Formulation and Evaluation of a Nutritionally Enriched Plant Protein-Based Matrix Using Low Temperature Extrusion Cooking—Air Drying Technology
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Experimental Design
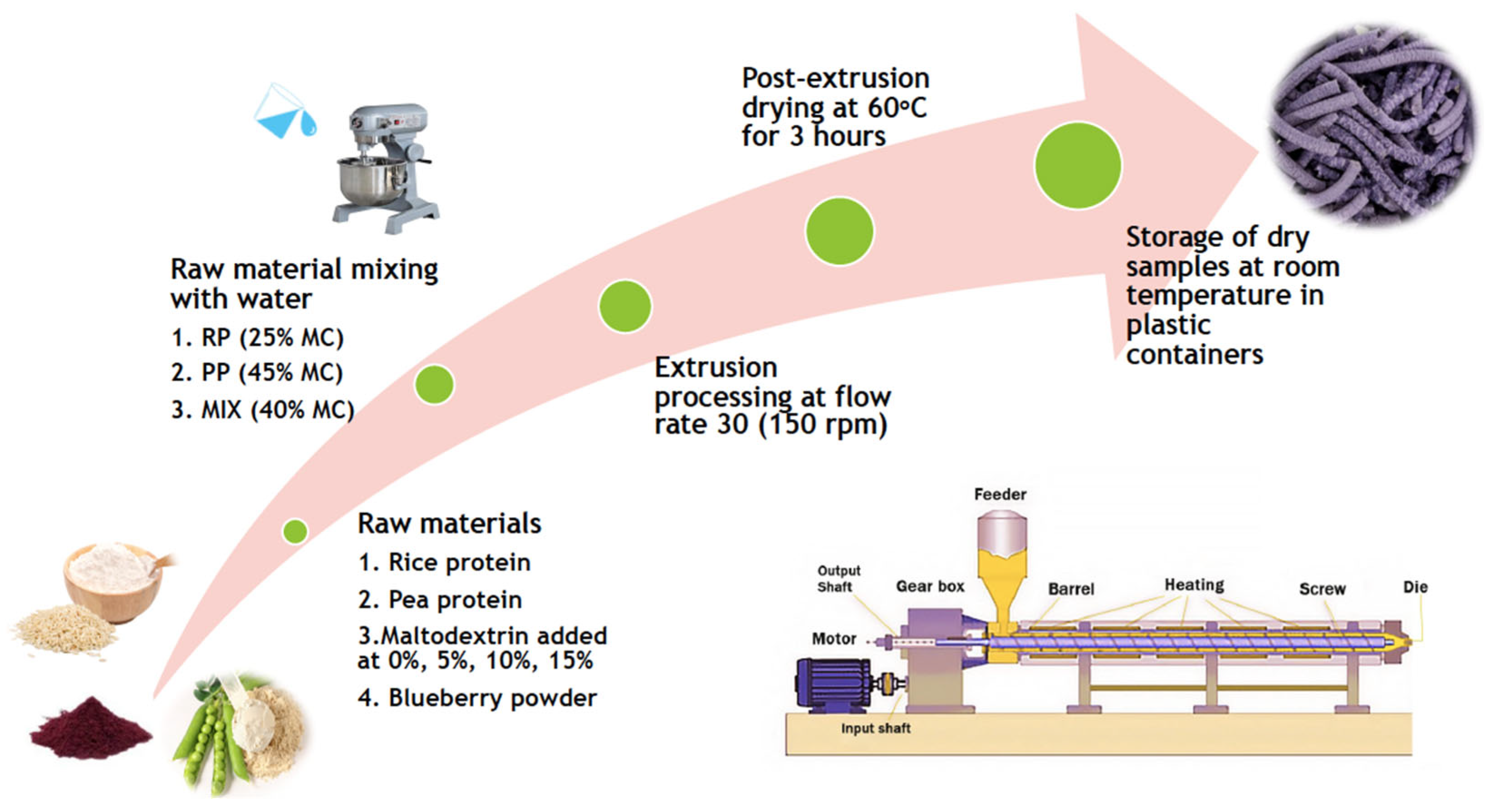
2.3. Feed Preparation
2.4. Extrusion Cooking and Air Drying
2.5. Sample Drying and Storage
3. Testing
3.1. Output Temperature
3.2. Expansion Ratio
3.3. Exit Moisture Content (%)
3.4. Water Holding Capacity (WHC)
3.5. Rehydration Ratio (RR)
3.6. Texture Analysis
3.7. Color Analysis and Appearance
3.8. Chemical Properties
3.8.1. Anthocyanin Content
3.8.2. Anthocyanin Retention Rate
3.8.3. Antioxidant Activity
3.9. Statistical Analysis
4. Results and Discussion
4.1. Output Temperature
4.2. Exit Moisture Content (%)
4.3. Expansion Ration (ER)
4.4. Rehydration Ratio (RR)
4.5. Water Holding Capacity (WHC)
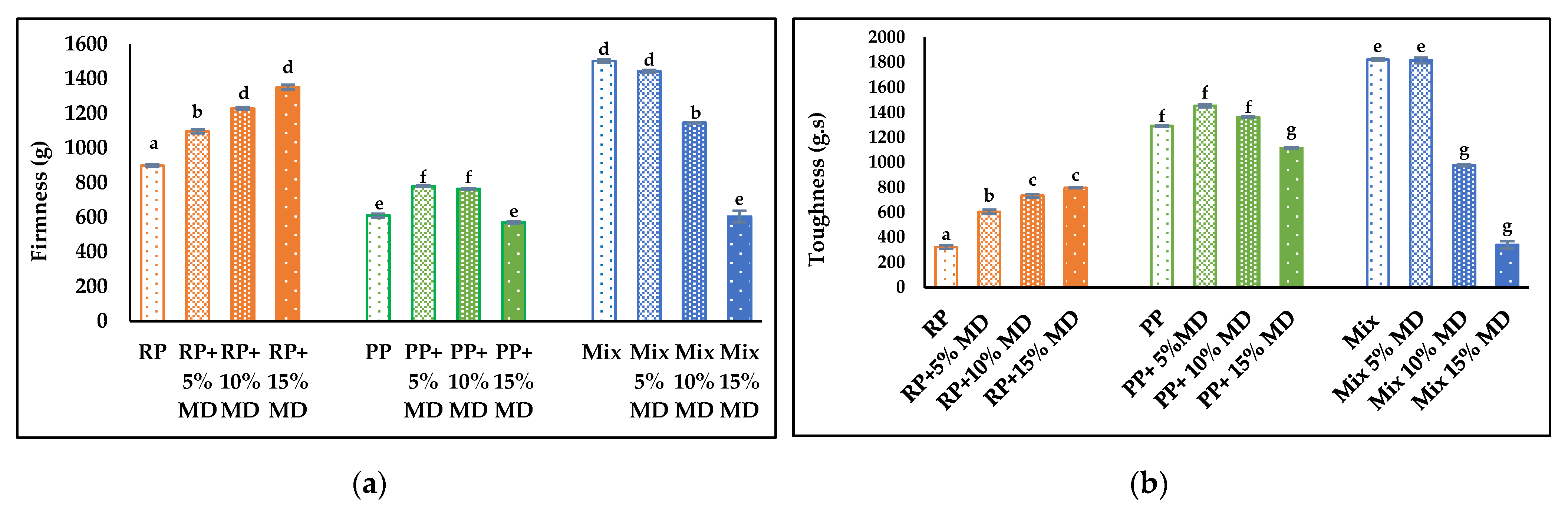
4.6. Textural Properties
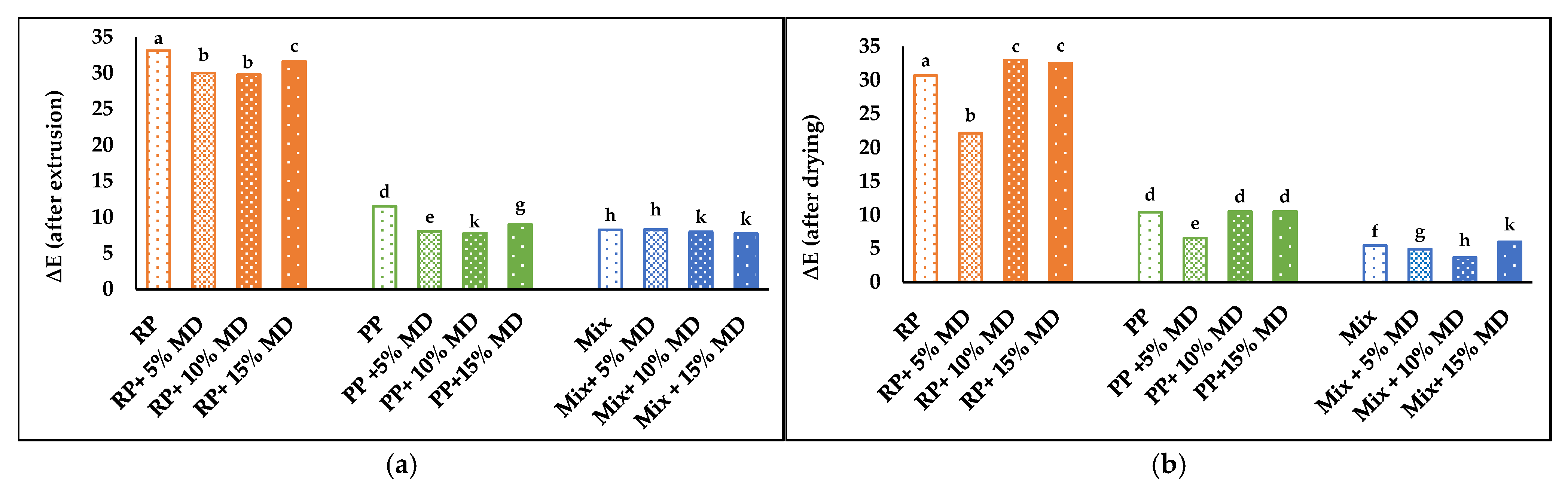
4.7. Appearance and Color Analysis
4.7.1. Appearance
4.7.2. Color Parameters
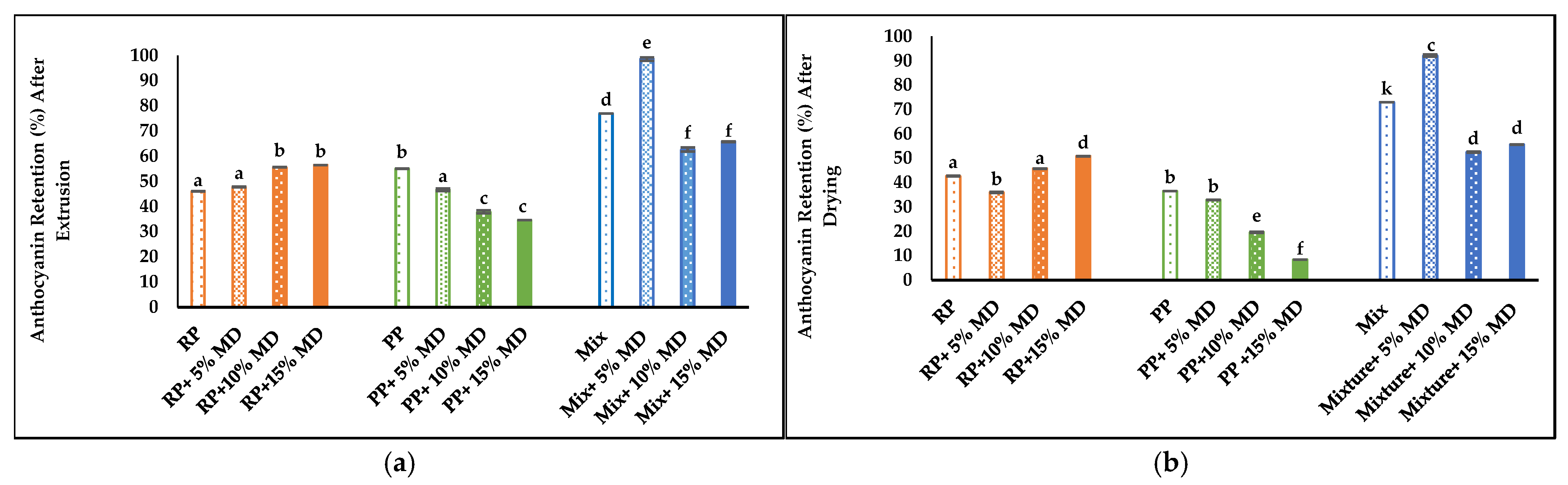
4.8. Chemical Components
