Abstract
Background/Objective: Type 2 diabetes mellitus (T2D) is a chronic metabolic disorder characterized by hyperglycemia. Plant-based interventions have gained attention as potential complementary treatments alongside conventional therapies. This systematic review evaluates the efficacy of plant-based interventions in improving glycemic control, insulin sensitivity, lipid profiles, and other outcomes such as GLUT-4, Tumor Necrosis Facto-alpha, dietary inflammation index, plasma lipopolysaccharide, total antioxidant capacity, and malondialdehyde in individuals with T2D. Methods: We conducted a systematic search of PubMed, Scopus, and ScienceDirect databases to identify randomized controlled trials (RCTs) and observational studies. RCTs were used as an additional screening criterion. The review included studies on the effects of plant-based interventions, encompassing fruits, vegetables, herbs, spices, and their extracts. We analyzed data on glycemic control, insulin sensitivity, lipid profiles, and other metabolic markers. Results: Twenty-six studies were included in our analysis. Various interventions showed potential benefits, with improved glycemic control, insulin sensitivity, and lipid profiles. Specific interventions such as Ziziphus jujuba juice, black tea, caper fruit extract, and balanced diets were linked with positive outcomes. Based on the Functional Food Claim framework, all 26 studies met the quality criteria for novel foods. However, the novel food score varied, and results were inconsistent across different interventions. Conclusion: Although some plant-based interventions appear promising in managing T2D, the evidence remains inconclusive due to variability in study quality and methodology. Further high-quality RCTs are necessary to confirm these findings and to establish the optimal dosage, duration, and combinations of interventions for effective T2D management. Despite inconclusive results, few plant-based diets have promising outcomes. Healthcare providers, especially nurse case managers, can incorporate the findings of this study into their practice protocol to support self-management for individuals with TD2.
1. Introduction
Type 2 diabetes mellitus (T2D) is a chronic metabolic disorder characterized by persistent hyperglycemia, resulting from a combination of insulin resistance and impaired insulin secretion. This condition is a major global health issue, leading to increased morbidity and mortality through its contribution to serious complications such as cardiovascular disease, kidney dysfunction, neuropathy, retinopathy, and diabetic foot problems. According to a recent report by the International Diabetes Federation (IDF), approximately 537 million adults worldwide were living with diabetes in 2021. This number is expected to rise to 783 million by 2045, underscoring the urgent need for effective management strategies. T2D disproportionately impacts low- and middle-income countries (LMICs), where limitations in healthcare accessibility further exacerbate the challenges faced by these populations [1,2,3].
Traditional management of T2D involves lifestyle interventions, such as dietary modifications, increased physical activity, and weight management, alongside pharmacological treatments, including metformin, sulfonylureas, and glucagon-like peptide-1 (GLP-1) receptor agonists [4]. Although these methods are established, there are ongoing challenges in maintaining long-term glycemic control and minimizing complications. Additionally, pharmacological options may pose side effects or remain out of reach for patients in resource-limited settings [1,2,3].
Self-management is widely recognized as a crucial aspect of improving self-management behaviors and clinical outcomes, particularly for individuals with non-communicable diseases (NCDs) such as diabetes and hypertension [5,6]. For individuals with T2D, self-management involves actively participating in self-care through lifestyle changes, including maintaining a healthy diet, engaging in regular exercise, monitoring blood glucose levels, adhering to medication regimens, and resolving diabetes-related issues [7,8]. Healthy food is essential for managing blood sugar levels and preventing complications, yet it remains a challenge for many. A case management approach provides support and guidance to individuals with T2D, empowering them to make better decisions and enhance their self-management skills [7]. Shin and colleagues reported that the case management program motivated patients to improve their knowledge, self-management skills, health behaviors, and healthcare utilization [9]. Patients’ self-care behaviors were found to have a positive association with provider recommendations, which were directly influenced by diabetes distress, health literacy, family history of diabetes, and prior diabetes education [7,8,9].
The Foods with Function Claims (FFC) system, introduced in Japan in April 2015, revolutionized the health food market by offering a more accessible pathway for manufacturers to make health claims. Unlike the stricter pre-existing systems (Foods for Specified Health Uses; FOSHU, and Foods with Nutrient Function Claims; FNFC), which involved lengthy and costly approval processes, the FFC allows companies, especially SMEs, to market health-benefiting products by simply notifying the Consumer Affairs Agency (CAA). Key to the FFC system is the manufacturer’s responsibility to possess and submit scientific evidence—derived from clinical trials or systematic reviews—to substantiate the product’s claimed health effects and safety. The CAA publicly disseminates this information and conducts post-market surveillance. This deregulation significantly boosted the health food sector, leading to over 3500 FFC-labeled products by 2020. Furthermore, the system encourages research into agricultural products with inherent health benefits, such as β-cryptoxanthin-rich Satsuma mandarins for bone health and O-methylated catechin-rich Bennifuki tea for allergy reduction, thereby enhancing the economic value and quality of local agricultural and food products from farm to consumer. Ultimately, the FFC serves as a crucial tool for promoting innovation and value-addition in Japan’s agricultural and food industries [10].
