Modulating Yogurt Fermentation Through Pulsed Electric Fields and Influence of Milk Fat Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Yogurt Manufacturing and Determination of Fermentation Endpoint
2.2. Pulsed Electric Field Treatment
2.3. Experimental Design
2.4. pH, Total Soluble Solids, and Conductivity Measurements
2.5. Microbial Count
2.6. Lactose and Organic Acids Extraction and Quantification
2.7. Riboflavin Extraction and Quantification
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of PEF Treatment on Fermentation Process
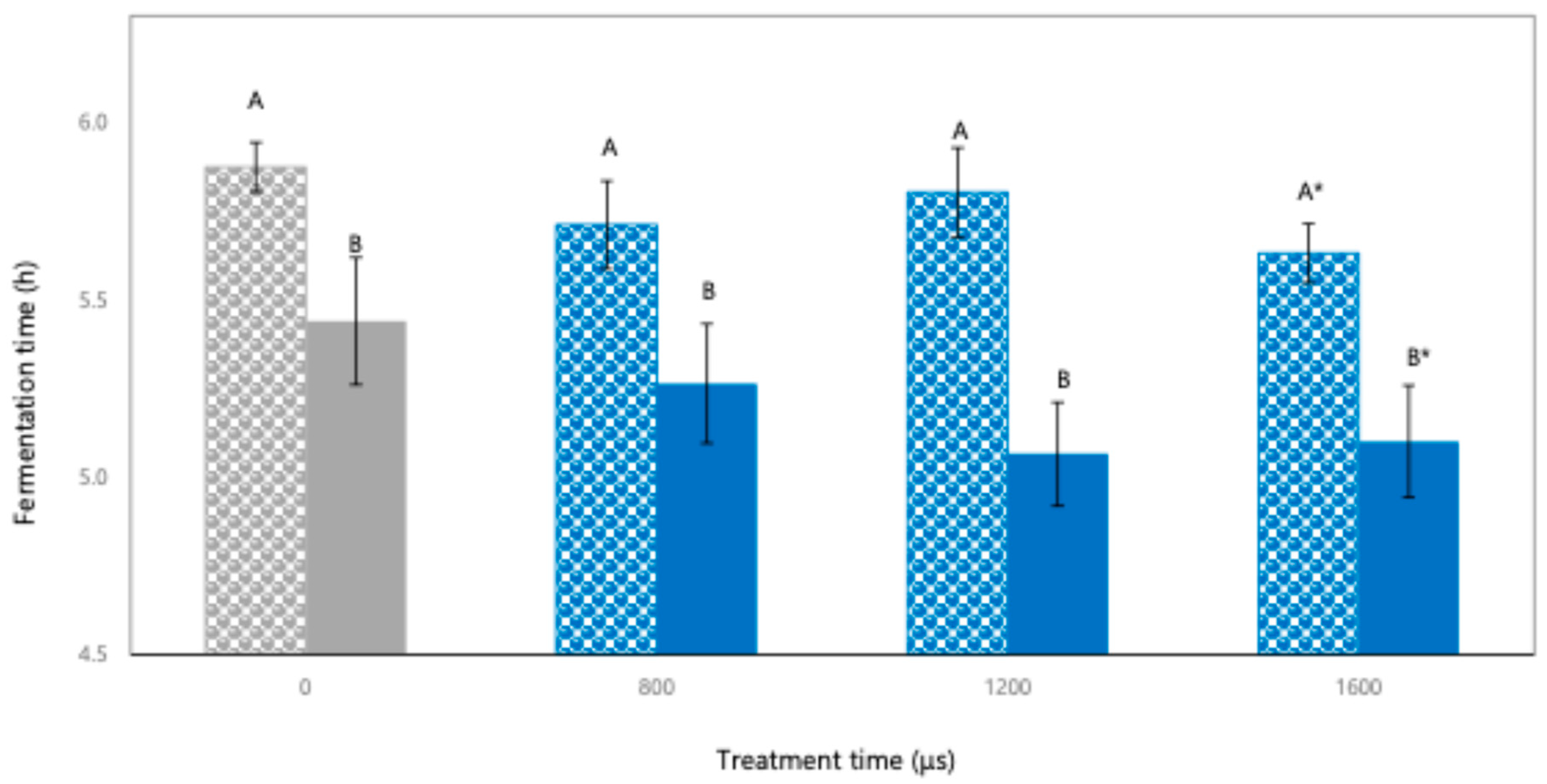
3.1.1. Fermentation Time
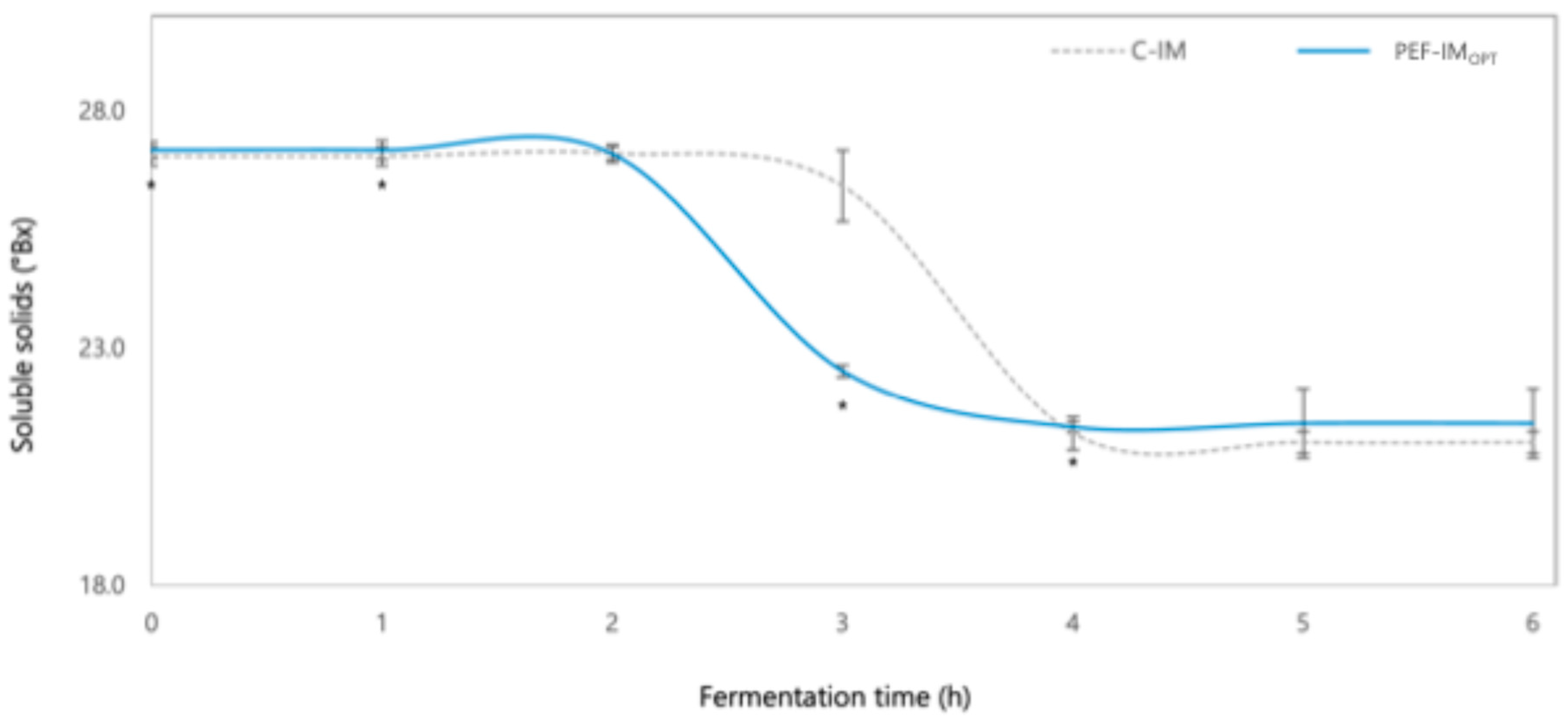
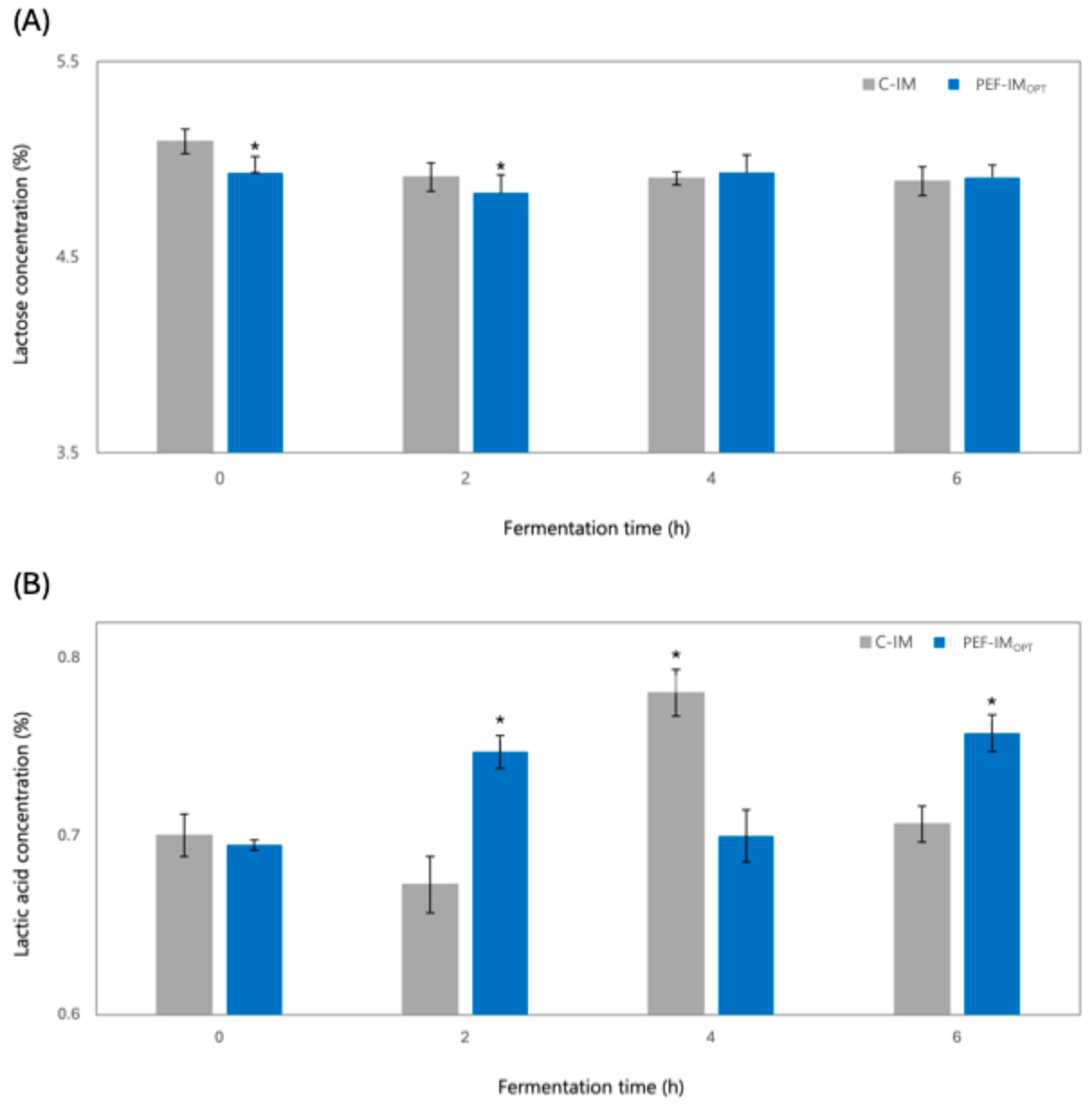
3.1.2. Changes in Soluble Solids, Lactose, and Organic Acids During Fermentation as Affected by PEFOPT
Soluble Solids
Lactose and Organic Acids
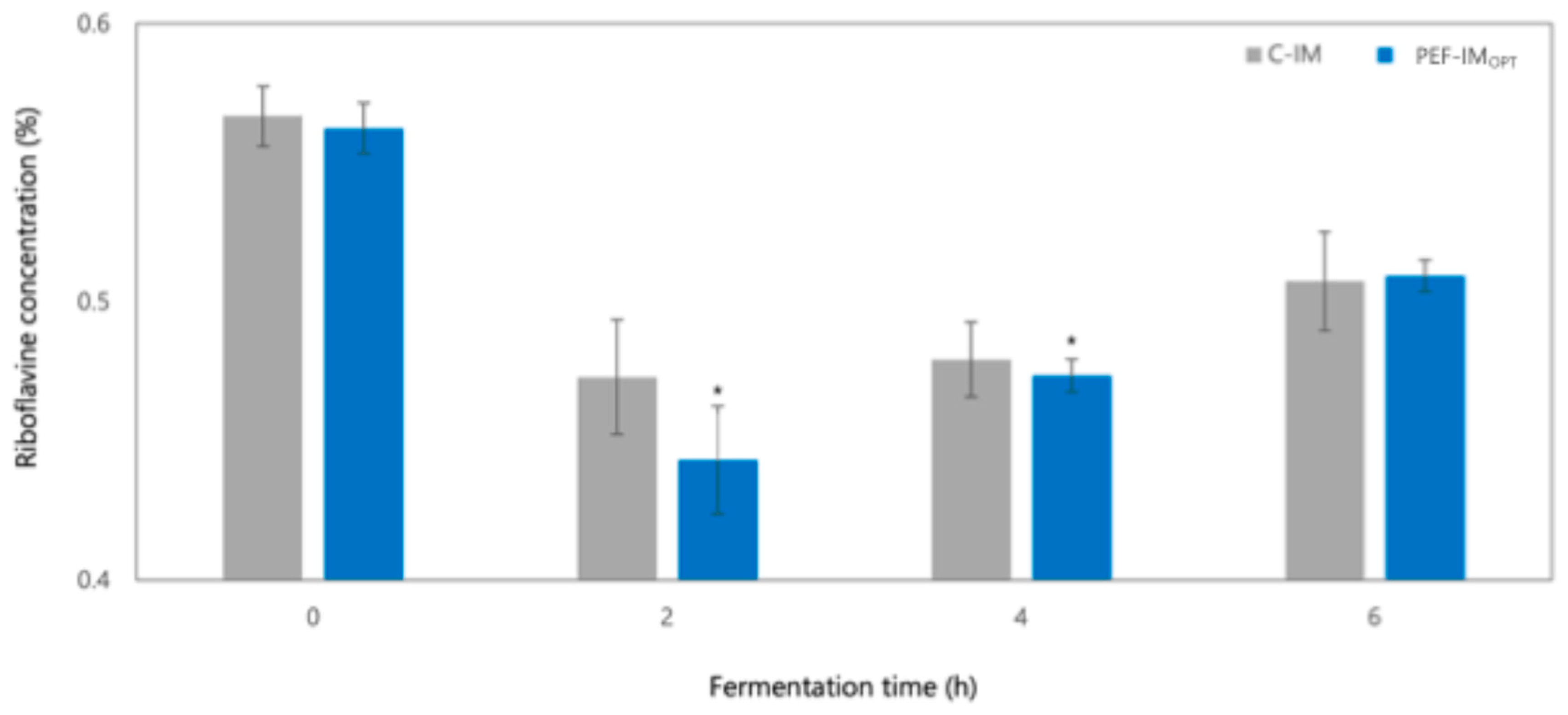
Riboflavin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEF | pulsed electric fields |
| IM | inoculum suspended in milk |
| PEF-IM | PEF-treated IM |
| C-IM | untreated-IM, as control |
| LAB | lactic acid bacteria |
| IM-0.5 | inoculum suspended in milk with 0.5% fat content |
| IM-2.8 | inoculum suspended in milk with 2.8% fat content |
