From Nutrition to Energy: Evaluating the Role of Rye (Secale cereale L.) Grain in Sustainable Food Systems and Biofuel Applications
Abstract
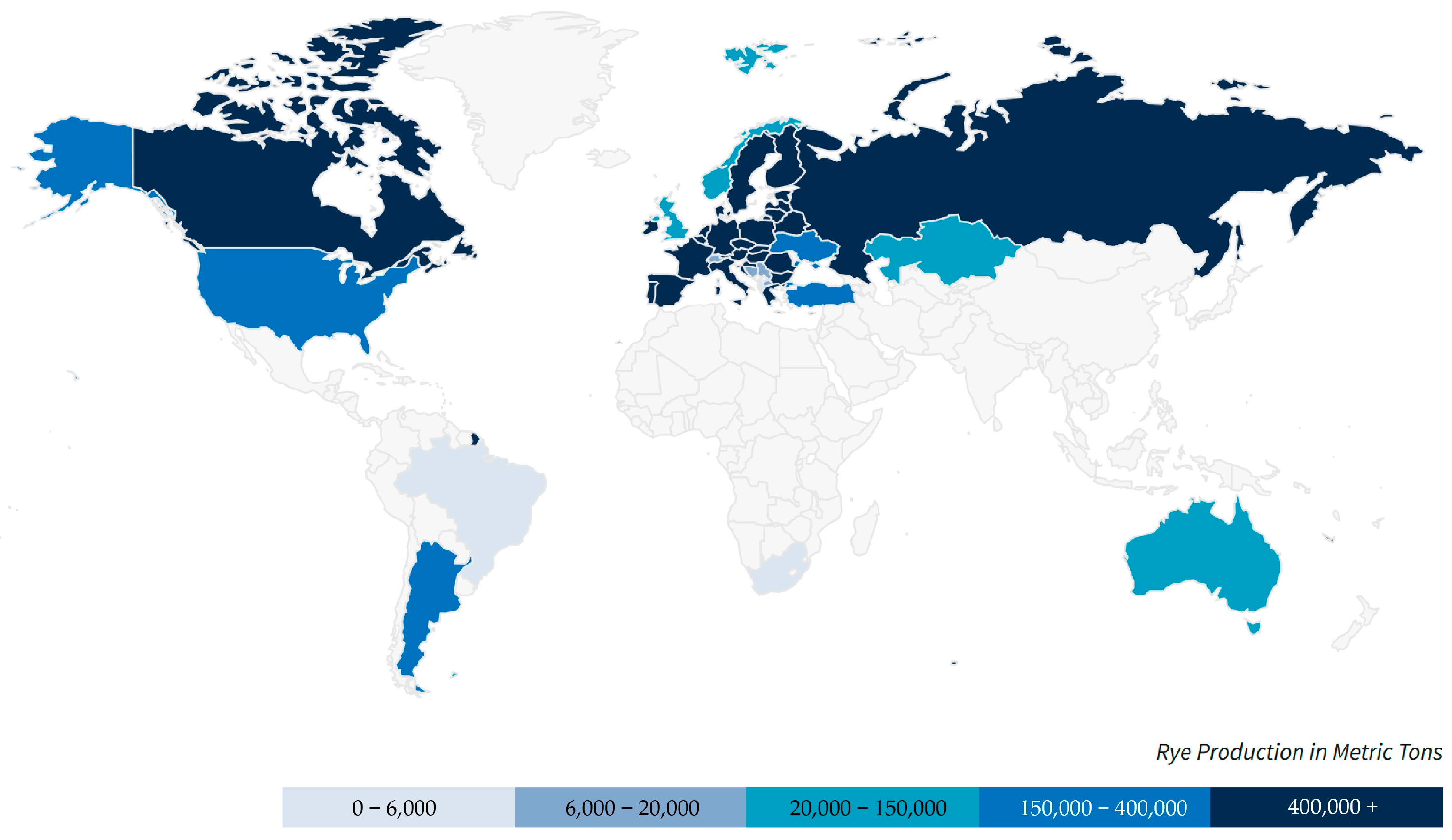
1. Introduction
2. Optimizing Grain Quality Through Production Technology
2.1. Selection of the Variety
2.2. Crop Rotation
2.3. Soil Tillage
2.4. Fertilization
2.5. Sowing Date and Density
2.6. Chemical Protection
2.7. Harvest Date
2.8. Organic Farming Practices
3. Grain Quality and Bioactive Substances in Rye Grains
3.1. Grain Quality
3.2. Bioactive Substances in Rye Grains
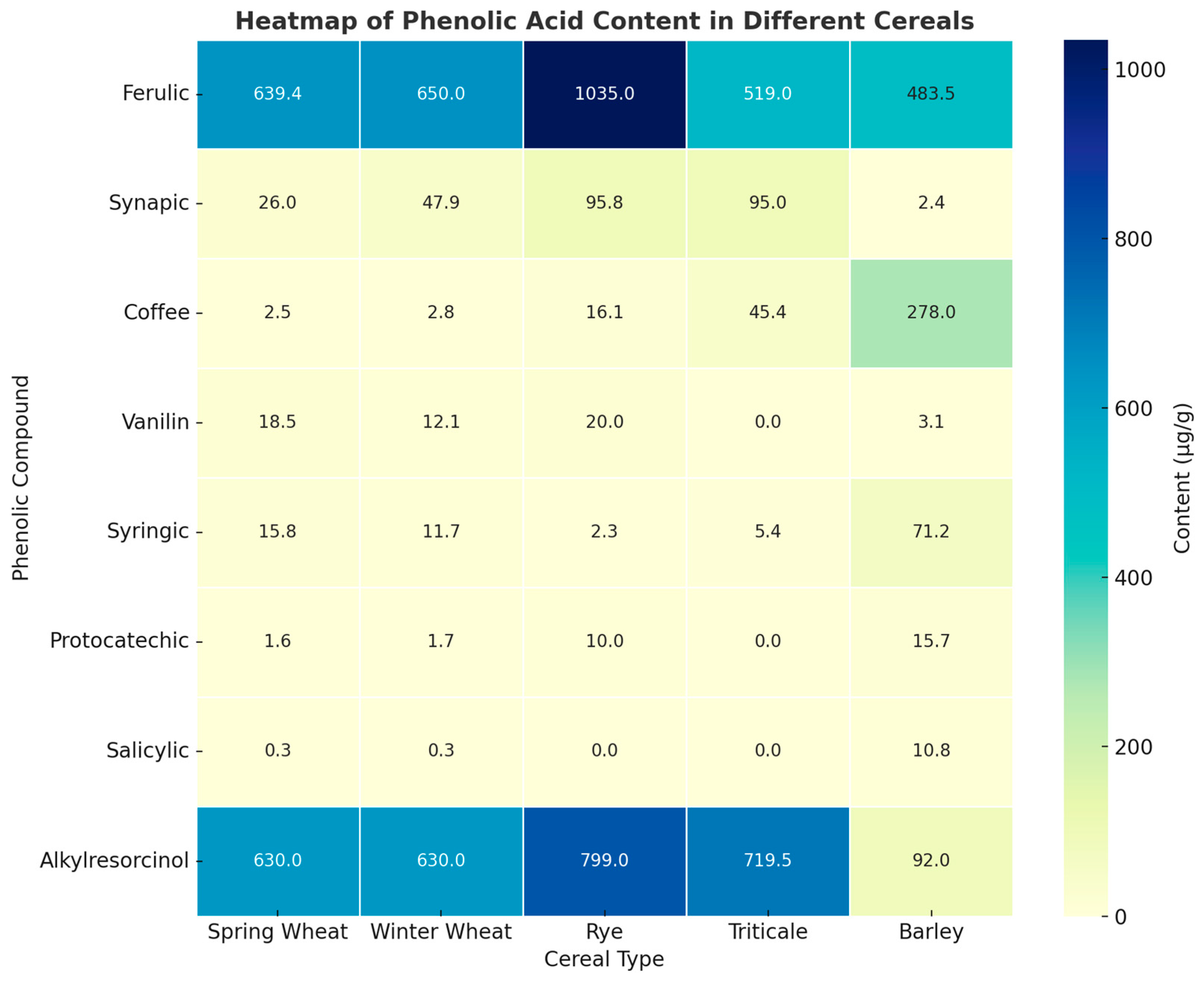
3.2.1. Phenolic Acids
3.2.2. Dietary Fiber
3.2.3. Tocols
3.2.4. Phytoestrogens
3.2.5. Alkylresorcinols
3.2.6. Benzoxazinoids
3.2.7. Carotenoids
3.2.8. Flavonoids
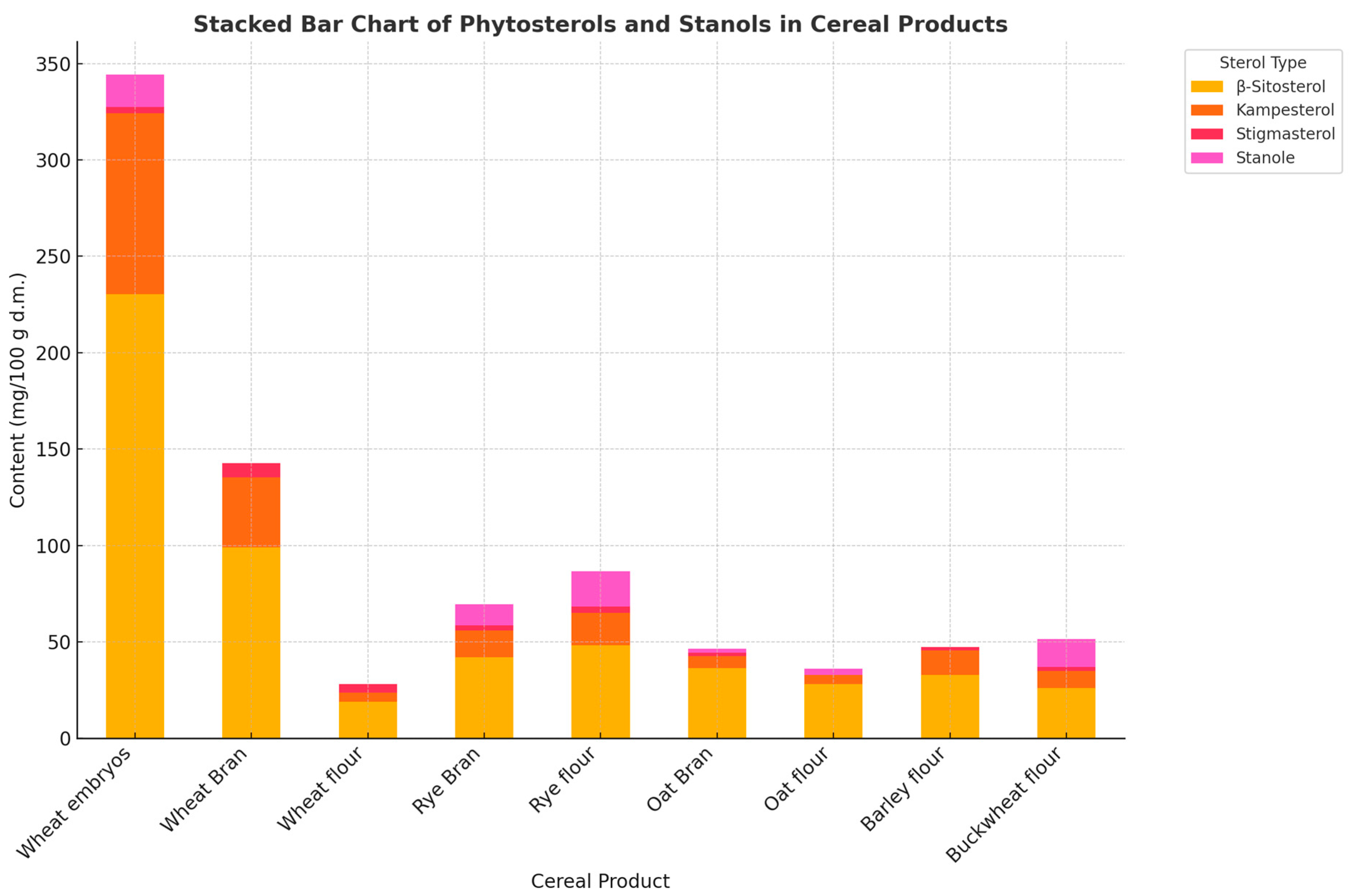
3.2.9. Phytosterols and Phytostanols
4. Alternative Use Scenario: Rye Grain Utilization as Biofuel
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sencer, H.A.; Hawkes, J.G. On the origin of cultivated rye. Biol. J. Linn. Soc. 1980, 13, 299–313. [Google Scholar] [CrossRef]
- Behre, K.-E. The history of rye cultivation in Europe. Veg. Hist. Archaeobotany 1992, 1, 141–156. [Google Scholar] [CrossRef]