Amid these challenges, functional foods have emerged as promising adjuncts in the management of T2D. Defined as foods that provide health benefits beyond basic nutrition, functional foods, particularly those from plant-based sources, have attracted interest for their potential to enhance glycemic control, insulin sensitivity, and lipid profiles. Such foods include fruits, vegetables, herbs, spices, and plant extracts rich in bioactive compounds like polyphenols, flavonoids, and dietary fibers. These compounds are known to modulate glucose metabolism, boost insulin sensitivity, and reduce inflammation—crucial factors in the pathophysiology of T2D [3].
However, the existing research on plant-based diets has significant limitations. Studies frequently differ in intervention type, dosage, duration, and participant characteristics, leading to inconsistent results and limited generalizability. Additionally, many studies do not meet high methodological standards, often lacking proper randomization, control groups, or comprehensive outcome measures, which makes drawing definitive conclusions challenging [11]. These issues underscore the need for systematic evaluations to ascertain the efficacy and practical applicability of plant-based interventions in managing T2D.
This systematic review addresses these limitations by synthesizing evidence from a broad spectrum of high-quality studies on plant-based dietary interventions for individuals with T2D. It specifically examines the effects of these interventions on glycemic control and insulin sensitivity as primary outcomes, and lipid profiles as secondary outcomes, providing a thorough analysis of their potential role in disease management. The healthcare Functional Food Claim Japan (FFC) rating system was employed to evaluate the quality of the studies included, ensuring a rigorous assessment of the evidence supporting novel food claims for industry applications [10,12]. The review provides a comprehensive analysis of the potential role of these interventions in managing the disease as well as regulatory landscape of novel food products, with a particular focus on the FFC Japan, where the regulatory framework for functional food claims is well-established and serves as a model for other countries [12].
- Aims
The primary objectives of this study were to identify plant-based diets that effectively reduce blood glucose levels in individuals with type 2 diabetes. Additionally, the secondary objective was to assess the quality of food claims based on the FFC of the Japanese Consumer Affairs Agency (CAA) [13].
- Research questions
Under the FFC standard, can a plant-based diet effectively reduce glycemic levels among individuals with T2D?
2. Materials and Methods
This review is guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify, select, appraise, and synthesize studies [14]. In addition, the review included a risk of bias assessment using the Cochrane Risk of Bias 2 (ROB 2) tool as well as the Japanese FFC rating system [10,11,12,13,14,15]. Details are given below.
2.1. Search Strategy
To identify relevant studies, we conducted a systematic literature review using the PubMed, Scopus, and ScienceDirect databases. The search focused on articles published between 1 January 2010, and 23 December 2023, to emphasize current research. Search terms were developed using the PICO framework (Population, Intervention, Comparison, Outcome) to ensure precision and relevance. Keywords such as “diabetes mellitus Type II”, “plants”, “extract”, and “clinical trial” were combined using Boolean operators (AND, OR) to create search strings like “(diabetes mellitus Type II OR T2DM) AND (plants OR plant-based interventions) AND (RCT OR clinical trial)”. The initial search was conducted on 23 July 2023, with updates on 15 November 2023 and 23 December 2023. Full search terms are detailed in Supplementary Materials.
Following the database searches, the Rayyan platform was used to manage and screen the results systematically. This tool helped in removing duplicates, organizing citations, and facilitating collaboration among reviewers during the study selection process.
Protocol Registration
This systematic review did not register under PRISMA but registered with Rayyan (Registration number: 844955).
2.2. Selection Criteria
This review included studies that met the following criteria: participants were adults (18 years or older) diagnosed with T2D. Interventions had to involve plant-based elements, such as whole plants, extracts, herbs, or functional foods. Studies were required to report outcomes related to glycemic management (e.g., glycated hemoglobin or hemoglobin A1C (HbA1c), fasting blood glucose), insulin sensitivity, or lipid profiles. Eligible study designs included randomized controlled trials (RCTs), non-RCTs, and observational studies with a comparator group. RCTs were used as an additional screening criterion. Only English-language publications providing sufficient data were included. Studies focusing on type 1 diabetes, gestational diabetes, or conditions co-occurring with T2D were excluded, as were studies without a comparison group, those lacking adequate data for analysis, or those evaluating non-plant-based diet.
2.3. Data Extraction
All titles from the database searches were initially screened by the primary researchers (ST, RO, CC, and NS) to discard irrelevant studies. Nine researchers (ST, KP, SL, CC, SC, AS, AO, NS, and RO) independently screened the titles and abstracts of potentially eligible studies using a predefined eligibility checklist to identify those suitable for full-text review. The full texts of 91 selected studies were then retrieved and reviewed by three reviewers (ST, KP, and RO) to determine final inclusion. Data extraction from these studies was performed by the primary researchers, with verification by secondary and tertiary researcher groups. To ensure data accuracy, any discrepancies were collaboratively discussed by all nine reviewers until a unanimous agreement was reached.
2.4. Quality Assessment
2.4.1. Risk of Bias Assessment
The ROB 2 tool will be used to assess the risk of bias in RCTs [16]. Domains include (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in measurement of the outcome, and (5) bias in selection of the reported result. Three reviewers independently assessed the risk of bias. Disagreements were resolved through discussion.
