Assessment of pH-Induced Conformational Changes in Whey Protein Isolate–Dextran Conjugate Using Spectral Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of WPI–Dextran Conjugates
2.3. Degree of Glycosylation
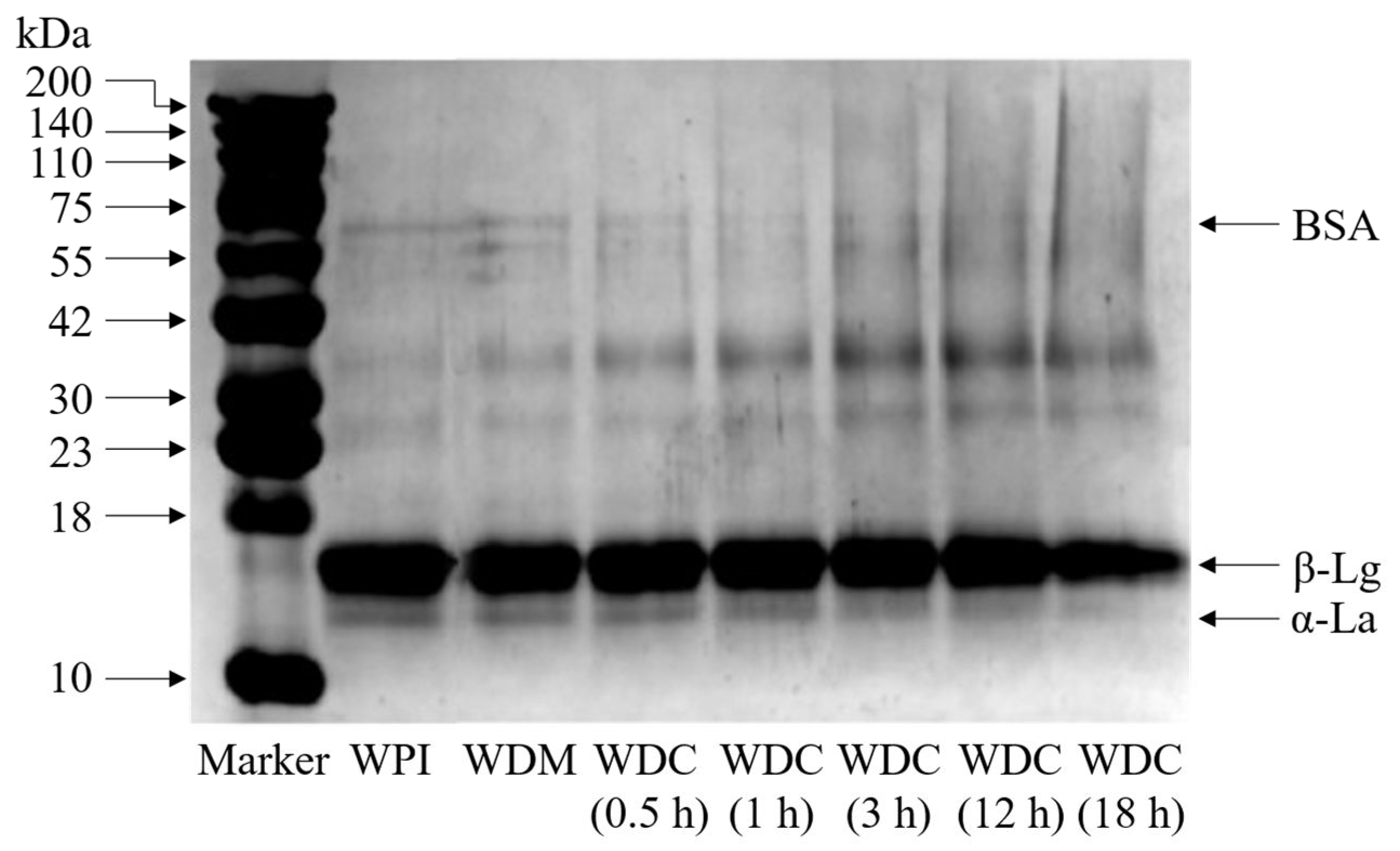
2.4. Electrophoresis
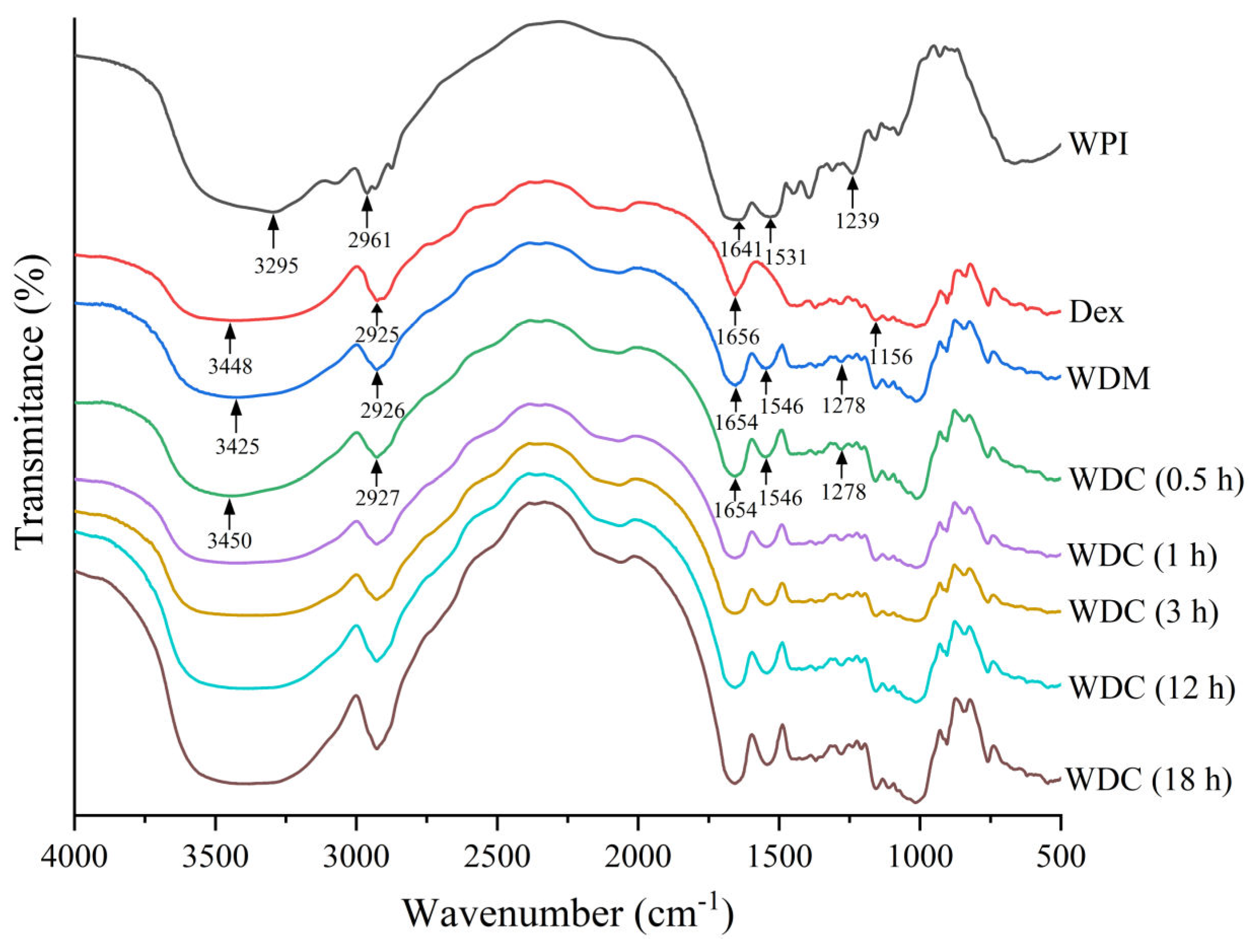
2.5. FTIR Spectroscopy
2.6. UV-Vis Absorption Spectroscopy
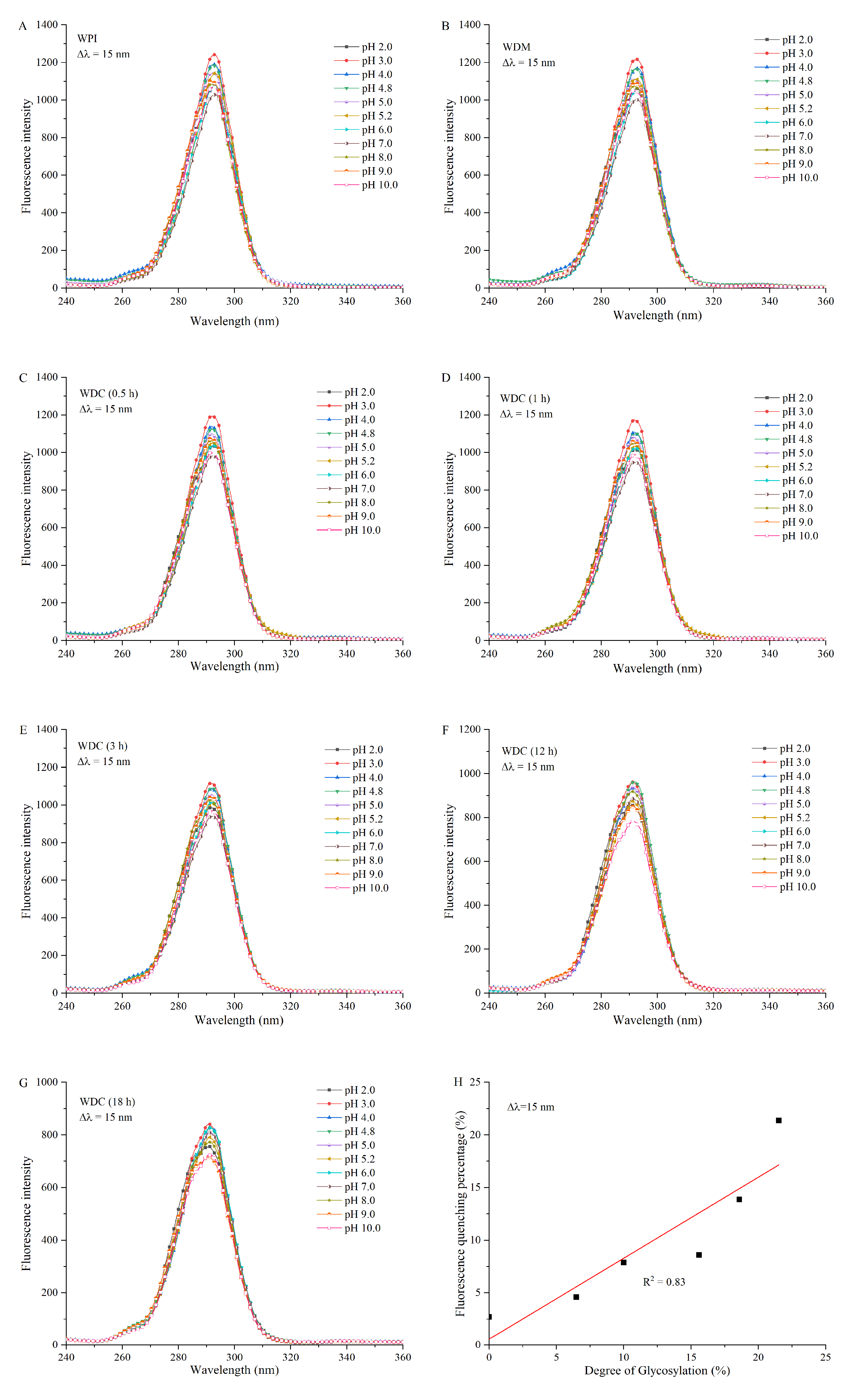
2.7. Intrinsic Fluorescence Emission Spectroscopy
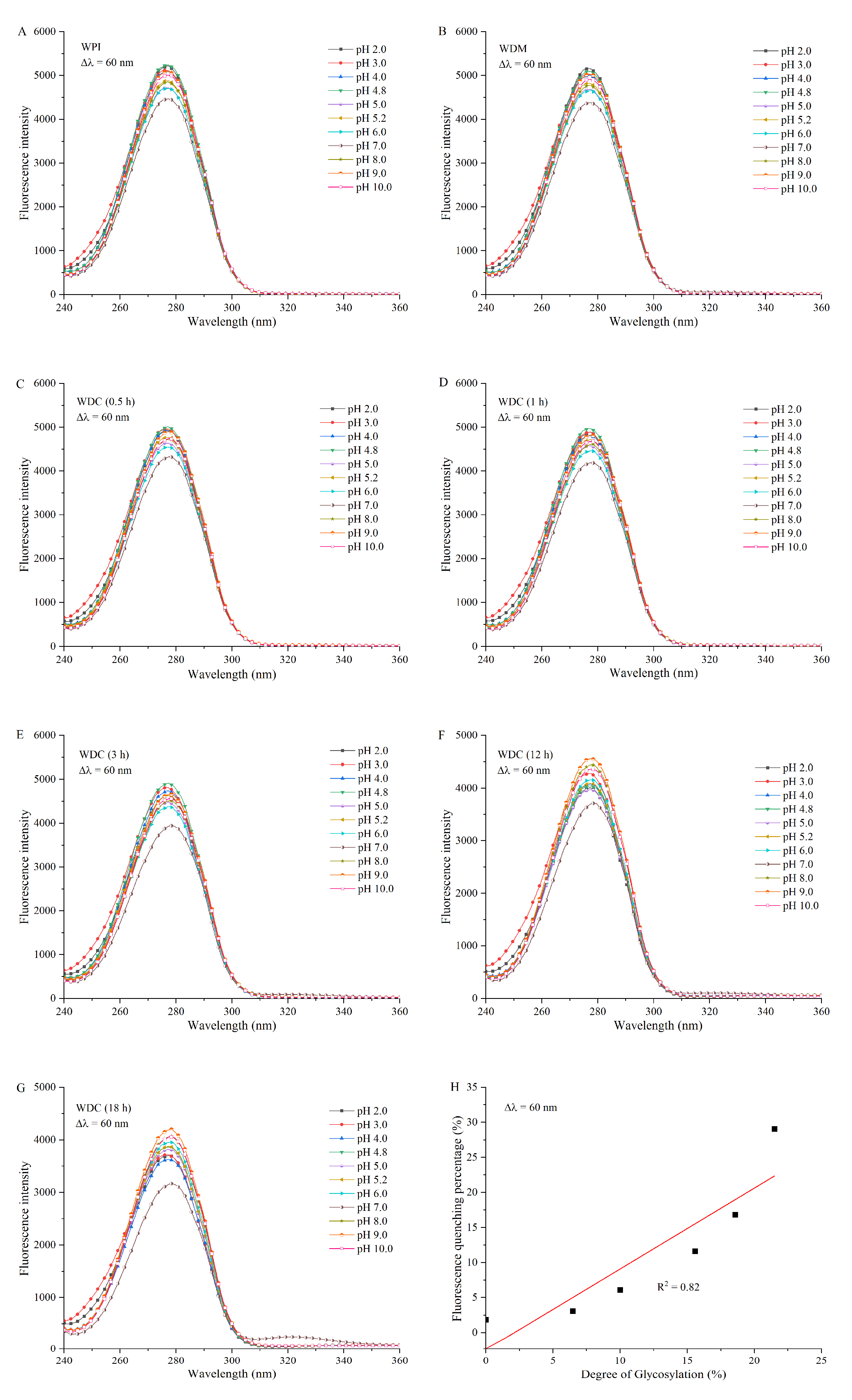
2.8. Synchronous Fluorescence Spectroscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Degree of Glycosylation Analysis
3.2. SDS-PAGE Analysis
3.3. FTIR Spectroscopy Analysis
3.4. UV-Vis Absorption Spectroscopy Analysis
3.5. Intrinsic Fluorescence Emission Spectroscopy Analysis
3.6. Synchronous Fluorescence Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, C.; Cao, D.; Ji, S.; Lin, W.; Zhang, X.; Muhoza, B. Whey protein isolate conjugated with xylo-oligosaccharides via Maillard reaction: Characterization, antioxidant capacity, and application for lycopene microencapsulation. LWT-Food Sci. Technol. 2020, 118, 108837. [Google Scholar] [CrossRef]
- Hong, Z.; Xiao, N.; Li, L.; Li, Y.; Xie, X. Glycation of whey protein isolate and emulsions prepared by conjugates. J. Food Eng. 2022, 316, 110852. [Google Scholar] [CrossRef]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. 2020, 60, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castaño, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Lv, X.; Huang, X.; Ma, B.; Chen, Y.; Batool, Z.; Fu, X.; Jin, Y. Modification methods and applications of egg protein gel properties: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2233–2252. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Han, J.; Sun, C.; Li, P. Structure and antioxidant activity of whey protein isolate conjugated with glucose via the Maillard reaction under dry-heating conditions. Food Struct. 