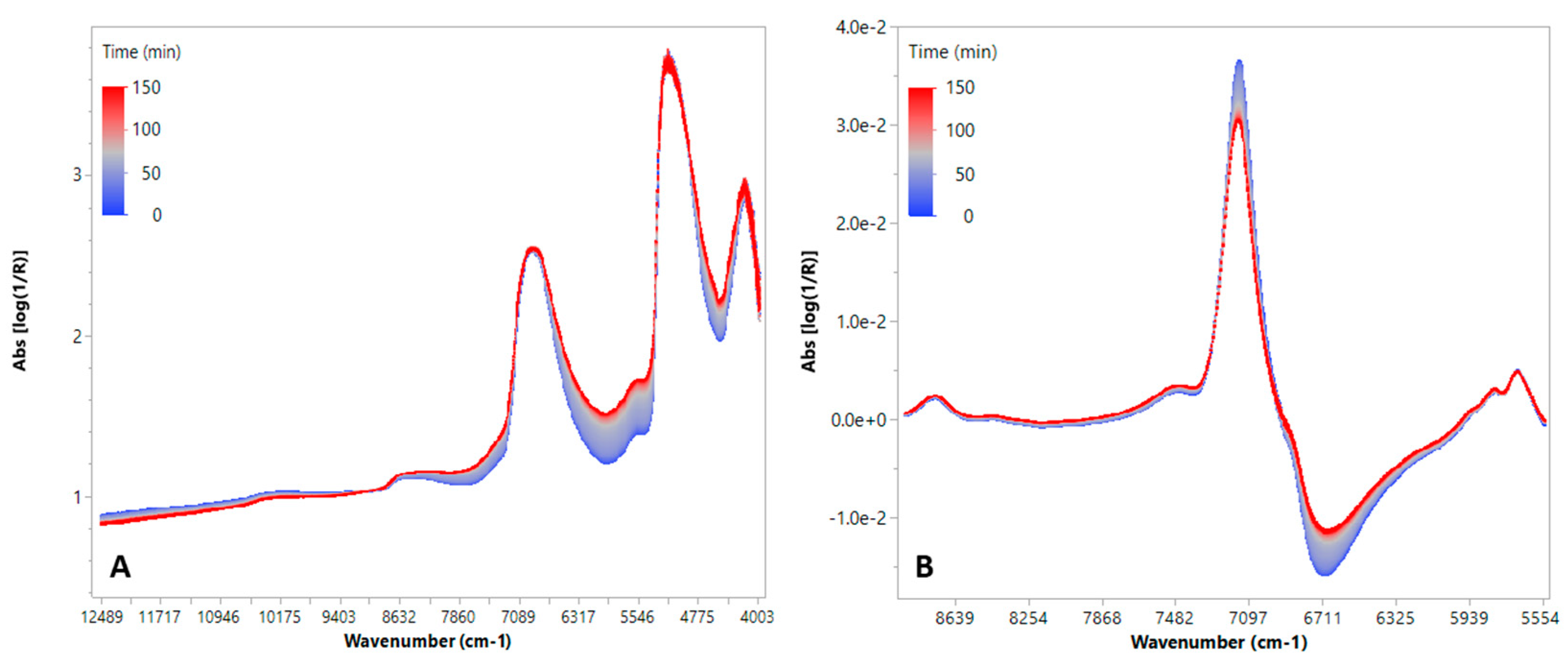
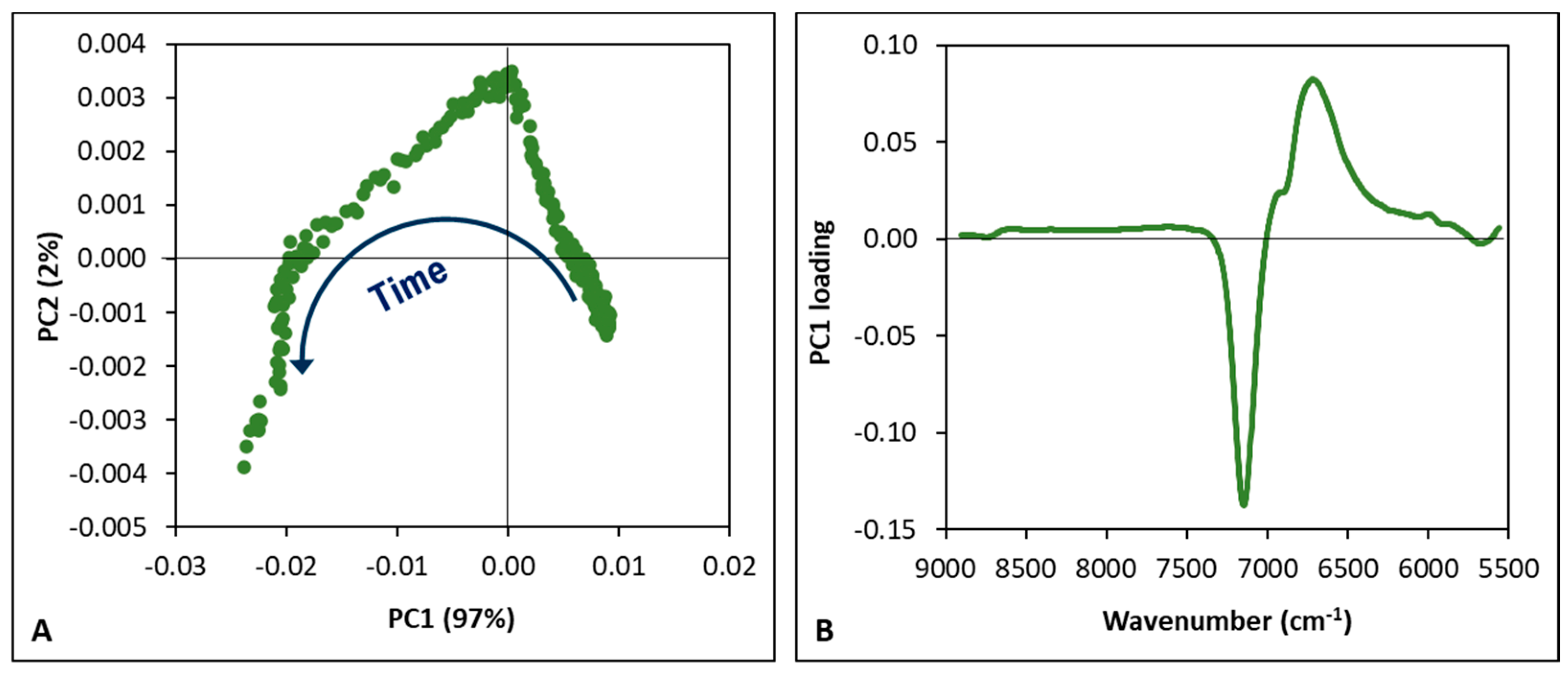
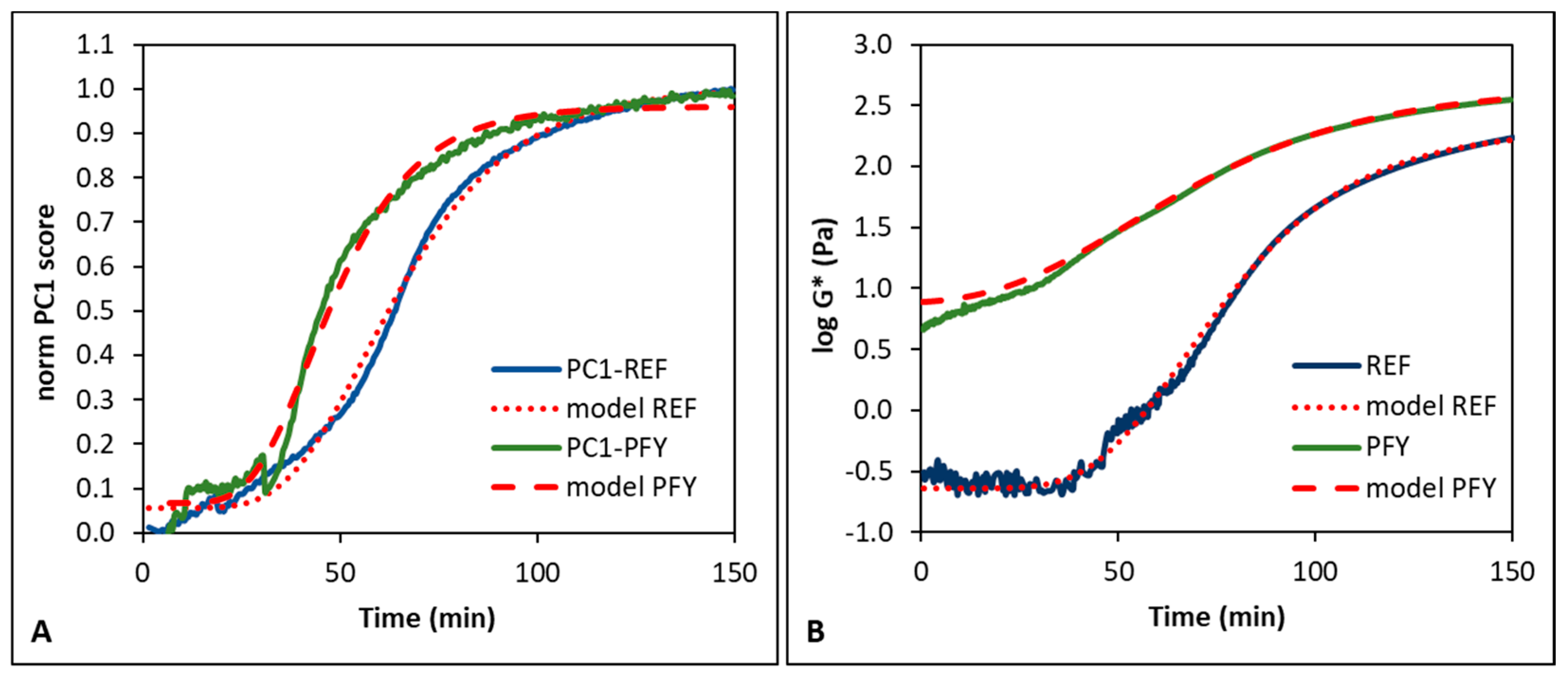
3.1. Fermentation Process Control
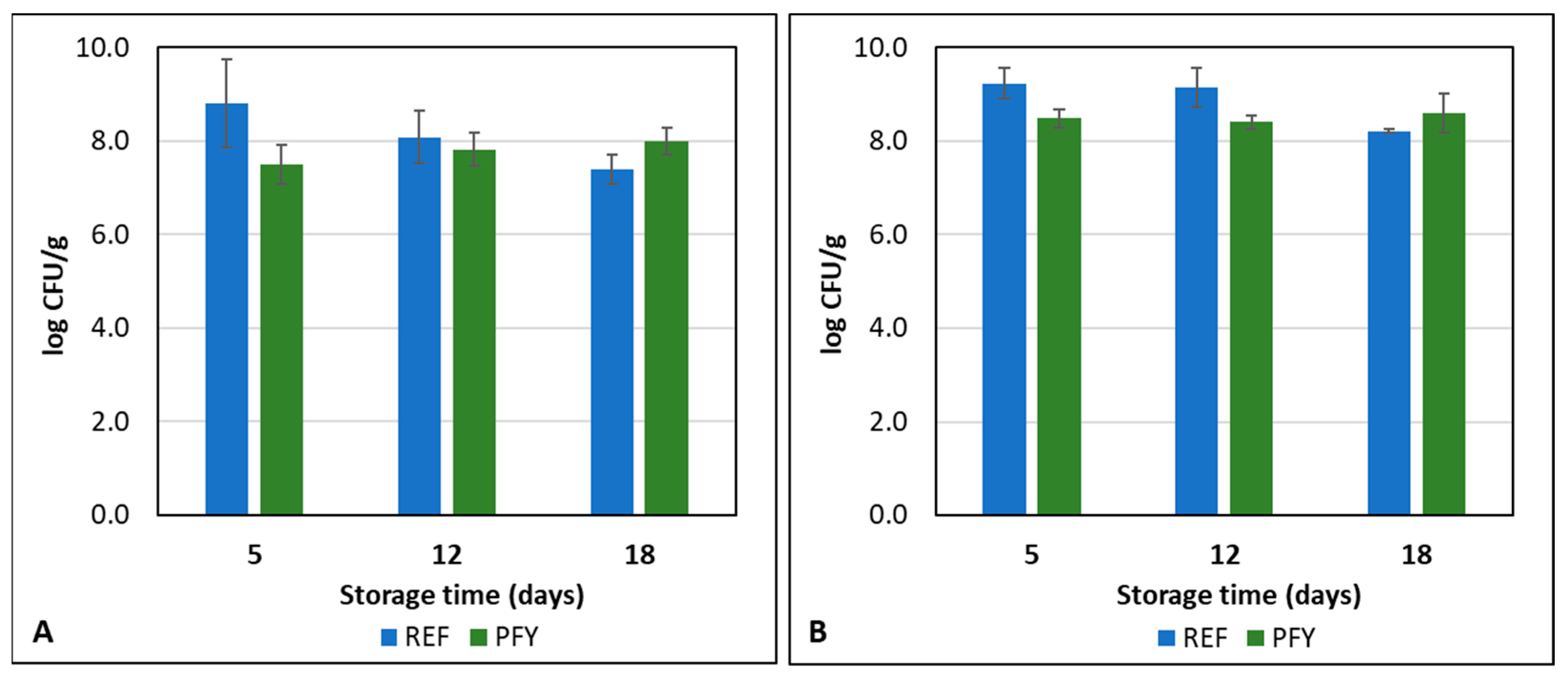
3.2. Yogurt Storage Monitoring
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tamime, Y.A.; Robinson, R.K. Yoghurt: Science and Technology, 3rd ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2007. [Google Scholar]
- Foegeding, A.; Vardhanabhuti, B.; Yang, X. Dairy systems. In Practical Food Rheology; Norton, I.T., Spyropoulos, F., Cox, P., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 133–172. [Google Scholar] [CrossRef]
- Gregersen, S.B.; Glover, Z.J.; Wiking, L.; Simonsen, A.C.; Bertelsen, K.; Pedersen, B.; Poulsen, K.R.; Andersen, U.; Hammershøj, M. Microstructure and rheology of acid milk gels and stirred yoghurts–quantification of process-induced changes by auto-and cross correlation image analysis. Food Hydrocoll. 2021, 111, 106269. [Google Scholar] [CrossRef]
- Laiho, S.; Williams, R.P.; Poelman, A.; Appelqvist, I.; Logan, A. Effect of whey protein phase volume on the tribology, rheology and sensory properties of fat-free stirred yoghurts. Food Hydrocoll. 2017, 67, 166–177. [Google Scholar] [CrossRef]
- Guenard-Lampron, V.; St-Gelais, D.; Villeneuve, S.; Turgeon, S.L. Individual and sequential effects of stirring, smoothing, and cooling on the rheological properties of nonfat yogurts stirred with a technical scale unit. J. Dairy Sci. 2019, 102, 190–201. [Google Scholar] [CrossRef]