- Yang, W.; Ma, C. Evolutionary Biology of Rye (Secale cereale): Domestication and Adaptation. Triticale Genomics and Genetics 2024, 15, 206–220. Available online: https://cropscipublisher.com/index.php/tgg/article/html/3979 (accessed on 16 May 2025).
- United States Department of Agriculture (USDA). Production: Rye. Available online: https://www.fas.usda.gov/data/production/commodity/0451000 (accessed on 16 May 2025).
- The World Ranking. Rye Production by Country. Available online: https://www.theworldranking.com/statistics/1068/rye-production-by-country/ (accessed on 16 May 2025).
- GUS. Rocznik Statystyczny Rolnictwa 2019 r; GUS: Warsaw, Poland, 2020. [Google Scholar]
- Poutanen, K.; Katina, K.; Heiniö, R.L. Rye. In Bakery Products Science and Technology; Wiley: Hoboken, NJ, USA, 2014; pp. 75–87. [Google Scholar] [CrossRef]
- Grabiński, J. Czynniki wpływające na cechy jakości żyta chlebowego. Stud. I Rap. IUNG-PIB 2015, 44, 181–191. [Google Scholar]
- Miedaner, T.; Lauenstein, S.; Lieberherr, B. Comparison of Hybrid Rye and Wheat for Grain Yield and Other Agronomic Traits under Less Favourable Environmental Conditions and Two Input Levels. Agriculture 2025, 15, 163. [Google Scholar] [CrossRef]
- Introspective Market Research. Rye Market—Global Industry Analysis and Forecast 2024–2032. Report ID: 17225; September 2024; 241+ Pages. Available online: https://introspectivemarketresearch.com/reports/rye-market/ (accessed on 16 May 2025).