2014, 1, 145–154. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Dai, Q.; Hong, Q.; Zhu, X.; Hornung, P.S.; Wang, H.; Fang, W.; Zhang, Y.; Beta, T. Formation of whey protein isolate–dextran conjugates by Maillard reaction with ethanol-water pretreatment. LWT-Food Sci. Technol. 2023, 185, 115142. [Google Scholar] [CrossRef]
- Du, X.; Zhao, M.; Pan, N.; Wang, S.; Xia, X.; Zhang, D. Tracking aggregation behaviour and gel properties induced by structural alterations in myofibrillar protein in mirror carp (Cyprinus carpio) under the synergistic effects of pH and heating. Food Chem. 2021, 362, 130222. [Google Scholar] [CrossRef]
- Ghaderi, F.; Monajjemzadeh, F. Review of the physicochemical methods applied in the investigation of the maillard reaction in pharmaceutical preparations. J. Drug Deliv. Sci. Tecnol. 2020, 55, 101362. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Wang, Y.; Li, Z.; Wang, Z.; Han, J. Effect of high hydrostatic pressure on solubility and conformation changes of soybean protein isolate glycated with flaxseed gum. Food Chem. 2020, 333, 127530. [Google Scholar] [CrossRef] [PubMed]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.S.; Gomes, A.J.; Lunardi, C.N.; Kaliaguine, S.; Patience, G.S. Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. Can. J. Chem. Eng. 2018, 96, 2512–2517. [Google Scholar] [CrossRef]
- Allahdad, Z.; Varidi, M.; Zadmard, R.; Saboury, A.A.; Haertlé, T. Binding of β-carotene to whey proteins: Multi-spectroscopic techniques and docking studies. Food Chem. 2019, 277, 96–106. [Google Scholar] [CrossRef]
- Ke, C.; Li, L. Influence mechanism of polysaccharides induced Maillard reaction on plant proteins structure and functional properties: A review. Carbohyd. Polym. 2023, 302, 120430. [Google Scholar] [CrossRef]
