Anaerobic Digestion as an Alternative to Improve the Industrial Production of MnP Economically and Environmentally Using Olive Mill Solid Waste as the Substrate
Abstract
1. Introduction
2. Materials and Methods
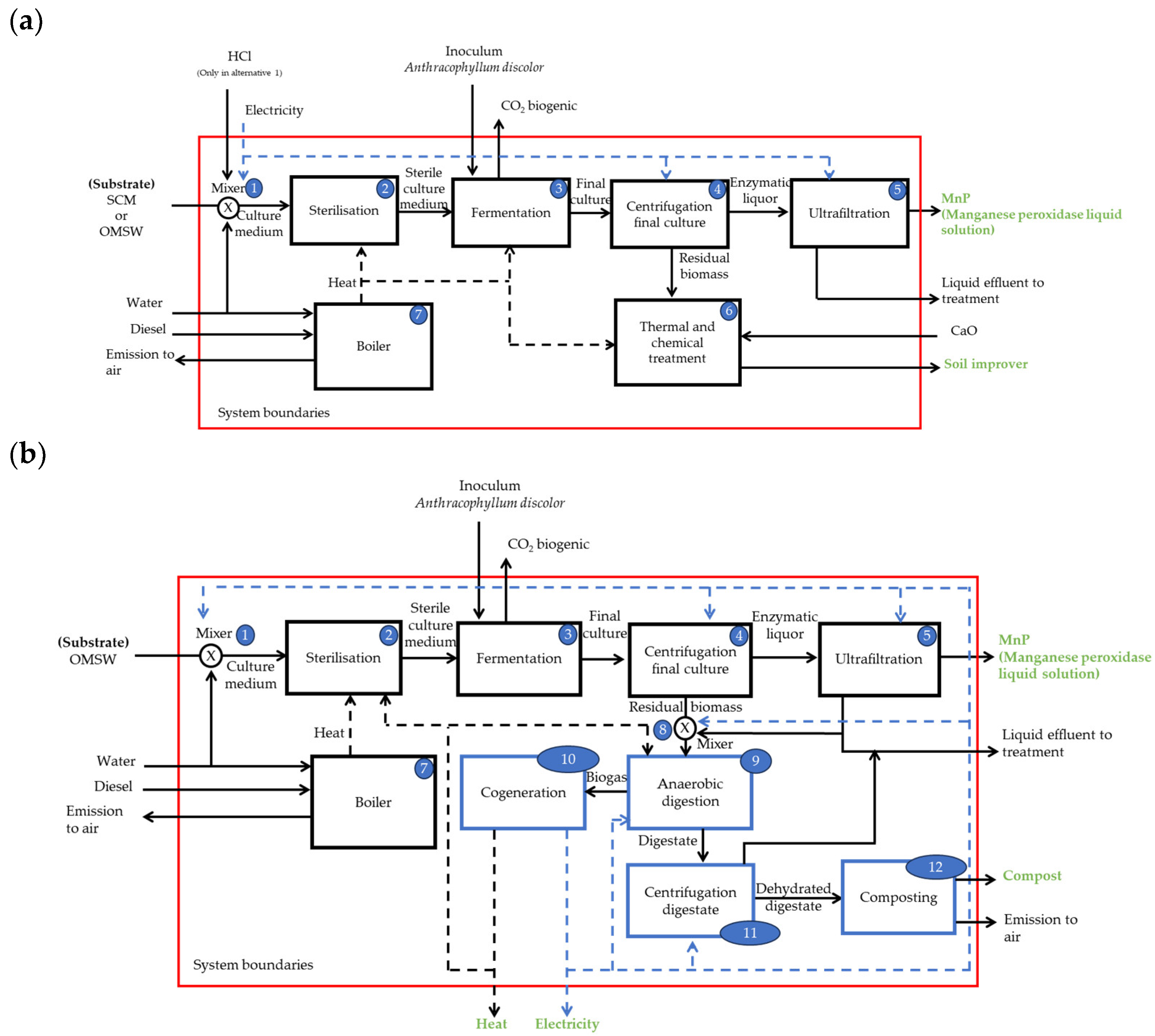
2.1. Description of the Alternatives for the Production of MnP by A. discolor
2.1.1. Boundaries of the MnP Production for Alternatives 1 and 2
2.1.2. Boundaries of the MnP Production for Alternative 3
2.2. Anaerobic Digestion (AD) of Residual Biomass from MnP Production Using OMSW
2.3. Methodology for the Economic Assessment of MnP Production Using A. discolor
2.4. Environmental Assessment
2.4.1. Goals and Scope of the Life Cycle Assessment (LCA)
2.4.2. Life Cycle Assessment (LCA) Inventory Data
2.4.3. Environmental Impact Assessment
3. Results and Discussion
3.1. Economic Assessment for MnP Production by A. discolor
3.1.1. Equipment Size and Investment Costs for the Different MnP Production Alternatives
3.1.2. Economic Assessment of Different MnP Production Alternatives
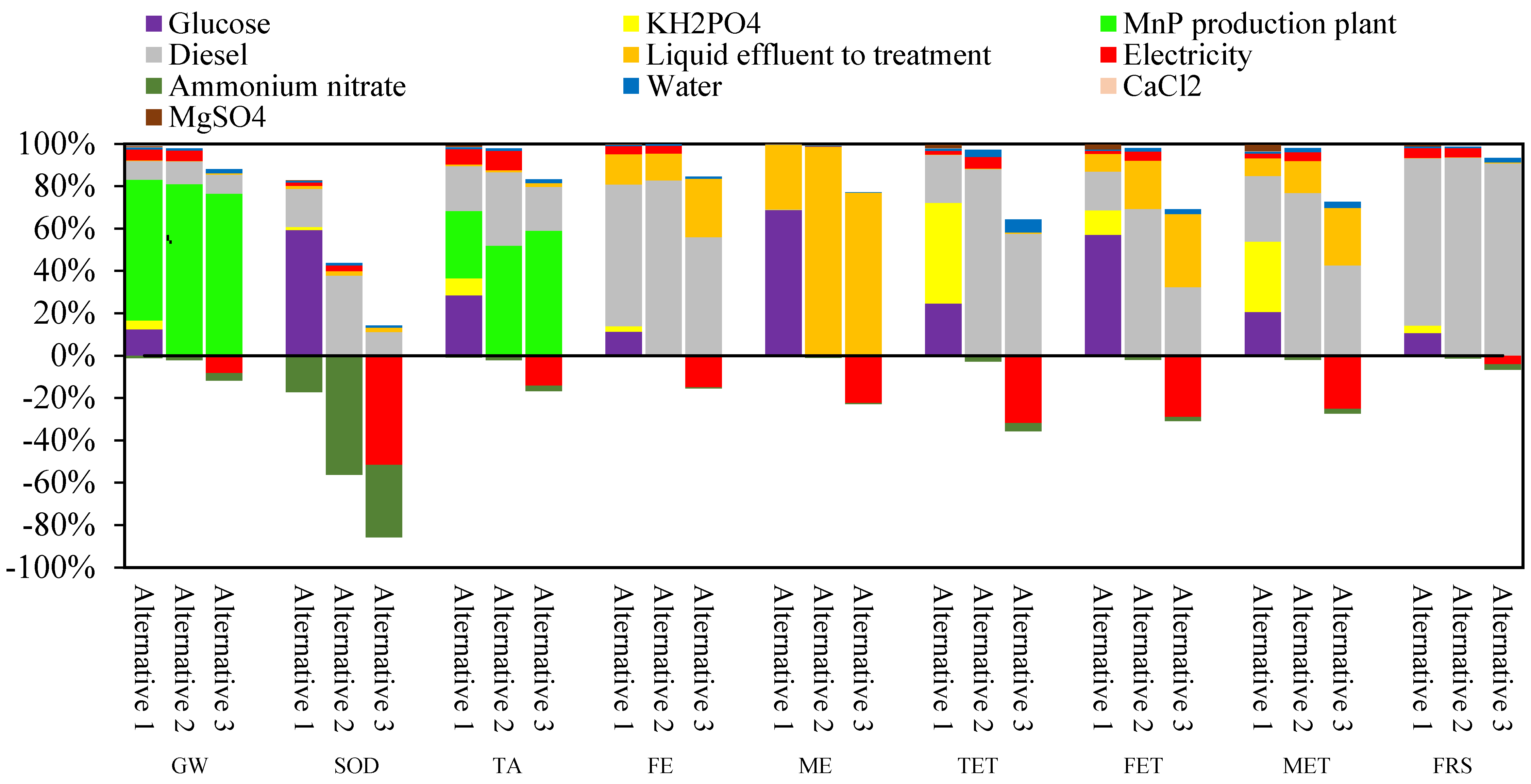
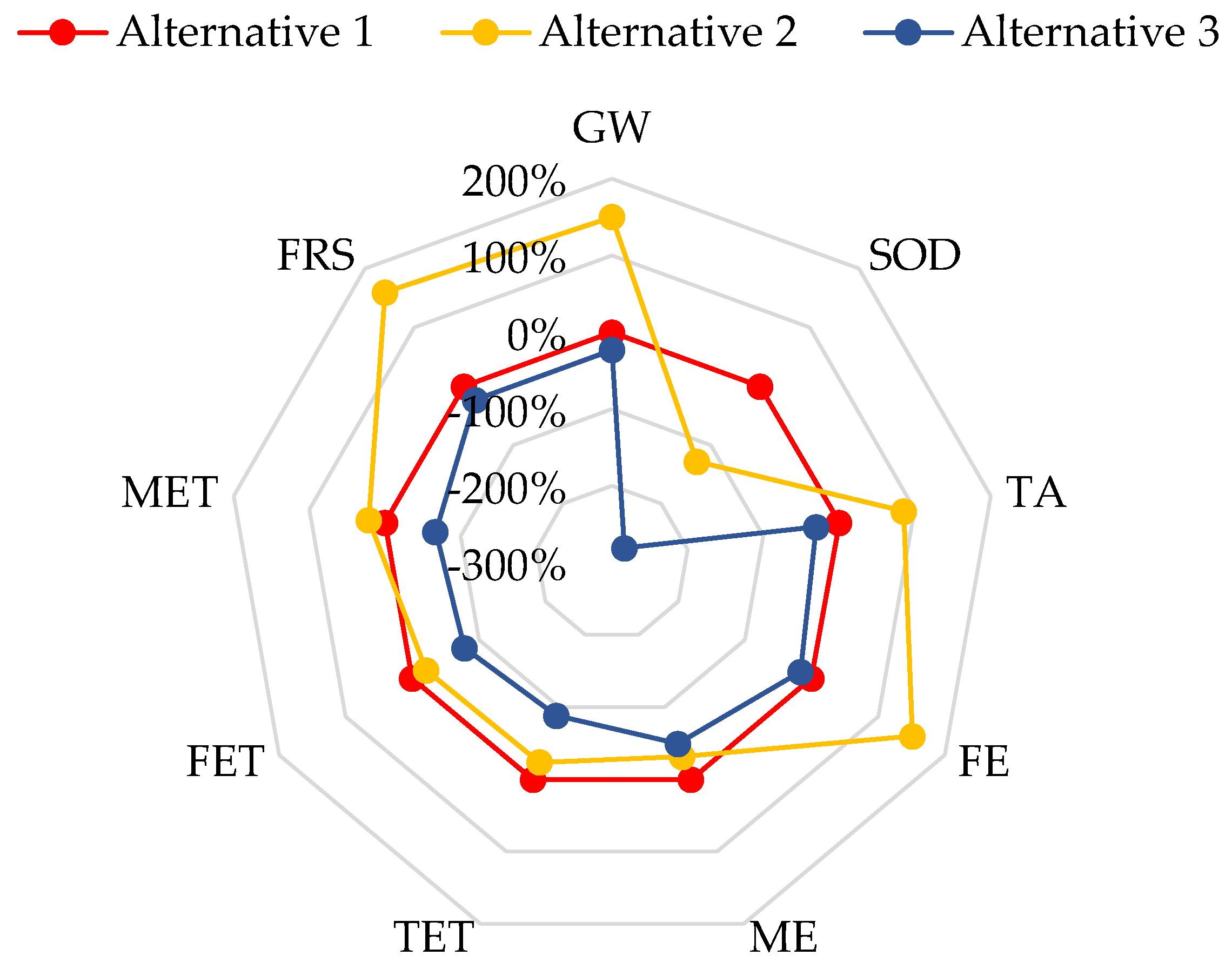
3.2. Life Cycle Assessment (LCA) for MnP Production by A. discolor
3.3. Final Remark and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Arora, P.K. Biotechnological Applications of Manganese Peroxidases for Sustainable Management. Front. Environ. Sci. 2022, 10, 875157. [Google Scholar] [CrossRef]
- Araneda, M.; Pinto-Ibieta, F.; Xu, X.; Rubilar, O.; Fermoso, F.G.; Ciudad, G. Aquaculture Sludge as Co-Substrate for Sustainable Olive Mill Solid Waste Pre-Treatment by Anthracophyllum Discolor. Agronomy 2023, 13, 724. [Google Scholar] [CrossRef]
- Bilal, M.; Zdarta, J.; Jesionowski, T.; Iqbal, H.M.N. Manganese Peroxidases as Robust Biocatalytic Tool—An Overview of Sources, Immobilization, and Biotechnological Applications. Int. J. Biol. Macromol. 2023, 234, 123531. [Google Scholar] [CrossRef]
- Chandra, M.R.G.S.; Madakka, M. Comparative Biochemistry and Kinetics of Microbial Lignocellulolytic Enzymes; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128163283. [Google Scholar]