- Królak, M.; Górska-Warsewicz, H.; Madra-Sawicka, M.; Rejman, K. Towards Sustainable Innovation in the Bakery Sector—An Example of Fibre-Enriched Bread. Sustainability 2022, 14, 2743. [Google Scholar] [CrossRef]
- Mucha, L. Applying the Theory of Planned Behavior to Examine the Customer Behavior Towards Craft Bakery Products: Evidence from Hungary. Humanit. Soc. Sci. Commun. 2024, 11, 1520. [Google Scholar] [CrossRef]
- Lindhauer, M.; Weipert, D. Die Qualitat der deutschen Roggenernte. Die Muhle+Mischfuttertechnik 2001, 138, 631–682. [Google Scholar]
- Hansen, H.B.; Møller, B.; Andersen, S.B.; Jørgensen, J.R.; Hansen, Å. Grain Characteristics, Chemical Composition, and Functional Properties of Rye (Secale cereale L.) as Influenced by Genotype and Harvest Year. J. Agric. Food Chem. 2004, 52, 2282–2291. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. 6—Rye. In Cereal Grains for the Food and Beverage Industries; Arendt, E.K., Zannini, E., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 220–238. [Google Scholar] [CrossRef]
- Targońska-Karasek, M.; Boczkowska, M.; Podyma, W.; Paśnik, M.; Niedzielski, M.; Rucińska, A.; Nowak-Życzynska, Z.; Rakoczy-Trojanowska, M. Multiplexed SSR and agronomic data used in an investigation of obsolete diversity of rye (Secale cereale L.). J. Appl. Genet. 2020, 61, 513–529. [Google Scholar] [CrossRef]
- Ponomareva, M.; Gorshkov, V.; Ponomarev, S.; Mannapova, G.; Askhadullin, D.; Askhadullin, D.; Gogoleva, O.; Meshcherov, A.; Korzun, V. Resistance to Snow Mold as a Target Trait for Rye Breeding. Plants 2022, 11, 2516. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, S.; Cacak-Pietrzak, G.; Fraś, A.; Jończyk, K.; Studnicki, M.; Wiśniewska, M.; Gzowska, M.; Salamon, A. Effect of Genotype and Environment on Yield and Technological and Nutrition Traits on Winter Rye Grain from Organic Production. Agriculture 2024, 14, 2249. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kostrzewska, M.K.; Marks, M.; Jastrzębski, W.P.; Treder, K.; Makowski, P. Crop Rotation Compared with Continuous Rye Cropping for Weed Biodiversity and Rye Yield. A Case Study of a Long-Term Experiment in Poland. Agronomy 2019, 9, 644. [Google Scholar] [CrossRef]
- Samuel, A.; Dines, L.; Finch, S.; Lane, G.P. Cereals. In Lockhart and Wiseman’s Crop Husbandry Including Grassland, 10th ed.; Samuel, A., Dines, L., Finch, S., Lane, G.P., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 349–394. [Google Scholar] [CrossRef]
- Dworakowski, T. Porównanie plonowania żyta z innymi gatunkami zbóż w stanowiskach po kłosowych. Pam. Puł. 2000, 128, 65–74. [Google Scholar]
- Butkevičienė, L.M.; Skinulienė, L.; Auželienė, I.; Bogužas, V.; Pupalienė, R.; Steponavičienė, V. The Influence of Long-Term Different Crop Rotations and Monoculture on Weed Prevalence and Weed Seed Content in the Soil. Agronomy 2021, 11, 1367. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V. Influence of Cover Crop, Tillage, and Crop Rotation Management on Soil Nutrients. Agriculture 2020, 10, 225. [Google Scholar] [CrossRef]
- Reeves, D.W. Cover Crops and Rotations. In Crops Residue Management; Hatfield, J.L., Stewart, B.A., Eds.; Advances in Soil Science; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 125–172. Available online: https://www.ars.usda.gov/ARSUserFiles/60100500/csr/ResearchPubs/reeves/reeves_94e.pdf?utm_source=chatgpt.com (accessed on 15 May 2025).
- Rezmerska-Piętka, J.; Łęgowiak, Z.; Radecki, A. Wpływ wieloletniego nawożenia mineralnego i organicznego na biologię dominujących chwastów w monokulturze żyta. Ann. Univ. Mariae Curie-Skłodowska Sect. E Agric. 2007, 62, 109–116. [Google Scholar] [CrossRef]
- Vakali, C.; Zaller, J.G.; Köpke, U. Reduced tillage in temperate organic farming: Effects on soil nutrients, nutrient content and yield of barley, rye and associated weeds. Renew. Agric. Food Syst. 2015, 30, 270–279. [Google Scholar] [CrossRef]
- Partlak, D.; Oliwa, T. Zmiany zachwaszczenia żyta ozimego w monokulturze pod wpływem zróżnicowanej uprawy pożniwnej i przedsiewnej. Fragm. Agron. 1997, 14, 43–49. [Google Scholar]
- Szczygielski, M.; Snarska, K. Zdrowotność i plonowanie wybranych odmian żyta ozimego uprawianego w dwóch technologiach. Prog. Plant Prot./Post. Ochr. Rośl. 2004, 44, 1135–1137. [Google Scholar]
- Pearsons, K.A.; Omondi, E.C.; Heins, B.J.; Zinati, G.; Smith, A.; Rui, Y. Reducing Tillage Affects Long-Term Yields but Not Grain Quality of Maize, Soybeans, Oats, and Wheat Produced in Three Contrasting Farming Systems. Sustainability 2022, 14, 631. [Google Scholar] [CrossRef]
- Podleśna, A.; Narolski, B. Efektywność Plonotwórcza Siarki i Azotu w Produkcji Żyta Jarego; IUNG-PIB: Puławy, Poland, 2021; ISBN 978-83-7562-368-0. [Google Scholar]
- Sułek, A.; Cacak-Pietrzak, G.; Studnicki, M.; Grabiński, J.; Nieróbca, A.; Wyzińska, M.; Różewicz, M. Influence of Nitrogen Fertilisation Level and Weather Conditions on Yield and Quantitative Profile of Anti-Nutritional Compounds in Grain of Selected Rye Cultivars. Agriculture 2024, 14, 418. [Google Scholar] [CrossRef]
- Grabiński, J.; Sułek, A.; Wyzińska, M.; Stuper-Szablewska, K.; Cacak-Pietrzak, G.; Nieróbca, A.; Dziki, D. Impact of Genotype, Weather Conditions and Production Technology on the Quantitative Profile of Anti-Nutritive Compounds in Rye Grains. Agronomy 2021, 11, 151. [Google Scholar] [CrossRef]
- Szara, E.; Mercik, S.; Sosulski, T. Bilans fosforu w trzech systemach nawożenia. Agron. Sci. 2004, 59, 599–606. [Google Scholar]
- Fotyma, E. Efektywność nawożenia azotem podstawowych roślin uprawy polowej. Fragm. Agron. 1997, 1, 46–65. [Google Scholar]
- Liszewski, M. Nawożenie azotem a wysokość i jakość plonów kilku odmian żyta ozimego. Zesz. Nauk. Akad. Rol. We Wrocławiu. Rolnictwo 1994, 60, 111–119. [Google Scholar]
- Klikocka, H.; Cybulska, M. Wpływ nawożenia azotem i siarką na plon ziarna i cechy jakościowe pszenicy jarej. Agron. Sci. 2020, 75, 65–74. [Google Scholar] [CrossRef]
- Bazhenov, M.; Litvinov, D.; Karlov, G.; Divashuk, M. Evaluation of phosphate rock as the only source of phosphorus for the growth of tall and semi-dwarf durum wheat and rye plants using digital phenotyping. PeerJ 2023, 11, e15972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-Y.; Zhang, X.-L.; Zhao, W.-F.; Zhao, S.-P.; Liu, G.-L.; Siddique, K.H.M. Root Distribution, Agronomic Performance, and Phosphorus Utilization in Wheat as Mediated by Phosphorus Placement under Rainfed Coastal Saline Conditions. Agronomy 2023, 13, 2700. [Google Scholar] [CrossRef]
- Stefanova-Dobreva, S.; Muhova, A. Thousand kernel weight of durum wheat (Triticum durum Desf.) over a 30-year period as affected by mineral fertilization and weather conditions. Bulg. J. Agric. Sci. 2024, 30, 247–253. Available online: https://www.agrojournal.org/30/02-07.pdf (accessed on 16 May 2025).