| PEF-IMOPT | PEF-IM at optimum conditions |
| MRS | de Man–Rogosa–Sharpe |
| t | treatment times |
| CFU/mL | colony forming units per milliliter |
| Le | lactose extracts |
| OAe | organic acid extracts |
| RID | refractive index detector |
| Lac | lactose |
| DAD | diode array detector |
| LA | lactic acid |
| PA | propionic acid |
| BA | butyric acid |
| HPLC | high-performance liquid chromatography |
| TCA | trichloroacetic acid |
| FLD | fluorescence detector |
| OSA | octane sulfonic acid |
| C-IM0.5 | untreated-IM, as control with 0.5% fat content |
| C-IM2.8 | untreated-IM, as control with 2.8% fat content |
References
- Drouin-Chartier, J.P.; Brassard, D.; Tessier-Grenier, M.; Côté, J.A.; Labonté, M.É.; Desroches, S.; Couture, P.; Lamarche, B. Systematic Review of the Association between Dairy Product Consumption and Risk of Cardiovascular-Related Clinical Outcomes. Adv. Nutr. 2016, 7, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.F.; Hernández, J.A.M.; Suárez, V.M.; Villares, J.M.M.; Yurrita, L.R.C.; Cabria, M.H.; Rey, F.J.M. Documento de Consenso: Importancia Nutricional y Metabólica de La Leche. Nutr. Hosp. 2015, 31, 92–101. [Google Scholar] [CrossRef]