4.8.1. Anthocyanin Content (AA) and Anthocyanin Retention (%)
4.8.2. Radical Scavenging Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirth, M.; Leiter, A.; Beck, S.M.; Schuchmann, H.P. Effect of extrusion cooking process parameters on the retention of bilberry anthocyanins in starch based food. J. Food Eng. 2014, 125, 139–146. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Vega, E.N.; García-Herrera, P.; Fernández-Tomé, S.; Pedrosa, M.M.; Arribas, C.; Berrios, J.D.J.; Pan, J.; Leal, P.; Cámara, M.; et al. New Gluten-Free Extruded Snack-Type Products Based on Rice and Chickpea and Fortified with Passion Fruit Skin: Extrusion Cooking Effect on Phenolic Composition, Non-Nutritional Factors, and Antioxidant Properties. Molecules 2025, 30, 1225. [Google Scholar] [CrossRef] [PubMed]
- Neder-Suárez, D.; Lardizabal-Gutiérrez, D.; Zazueta-Morales, J.d.J.; Meléndez-Pizarro, C.O.; Delgado-Nieblas, C.I.; Ramírez Wong, B.; Gutiérrez-Méndez, N.; Hernández-Ochoa, L.R.; Quintero-Ramos, A. Anthocyanins and Functional Compounds Change in a Third-Generation Snacks Prepared Using Extruded Blue Maize, Black Bean, and Chard: An Optimization. Antioxidants 2021, 10, 1368. [Google Scholar] [CrossRef]
- Jabeen, A.; Naik, H.; Jan, N.; Hussain, S.Z.; Amin, T.; Rafiq, A. Studying the effect of tomato pomace incorporation on physicochemical, nutritional and storage characteristics of corn-based extrudates using response surface approach. Br. Food J. 2022, 124, 3705–3723. [Google Scholar] [CrossRef]
- Korkerd, S.; Wanlapa, S.; Puttanlek, C.; Uttapap, D.; Rungsardthong, V. Expansion and functional properties of extruded snacks enriched with nutrition sources from food processing by-products. J. Food Sci. Technol. 2016, 53, 561–570. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J. Effect of Extrusion on the Antioxidant Capacity and Color Attributes of Expanded Extrudates Prepared from Purple Potato and Yellow Pea Flour Mixes. J. Food Sci. 2011, 76, C874–C883. [Google Scholar] [CrossRef] [PubMed]
- Salunke, S. Protein Rich Extruded Snack Food from Finger Millet Flour, Oat Flour, Potato Starch and Whey Protein Isolate Blends; McGill University Libraries: Montreal, QC, Canada, 2016; Available online: https://central.bac-lac.gc.ca/.item?id=TC-QMM-139268&op=pdf&app=Library (accessed on 5 May 2025).
