Effect of Ultrasound and Chemical Cross-Linking on the Structural and Physicochemical Properties of Malanga (Colocasia esculenta) Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Isolation
2.3. Ultrasound (US) Modification
2.4. Chemical Cross-Linking (CL) Modification
2.5. Physicochemical Properties
2.5.1. Viscosity Profile
2.5.2. Thermal Properties
2.5.3. Amylose Content
2.5.4. Swelling Power and Solubility
2.5.5. Refrigeration and Freezing Stability
2.5.6. Water Activity
2.6. Morphological Analysis
3. Results and Discussion
3.1. Starch Isolation Yield
3.2. Physicochemical Properties of Modified Starches
3.2.1. Viscosity Profile
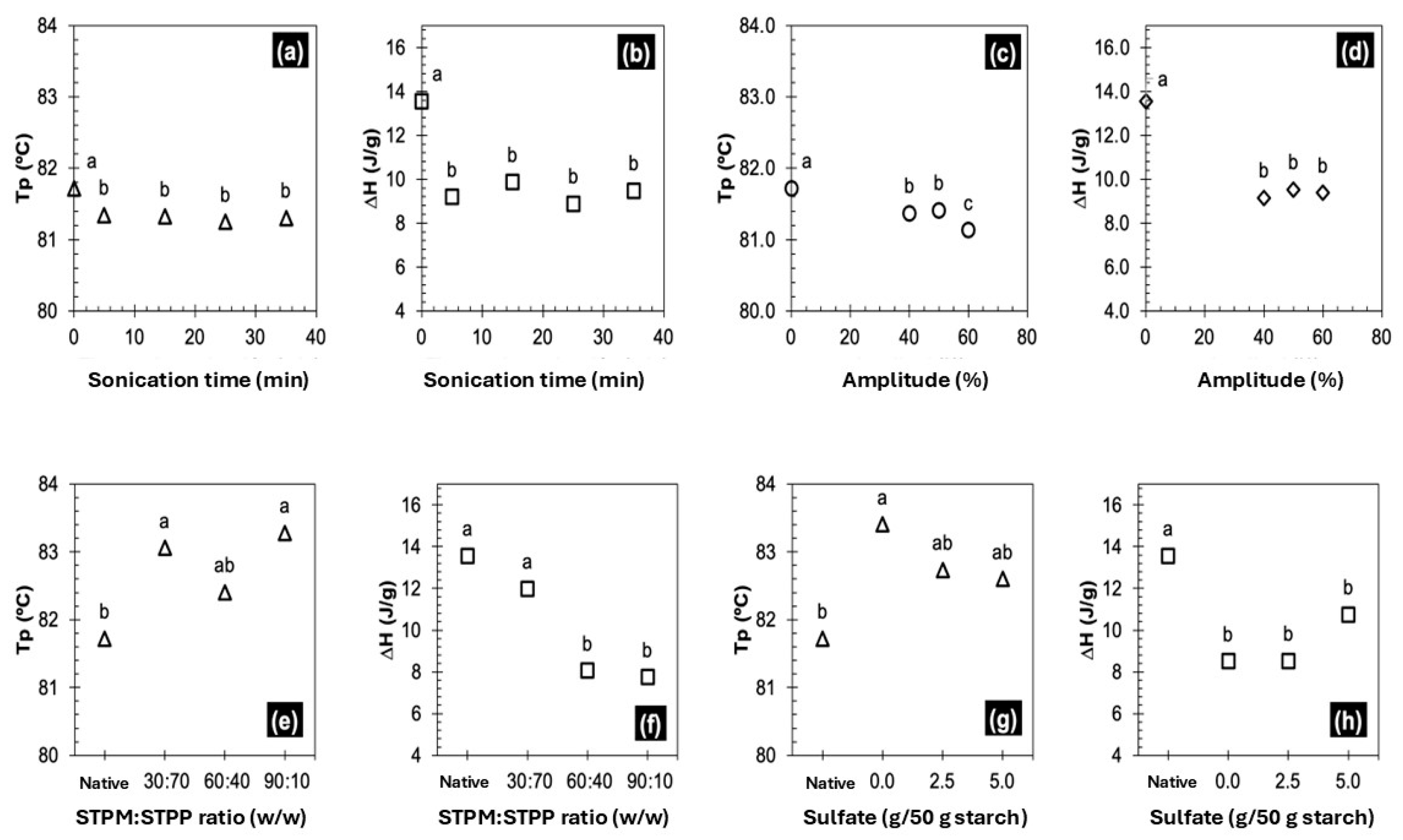
3.2.2. Thermal Properties
3.2.3. Apparent Amylose Content
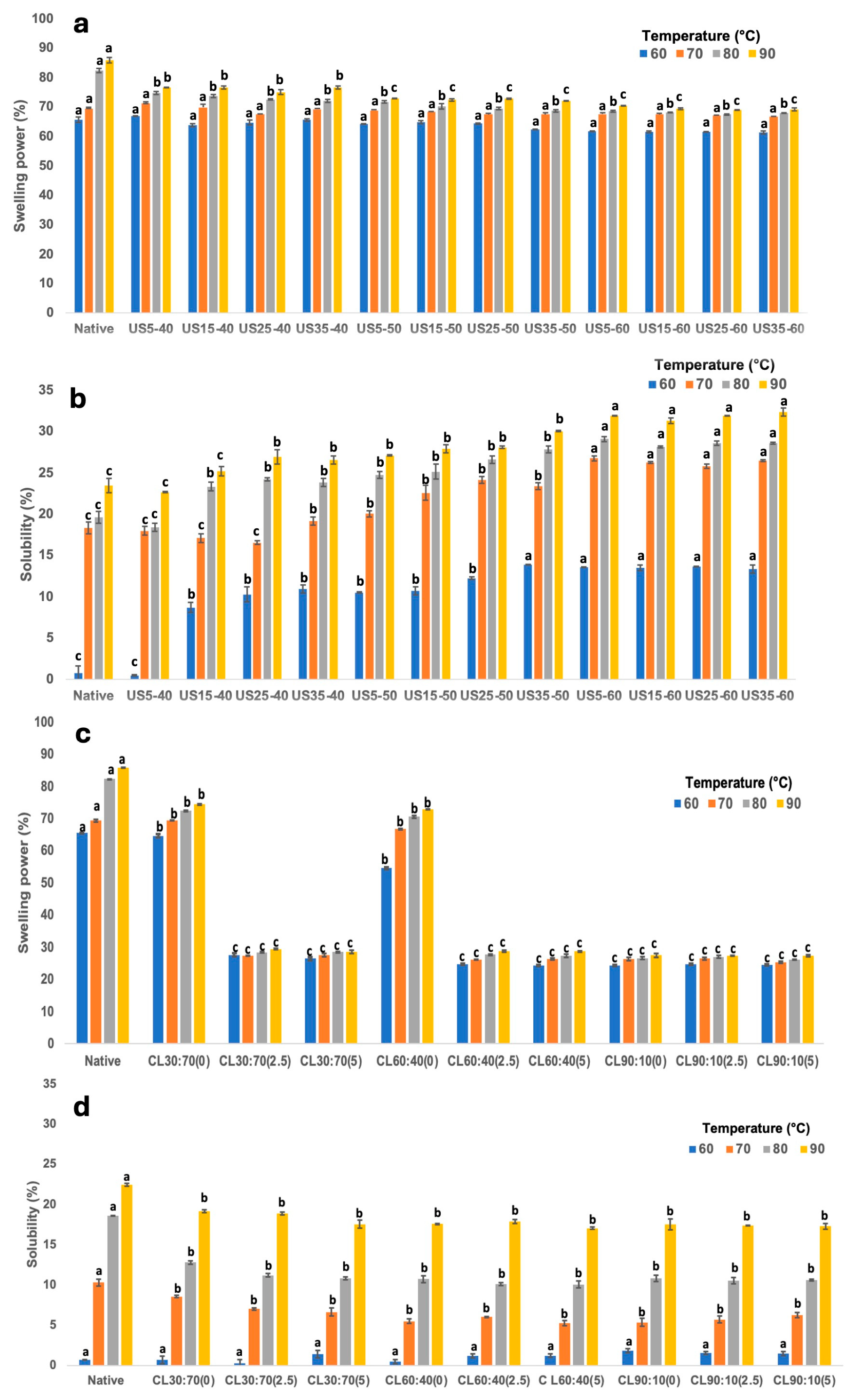
3.2.4. Swelling Power and Solubility
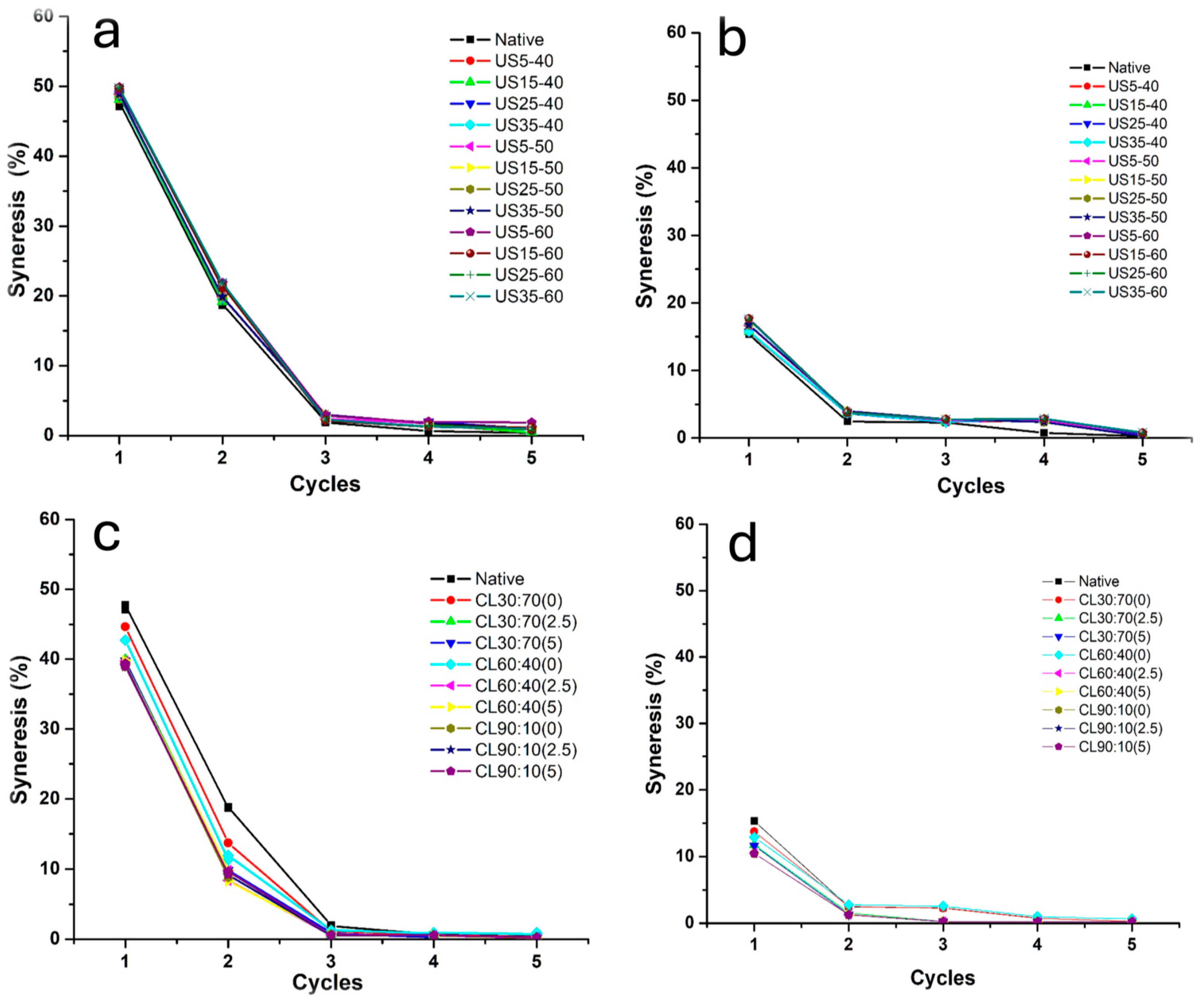
3.2.5. Freeze–Thaw Stability
3.2.6. Water Activity (aw)
3.3. Morphological Analysis
3.3.1. Particle Size Distribution (PSD)
3.3.2. Optimal Conditions
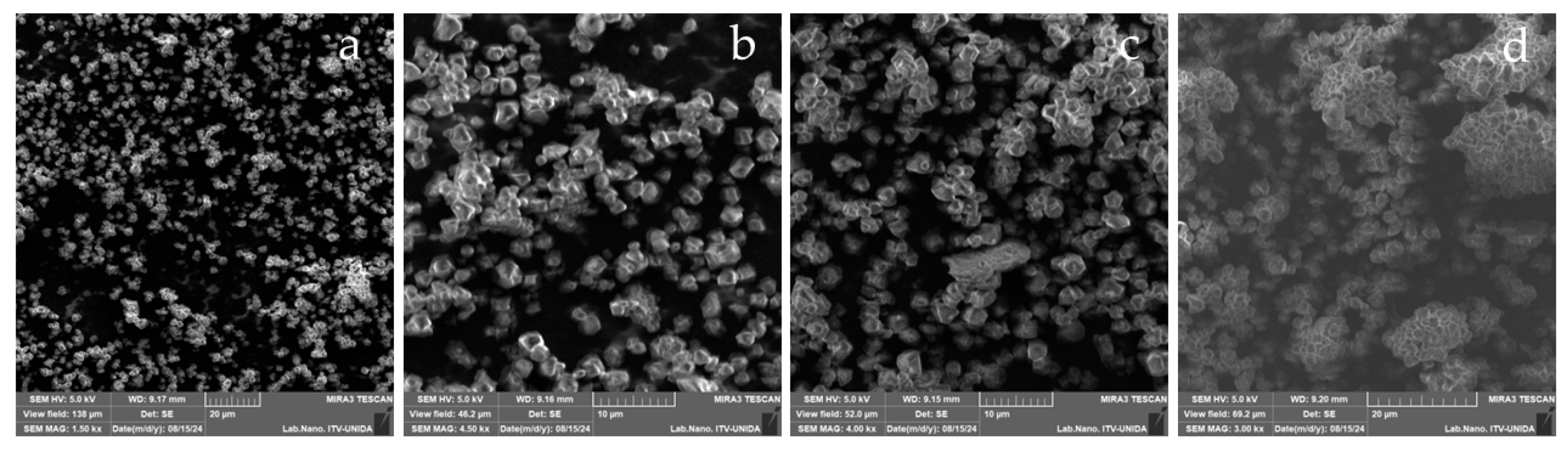
3.3.3. Scanning Electron Microscopy (SEM)