2.4.2. Functional Food Claim Japan Quality Assessment
Japan has a unique regulatory framework for functional foods. This system, launched in 2015 by the CAA, allows manufacturers to make health claims on food labels without needing pre-approval, provided they submit scientific evidence and notify the CAA. The FFC system requires that (1) companies must submit a dossier with scientific evidence supporting the claimed functionality; (2) the claims must be based on well-designed clinical trials or systematic reviews; and (3) transparency is maintained through public access to submitted data on the CAA website [10]. The system ensures that functional claims are evidence-based [12].
The quality of included studies was assessed based on five components: (1) the materials used, their preparation, and any relevant analytical methods; (2) study population; (3) study design, which refers to the overall structure of the study, such as an RCT or a case-control study; (4) research results and conclusions; and (5) health claims validation. The reliability of studies was ranked on a three-level scale (−2 to 0) for each component. Furthermore, the overall levels for each component were used to define the strength of the study, classified as A–E; A: clear and well-founded (all five components at level 0), B: positively grounded (all five components at level −1), C: suggestively grounded (all five components at level −2), D: insufficient evidence, and E: negative evidence [14,15]. Data curation followed the categorization by Mohamed et al., 2019 [4], alongside the FFC Bulletin. Criterion 3 of the FFC, which requires consensus among three food technology background authors for record selection, was applied to minimize selection bias [11,13].
Moreover, for nutrient function claims and other function claims submitted to the Thai FDA, robust evidence, primarily from human clinical trials, especially those focusing on specific population groups, is considered the most compelling. This may be supported by evidence from in vitro studies and epidemiological research [12].
3. Results
The search strategy identified 1850 unique publications. After initial screening, 300 abstracts were assessed for relevance. Ninety-one of these abstracts warranted full-text reviews. Ultimately, 30 studies met our inclusion criteria and were selected for detailed analysis (Figure 1). The interventions in these studies varied in terms of the number and duration of sessions; however, their primary focus was on the effects of plant or plant extract administration on blood chemistry, particularly HbA1c levels, in individuals with T2D.
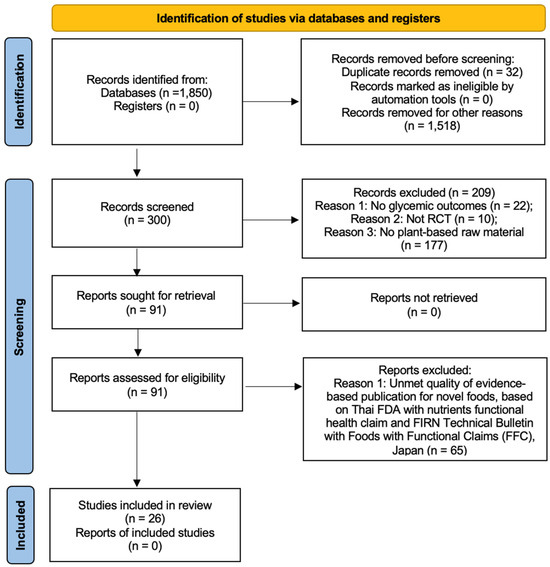
Figure 1.
PRISMA diagram [15].
This systematic review adhered to the methodological standards outlined in the PRISMA guidelines. Initially, 1850 non-duplicated records were screened. Following the assessment of 300 abstracts, 91 publications underwent full-text evaluation. Of these, 26 studies fulfilled the inclusion criteria for this review (Figure 1). Despite variation in the number and duration of treatment sessions among the studies, all interventions predominantly examined the impact of plants or plant extracts on blood chemistry, specifically targeting HbA1c levels in individuals with T2D.
3.1. Study Characteristics
Twenty-six studies were analyzed in this systematic review. All 26 studies [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] were RCTs. The duration of interventions ranged from 8 weeks to 2 years. A significant portion of this research has been conducted in diverse regions, with fifteen studies originating from Iran, significantly contributing to the existing knowledge base [16,17,19,21,22,23,26,27,28,29,30,32,33,34,35]. Additionally, research efforts have extended to the UK [36,37], Germany [38,39], and Canada [18,40], with each country contributing two studies. Furthermore, single studies have been conducted in China [20], USA [24], Ireland [25], Kuwait [31], and Italy [41]. Most participants were aged between 30 and 69 years (n = 21, 81%), while those aged 70 years and above constituted 19% (n = 5) of the study population [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,41].
In this review, the majority of studies (53%) used HbA1c as the primary outcome measure, with observed effect sizes (Cohen’s d) ranging from 0.1 to 1.0. Blood lipid profiles were commonly assessed as a secondary outcome.
3.2. Quality Assessment
Initially, 91 studies were evaluated for quality using the Functional Food Claim Japan (FFC) rating system [10,11]. The quality of the included studies was assessed based on the five components discussed previously. Applying FFC’s third criterion minimized selection bias, particularly ensuring consensus among three food technology background authors for record selection. Ultimately, twenty-six studies were selected for inclusion, with nine achieving a ‘Grade B’ [16,17,18,19,20,21,22,23] and seventeen classified as ‘Grade C’ [24,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,41].