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Application of extrusion technology in plant food processing byproducts: An overview. Compr. Rev. Food Sci. Food Saf. 2020, 19, 218–246. [Google Scholar] [CrossRef]
- Zambrano, Y.; Contardo, I.; Moreno, M.C.; Bouchon, P. Effect of Extrusion Temperature and Feed Moisture Content on the Microstructural Properties of Rice-Flour Pellets and Their Impact on the Expanded Product. Foods 2022, 11, 198. [Google Scholar] [CrossRef]
- Manzoor, S.; Hussain, S.Z.; Amin, T.; Bashir, O.; Naseer, B.; Jabeen, A.; Fayaz, U.; Nisar, N.; Mushtaq, A.; Yousouf, M.; et al. The use of extrusion technology for encapsulation of bioactive components for their improved stability and bioavailability. Nutr. Food Sci. 2023, 53, 959–976. [Google Scholar] [CrossRef]
- Espinoza-Moreno, R.J.; Reyes-Moreno, C.; Milán-Carrillo, J.; López-Valenzuela, J.A.; Paredes-López, O.; Gutiérrez-Dorado, R. Healthy Ready-to-Eat Expanded Snack with High Nutritional and Antioxidant Value Produced from Whole Amarantin Transgenic Maize and Black Common Bean. Plant Foods Hum. Nutr. 2016, 71, 218–224. [Google Scholar] [CrossRef]
- Singh, R.; Guerrero, M.; Nickerson, M.T.; Koksel, F. Effects of extrusion screw speed, feed moisture content, and barrel temperature on the physical, techno-functional, and microstructural quality of texturized lentil protein. J. Food Sci. 2024, 89, 2040–2053. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Rodas-Gonzalez, A.; Tulbek, M.; Koksel, F. Effects of protein formula and extrusion cooking conditions on the techno-functional properties of texturised pea proteins. Int. J. Food Sci. Technol. 2024, 59, 584–595. [Google Scholar] [CrossRef]
- Wan, Y.; Lin, C.; Li, Y.; Wang, R.; Feng, W.; Chen, Z.; Wang, T.; Luo, X.; Wu, X. Tuning the electrostatic interaction between rice protein and carboxymethyl cellulose toward hydrophilic composites with enhanced functional properties. Int. J. Biol. Macromol. 2023, 235, 123918. [Google Scholar] [CrossRef]
- Han, J.H. Innovations in Food Packaging; Elsevier Academic: San Diego, CA, USA; Elsevier Science: Oxford, UK, 2005. [Google Scholar]
- Pietrysiak, E.; Smith, D.M.; Smith, B.M.; Ganjyal, G.M. Enhanced functionality of pea-rice protein isolate blends through direct steam injection processing. Food Chem. 2018, 243, 338–344. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; Patel, B.; Silva, M.P.; Comunian, T.A.; Federici, E.; Jones, O.G.; Campanella, O.H. Microencapsulation as a tool to producing an extruded functional food. LWT-Food Sci. Technol. 2020, 128, 109433. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications–a review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Kurek, M.A.; Pratap-Singh, A. Plant-Based (Hemp, Pea and Rice) Protein-Maltodextrin Combinations as Wall Material for Spray-Drying Microencapsulation of Hempseed (Cannabis sativa) Oil. Foods 2020, 9, 1707. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Jusoh, Y.M.M.; Nawi, N.M.; Aziz, A.A.; Padzil, A.M.; Lian, H.L. Chapter 15-Advanced Natural Food Colorant Encapsulation Methods: Anthocyanin Plant Pigment. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 495–526. [Google Scholar]
- Shinde, B.; Ramaswamy, H.S. Kinetic modeling of microwave osmotic dehydration of mangoes under continuous flow medium spray conditions using sucrose and maltodextrin (10-18 DE) solute mixtures. Dry. Technol. 2021, 39, 713–725. [Google Scholar] [CrossRef]
- Mohamed Latif, N.A.; Mat Ropi, A.A.; Dos Mohamad, A.M.; Shaharuddin, S. Fat reduction and characteristic enhancement of edible composite coating (Pectin-Maltodextrin) on fried potato chips. Mater. Today: Proc. Part 1 2020, 31, A79–A84. [Google Scholar] [CrossRef]