- Bajpai, P. Green Chemistry and Sustainability in Pulp and Paper Industry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–258. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Bhardwaj, N.K. Biobleaching—An Ecofriendly and Environmental Benign Pulp Bleaching Technique: A Review. J. Carbohydr. Chem. 2019, 38, 87–108. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Nielsen, P.H. Environmental Assessment of Enzyme Use in Industrial Production-a Literature Review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Oxenbøll, K.M.; Wenzel, H. Enzyme Products LCA Case Studies 432 LCA Case Studies Cradle-to-Gate Environmental Assessment of Enzyme Products Produced Industrially in Denmark by Novozymes A/S. Int. J. Life Cycle Assess. 2007, 12, 432–438. [Google Scholar] [CrossRef]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of Lignocellulose Using Ligninolytic Enzymes from White-Rot Fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, D.; Li, R.; Wang, T.; Zhu, Y. Textile Dye Biodecolorization by Manganese Peroxidase: A Review. Molecules 2021, 26, 4403. [Google Scholar] [CrossRef]
- Emami, E.; Zolfaghari, P.; Golizadeh, M.; Karimi, A.; Lau, A.; Ghiasi, B.; Ansari, Z. Effects of Stabilizers on Sustainability, Activity and Decolorization Performance of Manganese Peroxidase Enzyme Produced by Phanerochaete Chrysosporium. J. Environ. Chem. Eng. 2020, 8, 104459. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Mnif, W.; Ben Rebah, F. Iron Oxide / Chitosan Magnetic Nanocomposite Immobilized Manganese Peroxidase For. J. Clean. Prod. 2020, 243, 118634. [Google Scholar]
- Thampraphaphon, B.; Phosri, C.; Pisutpaisal, N.; Thamvithayakorn, P.; Chotelersak, K.; Sarp, S.; Suwannasai, N. High Potential Decolourisation of Textile Dyes from Wastewater by Manganese Peroxidase Production of Newly Immobilised Trametes Hirsuta PW17-41 and FTIR Analysis. Microorganisms 2022, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Leng, Y.; Wan, D.; Chang, F.; Huang, Y.; Li, Z.; Xiong, W.; Wang, J. Transformation of Tetracycline by Manganese Peroxidase from Phanerochaete Chrysosporium. Molecules 2021, 26, 6803. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Jia, Y.; Li, J. Enzymatic Degradation of Tetracycline and Oxytetracycline by Crude Manganese Peroxidase Prepared from Phanerochaete Chrysosporium. J. Hazard. Mater. 2010, 177, 924–928. [Google Scholar] [CrossRef]