- Bazán, D.L.; del Río, P.G.; Domínguez, J.M.; Cortés-Diéguez, S.; Mejuto, J.C.; Pérez-Guerra, N. The Chemical, Microbiological and Volatile Composition of Kefir-like Beverages Produced from Red Table Grape Juice in Repeated 24-h Fed-Batch Subcultures. Foods 2022, 11, 3117. [Google Scholar] [CrossRef]
- Doleyres, Y.; Schaub, L.; Lacroix, C. Comparison of the Functionality of Exopolysaccharides Produced in Situ or Added as Bioingredients on Yogurt Properties. J. Dairy Sci. 2005, 88, 4146–4156. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial Yogurt Manufacture: Monitoring of Fermentation Process and Improvement of Final Product Quality. J. Dairy Sci. 2007, 90, 2641–2654. [Google Scholar] [CrossRef]
- Leblanc, J.G.; Laiño, J.E.; del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-Group Vitamin Production by Lactic Acid Bacteria—Current Knowledge and Potential Applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Juarez del Valle, M.; Laiño, J.E.; Savoy de Giori, G.; LeBlanc, J.G. Riboflavin Producing Lactic Acid Bacteria as a Biotechnological Strategy to Obtain Bio-Enriched Soymilk. Food Res. Int. 2014, 62, 1015–1019. [Google Scholar] [CrossRef]
- Sodini, I.; Montella, J.; Tong, P.S. Physical Properties of Yogurt Fortified with Various Commercial Whey Protein Concentrates. J. Sci. Food Agric. 2005, 85, 853–859. [Google Scholar] [CrossRef]