- Wach, D. Potas w glebie i roślinie—Aktualny stan wiedzy. Stud. I Rap. IUNG-PIB 2021, 63, 65–78. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Xue, Y.-F.; Li, Y.; Xue, X.-J.; Zhang, Z.-J.; Jin, Y.-X.; Zuo, Y.-M.; Wang, L.; Sun, C.-X. Biofortification of Different Maize Cultivars with Zinc, Iron and Selenium by Foliar Fertilizer Applications. Front. Plant Sci. 2023, 14, 1144514. [Google Scholar] [CrossRef]
- Sajedi, N.A.; Madani, H.; Naderi, A.; Nazeri, P.; Fazeli, F.; Miraghazadeh, A. The Effects of Selenium and Other Micronutrients on the Antioxidant Activities and Yield of Corn (Zea mays L.) under Drought Stress. Physiol. Mol. Biol. Plants 2011, 17, 215–222. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Grabiński, J.; Jaśkiewicz, B.; Podolska, G.; Sułek, A. Terminy siewu w uprawie zbóż. Stud. I Rap. IUNG PIB 2007, 9, 37–45. [Google Scholar]
- Lawson, A.; Cogger, C.; Bary, A.; Fortuna, A.-M. Influence of Seeding Ratio, Planting Date, and Termination Date on Rye-Hairy Vetch Cover Crop Mixture Performance under Organic Management. PLoS ONE 2015, 10, e0129597. [Google Scholar] [CrossRef]
- Yağmur, M. Effects of Seeding Rates and Sowing Times on Grain Yield and Yield Components in Rye (Secale cereale L.) Under Dry Condition. Manas. J. Agric. Vet. Life Sci. 2023, 13, 9–16. [Google Scholar] [CrossRef]
- Nye, C. Effects of Planting Date and Seeding Rate on Hybrid Winter Rye (Secale cereale L.) Grain Yield Across the U.S. Master’s Thesis, Ohio State University, Columbus, OH, USA, 2023. Available online: https://rave.ohiolink.edu/etdc/view?acc_num=osu1701196176826016 (accessed on 16 May 2025).
- Szuleta, E.; Phillips, T.; Knott, C.A.; Lee, C.D.; Van Sanford, D.A. Influence of Planting Date on Winter Rye Performance in Kentucky. Agronomy 2022, 12, 2887. [Google Scholar] [CrossRef]
- Altai, D.S.K.; Noaema, A.H.; Alhasany, A.R.; Hadházy, Á.; Mendler-Drienyovszki, N.; Abido, W.A.E.; Magyar-Tábori, K. Effect of Sowing Date on Some Agronomical Characteristics of Rye Cultivars in Iraq. Agronomy 2024, 14, 1995. [Google Scholar] [CrossRef]
- Grabiński, J.; Mazurek, J. Plonowanie żyta mieszańcowego odmiany Nawid w warunkach rzadkich siewów. Biul. Inst. Hod. Aklim. Roślin 2002, 223–224, 137–143. [Google Scholar]
- Blecharczyk, A.; Małecka, I.; Piechota, T. Wpływ płodozmianu, monokultury i nawożenia na zachwaszczenie żyta ozimego. Zesz. Probl. Postępów Nauk. Rol. 2003, 490, 17–23. [Google Scholar]
- Rola, H.; Sumisławska, J.; Marczewski, K. The Effect of Sulfonylurea Herbicides on Grain Yield and Technological Quality of Winter Rye Cultivars. J. Plant Prot. Res. 2009, 49, 178–184. [Google Scholar] [CrossRef]
- Xu, J.; Smith, S.; Smith, G.; Wang, W.; Li, Y. Glyphosate Contamination in Grains and Foods: An Overview. Food Control 2019, 106, 106710. [Google Scholar] [CrossRef]
- Kurowski, T.P.; Majchrzak, B. Patogeniczność i szkodliwość wybranych gatunków z rodzaju Fusarium dla żyta, pszenicy i owsa. Rocz. Akad. Rol. W Poznaniu. Ogrod. 2000, 30, 61–68. [Google Scholar]
- Burgieł, Z.J.; Klima, K. Skuteczność nowych zapraw nasiennych w ochronie żyta przed pleśnią śniegową. Prog. Plant Prot./Post. Ochr. Rośl. 1999, 39, 828–830. [Google Scholar]
- Chongo, G.; Gossen, B.D.; Kutcher, H.R.; Gilbert, J.; Turkington, T.K.; Fernandez, M.R.; Mclaren, D. Reaction of seedling roots of 14 crop species to Fusarium graminearum from wheat heads. Can. J. Plant Pathol. 2001, 23, 132–137. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Singh, R.P.; Sabraoui, A.; Ginkel, M.V.; Huerta-Espino, J.; Amri, A.; El-Bouhssini, M. Resistance to Insect Pests in Wheat—Rye and Aegilops speltoides Tausch Translocation and Substitution Lines. Euphytica 2019, 215, 123. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, H.; Wang, X.; Kang, Z. Prevalent Pest Management Strategies for Grain Aphids: Opportunities and Challenges. Front. Plant Sci. 2022, 12, 790919. [Google Scholar] [CrossRef]
- Łozowicka, B.; Miciński, J.; Zwierzchowski, G.; Kowalski, I.M.; Szarek, J. Monitoring Study of Pesticide Residues in Cereals and Foodstuff from Poland. Pol. J. Environ. Stud. 2012, 21, 1703–1712. Available online: https://www.pjoes.com/pdf-88920-22779?filename=22779.pdf (accessed on 18 May 2025).