- Bosso, L.; Lacatena, F.; Cristinzio, G.; Cea, M.; Diez, M.C.; Rubilar, O. Biosorption of Pentachlorophenol by Anthracophyllum Discolor in the Form of Live Fungal Pellets. New Biotechnol. 2015, 32, 21–25. [Google Scholar] [CrossRef]
- Cea, M.; Jorquera, M.; Rubilar, O.; Langer, H.; Tortella, G.; Diez, M.C. Bioremediation of Soil Contaminated with Pentachlorophenol by Anthracophyllum Discolor and Its Effect on Soil Microbial Community. J. Hazard. Mater. 2010, 181, 315–323. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Gianfreda, L.; Valenzuela, E.; Diez, M.C. Enzymatic Characterization of Chilean Native Wood-Rotting Fungi for Potential Use in the Bioremediation of Polluted Environments with Chlorophenols. World J. Microbiol. Biotechnol. 2008, 24, 2805–2818. [Google Scholar] [CrossRef]
- Elgueta, S.; Santos, C.; Lima, N.; Diez, M.C. Immobilization of the White-rot Fungus Anthracophyllum Discolor to Degrade the Herbicide Atrazine. AMB Express 2016, 6, 104. [Google Scholar] [CrossRef]
- Acevedo, F.; Pizzul, L.; Castillo, M.d.P.; Cuevas, R.; Diez, M.C. Degradation of Polycyclic Aromatic Hydrocarbons by the Chilean White-Rot Fungus Anthracophyllum Discolor. J. Hazard. Mater. 2011, 185, 212–219. [Google Scholar] [CrossRef]
- Adeola, A.O.; Forbes, P.B.C. Advances in Water Treatment Technologies for Removal of Polycyclic Aromatic Hydrocarbons: Existing Concepts, Emerging Trends, and Future Prospects. Water Environ. Res. 2021, 93, 343–359. [Google Scholar] [CrossRef]
- González-Rodríguez, S.; Arias, A.; Feijoo, G.; Moreira, M.T. Modelling and Environmental Profile Associated with the Valorization of Wheat Straw as Carbon Source in the Biotechnological Production of Manganese Peroxidase. Sustainability 2022, 14, 4842. [Google Scholar] [CrossRef]
- Lú-Chau, T.A.; Martínez-Patiño, J.C.; Gullón, B.; García-Torreiro, M.; Moreira, M.T.; Lema, J.M.; Eibes, G. Scale-up and Economic Analysis of the Production of Ligninolytic Enzymes from a Side-Stream of the Organosolv Process. J. Chem. Technol. Biotechnol. 2018, 93, 3125–3134. [Google Scholar] [CrossRef]