- Bose, A.; Thomas, I.; Kavitha, G.; Abraham, E. Fluorescence spectroscopy and its applications: A review. Int. J. Adv. Pharm. Anal. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Lv, N.; Ren, G.; Wu, R.; Wang, B.; Cao, Z.; Xie, H. Explore the interaction mechanism between zein and EGCG using multi-spectroscopy and molecular dynamics simulation methods. Food Hydrocoll. 2021, 120, 106906. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Fluorophores. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1999; pp. 63–93. [Google Scholar]
- Gong, W.; Guo, X.L.; Huang, H.B.; Li, X.; Xu, Y.; Hu, J.N. Structural characterization of modified whey protein isolates using cold plasma treatment and its applications in emulsion oleogels. Food Chem. 2021, 356, 129703. [Google Scholar] [CrossRef]
- Dong, X.; Du, S.; Deng, Q.; Tang, H.; Yang, C.; Wei, F.; Chen, H.; Quek, S.Y.; Zhou, A.; Liu, L. Study on the antioxidant activity and emulsifying properties of flaxseed gum-whey protein isolate conjugates prepared by Maillard reaction. Int. J. Biol. Macromol. 2020, 153, 1157–1164. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Maresca, P.; Pataro, G.; Ferrari, G. Influence of pulsed light treatment on the aggregation of whey protein isolate. Food Res. Int. 2017, 99, 419–425. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, Z.; Yang, C.; Dong, C.; Chen, F.; Liu, K. Smart antimicrobial system based on enzyme-responsive high methoxyl pectin-whey protein isolate nanocomplex for fresh-cut apple preservation. Int. J. Biol. Macromol. 2023, 253, 127064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, Y.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Impact of pH on the interaction between soybean protein isolate and oxidized bacterial cellulose at oil-water interface: Dilatational rheological and emulsifying properties. Food Hydrocoll. 2021, 115, 106609. [Google Scholar] [CrossRef]