- El Darra, N.; Turk, M.F.; Ducasse, M.A.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Changes in Polyphenol Profiles and Color Composition of Freshly Fermented Model Wine Due to Pulsed Electric Field, Enzymes and Thermovinification Pretreatments. Food Chem. 2016, 194, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Morales-de la Peña, M.; Miranda-Mejía, G.A.; Martín-Belloso, O. Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies. Beverages 2023, 9, 51. [Google Scholar] [CrossRef]
- Miranda-Mejía, G.A.; Martín del Campo-Barba, S.T.; Arredondo-Ochoa, T.; Tejada-Ortigoza, V.; la Peña, M.M. de Low-Intensity Pulsed Electric Fields Pre-Treatment on Yogurt Starter Culture: Effects on Fermentation Time and Quality Attributes. Innov. Food Sci. Emerg. Technol. 2024, 95, 103708. [Google Scholar] [CrossRef]
- Vazquez-Cabral, D.; Valdez-Fragoso, A.; Rocha-Guzman, N.E.; Moreno-Jimenez, M.R.; Gonzalez-Laredo, R.F.; Morales-Martinez, P.S.; Rojas-Contreras, J.A.; Mujica-Paz, H.; Gallegos-Infante, J.A. Effect of Pulsed Electric Field (PEF)-Treated Kombucha Analogues from Quercus Obtusata Infusions on Bioactives and Microorganisms. Innov. Food Sci. Emerg. Technol. 2016, 34, 171–179. [Google Scholar] [CrossRef]