3.3. Impact of the Plant or Plant Extract Intervention on Blood Sugar and HbA1c
Table 1 and Table 2 summarizes the findings from twelve studies graded as “Grade B” that explored the effects of functional food interventions on individuals with T2D. These studies encompassed various interventions, including pomegranate seed oil [16], saffron [17], sesame or canola oil [18], nano-curcumin [19], and a diet incorporating 100 g/d of germinated brown rice (GBR) [20]. The duration of these interventions varied. Pomegranate seed oil supplementation [16] significantly improved fasting blood sugar and insulin sensitivity. Nano-curcumin supplementation [19] reduced blood sugar, HbA1c, and neuropathy symptoms. The GBR intervention [20] decreased inflammation, blood sugar, and lipid levels while increasing beneficial fatty acids. In the control group, placebo rusk powder improved blood sugar control through SIRT1 activation. Additionally, oligofructose-enriched inulin reduced blood sugar, HbA1c, and inflammation markers.

Table 1.
Overview of the eligible studies examining the effects of functional foods interventions for people with type 2 diabetes mellitus (T2D) categorized as Grade B.

Table 2.
Overview of the eligible studies examining the effects of functional foods interventions for people with type 2 diabetes mellitus (T2D) categorized as Grade C.
The studies included a diverse group of participants, primarily adults diagnosed with T2D. The interventions tested were pomegranate seed oil [16], saffron [17], sesame or canola oil [18], nano-curcumin [19], and a diet incorporating 100 g/day of GBR [20]. These interventions varied in duration from one week to three months. Pomegranate seed oil supplementation [16] significantly improved key outcomes such as fasting blood sugar and insulin sensitivity. Saffron supplementation [17] effectively lowered blood sugar levels and reduced inflammation markers. Additionally, supplementation with sesame or canola oil [10] enhanced insulin sensitivity and improved lipid profiles.
The study conducted by Sauder in the United States in 2015 [24] was a randomized, crossover, controlled trial involving 30 adults with T2D. This four-week study compared the effects of a nutritionally balanced diet, including pistachios, to a similar diet, excluding pistachios. Although no significant differences were observed in primary outcomes such as fasting blood sugar, insulin levels, and inflammatory markers, the study did find that fructosamine levels were notably lower in the pistachio group. Additionally, the inclusion of pistachios resulted in significant reductions in secondary outcomes like total cholesterol, HDL cholesterol, and triglycerides compared to the diet without pistachios [24].
The 2021 randomized, double-blind, placebo-controlled crossover study involved 131 adults with T2D and investigated the impact of inulin-type fructan (ITF) prebiotic supplementation on glycemic control and incretin hormone levels. Participants were randomly assigned to receive 16 g/day of ITF or a placebo for six weeks. The results showed a significant decrease in glycated hemoglobin (HbA1c) in the ITF group compared to the placebo group (−0.56% vs. −0.07%, 95% CI: −0.25 to −0.86% vs. −0.15 to 0.29%), which was the primary endpoint. However, ITF supplementation did not significantly affect GLP-1, glucagon-like peptide-2 (GLP-2), fasting glucose, or insulin levels [22].
Figure 2 and Figure 3 show the quality assessment (Revised tool for Risk of Bias in randomized trials) for the included RCTs, generated by robvis [42]. RCT domain 1 (D1), Bias arising from the randomization process; D2, Bias due to deviation from the intended intervention; D3, Bias due to missing outcome data; D4, Risk of bias in measurement of the outcome; D5, Risk of bias in selection of the reported result [15]. Domains 1 and 3 show no risk of bias; the majority of domain 2 have some concern. Despite the risk of bias raised due to missing outcome data, none of the included studies were categorized as “high risk”.
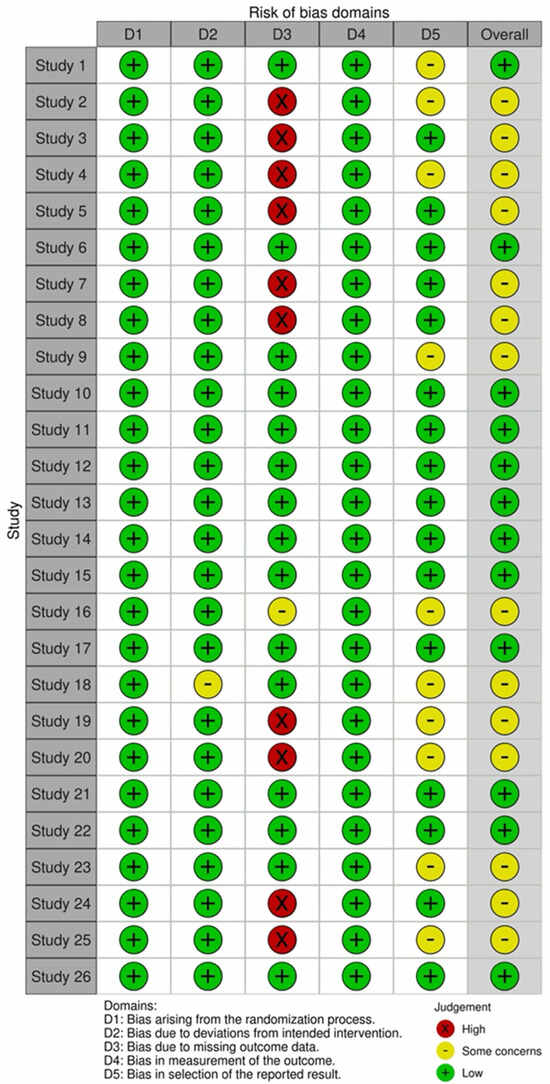
Figure 2.