- Benavides, V.; Pinto-Ibieta, F.; Serrano, A.; Rubilar, O.; Ciudad, G. Use of Anthracophyllum Discolor and Stereum Hirsutum as a Suitable Strategy for Delignification and Phenolic Removal of Olive Mill Solid Waste. Foods 2022, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Phenols Recovery after Steam Explosion of Olive Mill Solid Waste and Its Influence on a Subsequent Biomethanization Process. Bioresour. Technol. 2017, 243, 169–178. [Google Scholar] [CrossRef]
- Messineo, A.; Maniscalco, M.P.; Volpe, R. Biomethane Recovery from Olive Mill Residues through Anaerobic Digestion: A Review of the State of the Art Technology. Sci. Total Environ. 2020, 703, 135508. [Google Scholar] [CrossRef]
- Chile Oliva. Informe Anual del Mercado de Aceite de Oliva; Chile Oliva: Santiago, Chile, 2022. [Google Scholar]
- Becker, M.; Ziemińska-Stolarska, A.; Markowska, D.; Lütz, S.; Rosenthal, K. Comparative Life Cycle Assessment of Chemical and Biocatalytic 2’3’-Cyclic GMP-AMP Synthesis. ChemSusChem 2023, 16, e202201629. [Google Scholar] [CrossRef]
- Rajendran, K.; Murthy, G.S. Techno-Economic and Life Cycle Assessments of Anaerobic Digestion—A Review. Biocatal. Agric. Biotechnol. 2019, 20, 101207. [Google Scholar] [CrossRef]
- van den Oever, A.E.M.; Cardellini, G.; Sels, B.F.; Messagie, M. Life Cycle Environmental Impacts of Compressed Biogas Production through Anaerobic Digestion of Manure and Municipal Organic Waste. J. Clean. Prod. 2021, 306, 127156. [Google Scholar] [CrossRef]
- Hamedani, S.R.; Villarini, M.; Colantoni, A.; Carlini, M.; Cecchini, M.; Santoro, F.; Pantaleo, A. Environmental and Economic Analysis of an Anaerobic Co-Digestion Power Plant Integrated with a Compost Plant. Energies 2020, 13, 2724. [Google Scholar] [CrossRef]
- Mendieta, O.; Castro, L.; Escalante, H.; Garfí, M. Low-Cost Anaerobic Digester to Promote the Circular Bioeconomy in the Non-Centrifugal Cane Sugar Sector: A Life Cycle Assessment. Bioresour. Technol. 2021, 326, 124783. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Techno-Economic Assessment of Bioenergy and Fertilizer Production by Anaerobic Digestion of Brewer’s Spent Grains in a Biorefinery Concept. J. Clean. Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Biomethanization of Olive Mill Solid Waste after Phenols Recovery through Low-Temperature Thermal Pre-Treatment. Waste Manag. 