- Xie, S.-h.; Wang, Z.-j.; He, Z.-y.; Zeng, M.-m.; Qin, F.; Adhikari, B.; Chen, J. The effects of maltodextrin/starch in soy protein isolate–wheat gluten on the thermal stability of high-moisture extrudates. J. Integr. Agric. 2023, 22, 1590–1602. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Gu, X.; Luan, B.; Zhu, Y.; Huang, Y.; Zhu, X. High-moisture extrusion cooking on soybean-wheat protein mixtures: Effect of sodium alginate/xanthan gum/maltodextrin on promoting a fibrous structure. Front. Nutr. 2022, 9, 1077601. [Google Scholar] [CrossRef]
- Yu, L.; Ramaswamy, H.S.; Boye, J. Protein rich extruded products prepared from soy protein isolate-corn flour blends. LWT-Food Sci. Technol. 2013, 50, 279–289. [Google Scholar] [CrossRef]
- Schmid, E.-M.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High moisture extrusion cooking of meat analogs: A review of mechanisms of protein texturization. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.; Durrieu, V.; Raynaud, C.; Rouilly, A. Influence of DE-value on the physicochemical properties of maltodextrin for melt extrusion processes. Carbohydr. Polym. 2016, 144, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76 Pt 1, 31–38. [Google Scholar] [CrossRef]
- Delaney, W.; Hulya, D.; Yonghui, L.; Sajid, A. Physico-Chemical Properties and Texturization of Pea, Wheat and Soy Proteins Using Extrusion and Their Application in Plant-Based Meat. Foods 2023, 12, 1586. [Google Scholar] [CrossRef]
- Opaluwa, C.; De Angelis, D.; Summo, C.; Karbstein, H.P. Effect of different vegetable oils on extruded plant-based meat analogs: Evaluation of oxidative degradation, textural, rheological, tribological and sensory properties. Food Hydrocoll. 2025, 163, 111038. [Google Scholar] [CrossRef]
- Skamniotis, C.G.; Patel, Y.; Elliott, M.; Charalambides, M.N. Toughening and stiffening of starch food extrudates through the addition of cellulose fibres and minerals. Food Hydrocoll. 2018, 84, 515–528. [Google Scholar] [CrossRef]
- Azab, D.E.-S.H.; Almoselhy, R.I.M.; Mahmoud, M.H. Improving the quality characteristics of low fat toffee by using mango kernel fat, pectin, and high-speed homogenizer. J. Food Process. Preserv. 2022, 46, e17235. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Smith, D.E. Color analysis. In Food Analysis; Springer: Cham, Switzerland, 2017; pp. 545–555. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins: 2. Determination of Total Anthocyanin and Degradation Index for Cranberry Juice. J. Food Sci. 1968, 33, 78–83. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Matteoli, S.; Landi, M.; Guidi, L.; Massai, R.; Remorini, D. Measurements of Anthocyanin Content of Prunus Leaves Using Proximal Sensing Spectroscopy and Statistical Machine Learning. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Qing, X.; Bingjing, L.; Daibo, W.; Liping, L.; Guohua, L.; Yuanjing, Z. Microencapsulation and Stability Analysis of Blueberry Anthocyanins. IOP Conf. Ser. Earth Environ. Sci. 2019, 252, 052133. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Antioxidant activity of Cassia auriculata flowers. Fitoterapia 2007, 78, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B Biomed. Biotechnol. 2012, 13, 94–102. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C 2019, 94, 393–402. [Google Scholar] [CrossRef]
- Izalin, Z.; Ferawati, F.; Jeanette, K.P.; Marilyn, R.; Cecilia, A.; Amanda, H.; Karolina, Ö. Development and Characterization of Extrudates Based on Rapeseed and Pea Protein Blends Using High-Moisture Extrusion Cooking. Foods 2021, 10, 2397. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, T.; Zhang, Y.; Jiang, L.; Sui, X. High moisture extrusion of soy protein and wheat gluten blend: An underlying mechanism for the formation of fibrous structures. LWT 2022, 163, 113561. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Adhikari, B. Maillard reaction between pea protein isolate and maltodextrin via wet-heating route for emulsion stabilisation. Future Foods 2022, 6, 100193. [Google Scholar] [CrossRef]
- Valenzuela, C.; Aguilera, J.M. Effects of maltodextrin on hygroscopicity and crispness of apple leathers. J. Food Eng. 2015, 144, 1–9. [Google Scholar] [CrossRef]