- Nyarko, S.K.; Akyereko, Y.G.; Akowuah, J.O.; Wireko-Manu, F.D. Comparative Studies on Grain Quality and Pesticide Residues in Maize Stored in Hermetic and Polypropylene Storage Bags. Agriculture 2021, 11, 772. [Google Scholar] [CrossRef]
- McLeod, J.G.; Clarke, J.M. Effect of harvest time and drying method on quality and grade of winter rye. Can. J. Plant Sci. 1987, 67, 417–424. [Google Scholar] [CrossRef]
- Ronga, D.; Dal Prà, A.; Immovilli, A.; Ruozzi, F.; Davolio, R.; Pacchioli, M.T. Effects of Harvest Time on the Yield and Quality of Winter Wheat Hay Produced in Northern Italy. Agronomy 2020, 10, 917. [Google Scholar] [CrossRef]
- Ryniecki, A.; Szymański, P. (Eds.) Dobrze Przechowane Zboże; MR INFO oraz Towarzystwo Umiejętności Rolniczych w Poznaniu: Poznań, Poland, 2002. [Google Scholar]
- European Commission. The European Green Deal; COM(2019) 640 Final; European Commission: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52019DC0640 (accessed on 16 May 2025).
- European Commission. EU Biodiversity Strategy for 2030: Bringing Nature Back into Our Lives; COM(2020) 380 Final; European Commission: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0380 (accessed on 16 May 2025).
- European Commission. EU Soil Strategy for 2030: Reaping the Benefits of Healthy Soils for People, Food, Nature and Climate; COM(2021) 699 Final; European Commission: Brussels, Belgium, 2021; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52021DC0699 (accessed on 16 May 2025).
- Eurostat. Organic Farming Area in the EU Increased to 16.9 Million Hectares in 2022. 2024. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20240619-3 (accessed on 16 May 2025).
- Eurostat. Developments in Organic Farming—Statistics Explained. 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Developments_in_organic_farming (accessed on 16 May 2025).
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; COM(2020) 381 Final; European Commission: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0381 (accessed on 16 May 2025).
- Moore, E.B.; Wiedenhoeft, M.H.; Kaspar, T.C.; Cambardella, C.A. Rye Cover Crop Effects on Soil Quality in No-Till Corn Silage–Soybean Cropping Systems. Soil Sci. Soc. Am. J. 2014, 78, 968–976. [Google Scholar] [CrossRef]
- Reberg-Horton, S.C.; Burton, J.D.; Danehower, D.A.; Ma, G.Y.; Monks, D.W.; Murphy, J.P.; Ranells, N.N.; Williamson, J.D.; Creamer, N.G. Changes over Time in the Allelochemical Content of Ten Cultivars of Rye (Secale cereale L.). J. Chem. Ecol. 2005, 31, 179–193. [Google Scholar] [CrossRef]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil Fertility and Biodiversity in Organic Farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- Schatzmayr, G.; Streit, E. Global Occurrence of Mycotoxins in the Food and Feed Chain: Facts and Figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Bertholdsson, N.O. Early Vigour and Allelopathy—Two Useful Traits for Enhanced Barley and Wheat Competitiveness against Weeds. Weed Res. 2005, 45, 94–102. [Google Scholar] [CrossRef]
- Kozłowska, M.; Kosewska, A. Wpływ kąkolu polnego (Agrostemma githago L.) na zdrowie ludzi i zwierząt. Med. Wet. 2009, 65, 203–206. [Google Scholar]
- Wilson, C.E.; Castro, K.L.; Thurston, G.B.; Sissons, A. Pathway Risk Analysis of Weed Seeds in Imported Grain: A Canadian Perspective. NeoBiota 2016, 30, 49–74. Available online: https://neobiota.pensoft.net/article/7502/ (accessed on 16 May 2025).
- Berbeć, A.K.; Staniak, M.; Feledyn-Szewczyk, B.; Kocira, A.; Stalenga, J. Organic but Also Low-Input Conventional Farming Systems Support High Biodiversity of Weed Species in Winter Cereals. Agriculture 2020, 10, 413. [Google Scholar] [CrossRef]
- Haliniarz, M.; Gawęda, D.; Nowakowicz-Dębek, B.; Najda, A.; Chojnacka, S.; Łukasz, J.; Wlazło, Ł.; Różańska-Boczula, M. Evaluation of the Weed Infestation, Grain Health, and Productivity Parameters of Two Spelt Wheat Cultivars Depending on Crop Protection Intensification and Seeding Densities. Agriculture 2020, 10, 229. [Google Scholar] [CrossRef]
- Deveikyte, I.; Semaskiene, R.; Leistrumaite, A. The Competition between Cereals and Weeds under the Conditions of Organic Agriculture. Zemdirb.-Agric. 2008, 95, 3–15. [Google Scholar]
- Bernhoft, A.; Wang, J.; Leifert, C. Effect of Organic and Conventional Cereal Production Methods on Fusarium Head Blight and Mycotoxin Contamination Levels. Agronomy 2022, 12, 797. [Google Scholar] [CrossRef]
- Brodal, G.; Hofgaard, I.S.; Eriksen, G.S.; Bernhoft, A.; Sundheim, L. Mycotoxins in Organically versus Conventionally Produced Cereal Grains and Some Other Crops in Temperate Regions. World Mycotoxin J. 2016, 9, 755–770. [Google Scholar] [CrossRef]
- Wang, J.; Sufar, E.K.; Bernhoft, A.; Seal, C.; Rempelos, L.; Hasanaliyeva, G.; Zhao, B.; Iversen, P.O.; Baranski, M.; Volakakis, N.; et al. Mycotoxin Contamination in Organic and Conventional Cereal Grain and Products: A Systematic Literature Review and Meta-Analysis. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13363. [Google Scholar] [CrossRef]
- Borkowska, B.; Banach, D. Ocena wybranych cech fizykochemicznych pszenicy i żyta z północnego i południowego regionu Polski. Rocz. Nauk. Stowarzyszenia Ekon. Rol. Agrobiznesu 2018, 20, 17–22. [Google Scholar]
- PN-R-74108:1996; Zboża—Żyto konsumpcyjne. Polski Komitet Normalizacyjny: Warsaw, Poland, 1996.
- Boyd, N.S.; Brennan, E.B.; Smith, R.F.; Yokota, R. Effect of Seeding Rate and Planting Arrangement on Rye Cover Crop and Weed Growth. Agron. J. 2009, 101, 47–51. [Google Scholar] [CrossRef]
- Wyniki Porejestrowych Doświadczeń Odmianowych w Województwie Małopolskim. 2019. Available online: https://coboru.gov.pl/PlikiWynikow/39_2020_IN_1_.pdf (accessed on 18 May 2025).
- Wyniki Porejestrowych Doświadczeń Odmianowych w Województwie Małopolskim. 2023. Available online: https://coboru.gov.pl/PlikiWynikow/39_2024_IN_1_.pdf (accessed on 18 May 2025).