- Ma, T.X.; Zhang, L.; Xu, L.; Ye, Y.H.; Huang, T.; Zhou, Q.M.; Liu, H.L. Mitigation of isoquercitrin on β-lactoglobulin glycation: Insight into the mechanisms by mass spectrometry and interaction analysis. Int. J. Biol. Macromol. 2020, 155, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Saville, D.J. Multiple comparison procedures—Cutting the gordian knot. Agron. J. 2015, 107, 730–735. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, S.; Ren, X.; Oladejo, A.O.; Lu, F.; Ma, H. Effects and mechanism of ultrasound pretreatment of protein on the Maillard reaction of protein-hydrolysate from grass carp (Ctenopharyngodon idella). Ultrason. Sonochem. 2020, 64, 104964. [Google Scholar] [CrossRef]
- Dev, M.J.; Pandit, A.B.; Singhal, R.S. Ultrasound assisted vis-à-vis classical heating for the conjugation of whey protein isolate-gellan gum: Process optimization, structural characterization and physico-functional evaluation. Innov. Food Sci. Emerg. 2021, 72, 102724. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Wang, W.; Lv, R.; Guo, M.; Ding, T.; Ye, X.; Miao, S.; Liu, D. Preparation of modified whey protein isolate with gum acacia by ultrasound Maillard reaction. Food Hydrocoll. 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, M.; Wang, Y.; Zheng, J.; Tian, S.; Zhou, Y.; Xu, L.; Wang, T.; Ma, D.; Li, L.; et al. Physicochemical and functional properties of whey protein-Yeast beta-glucan conjugates formed by glycosylation. LWT-Food Sci Technol. 2025, 224, 117842. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of whey protein isolate (WPI)–dextran conjugates in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef]