Risk of bias assessment results using the Cochrane risk of bias (RoB 2) tool for randomized clinical trial studies [42].
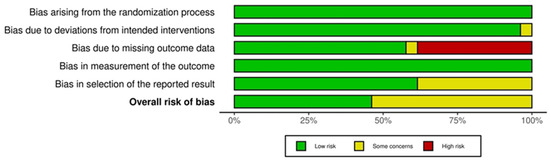
Figure 3.
Risk of bias summary graph for randomized controlled trials [42].
Functional food interventions such as beetroot juice supplementation [17], fenugreek seeds [18], pomegranate seed powder [19], okra powder [20], saffron supplementation [21], and chicory inulin [41] may improve glycemic control, insulin sensitivity, lipid profiles, and other metabolic parameters in individuals with T2D. The findings from these studies demonstrate various benefits, including improved glycemic control and reduced insulin resistance with ZJF juice [22], enhanced fasting blood glucose levels and HbA1c with black tea [23], and decreased fasting blood glucose alongside improved lipid profiles with caper fruit extract [24]. Additionally, increased regulatory T cells and reduced pro-inflammatory cytokines were noted with black tea consumption [23].
The analysis of the provided studies indicates that functional food interventions, such as flaxseed oil [26], soy nuts [27], aged garlic extract [28], and flavanol-rich cocoa powder [29], may positively affect lipid profiles, glycemic control, insulin sensitivity, blood pressure, endothelial function, blood flow, vascular function, muscle mass, and strength in individuals with T2D. However, gelatin capsules and aged garlic extract did not demonstrate significant benefits in the reviewed studies. Furthermore, the effects of flavanol-rich cocoa powder on T2D remain inconclusive.
The analysis of the provided studies suggests that functional food interventions—including resveratrol [30], modified oat [31], insoluble fiber, and oat-derived β-glucan—may improve glycemic control, insulin sensitivity, lipid profiles, and other metabolic parameters in individuals with T2D. Significant findings from these studies include enhanced glycemic control and insulin sensitivity with D-allulose; reduced blood glucose levels with resveratrol; improved control over glycemic levels and increased satiety with whey protein; better glycemic management and insulin responsiveness with modified oat; decreased glucose levels when using white rice; lowered HbA1c, fasting plasma glucose, and 2-h plasma glucose with specific carbohydrate and fat combinations; improved postprandial peak glucose levels with D-allulose; consistent reduction of blood glucose levels with resveratrol; diminished daily hyperglycemia and enhanced overall glycemic control with whey protein preloads; lowered total cholesterol with oat-derived β-glucan; and significant reductions in HbA1c levels, along with improved insulin sensitivity and hepatic insulin clearance with insoluble fiber.
4. Discussion
The provided research suggests that a variety of natural foods and dietary components hold promise for improving metabolic health in individuals with T2D. By targeting key mechanisms involved in the disease process, these foods may contribute to improved glycemic control, lipid profiles, and overall cardiovascular health. However, these findings should be interpreted with caution. There is a need for more rigorous research and a comprehensive approach to managing T2D. Individuals with T2D should consult their healthcare providers before making significant dietary changes.
The review offers an extensive overview of functional foods potentially beneficial for T2D management, yet the evidence is limited by significant flaws. The studies demonstrate considerable heterogeneity in design, population, dosages, and durations, which complicates comparisons and generalizations. Broad claims regarding improvements in glycemic control and inflammation are often nonspecific, with insufficient discussion of the underlying mechanisms or clinically significant outcomes. The lack of consistently reported control groups and the potential for publication and funding biases further undermine the strength of the evidence. Additionally, the emphasis on biomarker changes without corresponding long-term outcome data, along with an absence of standardized recommendations and consideration of individual variability, underscores the necessity for more rigorous research. This includes conducting large-scale RCTs to verify the efficacy and determine the optimal use of these functional foods.
The search strategy employed in this review exhibits several limitations that may affect its comprehensiveness. Primarily, the reliance solely on PubMed, Scopus, and ScienceDirect potentially excludes relevant studies available in other valuable databases such as Embase and the Cochrane Library, thus limiting the scope of the review. Additionally, restricting the search to English-language publications introduces a language bias, possibly overlooking significant research published in other languages. Although the PICO framework was used, the broad search terms like “plants” lacked the necessary specificity to retrieve targeted research effectively. A more detailed approach, using specific plant names or phytochemicals, would likely have yielded more precise results. Moreover, while updates were conducted, the intervals between these updates might have missed recent studies, especially in the rapidly evolving field of nutrition research.
The selection criteria employed in this review also raise several concerns regarding the validity and generalizability of its findings. Including a range of study designs from RCTs to observational studies introduces significant heterogeneity. Observational studies, in particular, are prone to confounding variables and generally offer weaker causal inferences. Excluding studies involving co-occurring conditions, while practical, limits the applicability of the review’s conclusions to real-world scenarios where comorbidities are common. The subjective criterion of “sufficient data” for inclusion lacks clarity and consistency, which could lead to biased study selection. A more objective and predefined definition of what constitutes sufficient data would have been preferable. Furthermore, the vague reference to the use of RCTs as “additional screening criteria” requires clarification. It remains unclear whether RCTs were used to refine initial screenings or to prioritize certain study designs, a distinction that could significantly influence the conclusions drawn from the review.