- Singh, B.; Rachna; Hussain, S.Z.; Sharma, S. Response Surface Analysis and Process Optimization of Twin Screw Extrusion Cooking of Potato-Based Snacks. J. Food Process. Preserv. 2015, 39, 270–281. [Google Scholar] [CrossRef]
- Lotfi Shirazi, S.; Koocheki, A.; Milani, E.; Mohebbi, M. Production of high fiber ready-to-eat expanded snack from barley flour and carrot pomace using extrusion cooking technology. J. Food Sci. Technol. 2020, 57, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, X.; Luo, S.; Liu, W.; Chen, J.; Zeng, Z.; Liu, C. Properties of Starch after Extrusion: A Review. Starch-Stärke 2018, 70, 1700110. [Google Scholar] [CrossRef]
- Ali, Y.; Hanna, M.A.; Chinnaswamy, R. Expansion Characteristics of Extruded Corn Grits. LWT-Food Sci. Technol. 1996, 29, 702–707. [Google Scholar] [CrossRef]
- Beigh, M.; Hussain, S.Z.; Qadri, T.; Naseer, B.; Raja, T.; Naik, H. Investigation of process and product parameters for physico-chemical properties of low Glycemic Index water chestnut and barley flour-based extruded snacks. Br. Food J. 2020, 122, 227–241. [Google Scholar] [CrossRef]
- Robin, F.; Schuchmann, H.P.; Palzer, S. Dietary fiber in extruded cereals: Limitations and opportunities. Trends Food Sci. Technol. 2012, 28, 23–32. [Google Scholar] [CrossRef]
- Ding, Q.-B.; Ainsworth, P.; Tucker, G.; Marson, H. The effect of extrusion conditions on the physicochemical properties and sensory characteristics of rice-based expanded snacks. J. Food Eng. 2005, 66, 283–289. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.; Jeong, Y.J.; Choi, I.; Han, J. Impact of interactions between soy and pea proteins on quality characteristics of high-moisture meat analogues prepared via extrusion cooking process. Food Hydrocoll. 2023, 139, 108567. [Google Scholar] [CrossRef]
- Khatkar, A.B.; Kaur, A.; Khatkar, S.K.; Bala, M.; Maan, S.; Tyagi, S.K. Valorization of ultrasound assisted restructured soy protein: Impact on the quality characteristics of instant noodles. LWT 2021, 147, 111599. [Google Scholar] [CrossRef]
- Lee, J.-S.; Choi, I.; Han, J. Construction of rice protein-based meat analogues by extruding process: Effect of substitution of soy protein with rice protein on dynamic energy, appearance, physicochemical, and textural properties of meat analogues. Food Res. Int. 2022, 161, 111840. [Google Scholar] [CrossRef] [PubMed]
- Kosińska-Cagnazzo, A.; Bocquel, D.; Marmillod, I.; Andlauer, W. Stability of goji bioactives during extrusion cooking process. Food Chem. 2017, 230, 250–256. [Google Scholar] [CrossRef]
- Camire, M.E.; Chaovanalikit, A.; Dougherty, M.P.; Briggs, J. Blueberry and Grape Anthocyanins as Breakfast Cereal Colorants. J. Food Sci. 2002, 67, 438–441. [Google Scholar] [CrossRef]
- Schmid, V.; Steck, J.; Mayer-Miebach, E.; Behsnilian, D.; Bunzel, M.; Karbstein, H.P.; Emin, M.A. Extrusion Processing of Pure Chokeberry (Aronia melanocarpa) Pomace: Impact on Dietary Fiber Profile and Bioactive Compounds. Foods 2021, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Dougherty, M.P.; Briggs, J.L. Functionality of fruit powders in extruded corn breakfast cereals. Food Chem. 2007, 101, 765–770. [Google Scholar] [CrossRef]
- Hirth, M.; Preiß, R.; Mayer-Miebach, E.; Schuchmann, H.P. Influence of HTST extrusion cooking process parameters on the stability of anthocyanins, procyanidins and hydroxycinnamic acids as the main bioactive chokeberry polyphenols. LWT-Food Sci. Technol. 2015, 62, 511–516. [Google Scholar] [CrossRef]
- O˝zer, E.A.; Herken, E.N.; Güzel, S.; Ainsworth, P.; İbanoğlu, S. Effect of extrusion process on the antioxidant activity and total phenolics in a nutritious snack food. Int. J. Food Sci. Technol. 2006, 41, 289–293. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Brownmiller, C.R.; Prior, R.L. Influence of Extrusion Processing on Procyanidin Composition and Total Anthocyanin Contents of Blueberry Pomace. J. Food Sci. 2009, 74, H52–H58. [Google Scholar] [CrossRef]