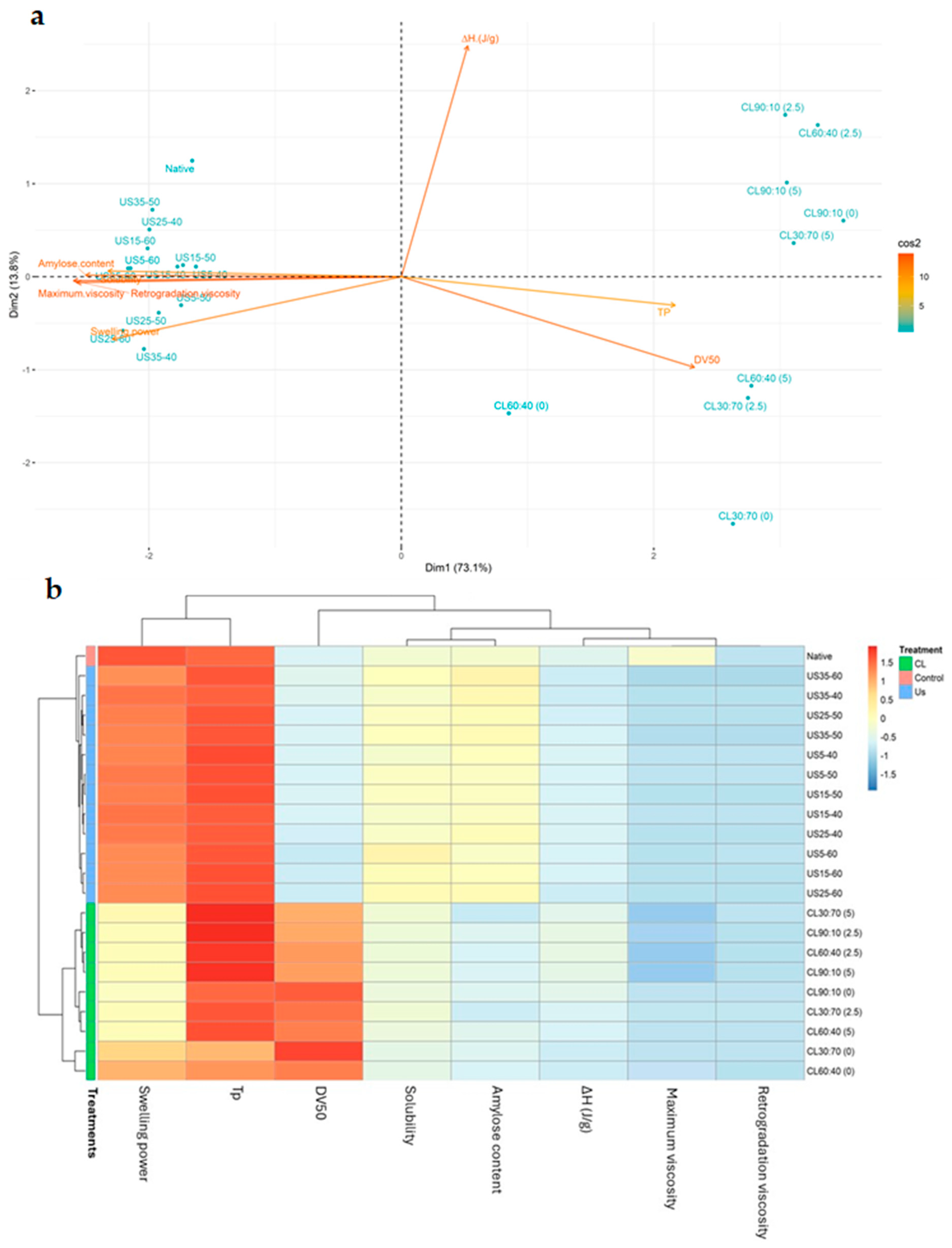
3.4. Chemometric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| US | Ultrasound |
| CL | Cross-linking |
| STMP | Sodium Trimetaphosphate |
| STPP | Sodium Tripolyphosphate |
| ECH | Epichlorohydrin |
| POCl3 | Phosphoryl Chloride |
| FDA | Food and Drug Administration |
| DSC | Differential Scanning Calorimetry |
| SP | Swelling Power |
| WS | Water Solubility |
| SEM | Scanning Electron Microscopy |
| PSD | Particle Size Distribution |
| Tp | Gelatinization Temperature |
| ∆H | Enthalpy |
| PCA | Principal Component Analysis |
References
- Gonzalez-Soto, R.A.; de la Vega, B.; García-Suarez, F.J.; Agama-Acevedo, E.; Bello-Pérez, L.A. Preparation of spherical aggregates of taro starch granules. LWT 2011, 44, 2064–2069. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Eun, J.-B. Physical and Functional Properties, Digestibility, and Storage Stability of Spray- and Freeze-Dried Microencapsulated Bioactive Products from Moringa stenopetala Leaves Extract. Ind. Crops Prod. 2020, 156, 112891. [Google Scholar] [CrossRef]
- Torres Rapelo, A.; Montero Castillo, P.; Duran Lengua, M. Propiedades fisicoquímicas, morfológicas y funcionales del almidón de malanga (Colocasia esculenta). Lasallista Investig. 2013, 10, 52–61. [Google Scholar]
- The Observatory of Economic Complexity (OEC). Available online: https://oec.world/es/profile/bilateral-product/vegetable-roots-and-tubers-taro-colocasia-spp-with-high-starch-or-inulin-content-fresh-chilled-frozen-or-dried-whether-or-not-sliced-or-in-the-form-of-pellets/reporter/mex (accessed on 23 June 2025).