- Yadav, J.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Fátima Poças, M.; Pintado, M.E.; Xavier Malcata, F. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. ACTA Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Singh, B.R. Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, G.; Wang, X.; Xu, Y.; Chen, S.; Wang, X.; Jiang, L. Improving the freeze-thaw stability of soy protein emulsions via combing limited hydrolysis and Maillard-induced glycation. LWT-Food Sci Technol. 2018, 91, 63–69. [Google Scholar] [CrossRef]
- Wang, W.D.; Li, C.; Bin, Z.; Huang, Q.; You, L.J.; Chen, C.; Fu, X.; Liu, R.H. Physicochemical properties and bioactivity of whey protein isolate-inulin conjugates obtained by Maillard reaction. Int. J. Biol. Macromol. 2020, 150, 326–335. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Q.; Diao, C.; Wang, J.; Wu, Z.; Wang, H. Enhanced physicochemical stability of lutein-enriched emulsions by polyphenol-protein-polysaccharide conjugates and fat-soluble antioxidant. Food Hydrocoll. 2020, 101, 105447. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Ushikubo, F.Y.; Furtado, G.d.F.; Cunha, R.L. Oil in water emulsions stabilized by Maillard conjugates of sodium caseinate-locust bean gum. J. Disper. Sci. Technol. 2019, 40, 634–645. [Google Scholar] [CrossRef]
- Wang, W.Q.; Bao, Y.H.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef]
- Hristova, S.H.; Zhivkov, A.M. Isoelectric point of free and adsorbed cytochrome c determined by various methods. Colloids Surf. B 2019, 174, 87–94. [Google Scholar] [CrossRef]
- Salgın, S.; Salgın, U.; Bahadır, S. Zeta potentials and isoelectric points of biomolecules: The effects of ion types and ionic strengths. Int. J. Electrochem. Sci. 2012, 7, 12404–12414. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, M.; Hu, J.; Zeng, S.; Bai, X. Correspondence analysis of antioxidant activity and UV–Vis absorbance of Maillard reaction products as related to reactants. LWT-Food. Sci. Technol. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Malyshev, D.; Öberg, R.; Landström, L.; Andersson, P.O.; Dahlberg, T.; Andersson, M. pH-induced changes in Raman, UV–vis absorbance, and fluorescence spectra of dipicolinic acid (DPA). Spectrochim. Acta A 2022, 271, 120869. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, D. Investigation on the pH-dependent binding of benzocaine and lysozyme by fluorescence and absorbance. Spectrochim. Acta A 2011, 82, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.E.; Bruzzone, L. Synchronous fluorescence spectrometry: Conformational investigation or inner filter effect? J. Lumin. 2013, 137, 138–142. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Cui, Q.; Li, R.; Wang, X.; Jiang, L. Effect of pH on freeze-thaw stability of glycated soy protein isolate. J. Oleo Sci. 2019, 68, 281–290. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yang, Y.; Zhao, J.; Zhang, Y.; Li, L.; Wang, Q.; Ming, J. Interaction between wheat gliadin and quercetin under different pH conditions analyzed by multi-spectroscopy methods. Spectrochim. Acta A 2020, 229, 117937. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Ma, X.; Lv, R.; Watharkar, R.B.; Ding, T.; Ye, X.; Liu, D. Effect of pH-shifting treatment on structural and functional properties of whey protein isolate and its interaction with (-)-epigallocatechin-3-gallate. Food Chem. 2019, 274, 234–241. [Google Scholar] [CrossRef]
- Renard, D.; Lefebvre, J.; Griffin, M.C.A.; Griffin, W.G. Effects of pH and salt environment on the association of β-lactoglobulin revealed by intrinsic fluorescence studies. Int. J. Biol. Macromol. 1998, 22, 41–49. [Google Scholar] [CrossRef]
- Malavašǐc, M.; Poklar, N.; Maček, P.; Vesnaver, G. Fluorescence studies of the effect of pH, guanidine hydrochloride and urea on equinatoxin II conformation. BBA-Biomembranes 1996, 1280, 65–72. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence study of the curcumin–casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef]