2017, 61, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Valenti, F.; Zhong, Y.; Sun, M.; Porto, S.M.C.; Toscano, A.; Dale, B.E.; Sibilla, F.; Liao, W. Anaerobic Co-Digestion of Multiple Agricultural Residues to Enhance Biogas Production in Southern Italy. Waste Manag. 2018, 78, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kuusik, A.; Pachel, K.; Kuusik, A.; Loigu, E. Anaerobic Co-Digestion of Sewage Sludge with Fish Farming Waste. In Proceedings of the 9th International Conference “Environmental Engineering”, Vilnius, Lithuania, 22–23 May 2014. [Google Scholar] [CrossRef]
- Coral-Velasco, D.A.; Correa, L.F.; Sánchez, Ó.J.; Gómez, J.A. Process Design and Techno-Economic Assessment of Cellulolytic Enzymes Production from Coffee Husk through Process Simulation. Biomass Convers. Biorefinery 2022, 14, 8353–8373. [Google Scholar] [CrossRef]
- Andess Chile. Precio Medio en Chile. 2023. Available online: https://www.andess.cl/el-agua-potable-en-chile-cuesta-entre-1-y-2-por-litro/ (accessed on 25 September 2023).
- BulkSupplements. Precio Thiamine. Available online: https://www.ubuy.cl/sp/product/4F3CZ7Q0C-bulksupplements-thiamine-hcl-vitamin-b1-powder-5-kilograms (accessed on 25 September 2023).
- Comisión Nacional de Energía (CNE), Chile. 2023. Available online: https://www.cne.cl/precio-medio-de-mercado-2/2023-2/ (accessed on 25 September 2023).
- Reachem. Precio Calcio Cloruro. Available online: https://www.reachem.cl/producto/calcio-cloruro-72-1-kg/ (accessed on 25 September 2023).
- SciencesLife, T. Precio Peptona. Available online: https://taag-ls.cl/products/medio-de-cultivo-peptona-de-soja (accessed on 25 September 2023).
- Servicio de Impuestos Internos, C. Dólar Observado, Septiembre. 2023. Available online: https://www.sii.cl/valores_y_fechas/dolar/dolar2023.htm (accessed on 25 September 2023).
- Banco Mundial. Tasa Tributaria Total (% de Utilidades Comerciales)—Chile. Available online: https://datos.bancomundial.org/indicator/IC.TAX.TOTL.CP.ZS?locations=CL (accessed on 25 September 2023).
- GlobalPetrolPrices. Chile, Precios Del Diesel. 2023. Available online: https://es.globalpetrolprices.com/Chile/diesel_prices/ (accessed on 25 September 2023).