- El Kantar, S.; Koubaa, M. Pulsed Electric Field Treatment for the Stimulation of Microorganisms: Applications in Food Production. Res. Agric. Eng. 2022, 68, 80–92. [Google Scholar] [CrossRef]
- Ulmer, A.; Erdemann, F.; Mueller, S.; Loesch, M.; Wildt, S.; Jensen, M.L.; Gaspar, P.; Zeidan, A.A.; Takors, R. Differential Amino Acid Uptake and Depletion in Mono-Cultures and Co-Cultures of Streptococcus Thermophilus and Lactobacillus Delbrueckii Subsp. Bulgaricus in a Novel Semi-Synthetic Medium. Microorganisms 2022, 10, 1771. [Google Scholar] [CrossRef]
- Chanos, P.; Warncke, M.C.; Ehrmann, M.A.; Hertel, C. Application of Mild Pulsed Electric Fields on Starter Culture Accelerates Yogurt Fermentation. Eur. Food Res. Technol. 2020, 246, 621–630. [Google Scholar] [CrossRef]
- Stühmeier-Niehe, C.; Lass, L.; Brocksieper, M.; Chanos, P.; Hertel, C. Pre-Treatment of Starter Cultures with Mild Pulsed Electric Fields Influences the Characteristics of Set Yogurt. Foods 2023, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Tremín, J. Inactivación Microbiana Por Pulsos Eléctricos de Alto Voltaje. 2014, pp. 1–45. Available online: https://zaguan.unizar.es/record/16002/files/TAZ-TFG-2014-1503.pdf (accessed on 15 April 2025).