The data extraction and quality assessment processes within this review reveal several weaknesses that raise concerns about the reliability of the synthesized evidence. The use of the Functional Food Claim Japan (FFC) rating system provides a framework but may not be universally suitable for all nutrition research and is susceptible to subjective biases. The failure of the review to explicitly report inter-rater reliability scores, such as kappa statistics, for data extraction and quality assessment undermines its credibility. Moreover, while the FFC system assesses study quality, the absence of detailed critical appraisals for each study limits the evaluation of bias risk within individual studies.
The findings of this review, which highlight the potential benefits of various functional foods for T2D, necessitate a cautious approach in clinical practice, policy formulation, and future research. Clinically, these functional foods should be considered complementary therapies, requiring personalized recommendations and careful monitoring, complemented by robust patient education. From a policy perspective, the development of evidence-based dietary guidelines and regulated food labeling should be considered, albeit with a strong emphasis on securing further research funding. Future research should prioritize rigorous, large-scale RCTs to confirm efficacy, investigate mechanisms of action, assess long-term outcomes, determine optimal dosages, and explore effects specific to different populations. It is also essential to standardize quality assessment tools. Ultimately, translating these promising findings into tangible clinical and public health benefits depends on robust, well-designed research.
This review on functional foods for type 2 diabetes (T2D) management is significantly limited by the heterogeneity and methodological flaws in the underlying evidence. Key limitations include considerable variability in study designs (from RCTs to observational), diverse populations, dosages, and durations, which hinder direct comparisons and generalizability. Furthermore, the outcomes reported often lack specificity and long-term clinical relevance, focusing on biomarker changes without corresponding patient outcomes. The search strategy itself is limited by relying on a few databases and English-only publications, potentially omitting significant research. Concerns also arise from the broad and subjective selection criteria, including studies prone to confounding and unclear definitions of “sufficient data”. Finally, weaknesses in data extraction and quality assessment, such as the absence of inter-rater reliability, further compromise the synthesized evidence. These collective limitations underscore the urgent need for rigorous, large-scale RCTs to robustly confirm efficacy, investigate mechanisms, and determine optimal use across diverse T2D populations.
Under the FFC framework, several studies have presented practical strategies for using plant-based diets in T2D clinical management, which have been found to be highly acceptable in medical contexts [43]. Clinicians may consider suggesting that their T2D patients adopt a plant-based diet, such as nano-curcumin, green cardamom, and pistachio. The likelihood of certain nano-curcumin formulations effectively treating T2D is cautiously optimistic, with significant potential. Traditional curcumin faces challenges in achieving good bioavailability, which nanotechnology aims to address. Clinical trials on nano-curcumin have yielded promising results, demonstrating significant reductions in fasting blood glucose (FBG), HbA1c, and inflammatory markers (such as CRP). These trials have also shown improvements in lipid profiles and suggest potential benefits for managing diabetic complications like neuropathy. However, more extensive, long-term trials are necessary to standardize the formulations, confirm optimal dosing, and establish its role as a complementary therapy in conjunction with conventional treatments. Ongoing self-management support, education, and follow-up can help patients achieve and maintain dietary changes for glycemic control.
5. Conclusions
This systematic review aimed to evaluate the efficacy of plant-based interventions in managing T2D. A total of 30 studies, primarily RCTs, were included in the analysis. These studies covered a wide range of plant-based products, including fruits, vegetables, herbs, spices, and their extracts.
The findings suggest that certain plant-based interventions may beneficially impact glycemic control, insulin sensitivity, lipid profile, and other metabolic parameters in individuals with T2D. However, the evidence remains inconsistent, and the quality of the studies varies. Some interventions, such as pomegranate seed oil, nano-curcumin, and GBR, showed promising results in improving blood sugar control and insulin sensitivity. Additionally, other interventions, including saffron, sesame or canola oil, and pistachios, also demonstrated potential benefits for glycemic control and lipid profiles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14111919/s1, Full Search Terms.
Author Contributions
Conceptualization, R.O., N.S.-D. and S.T.; methodology, R.O, N.S.-D., S.T., S.L., C.C., S.C., A.S. and K.P.; validation, S.L. and R.O.; formal analysis, R.O, N.S.-D., S.T., S.L., C.C., S.C., A.S. and A.O.; investigation, R.O. and N.S.-D.; resources, N.S.-D.; data curation, R.O., K.P. and S.T.; writing—original draft preparation, R.O., K.P., S.L., N.S.-D. and S.T.; writing—review and editing, R.O., N.S.-D., S.T., S.L., C.C., S.C., A.S. and A.O.; visualization, R.O., S.T. and N.S.-D.; supervision, R.O.; project administration, R.O.; funding acquisition, N.S.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported in part by Suranaree University of Technology [grant number HS8-807-66-12-05] and Health and Wellness research group [grant number RU8-807-61-09].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article: and further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank Suranaree University of Technology for providing the facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- American Diabetes Association. Releases 2023 Standards of Care in Diabetes to Guide Prevention, Diagnosis, and Treatment for People Living with Diabetes. Available online: https://diabetes.org/newsroom/american-diabetes-association-2023-standards-care-diabetes-guide-for-prevention-diagnosis-treatment-people-living-with-diabetes (accessed on 1 December 2024).