- Productos Químicos, Chile. Precio Fosfato Monopotásico. Available online: https://productosquimicos.cl/producto/fosfato-monopotasico-1-kg/ (accessed on 25 September 2023).
- Productos Químicos, Chile. Precio Sulfato de Manganeso. Available online: https://productosquimicos.cl/producto/sulfato-de-manganeso-1-kg/ (accessed on 25 September 2023).
- Productos Químicos, Chile. Precio Ácido Clorhídrico. Available online: https://productosquimicos.cl/producto/acido-clorhidrico-30/ (accessed on 25 September 2023).
- Micosecha. Precio Oxido de Calcio. Available online: https://www.micosecha.cl/product-page/cal-viva-oxido-de-calcio-1-5-kilos (accessed on 25 September 2023).
- Karimi Alavijeh, M.; Meyer, A.S.; Gras, S.L.; Kentish, S.E. Simulation and Economic Assessment of Large-Scale Enzymatic N-Acetyllactosamine Manufacture. Biochem. Eng. J. 2020, 154, 107459. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment: Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044; Environmental Management—Life Cycle Assessment: Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Gobierno Regional de Atacama. Fertilización del Olivo en El Valle del Huasco; Gobierno Regional de Atacama: Atacama, Chile, 2000.
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.D.M.; Hollander, A.; Zijp, M.; van Zelm, R. ReCiPe 2016: A Harmonized Life Cycle Impact Assessment Method at Midpoint and Endpoint Level Report I: Characterization. Int. J. Life Cycle Assess. 2016, 22, 138–147. [Google Scholar] [CrossRef]
- Acevedo, F.; Pizzul, L.; Castillo, M.d.P.; Rubilar, O.; Lienqueo, M.E.; Tortella, G.; Diez, M.C. A Practical Culture Technique for Enhanced Production of Manganese Peroxidase by Anthracophyllum Discolor Sp4. Braz. Arch. Biol. Technol. 2011, 54, 1175–1186. [Google Scholar] [CrossRef]
- Serrano, A.; Villa-Gomez, D.; Fermoso, F.G.; Alonso-Fariñas, B. Is Anaerobic Digestion a Feasible Alternative to the Combustion of Olive Mill Solid Waste in Terms of Energy Production? A Critical Review. Biofuels Bioprod. Biorefining 2021, 15, 150–162. [Google Scholar] [CrossRef]
- Suhartini, S.; Lestari, Y.P.; Nurika, I. Estimation of Methane and Electricity Potential from Canteen Food Waste. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 1–6. [Google Scholar] [CrossRef]
- Catalán, E.; Komilis, D.; Sánchez, A. Environmental Impact of Cellulase Production from Coffee Husks by Solid-State Fermentation: A Life-Cycle Assessment. J. Clean. Prod. 2019, 233, 954–962. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current Perspective on Production and Applications of Microbial Cellulases: A Review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Lütz, S.; Rosenthal, K. Environmental Assessment of Enzyme Production and Purification. Molecules 2021, 26, 573. [Google Scholar] [CrossRef] [PubMed]
- Campello, L.D.; Barros, R.M.; Filho, G.L.T.; dos Santos, I.F.S. Analysis of the Economic Viability of the Use of Biogas Produced in Wastewater Treatment Plants to Generate Electrical Energy. Environ. Dev. Sustain. 2021, 23, 2614–2629. [Google Scholar] [CrossRef]
- Gaffey, J.; Collins, M.N.; Styles, D. Review of Methodological Decisions in Life Cycle Assessment (LCA) of Biorefinery Systems across Feedstock Categories. J. Environ. Manag. 2024, 358, 120813. [Google Scholar] [CrossRef]
- Bustamante, M.; González, M.E.; Cartes, A.; Diez, M.C. Effect of Soya Lecithin on the Enzymatic System of the White-Rot Fungi Anthracophyllum Discolor. J. Ind. Microbiol. Biotechnol. 2011, 38, 189–197. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Separation Process Principles; Prentice Hall Professional Technical Reference: Hoboken, NJ, USA, 2003. [Google Scholar]
- Rubilar, O.; Elgueta, S.; Tortella, G.; Gianfreda, L.; Diez, M.C. Pelletization of Anthracophyllum Discolor for Water and Soil Treatment Contaminated with Organic Pollutants. Rev. Cienc. Suelo Nutr. Veg. 2009, 9, 161–175. [Google Scholar] [CrossRef]