- Bombón-Pilliza, L.A. Influencia de La Aplicación de Pulsos Eléctricos de Alta Intensidad (Peai) Sobre La Carga Microbiana Del Néctar de Fresa. 2012. Available online: https://repositorio.uta.edu.ec/items/68f896b9-4c8b-4920-8f9b-027d46de448e (accessed on 15 April 2025).
- Pineda-Posadas, J.A.; Ortiz-Rodríguez, E.; Ortiz-Rodríguez, L. Campos Eléctricos Pulsados. Handbook Tecnologías Emergentes Aplicadas en Alimentos. 2022, pp. 53–61. Available online: https://www.ecorfan.org/handbooks/Handbooks_Tecnologias_Emergentes_Aplicadas_en_Alimentos_TI/Handbooks_Tecnologias_Emergentes_Aplicadas_en_Alimentos_TI_6.pdf (accessed on 26 May 2025).
- Novonesis Internal Technical Protocol for Thermophilic Culture YF-L904; Document provided by the company uopon request; Novonesis: Gabsvaerd, Capital Region, Denmark, 2020.
- Turengano-Roldan, C. NORMA Oficial Mexicana NOM-181-SCFI-2010, Yogurt-Denominación, Especificaciones Fisicoquímicas y Mic. Available online: https://www.dof.gob.mx/normasOficiales/4209/seeco/seeco.htm (accessed on 18 August 2020).
- Kang, S.S.; Kim, M.K.; Kim, Y.J. Comprehensive Evaluation of Microbiological and Physicochemical Properties of Commercial Drinking Yogurts in Korea. Food Sci. Anim. Resour. 2019, 39, 820–830. [Google Scholar] [CrossRef]
- Leclercq-Perlat, M.-N.; Oumer, A.; Bergere, J.-L.; Spinnler, H.; Corrieu, G. Growth of Debaryomyces Hansenii on a Bacterial Surface-Ripened Soft Cheese. J. Dairy Res. 1999, 66, 271–281. [Google Scholar] [CrossRef]
- Picque, D.; Lefier, D.; Grappin, R.; Corrieu, G. Monitoring of Fermentation by Infrared Spectrometry: Alcoholic and Lactic Fermentations. Anal. Chim. Acta 1993, 279, 67–72. [Google Scholar] [CrossRef]
- Albalá-Hurtado, S.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Mariné-Font, A. Determination of Water-Soluble Vitamins in Infant Milk by High-Performance Liquid Chromatography. J. Chromatogr. A 1997, 778, 247–253. [Google Scholar] [CrossRef]
- Gliszczyńska-Świglo, A.; Koziolowa, A. Chromatographic Determination of Riboflavin and Its Derivatives in Food. J. Chromatogr. A 2000, 881, 285–297. [Google Scholar] [CrossRef]
- Wherry, B.; Barbano, D.M.; Drake, M.A. Use of Acid Whey Protein Concentrate as an Ingredient in Nonfat Cup Set-Style Yogurt. J. Dairy Sci. 2019, 102, 8768–8784. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, T.; Zhang, Y.; Zhang, A.; Gai, L.; Niu, D. Potential Applications of Pulsed Electric Field in the Fermented Wine Industry. Front. Nutr. 2022, 9, 1048632. [Google Scholar] [CrossRef]
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Effect of Pulsed Electric Fields on the Growth and Acidification Kinetics of Lactobacillus Delbrueckii Subsp. Bulgaricus. Foods 2020, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, D.; Ardiles, P.; Castillo, D.; Puente, L. Emerging Technology: Pulsed Electric Fields (Pef) for Food Treatment and Its Effect on Antioxidant Content. Rev. Chil. Nutr. 2021, 48, 609–619. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.E.; Gardini, F. Use of Natural Aroma Compounds to Improve Shelf-Life and Safety of Minimally Processed Fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. The Evolution of Fermented Milks, from Artisanal to Industrial Products: A Critical Review. Fermentation 2022, 8, 679. [Google Scholar] [CrossRef]