- Guenard-Lampron, V.; Villeneuve, S.; St-Gelais, D.; Turgeon, S.L. Relationship between smoothing temperature, storage time, syneresis and rheological properties of stirred yogurt. Int. Dairy J. 2020, 109, 104742. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Wellness 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Wazzan, H. Fortification of dairy products using plant-derived bioactive compounds. Nutr. Food Sci. 2024, 12, 561–571. [Google Scholar] [CrossRef]
- Satkın, B.; Aktas, A.B. The optimisation of processing and storage conditions of lyophilised purslane-fortified yoghurts by central composite design. Int. J. Dairy Technol. 2024, 77, 282–291. [Google Scholar] [CrossRef]
- Al-Quwaie, D.A.; Allohibi, A.; Aljadani, M.; Alghamdi, A.M.; Alharbi, A.A.; Baty, R.S.; Qahl, S.H.; Saleh, O.; Shakak, A.O.; Alqahtani, F.S.; et al. Characterization of Portulaca oleracea whole plant: Evaluating antioxidant, anticancer, antibacterial, and antiviral activities and application as quality enhancer in yogurt. Molecules 2023, 28, 5859. [Google Scholar] [CrossRef]
- Anli, E.A.; Gursel, A.; Sert, D. Physicochemical and textural properties of ready-to-eat yogurt fortified with dried purslane (Portulaca oleracea L.). Gida 2021, 46, 229–242. [Google Scholar] [CrossRef]