- Secretaría de Agricultura y Desarrollo Rural. Available online: https://www.gob.mx/agricultura/prensa/promueve-agricultura-cultivo-de-malanga-en-beneficio-de-productores-del-sur-sureste-del-pais (accessed on 13 January 2023).
- Pachuau, L.; Dutta, R.S.; Devi, T.B.; Deka, D.; Hauzel, L. Taro starch (Colocasia esculenta) and citric acid modified taro starch as tablet disintegrating agents. Int. J. Biol. Macromol. 2018, 118, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Nagar, C.K.; Dash, S.K.; Rayaguru, K.; Pal, U.S.; Nedunchezhiyan, M. Isolation, characterization, modification and uses of taro starch: A review. Int. J. Biol. Macromol. 2021, 192, 574–589. [Google Scholar] [CrossRef] [PubMed]
- França Lemos, P.F.; Rodrigues Marcelino, H.; Guimarães Cardoso, L.; Oliveira de Souza, C.; Izabel Druzian, J. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Łozińska, N.; Głowacz-Różyńska, A.; Artichowicz, W.; Lu, Y.; Jungnickel, C. Microencapsulation of fish oil—Determination of optimal wall material and encapsulation methodology. J. Food Eng. 2020, 268, 109730. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Ma, Y.; Liang, Y.; Zhang, H.; Chen, L. Impact of ultrasonication on the aggregation structure and physicochemical characteristics of sweet potato starch. Ultrason. Sonochem. 2020, 63, 104868. [Google Scholar] [CrossRef] [PubMed]
- Yusnita, H.; Siti, A.R. Effect of Heat Moisture Treatment on Molecular Structure and Thermal Properties of Taro Starch (Colocasia esculenta sp.). In Proceedings of the IAS-SSM, International Annual Symposium on Sustainability Science and Management, Kuala Terengganu, Malaysia, 9–11 July 2012; pp. 526–531. [Google Scholar]
- Martins, A.; Beninca, C.; Delinski Bet, C.; Bassetto Bisinella, R.Z.; de Oliveira, C.S.; Silveira Hornung, P.; Schnitzler, E. Ultrasonic modification of purple taro starch (Colocasia esculenta B. Tini): Structural, psychochemical and thermal properties. J. Therm. Anal. Calorim. 2020, 142, 819–828. [Google Scholar] [CrossRef]
- Das, A.; Sit, N. Modification of Taro Starch and Starch Nanoparticles by Various Physical Methods and their Characterization. Starch/Stärke 2021, 73, 2000227. [Google Scholar] [CrossRef]
- Yıldırım-Yalçın, M.; Şeker, M.; Sadıkoğlu, H. Development and characterization of edible films based on modified corn starch and grape juice. Food Chem. 2019, 292, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Vela, A.J.; Villanueva, M.; Ronda, F. Low-frequency ultrasonication modulates the impact of annealing on physicochemical and functional properties of rice flour. Food Hydrocoll. 2021, 120, 106933. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Yan, X.-H.; Zhang, J.-L.; Wang, L.-Y.; Xue, H.; Jiang, G.-C.; Ma, X.-T.; Liu, X.-J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, C.A.; Constenla, D.; Ramírez-Rigo, M.V.; Piña, J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Aparicio-Saguilán, A.; Vázquez-León, L.A.; Martínez-Cigarroa, A.S.; Carpintero-Tepole, V.; Barbero, G.F.; Acosta-Osorio, A.A.; Páramo-Calderón, D.E. Characterization of Starch from Jinicuil (Inga jinicuil) Seeds and Its Evaluation as Wall Material in Spray Drying. Agronomy 2024, 14, 272. [Google Scholar] [CrossRef]