- Mastrocola, D.; Munari, M. Progress of the Maillard reaction and antioxidant action of Maillard reaction products in preheated model systems during storage. J. Agric. Food Chem. 2000, 48, 3555–3559. [Google Scholar] [CrossRef]
- García-Tejeda, Y.V.; Salinas-Moreno, Y.; Hernández-Martínez, Á.R.; Martínez-Bustos, F. Encapsulation of Purple Maize Anthocyanins in Phosphorylated Starch by Spray Drying. Cereal Chem. 2016, 93, 130–137. [Google Scholar] [CrossRef]



| Sample | Moisture Content (%) | Output Temperature (°C) at 100 rpm | Output Temperature (°C) at 150 rpm | Output Temperature (°C) at 200 rpm |
|---|---|---|---|---|
| Rice protein (RP) | 25 | 55.2 ± 1.28 aA | 62.0 ± 1.40 bD | 72.0 ± 1.20 cF |
| Pea protein (PP) | 45 | 48.9 ± 1.12 aB | 50.9 ± 0.93 aE | 60.9 ± 1.10 bK |
| Mixture (Mix) | 40 | 46.2 ± 2.33 aC | 55.0 ± 0.61 bE | 61.2 ± 0.87 bK |
| Sample Name | MC (% Wet Basis) | |
|---|---|---|
| Wet Samples | Dry Samples | |
| RP-based samples | ||
| RP | 16.43 ± 0.82 a | 4.96 ± 0.52 a |
| RP + 5% MD | 29.13 ± 1.92 b | 11.88 ± 1.12 b |
| RP + 10% MD | 26.20 ± 4.84 c | 11.61 ± 0.94 b |
| RP + 15% MD | 28.53 ± 2.78 f | 10.81 ± 0.30 f |
| PP-based samples | ||
| PP | 44.21 ± 0.21 d | 13.99 ± 0.05 c |
| PP + 5% MD | 44.36 ± 0.24 d | 14.31 ± 0.22 c |
| PP + 10% MD | 44.86 ± 0.16 d | 14.57 ± 0.24 c |
| PP + 15% MD | 44.08 ± 0.54 d | 14.81 ± 0.08 c |
| Mixture-based samples | ||
| Mix | 40.75 ± 0.40 e | 10.01 ± 0.42 f |
| Mix + 5% MD | 39.42 ± 1.43 n | 9.84 ± 0.05 f |
| Mix + 10% MD | 38.16 ± 1.01 k | 9.54 ± 0.20 f |
| Mix + 15% MD | 36.81 ± 0.24 m | 9.15 ± 0.65 e |
| Sample Name | ER | RR (%) | WHC (g/g) |
|---|---|---|---|
| RP-based samples | |||
| RP | 1.26 ± 0.60 a | 78.70 ± 1.21 a | 1.44 ± 0.40 a |
| RP + 5% MD | 1.20 ± 0.40 a | 49.00 ± 2.31 b | 1.61 ± 0.10 b |
| RP + 10% MD | 1.16 ± 0.04 b | 34.00 ± 2.12 c | 1.70 ± 0.04 c |
| RP + 15% MD | 1.00 ± 0.05 d | 18.00 ± 1.60 d | 1.90 ± 0.04 d |
| PP-based samples | |||
| PP | 1.10 ± 0.05 c | 39.00 ± 2.11 f | 2.71 ± 0.08 f |
| PP + 5% MD | 1.10 ± 0.05 c | 59.00 ±1.73 m | 2.75 ± 0.20 f |
| PP + 10% MD | 1.10 ± 0.05 c | 86.50 ±1.85 k | 2.78 ± 0.03 g |
| PP + 15% MD | 1.10 ± 0.05 c | 91.20 ±2.20 n | 2.81 ± 0.04 g |
| Mix-based samples | |||
| Mix | 1.00 ± 0.05 d | 31.90 ±1.14 c | 2.28 ± 0.11 k |
| Mix + 5% MD | 1.00 ± 0.05 d | 58.00 ±2.20 m | 2.26 ± 0.20 k |
| Mix + 10% MD | 1.00 ± 0.05 d | 86.00 ±1.75 k | 2.82 ± 0.10 m |
| Mix + 15% MD | 1.00 ± 0.05 d | 88.00 ±1.70 k | 2.85 ± 0.10 m |
| L* Value | a* Value | b* Value | ||||
|---|---|---|---|---|---|---|
| Sample | Wet | Dry | Wet | Dry | Wet | Dry |
| RP before extrusion | 50.04 ± 1.11 a | NE | 1.50 ± 0.10 a | NE | 9.45 ± 0.90 a | NE |
| RP | 24.50 ± 1.32 bA | 22.82 ± 0.80 aB | 1.98 ± 0.22 bC | 2.68 ± 0.14 bD | −3.35 ± 0.31 bE | −4.74 ± 0.09 bG |
| RP + 5% MD | 24.39 ± 1.10 bA | 21.97 ± 1.00 bB | 2.43 ± 0.72 cC | 2.32 ± 0.21 cD | −3.07± 1.11 cE | −4.8 ± 0.72 bG |
| RP + 10% MD | 23.40 ± 1.58 cA | 20.20 ± 1.31 cB | 2.50 ± 0.10 cC | 2.10 ± 0.20 dD | −3.89 ± 0.19 dE | −4.59 ± 0.55 cG |
| RP + 15% MD | 21.03 ± 0.61 dA | 20.45 ± 1.12 kB | 2.84 ± 0.06 dC | 2.3 ± 0.10 cD | −3.14 ± 0.12 cE | −4.10 ± 0.10 dG |
| PP before extrusion | 21.47 ± 1.13 d | NE | −0.28 ± 0.03 e | NE | −1.32 ± 0.21 e | NE |
| PP | 11.51 ± 0.50 fA | 11.12 ± 1.10 vA | −0.47 ± 0.08 kC | 0.22 ± 0.04 eD | −7.12 ± 0.13 fE | −0.49 ± 0.05 eG |
| PP + 5% MD | 15.00 ± 1.11 gA | 11.20 ± 0.22 wB | −0.62 ± 0.05 gC | 0.43 ± 0.6 fD | −7.27 ± 0.20 gE | −0.85 ± 1.12 fG |
| PP + 10% MD | 16.51 ± 1.11 hA | 11.01 ± 0.50 dB | −0.62 ± 0.06 gC | 0.41± 0.04 fD | −7.32 ± 0.29 hE | −0.55 ± 0.41 kG |
| PP + 15% MD | 16.27 ± 1.30 hA | 11.00 ± 0.88 dB | −0.60 ± 0.08 gC | 0.40 ± 0.10 fD | −8.73 ± 0.40 kE | −0.62 ± 0.40 mG |
| Mix before extrusion | 33.10 ± 1.10 k | NE | 0.80 ± 0.11 f | NE | −6.10 ± 0.30 m | NE |
| Mix | 24.89 ± 0.59 wA | 28.09 ± 1.00 jB | 0.57 ± 0.65 hC | 1.24 ± 0.24 gD | −5.39 ± 1.00 nE | −8.27 ± 0.40 nG |