- Salehi, M.; Ghorbani, M.; Mahoonk, A.S.; Khomeiri, M. Physicochemical, antioxidant and sensory properties of yogurt fortified with common purslane (Portulaca oleracea) extract. J. Food Meas. Charact. 2021, 15, 4288–4296. [Google Scholar] [CrossRef]
- Montoya-Garcia, C.O.; Garcia-Mateos, R.; Becerra-Martinez, E.; Toledo-Aguilar, R.; Volke-Haller, V.H.; Magdaleno-Villar, J.J. Bioactive compounds of purslane (Portulaca oleracea L.) according to the production system: A review. Sci. Hortic. 2023, 308, 111584. [Google Scholar] [CrossRef]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose benefits of an underexplored species purslane (Portulaca oleracea L.): A critical review. Environ. Manag. 2023, 72, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.; Un, A.; Ali, M.E.; Rahman, M.M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Fernandes, A.; Barros, L.; Ferreira, I.C.; Ntatsi, G.; Petrotos, K.; Lykas, C.; Khah, E. Chemical composition and yield of six genotypes of common purslane (Portulaca oleracea L.): An alternative source of omega-3 fatty acids. Plant Foods Hum. Nutr. 2015, 70, 420–426. [Google Scholar] [CrossRef]
- Santiago-Saenz, Y.O.; Hernandez-Fuentes, A.D.; Monroy-Torres, R.; Carino-Cortes, R.; Jimenez-Alvarado, R. Physicochemical, nutritional and antioxidant characterization of three vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as potential sources of phytochemicals and bioactive compounds. J. Food Meas. Charact. 2018, 12, 2855–2864. [Google Scholar] [CrossRef]