- National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes. Available online: https://www.niddk.nih.gov/health-information/diabetes (accessed on 2 October 2024).
- Centers for Disease Control and Prevention. Preventing Type 2 Diabetes. Available online: https://www.cdc.gov/diabetes/prevention-type-2/index.html (accessed on 1 December 2024).
- Mohamed, A.; Staite, E.; Ismail, K.; Winkley, K. A systematic review of diabetes self-management education interventions for people with type 2 diabetes mellitus in the Asian western pacific (AWP) region. Nurs. Open 2019, 6, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Shahaj, O.; Denneny, D.; Schwappach, A.; Pearce, G.; Epiphaniou, E.; Parke, H.L.; Taylor, S.J.C.; Pinnock, H. Supporting self-management for people with hypertension: A meta-review of quantitative and qualitative systematic reviews. J. Hypertens. 2019, 37, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Mahlare, S.S.; Rasweswe, M.M.; Ramavhoy, T.I. Self-management challenges of adults with type 2 diabetes mellitus in Ekurhuleni district primary health care facilities amid COVID-19 lockdown. Afr. J. Prim. Health Care Fam. Med. 2024, 16, e1–e7. [Google Scholar] [CrossRef]
- Misra, R.; Adelman, M.M.; Kirk, B.; Sambamoorthi, U. Relationship Among Diabetes Distress, Health Literacy, Diabetes Education, Patient-Provider Communication and Diabetes Self-Care. Am. J. Health Behav. 2022, 46, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Cangelosi, G.; Mancin, S.; Pantanetti, P.; Nguyen, C.T.T.; Morales Palomares, S.; Biondini, F.; Sguanci, M.; Petrelli, F. Lifestyle Medicine Case Manager Nurses for Type Two Diabetes Patients: An Overview of a Job Description Framework—A Narrative Review. Diabetology 2024, 5, 375–388. [Google Scholar] [CrossRef]
- Shin, S.A.; Kim, H.; Lee, K.; Lin, V.; Liu, G.; Shin, E. Effects of diabetic case management on knowledge, self-management abilities, health behaviors, and health service utilization for diabetes in Korea. Yonsei Med. J. 2015, 56, 244–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- FIRN. Technical Bulletin. Available online: https://firn.or.th/technical-bulletins-main (accessed on 1 May 2024).
- Guidelines for Use of Nutrition and Health Claims (CAC/GL 23-1997) Revised in 2004. Available online: https://www.fao.org/ag/humannutrition/32444-09f5545b8abe9a0c3baf01a4502ac36e4.pdf (accessed on 1 May 2024).
- Shimizu, T. Health claims on functional foods: The Japanese regulations and an international comparison. Nutr. Res. Rev. 2003, 16, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Consumer Affairs Agency, Government of Japan. Foods with Function Claims. Available online: https://www.caa.go.jp/policies/policy/food_labeling/information/ffc/ (accessed on 1 December 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Hamishehkar, H.; Mobasseri, M.; Ebrahimzadeh, V.; Alipour, M.; Alipour, B. Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: A randomized controlled clinical trial. J. Cell. Physiol. 2019, 234, 19621–19628. [Google Scholar] [CrossRef]
- Mobasseri, M.; Ostadrahimi, A.; Tajaddini, A.; Asghari, S.; Barati, M.; Akbarzadeh, M.; Nikpayam, O.; Houshyar, J.; Roshanravan, N.; Alamdari, N.M. Effects of saffron supplementation on glycemia and inflammation in patients with type 2 diabetes mellitus: A randomized double-blind, placebo-controlled clinical trial study. Diabetes Metab Syndr. 2020, 14, 527–534. [Google Scholar] [CrossRef]
- Raeisi-Dehkordi, H.; Amiri, M.; Zimorovat, A.; Moghtaderi, F.; Zarei, S.; Forbes, S.C.; Salehi-Abargouei, A. curcumin compared with sesame and sesame-canola oil on glycaemic control and liver function in patients with type 2 diabetes: A three-way randomized triple-blind crossover trial. Diabetes Metab. Res. Rev. 2020, 37, e3399. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Na, G.; Zhang, J.; Lv, D.; Chen, P.; Song, X.; Cai, F.; Zheng, S.; Wan, W.; Shan, Y. Germinated brown rice enhanced n-3 PUFA metabolism in type 2 diabetes patients: A randomized controlled trial. Clin. Nutr. 2023, 42, 579–589. [Google Scholar] [CrossRef]
- Aghasi, M.; Koohdani, F.; Qorbani, M.; Nasli-Esfahani, E.; Ghazi-Zahedi, S.; Khoshamal, H.; Keshavarz, A.; Sotoudeh, G. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: A randomized double-blind placebo controlled clinical trial. J. Sci. Food Agric. 2019, 99, 3933–3940. [Google Scholar] [CrossRef]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Sauder, K.A.; McCrea, C.E.; Ulbrecht, J.S.; Kris-Etherton, P.M.; West, S.G. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: A randomized trial. Metabolism 2015, 64, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, L.; Sohrab, G.; Hedayati, M.; Ebrahimof, S.; Emami, G.; Razavion, T. Effects of concentrated beetroot juice consumption on glycemic control, blood pressure, and lipid profile in type 2 diabetes patients: Randomized clinical trial study. Ir. J. Med. Sci. 2023, 192, 1143–1153. [Google Scholar] [CrossRef]