| Mix + 5% MD | 24.81 ± 1.00 wA | 28.36 ± 0.70 eB | 0.64 ± 1.10 vC | 0.58 ± 1.00 hD | −5.78 ± 0.58 wE | −7.40 ± 0.62 wG |
| Mix + 10% MD | 25.13 ± 0.22 nA | 29.61 ± 0.21 fB | 0.69 ± 0.43 vC | 0.72 ± 0.54 wD | −6.16 ± 1.31 mE | −7.38 ± 1.10 wG |
| Mix + 15% MD | 25.39 ± 1.00 kA | 27.87 ± 0.55 gB | 0.31 ± 1.22 wC | 1.27 ± 0.81 nD | −5.84 ± 0.23 wE | −8.91 ± 1.28 vG |
| C* | H | |||
|---|---|---|---|---|
| Sample | Wet | Dry | Wet | Dry |
| RP before extrusion | 9.57 ± 0.18 a | NE | 80.68 ± 0.70 a | NE |
| RP | 3.89 ± 0.38 bA | 5.44 ± 0.10 bB | 300.53 ± 0.8 bC | 299.56 ± 0.79 bC |
| RP + 5% MD | 4.54 ± 0.72 cA | 5.95 ± 0.10 cB | 300.82 ± 0.2 bC | 282.84 ± 0.65 cD |
| RP + 10% MD | 4.62 ± 0.21 dA | 4.93 ± 0.55 dB | 302.69 ± 0.51 bC | 291.46 ± 1.53 eD |
| RP + 15% MD | 4.24 ± 0.08 vA | 4.70 ± 0.14 eB | 312.10 ± 1.46 eC | 299.29 ± 0.58 bD |
| PP before extrusion | 1.36 ± 0.20 f | NE | 257.95 ± 1.8 d | NE |
| PP | 7.13 ± 0.08 eA | 0.54 ± 0.12 fB | 266.22 ± 0.64 eC | 294.61 ± 2.32 dD |
| PP + 5% MD | 7.09 ± 0.40 eA | 0.77 ± 0.32 gB | 266.39 ± 1.23 eC | 284.29 ± 0.70 cD |
| PP + 10% MD | 7.35 ± 0.27 kA | 0.76 ± 0.32 gB | 265.15 ± 0.84 eC | 293.23 ± 0.70 fD |
| PP + 15% MD | 8.75 ± 0.44 gA | 1.10 ± 0.54 kB | 266.06 ± 0.69 eC | 294.07 ± 0.54 dD |
| Mix before extrusion | 5.50 ± 0.40 k | NE | 276.00 ± 0.55 d | NE |
| Mix | 5.42 ± 1.00 kA | 8.36 ± 1.10 wB | 276.02 ± 1.19 dC | 278.55 ± 0.55 wD |
| Mix + 5% MD | 5.81 ± 0.40 wA | 7.41 ± 1.10 vB | 276.38 ± 2.19 dC | 274.49 ± 0.98 kD |
| Mix + 10% MD | 6.20 ± 0.57 vA | 7.42 ± 0.81 vB | 276.42 ± 0.64 dC | 275.57 ± 1.11 kD |
| Mix + 15% MD | 5.85 ± 1.10 wA | 9.01 ± 0.81 mB | 273.03 ± 1.64 dC | 278.11 ± 0.98 wD |
| Sample | Anthocyanin Content (mg/g) | Radical Scavenging Activity (%) | ||
|---|---|---|---|---|
| Wet Sample | Dry Sample | Wet Sample | Dry Sample | |
| Control | 3.56 ± 0.03 a | NE | 73.54 ± 0.26 a | NE |
| RP-based samples | ||||
| RP | 1.64 ± 0.03 bA | 1.52 ± 0.07 bB | 64.33 ± 0.18 cA | 55.02 ± 0.11 aB |
| RP + 5% MD | 1.70 ± 0.06 fA | 1.29 ± 0.10 cB | 65.62 ± 0.90 dA | 65.69 ± 0.28 bA |
| RP + 10% MD | 1.98 ± 0.02 cA | 1.63 ± 0.03 dB | 66.90 ± 0.90 mA | 67.17 ± 0.22 cB |
| RP + 15% MD | 2.01 ± 0.01 cA | 1.81 ± 0.01 eB | 69.71 ± 0.18 bA | 72.30 ± 1.10 kB |
| PP-based samples | ||||
| PP | 1.96 ± 0.01 cA | 1.30 ± 0.04 cB | 73.54 ± 1.10 aA | 57.65 ± 0.69 dB |
| PP + 5% MD | 1.66 ± 0.1 bA | 1.17 ± 0.2 kB | 72.40 ± 0.11 wA | 55.14 ± 1.69 aB |
| PP + 10% MD | 1.35 ± 0.04 dA | 0.70 ± 0.02 fB | 65.23 ± 1.62 dA | 50.80 ± 1.82 mB |
| PP + 15% MD | 1.23 ± 0.07 kA | 0.30 ± 0.02 wB | 60.96 ± 0.23 kA | 49.47 ± 0.70 mB |
| Mix-based samples | ||||
| Mix | 2.74 ± 0.02 mA | 2.60 ± 0.01 kB | 70.10 ± 1.27 bA | 74.43 ± 1.29 gB |
| Mix + 5% MD | 3.51 ± 0.10 aA | 3.28 ± 0.38 mB | 72.70 ± 1.48 jA | 78.83 ± 0.90 vB |
| Mix + 10% MD | 2.23 ± 0.02 gA | 1.87 ± 0.19 eB | 73.51 ± 1.48 aA | 74.62 ± 0.22 gB |
| Mix + 15% MD | 2.34 ± 0.06 hA | 1.98 ± 0.19 jB | 73.75 ± 1.80 aA | 74.20 ± 1.17 gB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharaty, G.; Ramaswamy, H.S. Formulation and Evaluation of a Nutritionally Enriched Plant Protein-Based Matrix Using Low Temperature Extrusion Cooking—Air Drying Technology. Foods 2025, 14, 1846. https://doi.org/10.3390/foods14111846
Alharaty G, Ramaswamy HS. Formulation and Evaluation of a Nutritionally Enriched Plant Protein-Based Matrix Using Low Temperature Extrusion Cooking—Air Drying Technology. Foods. 2025; 14(11):1846. https://doi.org/10.3390/foods14111846
Chicago/Turabian StyleAlharaty, Ghaidaa, and Hosahalli S. Ramaswamy. 2025. "Formulation and Evaluation of a Nutritionally Enriched Plant Protein-Based Matrix Using Low Temperature Extrusion Cooking—Air Drying Technology" Foods 14, no. 11: 1846. https://doi.org/10.3390/foods14111846
APA StyleAlharaty, G., & Ramaswamy, H. S. (2025). Formulation and Evaluation of a Nutritionally Enriched Plant Protein-Based Matrix Using Low Temperature Extrusion Cooking—Air Drying Technology. Foods, 14(11), 1846. https://doi.org/10.3390/foods14111846