- Gatea, F.; Teodor, D.E.; Seciu, A.M.; Nagoda, E.; Radu, G.L. Chemical constituents and bioactive potential of Portulaca pilosa L. vs. Portulaca oleracea L. Med. Chem. Res. 2017, 26, 1516–1527. [Google Scholar] [CrossRef]
- Ray, A.; Sinha, C.; Minz, P.S.; Dabas, J.K.; John, H.; Kumari, K. Automatic pH-controlled thermal modulation unit for yoghurt production. J. Food Proc. Engin. 2025, 48, e70050. [Google Scholar] [CrossRef]
- Alouache, B.; Touat, A.; Boutkedjirt, T.; Bennamane, A. Monitoring of lactic fermentation process by ultrasonic technique. Phys. Procedia 2015, 70, 1057–1060. [Google Scholar] [CrossRef][Green Version]
- Benozzi, E.; Romano, A.; Capozzi, V.; Makhoul, S.; Cappellin, L.; Khomenko, I.; Aprea, E.; Scampicchio, M.; Spano, G.; Märk, T.D.; et al. Monitoring of lactic fermentation driven by different starter cultures via direct injection mass spectrometric analysis of flavour-related volatile compounds. Food Res. Int. 2015, 76, 682–688. [Google Scholar] [CrossRef]
- Svendsen, C.; Skov, T.; van den Berg, F.W.J. Monitoring fermentation processes using in-process measurements of different orders. J. Chem. Technol. Biotechnol. 2015, 90, 244–254. [Google Scholar] [CrossRef]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial yogurt manufacture: Monitoring of fermentation process and improvement of final product quality. J. Dairy Sci. 2007, 90, 2641–2654. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Svendsen, C.; Cieplak, T.; van den Berg, F.W.J. Exploring process dynamics by near infrared spectroscopy in lactic fermentations. J. Near Infr. Spectr. 2016, 24, 443–451. [Google Scholar] [CrossRef]
- Grassi, S.; Alamprese, C.; Bono, V.; Casiraghi, E.; Amigo, J.M. Modelling milk lactic acid fermentation using multivariate curve resolution-alternating least squares (MCR-ALS). Food Bioprocess. Technol. 2014, 7, 1819–1829. [Google Scholar] [CrossRef]
- Grassi, S.; Alamprese, C.; Bono, V.; Picozzi, C.; Foschino, R.; Casiraghi, E. Monitoring of lactic acid fermentation process using Fourier transform near infrared spectroscopy. J. Near Infr. Spectrosc. 2013, 21, 417–425. [Google Scholar] [CrossRef]
- Muncan, J.; Tei, K.; Tsenkova, R. Real-time monitoring of yogurt fermentation process by aquaphotomics near-infrared spectroscopy. Sensors 2020, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Niu, S.; Wu, M.; Li, H.; Yang, C.; Liu, C.; Dai, C.; He, R.; Xu, Y. Research progress on quality characteristics of yoghurt and its fermentation enhancement technology: A review. J. Food Process. Eng. 2025, 48, e70042. [Google Scholar] [CrossRef]
- ISO 4833-2:2022; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. International Organization for Standardization: Geneva, Switzerland, 2022.