- Salamon, A. Ocena wartości technologicznej ziarna żyta z krajowych zbiorów 2024 roku. Przegląd Zbożowo-Młynarski 2024, 6, 2. [Google Scholar] [CrossRef]
- Linina, A.; Kunkulberga, D.; Kronberga, A.; Locmele, I. Winter Rye Grain Quality of Hybrid and Population Cultivars. Agron. Res. 2019, 17, 1956–1965. [Google Scholar] [CrossRef]
- Król, B.; Grzelak, K. Qualitative and quantitative composition of fructooligosaccharides in bread. Eur. Food Res. Technol. 2006, 223, 755–758. [Google Scholar] [CrossRef]
- Gąsiorowski, H. (Ed.) Żyto: Chemia i Technologia; PWRiL: Poznań, Poland, 1994. [Google Scholar]
- Fomsgaard, I.S.; Mortensen, A.G.; Holm, P.B.; Gregersen, P. Use of Benzoxazinoids-Containing Cereal Grain Products for Health-Improving Purposes. International Patent WO2009115093A1, 24 September 2009. [Google Scholar]
- Rosenfeld, M.J.; Forsberg, S.R. Compounds for Use in Weight Loss and Appetite Suppression in Humans. U.S. Patent 7,524,877 B2, 28 April 2009. [Google Scholar]
- Majewska, M.; Zajac, K.; Zemelka, M.; Wrotek, S.; Brzozowska, A.; Skwarlo-Sonta, K. Influence of melatonin and its precursor L-tryptophan on Th1 dependent contact hypersensitivity. J. Physiol. Pharmacol. 2007, 58 (Suppl. S6), 125–132. [Google Scholar] [PubMed]
- Zieliński, H.; Achremowicz, B.; Przygocki, M. Przeciwutleniacze ziarniaków zbóż. Żywność Nauka Technol. Jakość 2012, 1, 5–26. [Google Scholar]
- Harasym, J. Wybrane substancje bioaktywne obecne w ziarnie żyta. Przegląd Zbożowo-Młynarski 2015, 4, 5–7. [Google Scholar]
- Thompson, L.U. Potential health benefis of whole grains and their components. Contemp. Nutr. 1992, 17, 1–2. [Google Scholar]
- Liang, H.; Zhou, J.; Chen, C. The aleurone layer of cereal grains: Development, genetic regulation, and breeding applications. Plant Commun. 2025, 6, 101283. [Google Scholar] [CrossRef]
- Rakha, A.; Åman, P.; Andersson, R. Characterisation of dietary fibre components in rye products. Food Chem. 2010, 119, 859–867. [Google Scholar] [CrossRef]
- Järvan, M.; Lukme, L.; Tupits, I.; Akk, A. The productivity, quality and bread- making properties of organically and conventionally grown winter rye. Zemdirb.-Agric. 2018, 105, 323–330. [Google Scholar] [CrossRef]
- Andersson, U.; Rosén, L.; Östman, E.; Ström, K.; Wierup, N.; Björck, I.; Holm, C. Metabolic effects of whole grain wheat and whole grain rye in the C57BL/6J mouse. Nutrition 2010, 26, 230–239. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B. Selen w Roślinnych Surowcach Leczniczych, Zawartość, Rozmieszczenie i Wzajemne Relacje z Innymi Pierwiastkami. Ph.D. Thesis, Akademia Medyczna w Gdańsku, Gdańsk, Poland, 2008. [Google Scholar]
- Eurola, M.; Ekholm, P.; Ylinen, M.; Koivistoinen, P.; Varo, P. Effects of Selenium Fertilization on the Selenium Content of Cereal Grains, Flour, and Bread Produced in Finland. Cereal Chem. 1990, 67, 334–337. [Google Scholar]
- Kariluoto, S.; Vahteristo, L.; Salovaara, H.; Katina, K.; Liukkonen, K.H.; Piironen, V. Effect of Baking Method and Fermentation on Folate Content of Rye and Wheat Breads. Cereal Chem. 2004, 81, 134–139. [Google Scholar] [CrossRef]
- Hefni, M.; Witthöft, C. Effect of germination and subsequent oven-drying on folate content in different wheat and rye cultivars. J. Cereal Sci. 2012, 56, 74–378. [Google Scholar] [CrossRef]
- Németh, R.; Tömösközi, S. Rye: Current state and future trends in research and applications. Acta Aliment. 2021, 50, 620–640. [Google Scholar] [CrossRef]
- Piłat, B.; Zadernowski, R. Substancje bioaktywne pozytywne i negatywne skutki dodawania do żywności. Przemysł Spożywczy 2017, 71, 24–27. [Google Scholar] [CrossRef]
- Świderski, F.; Kolanowski, W. Żywność funkcjonalna i dietetyczna. In Żywność Wygodna i Żywność Funkcjonalna; WNT: Warsaw, Poland, 1999; p. 28. [Google Scholar]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and Environmental Variation in Phenolic Content, Phenolic Acid Composition, and Antioxidant Activity of Hard Spring Wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Zieliński, H.; Michalska, A.; Amigo-Benavent, M.; del Castillo, M.D.; Piskula, M.K. Changes in protein quality and antioxidant properties of buckwheat seeds and groats inducted by roasting. J. Agric. Food Chem. 2009, 57, 771–776. [Google Scholar] [CrossRef]
- Karamać, M.; Amarowicz, R.; Weider, S. Anioxidant activity of rye caryopses and embroyes extracts. Czech J. Food Sci. 2001, 20, 209–214. [Google Scholar] [CrossRef]
- Rybka, K.; Sitarska, J.; Raczyńska-Bojanowska, K. Feluric acid in rye and wheat grain and grain dietary fiber. Cereal Chem. 1993, 70, 55–59. [Google Scholar]
- Lempereur, I.; Rouaur, X.; Abecassis, J. Arabinoxylan and ferulic acid variation in durum wheat (Triticum durum) and distribution in miling fractions. J. Cereal Sci. 1997, 25, 103–107. [Google Scholar] [CrossRef]
- Zieliński, H.; Ceglińska, A. Wpływ obróbki mechanicznej na zawartość przeciwutleniaczy w ziarniakach zbóż. In Przeciwutleniacze w Żywności—Aspekty Zdrowotne, Technologiczne, Molekularne i Analityczne; Gajek, W., Ed.; WNT: Warsaw, Poland, 2007; pp. 467–470. [Google Scholar]
- Czaban, J.; Sułek, A.; Pecio, Ł.; Żuchowski, J.; Podolska, G. Effect of genotype and crop management systems on phenolic acid content in winter wheat grain. J. Food Agric. Environ. 2013, 11, 1201–1206. [Google Scholar]
- Covas, M.I. Bioactive effects of olive oil phenolic compounds in humans: Reduction of heart disease factors and oxidative damage. Inflammopharmacology 2008, 16, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Atkinson, C.; Liggins, J.; Bluck, L.; Coward, A. Phyto-oestrogens: Where are we now? Br. J. Nutr. 1998, 79, 393–406. [Google Scholar] [CrossRef]