- Vela-Gutiérrez, G.; Velázquez López, A.A.; Tacias Pascacio, V.G.; Vidal López, D.G.; León García, E.; De La Cruz Medina, J. Effect of heat treatment on oxalate and hydrocyanic acid levels of malanga corms of two cultivars (Xanthosoma sagittifolium and Colocasia esculenta) in a murine model. J. Food Sci. Technol. 2022, 59, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Seib, P.A. Cross-linking pf wheat starch and hydroxypropylated wheat starch in alkaline slurry with sodium trimetaphosphate. Carbohydr. Polym. 1997, 33, 263–271. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Starch characteristics of black bean, chick pea, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem. 2002, 78, 489–498. [Google Scholar] [CrossRef]
- Sathe, S.K.; Salunkhe, D.K. Isolation, partial characterization and modification of the great north bean (Phaseolus bulgaris) starch. J. Food Sci. 1981, 46, 617–621. [Google Scholar] [CrossRef]
- Eliasson, A.-C.; Ryang Kim, H. Changes in rheological properties of hydroxypropyl potato starch pastes during freeze-thaw treatments I. A rheological approach for evaluation of freeze-thaw stability. J. Texture Stud. 1992, 23, 279–295. [Google Scholar] [CrossRef]
- Vázquez-León, L.A.; Aparicio-Saguilán, A.; Martínez-Medinilla, R.M.; Utrilla-Coello, R.G.; Torruco-Uco, J.G.; Carpintero-Tepole, V.; Páramo-Calderón, D.E. Physicochemical and morphological characterization of black bean (Phaseolus vulgaris L.) starch and potential application in nano-encapsulation by spray drying. J. Food Meas. Charact. 2022, 16, 547–560. [Google Scholar] [CrossRef]
- Chávez-Salazar, A.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Pacheco-Vargas, G. Isolation and partial characterization of starch from banana cultivars grown in Colombia. Int. J. Biol. Macromol. 2017, 98, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Sit, N.; Misra, S.; Baruah, D.; Badwaik, L.S.; Deka, S.C. Physicochemical properties of taro and maize starch and their effect on texture, colour and sensory quality of tomato ketchup. Starch/Stärke 2014, 66, 294–302. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Isolation of starches from different tubers and study of their physicochemical, thermal, rheological and morphological characteristics. Starch/Stärke 2016, 68, 160–168. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and techno-functional properties of cassava starch modified by ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Parra, E.J.; Palavecino, P.; Ribotta, P.D.; Penci, M.C. Effect of Ultrasound Treatment on Structural and Physical Properties of Native Maize Starch. Plant Foods Hum. Nutr. 2025, 80, 50. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wei, B.; Tian, Y.; Ma, R.; Chen, L.; Qiu, L.; Jin, Z. Structural changes of chemically modified rice starch by one-step reactive extrusion. Food Chem. 2019, 288, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Huang, Q.; Zhong, S.; Li, X.; Xiong, S.; Xie, J.; Yin, T.; Zhang, B.; Zhao, S. Gel properties of myofibrillar protein as affected by gelatinization and retrogradation behaviors of modified starches with different crosslinking and acetylation degrees. Food Hydrocoll. 2019, 96, 604–616. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Liu, Y.; Liu, B.; Lv, X.; Sun, P.; Zhang, Z.; Zhang, F.; Yin, S.; Liu, Z. Preparation and application of starch phosphate with a low degree of substitution. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 974–982. [Google Scholar] [CrossRef]