- Enevoldsen, A.D.; Hansen, E.B.; Jonsson, G. Electro-Ultrafiltration of Amylase Enzymes: Process Design and Economy. Chem. Eng. Sci. 2007, 62, 6716–6725. [Google Scholar] [CrossRef]
- Paszczyński, A.; Crawford, R.L.; Huynh, V.B. Manganese Peroxidase of Phanerochaete Chrysosporium: Purification. Methods Enzymol. 1988, 161, 264–270. [Google Scholar] [CrossRef]
- Alonso-Fariñas, B.; Oliva, A.; Rodríguez-Galán, M.; Esposito, G.; García-Martín, J.F.; Rodríguez-Gutiérrez, G.; Serrano, A.; Fermoso, F.G. Environmental Assessment of Olive Mill Solid Waste Valorization via Anaerobic Digestion versus Olive Pomace Oil Extraction. Processes 2020, 8, 626. [Google Scholar] [CrossRef]
- Saer, A.; Lansing, S.; Davitt, N.H.; Graves, R.E. Life Cycle Assessment of a Food Waste Composting System: Environmental Impact Hotspots. J. Clean. Prod. 2013, 52, 234–244. [Google Scholar] [CrossRef]
- Sampedro, I.; Marinari, S.; D’Annibale, A.; Grego, S.; Ocampo, J.A.; García-Romera, I. Organic Matter Evolution and Partial Detoxification in Two-Phase Olive Mill Waste Colonized by White-Rot Fungi. Int. Biodeterior. Biodegrad. 2007, 60, 116–125. [Google Scholar] [CrossRef]
- Gilpin, G.S.; Andrae, A.S.G. Comparative Attributional Life Cycle Assessment of European Cellulase Enzyme Production for Use in Second-Generation Lignocellulosic Bioethanol Production. Int. J. Life Cycle Assess. 2017, 22, 1034–1053. [Google Scholar] [CrossRef]
- Kopsahelis, A.; Kourmentza, C.; Zafiri, C.; Kornaros, M. Gate-to-Gate Life Cycle Assessment of Biosurfactants and Bioplasticizers Production via Biotechnological Exploitation of Fats and Waste Oils. J. Chem. Technol. Biotechnol. 2018, 93, 2833–2841. [Google Scholar] [CrossRef]
- Catalán, E.; Sánchez, A. Solid-State Fermentation (SSF) versus Submerged Fermentation (SmF) for the Recovery of Cellulases from Coffee Husks: A Life Cycle Assessment (LCA) Based Comparison. Energies 2020, 13, 2685. [Google Scholar] [CrossRef]
| Item | Price | Unit | Reference |
|---|---|---|---|
| Electricity | 0.1 | USD/kWh | [40] |
| Diesel | 1.2 | USD/kg | [45] |
| Water | 1.7 1 | USD/m3 | [38] |
| Glucose | 17.9 | USD/kg | Sigma-Aldrich, St Louis, MO, USA (G8270-25KG) |
| Peptone | 68.6 | USD/kg | [42] |
| KH2PO4 | 20.5 | USD/kg | [46] |
| MgSO4 | 7.7 | USD/kg | [47] |
| CaCl2 | 3.5 | USD/kg | [41] |
| Thiamine | 95.2 | USD/kg | [39] |
| HCl | 14.4 | USD/L | [48] |
| CaO | 2.6 | USD/kg | [49] |
| Parameters | Value/Description | ||
| Currency | US dollar | ||
| Conversion factor | USD = CLP 889.21 | [43] | |
| Year of analysis | 2023 | ||
| Production | 1200 kg of MnP/year | ||
| Project lifetime | 25 years | ||
| Annual operating time | 330 days | ||
| Discount rate | 13.6% | [37] | |
| Taxation rate (year 2019) | 35% | [44] | |
| Depreciation | Straight line method over 10 years | [37] | |
| Equipment Size | Cost of Scaling for Each Equipment (USD) | Reference | |||||
|---|---|---|---|---|---|---|---|
| Alternative | Alternative | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| Production plant MnP | |||||||
| Tank for culture medium (m3) | 400 | 1 | 1 | 318,215 | 587,949 | 587,949 | [37] |
| Pumping flow (m3/h) | 25 | 62.5 | 62.5 | 10 | 10 | 10 | LS |
| Sterilizer (m3/h) | 25 | 62.5 | 62.5 | 103,047 | 154,215 | 154,215 | [37] |
| Fermenter (m3) | 200 | 500 | 500 | 355,036 | 484,811 | 484,811 | [37] |
| Centrifugal decanter (m3/h) | 25 | 62.5 | 62.5 | 130,009 | 251,472 | 251,472 | [37] |
| Ultrafiltration unit (m3/h) | 25 | 62.5 | 62.5 | 307,224 | 675,590 | 675,590 | [37] |
| Seed fermenter (m3) | 5 | 5 | 5 | 40,571 | 40,571 | 40,571 | [37] |
| Tank for biomass (m3) | 20 | 277 | 277 | 26,989 | 197,992 | 197,992 | [37] |