- Simion, A.M.; Aprodu, I.; Dumitrașcu, L.; Bahrim, G.E.; Alexe, P.; Stănciuc, N. Probing thermal stability of the β-lactoglobulin–oleic acid complex by fluorescence spectroscopy and molecular modeling. J. Mol. Struct. 2015, 1095, 26–33. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, R.; Xu, X.; Du, M.; Zhu, B.; Wu, C. Structural interplay between curcumin and soy protein to improve the water-solubility and stability of curcumin. Int. J. Biol. Macromol. 2021, 193, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Boggione Santos, I.J.; Hernandez Hernandez, H.L.; Cardoso Costa, M.H.; de Queiroz Lafeta Júnior, J.A.; dos Reis Coimbra, J.S. Conjugates of α-lactalbumin, β-lactoglobulin, and lysozyme with polysaccharides: Characterization and techno-functional properties. Food Res. Int. 2019, 116, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Jin, Y.; Ma, M. Synthesis and structural characterization of lysozyme–pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Shahri, P.A.; Rad, A.S.; Beigoli, S.; Saberi, M.R.; Chamani, J. Human serum albumin-amlodipine binding studied by multi-spectroscopic, zeta-potential, and molecular modeling techniques. J. Iran. Chem. Soc. 2018, 15, 223–243. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef]
- Karbasi, M.; Askari, G.; Madadlou, A. Surface decoration of whey protein microgels through the Maillard conjugation with maltodextrin. Food Hydrocoll. 2019, 91, 190–197. [Google Scholar] [CrossRef]
- Huang, C.; Cui, H.; Hayat, K.; Zhang, X.; Ho, C.T. Variation of moisture state and taste characteristics during vacuum drying of Maillard reaction intermediates of hydrolyzed soybean protein and characterization of browning precursors via fluorescence spectroscopy. Food Res. Int. 2022, 162, 112086. [Google Scholar] [CrossRef]