- Alamprese, C.; Mariotti, M. Effects of different milk substitutes on pasting, rheological and textural properties of puddings. LWT-Food Sci. Technol. 2011, 44, 2019–2025. [Google Scholar] [CrossRef]
- Aberoumand, A. Nutritional evaluation of edible Portulaca oleracia as plant food. Food Anal. Methods 2009, 2, 204–207. [Google Scholar] [CrossRef]
- McCann, H.; Fabre, F.; Day, L. Microstructure, rheology and storage stability of low-fat yoghurt structured by carrot cell wall particles. Food Res. Int. 2011, 44, 884–892. [Google Scholar] [CrossRef]
- Beattie, R.J.; Esmonde-White, F.W.L. Exploration of Principal Component Analysis: Deriving Principal Component Analysis visually using spectra. Appl. Spectrosc. 2021, 75, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. The Gompertz model and its applications in microbial growth and bioproduction kinetics: Past, present and future. Biotechnol. Adv. 2024, 72, 108335. [Google Scholar] [CrossRef] [PubMed]
- CXS 243-2003; Codex Alimentarius Standard for Fermented Milks. Codex Alimentarius Commission: Rome, Italy, 2003; (Adopted in 2003. Revised in 2008, 2010, 2018. Amended in 2022).
- Salvador, A.; Fiszman, S.M. Textural and sensory characteristics of whole and skimmed flavored set-type yogurt during long storage. J. Dairy Sci. 2004, 87, 4033–4041. [Google Scholar] [CrossRef]
- Grassi, S.; Alamprese, C. Advances in NIR spectroscopy applied to process analytical technology in food industries. Curr. Opin. Food Sci. 2018, 22, 17–21. [Google Scholar] [CrossRef]
- Casson, A.; Beghi, R.; Giovenzana, V.; Fiorindo, I.; Tugnolo, A.; Guidetti, R. Visible Near Infrared spectroscopy as a green technology: An environmental impact comparative study on olive oil analyses. Sustainability 2019, 11, 2611. [Google Scholar] [CrossRef]

| Yogurt Type | Lb. bulgaricus | Str. thermophilus | ||
|---|---|---|---|---|
| t0 | t2.5 | t0 | t2.5 | |
| REF | 7.4 ± 1.0 a | 7.3 ± 0.1 a | 8.3 ± 1.1 a | 8.3 ± 0.1 b |
| PFY | 7.2 ± 1.1 a | 7.4 ± 0.4 a | 8.3 ± 1.0 a | 8.3 ± 0.1 a |
| Yogurt Type | Norm PC1 Curve | log G* Curve | ||||
|---|---|---|---|---|---|---|
| Max d2 (min) | Max d1 (min) | Min d2 (min) | Max d2 (min) | Max d1 (min) | Min d2 (min) | |
| REF | 32.4 | 53.6 | 75.5 | 60.1 | 77.6 | 92.3 |
| PFY | 27.9 | 41.2 | 54.2 | 19.3 | 52.4 | 83.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktas, A.B.; Grassi, S.; Picozzi, C.; Alamprese, C. Purslane-Fortified Yogurt: In-Line Process Control by FT-NIR Spectroscopy and Storage Monitoring. Foods 2025, 14, 2053. https://doi.org/10.3390/foods14122053
Aktas AB, Grassi S, Picozzi C, Alamprese C. Purslane-Fortified Yogurt: In-Line Process Control by FT-NIR Spectroscopy and Storage Monitoring. Foods. 2025; 14(12):2053. https://doi.org/10.3390/foods14122053
Chicago/Turabian StyleAktas, Ayse Burcu, Silvia Grassi, Claudia Picozzi, and Cristina Alamprese. 2025. "Purslane-Fortified Yogurt: In-Line Process Control by FT-NIR Spectroscopy and Storage Monitoring" Foods 14, no. 12: 2053. https://doi.org/10.3390/foods14122053
APA StyleAktas, A. B., Grassi, S., Picozzi, C., & Alamprese, C. (2025). Purslane-Fortified Yogurt: In-Line Process Control by FT-NIR Spectroscopy and Storage Monitoring. Foods, 14(12), 2053. https://doi.org/10.3390/foods14122053