| Tank for fuel (m3) | 9 | 27 | 9 | 15,612 | 42,016 | 15,640 | [37] |
| Boiler (ton/h) | 21 | 147 | 13 | 20 | 40 | 20 | LS |
| Piping and electrical projects | 185,738 | 347,846 | 341,353 | [37] | |||
| Production plant energy | |||||||
| Anaerobic reactor and cogeneration engine (kWe) | 142 | 451,560 | [25] | ||||
| Total investment (USD) | 1,512,441 | 2,832,464 | 3,231,155 | ||||
| Item | Alternative 1 | Alternative 2 | Alternative 3 | MnP Production Using SCM and Side-Stream of the Organosolv Process [23] | Cellulase Using Coffee Husk as Substrate [58] |
|---|---|---|---|---|---|
| Variable costs (USD/kg MnP) | 1573 | 299 | 103 | n.r. | 28.7 |
| Minimum selling price (MSP) (USD/kg MnP) | 2083 | 1.06 | 931 | n.r. | n.r. |
| Assumed selling price (ASP) (USD/kg MnP) | 2291 | 1166 | 1166 | 7597 | 42 |
| NPV (USD) | 873,444 | 442,987 | 984,464 | n.r. | 32,958 |
| IRR (%) | 27.1 | 17.5 | 20.9 | n.r. | 61.02 |
| Payback period (year) | 3.4 | 4.6 | 4.1 | n.r. | 2.27 |
| Annual profit margin (APM) (USD/year) | 602,299 | 780,866 | 1,016,280 | n.r. | 11,956 |
| Energy | Stage | Alternative 1 | Alternative 2 | Alternative 3 |
|---|---|---|---|---|
| Electricity (kWh) | Mixer (1) | 15.2 | 38.0 | 38.0 |
| Centrifugation final culture (4) | 12.0 | 30.0 | 30.0 | |
| Ultrafiltration (5) | 5.8 | 12.7 | 12.7 | |
| Mixer (8) | 38.0 | |||
| Anaerobic digestion (9) | 38.0 | |||
| Centrifugation digestate (11) | 25.3 | |||
| Total electricity | 33.0 | 80.7 | 182.0 | |
| Heat (MJ) | Sterilization (steam) (2) | 1625 (92%) | 4219 (88%) | 4219 (97%) |
| Fermentation (3) | 84.6 | |||
| Thermal treatment residual biomass (6) | 50.8 | 593.9 | ||
| Anaerobic digestion (9) | 133.4 | |||
| Total heat | 1760 (100%) | 4813 (100%) | 4352 (100%) | |
| Impact Category | Unit | Alternative 1 | Alternative 2 | Alternative 3 | MnP Production Using SCM and Wheat Straw as Substrate [22] | Cellulase Production Using Coffee Husk as Substrate [58] |
|---|---|---|---|---|---|---|
| GW | kg CO2 eq | 351.3 | 879.0 | 271.5 | 2852 | 425,722 |
| SOD | Kg CFC-11 eq | 2.4 × 10−4 | −6.6 × 10−5 | −4.2 × 10−4 | 2.0 × 10−3 | 1.9 × 10−1 |
| TA | kg SO2 eq | 1.3 | 2.5 | 0.9 | 11.2 | 2.6 |
| FE | kg P eq | 0.05 | 0.12 | 0.04 | 0.74 | 16.00 |
| ME | kg N eq | 0.07 | 0.05 | 0.04 | 0.39 | 1.44 |
| TET | kg 1.4-DCB | 487 | 370 | 56 | 2.28 | 244,249 |
| FET | kg 1.4-DCB | 0.43 | 0.34 | 0.09 | 22.1 | 63.9 |
| MET | kg 1.4-DCB | 0.58 | 0.71 | 0.19 | 31.13 | 228.00 |
| FRS | kg oil eq | 107 | 278 | 82 | 864 | 100,436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araneda, M.; Pinto-Ibieta, F.; Alonso-Fariñas, B.; Fermoso, F.G.; Ciudad, G. Anaerobic Digestion as an Alternative to Improve the Industrial Production of MnP Economically and Environmentally Using Olive Mill Solid Waste as the Substrate. Foods 2025, 14, 1918. https://doi.org/10.3390/foods14111918
Araneda M, Pinto-Ibieta F, Alonso-Fariñas B, Fermoso FG, Ciudad G. Anaerobic Digestion as an Alternative to Improve the Industrial Production of MnP Economically and Environmentally Using Olive Mill Solid Waste as the Substrate. Foods. 2025; 14(11):1918. https://doi.org/10.3390/foods14111918
Chicago/Turabian StyleAraneda, Michael, Fernanda Pinto-Ibieta, Bernabé Alonso-Fariñas, Fernando G. Fermoso, and Gustavo Ciudad. 2025. "Anaerobic Digestion as an Alternative to Improve the Industrial Production of MnP Economically and Environmentally Using Olive Mill Solid Waste as the Substrate" Foods 14, no. 11: 1918. https://doi.org/10.3390/foods14111918
APA StyleAraneda, M., Pinto-Ibieta, F., Alonso-Fariñas, B., Fermoso, F. G., & Ciudad, G. (2025). Anaerobic Digestion as an Alternative to Improve the Industrial Production of MnP Economically and Environmentally Using Olive Mill Solid Waste as the Substrate. Foods, 14(11), 1918. https://doi.org/10.3390/foods14111918