- Zupfer, J.K.; Churchill, K.E.; Rasmusson, D.C.; Gary Fulcher, R. Variation in Ferulic Acid Concentration among Diverse Barley Cultivars Measured by HPLC and Microspectrophotometry. J. Agric. Food Chem. 1998, 46, 1350–1354. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Christensen, L.P.; Meyer, A.S.; Hansen, A. Content of phenolic acids and ferulic acid dehydrodimers in 17 rye (Secale cereale L.) varieties. J. Agric. Food Chem. 2000, 48, 2837–2842. [Google Scholar] [CrossRef]
- Weidner, S.; Frączek, E.; Amarowicz, R.; Abe, S. Alternations in phenolic acids content in developing rye grains in normal environment and during enforced dehydration. Acta Physiol. Plant. 2001, 23, 475–482. [Google Scholar] [CrossRef]
- Quinde-Axtell, Z.; Baik, B.K. Phenolic compounds of barley grain and their implication in food product discoloration. J. Agric Food Chem. 2006, 54, 9978–9984. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Aura, A.M.; Vuorela, S.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Rye phenolics in nutrition and health. J. Cereal Sci. 2009, 49, 323–336. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Czechowska, K.; Bartoń, H.; Fołta, M. The impact of extrusion on the content of poliphenols and antioxidant activity of rye grains (Secale cereal L.). Acta Sci. Pol. Technol. Aliment. 2010, 9, 319–330. [Google Scholar]
- Boros, D. Alkilorezorcynole ziarna zbóż—Ich znaczenie w żywności i paszy. Biul. Inst. Hod. Aklim. Roślin 2015, 277, 7–20. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2016, 25, 148–161. [Google Scholar] [CrossRef]
- Sułek, A. Właściwości prozdrowotne wybranych substancji bioaktywnych obecnych w ziarnie zbóż. Apl. Teoretyczne Teoretyczne Probl. Przemyśle Rolno-Spożywczym—Postęp Nauk.-Technol. 2016, 465, 317–328. [Google Scholar]
- Antonenko, K.; Kreicbergs, V.; Cinkmanis, I.; Cinkmanis, I. Influence of selenium, copper and zinc on phenolic compounds in rye malt. In Proceedings of the 11th Baltic Conference on Food Science and Technology “Food Science and Technology in a Changing World”, Jelgava, Latvia, 27–28 April 2017. Conference Paper. [Google Scholar] [CrossRef]
- Yang, X.-J.; Dang, B.; Fan, M.-T. Free and Bound Phenolic Compound Content and Antioxidant Activity of Different Cultivated Blue Highland Barley Varieties from the Qinghai-Tibet Plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef]
- Yoshida, A.; Sonoda, K.; Nogata, Y.; Nagamine, T.; Sato, M.; Oki, T.; Hashimoto, S.; Ohta, H. Determination of Free and Bound Phenolic Acids, and Evaluation of Antioxidant Activities and Total Polyphenolic Contents in Selected Pearled Barley. Food Sci. Technol. Res. 2010, 16, 215–224. [Google Scholar] [CrossRef]
- Hernanz, D.; Nuñez, V.; Sancho, A.I.; Faulds, C.B.; Williamson, G.; Bartolomé, B.; Gómez-Cordovés, C. Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley. J. Agric. Food Chem. 2001, 49, 4884–4888. [Google Scholar] [CrossRef]
- Kaszuba, J.; Kapusta, I.; Posadzka, Z. Content of Phenolic Acids in the Grain of Selected Polish Triticale Cultivars and Its Products. Molecules 2021, 26, 562. [Google Scholar] [CrossRef]
- Jin, H.M.; Dang, B.; Zhang, W.G.; Zheng, W.C.; Yang, X.J. Polyphenol and Anthocyanin Composition and Activity of Highland Barley with Different Colors. Molecules 2022, 27, 3411. [Google Scholar] [CrossRef] [PubMed]
- Jańczak-Pieniążek, M.; Horvat, D.; Viljevac Vuletić, M.; Kovačević Babić, M.; Buczek, J.; Szpunar-Krok, E. Antioxidant Potential and Phenolic Acid Profiles in Triticale Grain under Integrated and Conventional Cropping Systems. Agriculture 2023, 13, 1078. [Google Scholar] [CrossRef]
- Zhang, W.; Lan, Y.; Dang, B.; Zhang, J.; Zheng, W.; Du, Y.; Yang, X.; Li, Z. Polyphenol Profile and In Vitro Antioxidant and Enzyme Inhibitory Activities of Different Solvent Extracts of Highland Barley Bran. Molecules 2023, 28, 1665. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Gul, P.; Tayyab Rashid, M.; Li, Q.; Liu, K. Composition of Whole Grain Dietary Fiber and Phenolics and Their Impact on Markers of Inflammation. Nutrients 2024, 16, 1047. [Google Scholar] [CrossRef]
- Jasińska, I.; Kołodziejczyk, P.; Michniewicz, J. Ziarno żyta jako potencjalne źródło składników prozdrowotnych w diecie. Żywność Nauka Technol. Jakość 2006, 2, 85–92. [Google Scholar]
- Härkönen, H.; Pessa, E.; Suortti, T.; Poutanen, K. Distribution and some properties of cell wall polysaccharides in rye milling fractions. J. Cereal Sci. 1997, 26, 95–104. [Google Scholar] [CrossRef]
- Nilsson, M.; Aman, P.; Harkonen, H.; Hallmans, G.; Knudsen, K.E.B.; Mazur, W.; Adlercreutz, H. Content of nutrients and lignans in roller milled fractions of rye. J. Sci. Food Agric. 1997, 73, 143–148. [Google Scholar] [CrossRef]
- Badowski, P.; Urbanek-Karłowska, B. Fitoestrogeny—Występowanie w żywności. Rocz. Państwowego Zakładu Hig. 2001, 52, 203–212. [Google Scholar]
- Knight, D.C.; Eden, J.A. Phytoestrogens—A short review. Maturitas 1995, 22, 167–175. [Google Scholar] [CrossRef]
- Kozubek, A.; Pietr, S.; Czerwonka, A. Alkyloresorcinols are abundant lipid component in Azotobacter and Pseudomonas strains. J. Bacteriol. 1996, 178, 4027–4030. [Google Scholar] [CrossRef]
- Wenkert, E.; Loeser, E.M.; Mahapatra, N.; Schenker, F.; Wilson, E.M. Wheat grain phenols. J. Org. Chem. 1964, 29, 435–439. [Google Scholar] [CrossRef]
- Wieringa, G.W. On Occurrence of Growth Inhibiting Substances in Rye. Institute for Storage and Processing of Agricultural Produce. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 1967; p. 68. Available online: https://core.ac.uk/download/pdf/29391882.pdf (accessed on 16 May 2025).