- Iftikhar, M.A.; Pasha, T.N.; Inayat, S.; Javed, K.; Ullah, R. Effect of Different Milk Composition on Physico-Chemical Characteristics of Set Type Yoghurt. Pak. J. Zool. 2022, 56, 1297–1304. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, E.; Shi, T.; Ye, L.; Konno, T.; Oda, M.; Ji, Z.S. Strand-Specific RNA-Seq Analysis of the Lactobacillus Delbrueckii Subsp. Bulgaricus Transcriptome. Mol. Biosyst. 2016, 12, 508–519. [Google Scholar] [CrossRef]
- Emanuel, E.; Dubrovin, I.; Pogreb, R.; Pinhasi, G.A.; Cahan, R. Resuscitation of Pulsed Electric Field-Treated Staphylococcus Aureus and Pseudomonas Putida in a Rich Nutrient Medium. Foods 2021, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Bamforth, W. The Microbiology of Malting and Brewing. Microbiol. Mol. Biol. Rev. 2013, 77, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Bücher, C.; Burtscher, J.; Domig, K.J. Propionic Acid Bacteria in the Food Industry: An Update on Essential Traits and Detection Methods. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4299–4323. [Google Scholar] [CrossRef] [PubMed]
- Peruzy, M.F.; Blaiotta, G.; Aponte, M.; De Sena, M.; Murru, N. Late Blowing Defect in Grottone Cheese: Detection of Clostridia and Control Strategies. Ital. J. Food Saf. 2022, 11, 96–102. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; De, S. Lactic Acid Bacteria as a Cell Factory for Riboflavin Production. Microb. Biotechnol. 2016, 9, 441–451. [Google Scholar] [CrossRef]
- Djukić-Vuković, A.; Meglič, S.H.; Flisar, K.; Mojović, L.; Miklavčič, D. Pulsed Electric Field Treatment of Lacticaseibacillus Rhamnosus and Lacticaseibacillus Paracasei, Bacteria with Probiotic Potential. LWT 2021, 152, 112304. [Google Scholar] [CrossRef]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of Vitamin B2 (Riboflavin) by Microorganisms: An Overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef]
| Treatment | Fat (%) | t (µs) |
|---|---|---|
| C-IM0.5 | 0.5 | 0 |
| PEF-IM0.5-800 | 800 | |
| PEF-IM0.5-1200 | 1200 | |
| PEF-IM0.5-1600 | 1600 | |
| C-IM2.8 | 2.8 | 0 |
| PEF-IM2.8-800 | 800 | |
| PEF-IM2.8-1200 | 1200 | |
| PEF-IM2.8-1600 | 1600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Mejía, G.A.; Cardador-Martínez, A.; Tejada-Ortigoza, V.; Morales-de la Peña, M.; Martín-Belloso, O. Modulating Yogurt Fermentation Through Pulsed Electric Fields and Influence of Milk Fat Content. Foods 2025, 14, 1927. https://doi.org/10.3390/foods14111927
Miranda-Mejía GA, Cardador-Martínez A, Tejada-Ortigoza V, Morales-de la Peña M, Martín-Belloso O. Modulating Yogurt Fermentation Through Pulsed Electric Fields and Influence of Milk Fat Content. Foods. 2025; 14(11):1927. https://doi.org/10.3390/foods14111927
Chicago/Turabian StyleMiranda-Mejía, Graciela A., Anaberta Cardador-Martínez, Viridiana Tejada-Ortigoza, Mariana Morales-de la Peña, and Olga Martín-Belloso. 2025. "Modulating Yogurt Fermentation Through Pulsed Electric Fields and Influence of Milk Fat Content" Foods 14, no. 11: 1927. https://doi.org/10.3390/foods14111927
APA StyleMiranda-Mejía, G. A., Cardador-Martínez, A., Tejada-Ortigoza, V., Morales-de la Peña, M., & Martín-Belloso, O. (2025). Modulating Yogurt Fermentation Through Pulsed Electric Fields and Influence of Milk Fat Content. Foods, 14(11), 1927. https://doi.org/10.3390/foods14111927