- Gu, H.; Hu, L.; Dong, Y.; Chen, Q.; Wei, Z.; Lv, R.; Zhou, Q. Evolving trends in fluorescence spectroscopy techniques for food quality and safety: A review. J. Food Compos. Anal. 2024, 131, 106212. [Google Scholar] [CrossRef]
- Liao, H.; Chao, L.; Xie, D.; Zhu, X.; Zhuang, Q.; Zhang, J. Application of fluorescence spectroscopy in meat analysis. J. Food Compos. Anal. 2025, 143, 107613. [Google Scholar] [CrossRef]


| Reaction Time (h) | Degree of Glycosylation (%) |
|---|---|
| 0.5 | 6.47 ± 0.31 e |
| 1 | 9.99 ± 0.16 d |
| 3 | 15.59 ± 0.16 c |
| 12 | 18.57 ± 0.31 b |
| 18 | 21.51 ± 0.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Wang, H.; Zhu, X.; Hornung, P.S.; Zhang, Y.; Hu, W.; Lin, A.; Yao, A.; Beta, T. Assessment of pH-Induced Conformational Changes in Whey Protein Isolate–Dextran Conjugate Using Spectral Technology. Foods 2025, 14, 1952. https://doi.org/10.3390/foods14111952
Dai Q, Wang H, Zhu X, Hornung PS, Zhang Y, Hu W, Lin A, Yao A, Beta T. Assessment of pH-Induced Conformational Changes in Whey Protein Isolate–Dextran Conjugate Using Spectral Technology. Foods. 2025; 14(11):1952. https://doi.org/10.3390/foods14111952
Chicago/Turabian StyleDai, Qingyuan, Huiqin Wang, Xiuling Zhu, Polyanna Silveira Hornung, Yuru Zhang, Wenxuan Hu, Anqi Lin, Anyi Yao, and Trust Beta. 2025. "Assessment of pH-Induced Conformational Changes in Whey Protein Isolate–Dextran Conjugate Using Spectral Technology" Foods 14, no. 11: 1952. https://doi.org/10.3390/foods14111952
APA StyleDai, Q., Wang, H., Zhu, X., Hornung, P. S., Zhang, Y., Hu, W., Lin, A., Yao, A., & Beta, T. (2025). Assessment of pH-Induced Conformational Changes in Whey Protein Isolate–Dextran Conjugate Using Spectral Technology. Foods, 14(11), 1952. https://doi.org/10.3390/foods14111952