- Tłuścik, F.; Kozubek, A.; Mejbaum-Katzenellebogen, W. Alkylresorcinols in rye (Secale cereal L.) grains. VI. Colorimetric micromethod for the determination of alkylresorcinols with the use of diazonium salt. Fast Blue B. Acta Soc. Bot. Pol. 1981, 50, 645–651. [Google Scholar] [CrossRef][Green Version]
- Evans, L.E.; Dedio, W.; Hill, R.D. Variability in the alkylresorcinol content of rye grain. Can. J. Plant Sci. 1973, 53, 485–488. [Google Scholar] [CrossRef]
- Sałek, M. Oznaczanie zawartości 5-alkilorezorcyn w ziarnie i produktach przemiału żyta. Rocz. Państwowego Zakładu Hig. 1978, 29, 205–211. [Google Scholar]
- Gajek, W. (Ed.) Przeciwutleniacze w Żywności—Aspekty Zdrowotne, Technologiczne, Molekularne i Analityczne; WNT: Warsaw, Poland, 2007. [Google Scholar]
- Konopka, I.; Czaplicki, S.; Rotkiewicz, D. Differences in content and composition of fee lipids and carotenoids in flour of spring and winter wheat cultivated in Poland. Food Chem. 2006, 95, 290–300. [Google Scholar] [CrossRef]
- Gąsiorowski, H. (Ed.) Jęczmień—Chemia i Technologia; PWRiL: Poznań, Poland, 1997. [Google Scholar]
- Lagarda, M.J. Analysis of phytosterols in food. J. Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Hallikainen, M.; Nissinen, M.; Miettinen, T.A. The effect of very high daily plant stanol ester intake on serum lipids, carotenoids, and fat-soluble vitamins. Clin. Nutr. 2010, 29, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Miettinen, T.A. Plants sterols in nutrition. Scand. J. Nutr. 2000, 44, 155–157. [Google Scholar]
- Bryngelsson, S.; Johnsson, M.; Normen, L.; Dutta, P.; Andersson, H. Plant sterols in cereals products. In Bioactive Inositol Phosphates and Phytosterols in Foods—COST 916, Proceedings of the Second Workshop, Goteborg, Sweden, 23–25 October 1997; Office for Official Publications of the European Communities: Luxembourg, 1999; pp. 131–134. [Google Scholar]
- Parke, D.V. Nutritional antioxidants and disease prevention: Mechanisms of action. In Antioxidants in Human Health and Disease; Biddles Ltd.: Wallingford, UK, 1999; pp. 1–13. [Google Scholar]
- Quilez, J.; Garcia-Lorda, P.; Salas-Salvado, J. Potential uses and benefits of phytosterols in diet: Present situation and future directions. Clin. Nutr. 2003, 22, 343–351. [Google Scholar] [CrossRef]
- Malone, R.W.; Obrycki, J.F.; Karlen, D.L.; Ma, L.; Kaspar, T.C.; Jaynes, D.B.; Parkin, T.B.; Lence, S.H.; Feyereisen, G.W.; Fang, Q.X.; et al. Harvesting fertilized rye cover crop: Simulated revenue, net energy, and drainage nitrogen loss. Agric. Environ. Lett. 2018, 3, 170041. [Google Scholar] [CrossRef]
- Żabiński, A.; Sadowska, U.; Wcisło, G. Ciepło spalania ziarniaków zbóż o obniżonych cechach jakościowych. Inżynieria Rol. 2012, 16, 353–359. [Google Scholar]
- Esteves, B.; Sen, U.; Pereira, H. Influence of Chemical Composition on Heating Value of Biomass: A Review and Bibliometric Analysis. Energies 2023, 16, 4226. [Google Scholar] [CrossRef]
- Keppel, A.; Finnan, J.; Rice, B.; Owende, P.; MacDonnell, K. Cereal grain combustion in domestic boilers. Biosyst. Eng. 2013, 115, 136–143. [Google Scholar] [CrossRef]
- Darguža, M.; Gaile, Z. Productivity of crop rotation measured as energy produced by included plants: A review. Res. Rural Dev. 2018, 2, 20–27. [Google Scholar]
- Tobiasz-Salach, R.; Stadnik, B.; Bajcar, M. Oat as a potential source of energy. Energies 2023, 16, 6019. [Google Scholar] [CrossRef]
- Głowacki, S.; Salamon, A.; Sojak, M.; Tulej, W.; Bryś, A.; Hutsol, T.; Salamon, M.; Kukharets, S.; Janaszek-Mańkowska, M. The use of brewer’s spent grain after beer production for energy purposes. Materials 2022, 15, 3703. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; DiMarco, K.; Richard, T.; Lynd, L. Winter rye as a bioenergy feedstock: Impact of crop maturity on composition, biological solubilization and potential revenue. Biotechnol. Biofuels 2015, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Herbstritt, S.; Richard, T.L.; Lence, S.H.; Wu, H.; O’brien, P.L.; Emmett, B.D.; Kaspar, T.C.; Karlen, D.L.; Kohler, K.; Malone, R.W. Rye as an energy cover crop: Management, forage quality, and revenue opportunities for feed and bioenergy. Agriculture 2022, 12, 1691. [Google Scholar] [CrossRef]
- Mattison, E.H.A.; Norris, K. Intentions of UK farmers toward biofuel crop production. Environ. Sci. Technol. 2007, 41, 4995–5000. [Google Scholar] [CrossRef]
- Barrett, E.M.; Amoutzopoulos, B.; Batterham, M.; Ray, S.; Beck, E.J. Whole grain intake compared with cereal fibre intake in association to cvd risk factors: A cross-sectional analysis of the national diet and nutrition survey (UK). Public Health Nutr. 2020, 23, 1392–1403. [Google Scholar] [CrossRef]
- Dyganova, R.; Gordeeva, A. Greening by grain waste recycling in cereal industry. E3S Web Conf. 2020, 161, 01098. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Cotana, F.; Nicolini, A.; Coccia, V.; Petrozzi, A.; Formica, A.; Bertini, A. Characterization of various biomass feedstock suitable for small-scale energy plants as preliminary activity of biocheaper project. Sustainability 2020, 12, 6678. [Google Scholar] [CrossRef]
- Marks-Bielska, R.; Bielski, S.; Novikova, A.; Romaneckas, K. Straw stocks as a source of renewable energy. A case study of a district in poland. Sustainability 2019, 11, 4714. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y.; Soanes, B.K.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.A.; Martin, A.C.; et al. Enhancing climate change resilience in agricultural crops. Curr. Biol. 2023, 33, R1246–R1261. [Google Scholar] [CrossRef]
| Component | Whole Grains | Flour | References |
|---|---|---|---|
| Aleuren fraction | 11–14% | <0.1% | [98,99] |
| Embryo | 3% | <0.1% | [98] |
| Total fiber | 13.0–22.2% | 3% | [98,100] |
| Insoluble fiber | 11.5% | 1.90% | [98] |
| Soluble fiber | 1% | 1.00% | [98] |
| Protein | 8–14% | 14% | [98,101] |
| Fat | 1.5–3% | 1.40% | [98,102] |
| Starch and other sugars | 70% | 83% | [98] |
| Minerals | 2% | 0.6% | [98] |
| Zinc (µg g−1) | 29 | 8 | [98] |
| Iron (µg g−1) | 35 | 13 | [98] |
| Selenium (µg g−1) | 1.4–7 | 0.02 | [98,103,104] |
| B-tocotrienol (µg g−1) | 33 | 5.7 | [98] |
| Vitamin B6 (mg g−1) | 8 | 1.4 | [98] |
| Folic acid (mg g−1) | 23–140 | 0.11 | [98,105,106] |
| Feluric acid (mg g−1) | 5 | 0.4 | [99] |
| Phosphorus (mg g−1) | 1.8–4.2 | 0.1 | [91,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berbeć, A.K.; Wyzińska, M. From Nutrition to Energy: Evaluating the Role of Rye (Secale cereale L.) Grain in Sustainable Food Systems and Biofuel Applications. Foods 2025, 14, 1971. https://doi.org/10.3390/foods14111971
Berbeć AK, Wyzińska M. From Nutrition to Energy: Evaluating the Role of Rye (Secale cereale L.) Grain in Sustainable Food Systems and Biofuel Applications. Foods. 2025; 14(11):1971. https://doi.org/10.3390/foods14111971
Chicago/Turabian StyleBerbeć, Adam Kleofas, and Marta Wyzińska. 2025. "From Nutrition to Energy: Evaluating the Role of Rye (Secale cereale L.) Grain in Sustainable Food Systems and Biofuel Applications" Foods 14, no. 11: 1971. https://doi.org/10.3390/foods14111971
APA StyleBerbeć, A. K., & Wyzińska, M. (2025). From Nutrition to Energy: Evaluating the Role of Rye (Secale cereale L.) Grain in Sustainable Food Systems and Biofuel Applications. Foods, 14(11), 1971. https://doi.org/10.3390/foods14111971