- Rafraf, M.; Malekiyan, M.; Asghari-Jafarabadi, M.; Aliasgarzadeh, A. Effect of fenugreek seeds on serum metabolic factors and adiponectin levels in Type 2 diabetic patients. Int. J. Vitam. Nutr. Res. 2014, 84, 196–205. [Google Scholar] [CrossRef]
- Seyed Hashemi, M.; Namiranian, N.; Tavahen, H.; Dehghanpour, A.; Rad, M.H.; Jam-Ashkezari, S.; Emtiazy, M.; Hashempur, M.H. Efficacy of pomegranate seed powder on glucose and lipid metabolism in patients with type 2 diabetes: A prospective randomized double-blind placebo-controlled clinical trial. Complement. Med. Res. 2021, 28, 226–233. [Google Scholar] [CrossRef]
- Moradi, A.; Tarrahi, M.-J.; Ghasempour, S.; Shafiepour, M.; Clark, C.C.T.; Safavi, S.-M. The effect of okra (Abelmoschus esculentus) on lipid profiles and glycemic indices in Type 2 diabetic adults: Randomized double blinded trials. Phytother. Res. 2020, 34, 3325–3332. [Google Scholar] [CrossRef]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Haleem Al-Qaim, Z.; Hadi, A.; Aeinehchi, A.; Sefid-Mooye Azar, P.; Ostadrahimi, A. The effect of saffron (Crocus sativus L.) on glycemia, lipid profile, and antioxidant status in patients with type-2 diabetes mellitus: A randomized placebo-controlled trial. Phytother. Res. 2023, 37, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Ghadiri-Anari, A.; Mehrjardi, A.V.; Dehghani, A.; Zardini, H.Z.; Nadjarzadeh, A. Effect of Ziziphus jujube fruit infusion on lipid profiles, glycaemic index and antioxidant status in type 2 diabetic patients: A randomized controlled clinical trial. Phytother. Res. 2017, 31, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Haines, D.; Al-Ozairi, E.; Dashti, A. Effect of black tea consumption on intracellular cytokines, regulatory T cells and metabolic biomarkers in type 2 diabetes patients. Phytother. Res. 2016, 30, 454–462. [Google Scholar] [CrossRef]
- Huseini, H.F.; Hasani-Rnjbar, S.; Nayebi, N.; Heshmat, R.; Sigaroodi, F.K.; Ahvazi, M.; Alaei, B.A.; Kianbakht, S. Capparis spinosa L. (Caper) fruit extract in treatment of type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2013, 21, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.; Shahbazian, H.; Rezazadeh, A.; Haidari, F.; Jahanshahi, A.; Mahmoud Latifi, S.M.; Shirbeigi, E. The effect of soy nut on serum total antioxidant, endothelial function and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 1387–1391. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Seyyedebrahimi, S.; Esfahani, E.N.; Razi, F.; Meshkani, R. Resveratrol supplementation decreases blood glucose without changing the circulating CD14+CD16+ monocytes and inflammatory cytokines in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Nutr. Res. 2018, 54, 40–51. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Javid, A.Z.; Dehghan, P. The effect of enriched chicory inulin on liver enzymes, calcium homeostasis and hematological parameters in patients with type 2 diabetes mellitus: A randomized placebo-controlled trial. Prim. Care Diabetes 2016, 10, 265–271. [Google Scholar] [CrossRef]
- Atkin, M.; Laight, D.; Cummings, M.H. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo-controlled trial. J. Diabetes Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef]
- McGeoch, S.C.; Johnstone, A.M.; Lobley, G.E.; Adamson, J.; Hickson, K.; Holtrop, G.; Fyfe, C.; Clark, L.F.; Pearson, D.W.; Abraham, P.; et al. A randomized crossover study to assess the effect of an oat-rich diet on glycaemic control, plasma lipids and postprandial glycaemia, inflammation and oxidative stress in type 2 diabetes. Diabet. Med. 2013, 30, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.; Kirch, N.; Gronwald, D.; Wernken, K.; Zimmermann, B.F.; Helfrich, H.P.; Ellinger, S. Regular intake of a usual serving size of flavanol-rich cocoa powder does not affect cardiometabolic parameters in stably treated patients with type 2 diabetes and hypertension-A double-blinded, randomized, placebo-controlled trial. Nutrients 2018, 10, 1435. [Google Scholar] [CrossRef]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef]
- Barre, D.E.; Mizier-Barre, K.A.; Griscti, O.; Hafez, K. Flaxseed oil supplementation manipulates correlations between serum individual mol % free fatty acid levels and insulin resistance in type 2 diabetics. Insulin resistance and percent remaining pancreatic β-cell function are unaffected. Endocr. Regul. 2016, 50, 183–193. [Google Scholar] [CrossRef]
- Tessari, P.; Lante, A. A multifunctional bread rich in beta glucans and low in starch improves metabolic control in type 2 diabetes: A controlled trial. Nutrients 2017, 9, 297. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).