- Gałkowska, D.; Juszczak, L. Effects of amino acids on gelatinization, pasting and rheological properties of modified potato starches. Food Hydrocoll. 2019, 92, 143–154. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Lin, L.; Zhang, J.; Liu, W.; Xie, J.; Ding, Y. Functional, physicochemical properties and structure of cross-linked oxidized maize starch. Food Hydrocoll. 2014, 36, 45–52. [Google Scholar] [CrossRef]
- Li, E.; Dhital, S.; Hasjim, J. Effects of grain milling on starch structures and flour/starch properties. Starch/Stärke 2014, 66, 15–27. [Google Scholar] [CrossRef]
- Bustillos-Rodríguez, J.C.; Tirado-Gallegos, J.M.; Ordóñez-García, M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.J.; Acosta-Muñiz, C.H.; Gallegos-Morales, G.; Páramo-Calderón, D.E.; Rios-Velasco, C. Physicochemical, thermal and rheological properties of three native corn starches. Food Sci. Technol. 2019, 39, 149–157. [Google Scholar] [CrossRef]
- Aparicio-Saguilán, A.; Méndez-Montealvo, G.; Solorza-Feria, J.; Bello-Pérez, L.A. Thermal and viscoelastic properties of starch gels from maize varieties. J. Sci. Food Agric. 2006, 86, 1078–1086. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and potential applications: A review of the modification of starches by heat-moisture treatment. Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch/Stärke 2014, 67, 55–68. [Google Scholar] [CrossRef]
- Zortéa-Guidolin, M.E.B.; Demiate, I.M.; de Godoy, R.C.B.; Scheer, A.P.; Grewell, D.; Jane, J.-l. Structural and functional characterization of starches from Brazilian pine seeds (Araucaria angustifolia). Food Hydrocoll. 2017, 63, 19–26. [Google Scholar] [CrossRef]
- Pérez-Pacheco, E.; Moo-Huchin, V.M.; Estrada-León, R.J.; Ortiz-Fernández, A.; May-Hernández, L.H.; Ríos-Soberanis, C.R.; Betancur-Ancona, D. Isolation and characterization of starch obtained from Brosimum alicastrum Swarts Seeds. Carbohydr. Polym. 2014, 101, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-drying performance and thermal stability of L-ascorbic acid microencapsulated with sodium alginate and gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef] [PubMed]
- Silva Faria, W.C.; da Conceição, E.C.; de Melo Moura, W.; de Barros, W.M.; Converti, A.; Bragagnolo, N. Design and evaluation of microencapsulated systems containing extract of whole green coffee fruit rich in phenolic acids. Food Hydrocoll. 2020, 100, 105437. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of physically modified oat starch with different sonication treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- Rahaman, A.; Kumari, A.; Zeng, X.-A.; Adil Farooq, M.; Siddique, R.; Khalifa, I.; Siddeeg, A.; Ali, M.; Manzoor, M.F. Ultrasound based modification and structural-functional analysis of corn and cassava starch. Ultrason. Sonochem. 2021, 80, 105795. [Google Scholar] [CrossRef] [PubMed]
| Code | Amplitude (%) | Time (min) |
|---|---|---|
| Native | - | - |
| US5-40 | 40 | 5 |
| US15-40 | 40 | 15 |
| US25-40 | 40 | 25 |
| US35-40 | 40 | 35 |
| US5-50 | 50 | 5 |
| US15-50 | 50 | 15 |
| US25-50 | 50 | 25 |
| US35-50 | 50 | 35 |
| US5-60 | 60 | 5 |
| US15-60 | 60 | 15 |
| US25-60 | 60 | 25 |
| US35-60 | 60 | 35 |
| Independent Variables | Evaluated Levels | ||
|---|---|---|---|
| STMP–STPP ratio (w/w) * | 30:70 | 60:40 | 90:10 |
| STMP (g per 50 g of starch) | 0.9 | 1.8 | 2.7 |
| STPP (g per 50 g of starch) | 2.1 | 1.2 | 0.3 |
| Sodium sulfate (g per 50 g of starch) | 0 | 2.5 | 5 |
| US-Treated Sample | Maximum Viscosity (Pa·s) | Retrogradation Viscosity (Pa·s) | CL-Treated Sample | Maximum Viscosity (Pa·s) | Retrogradation Viscosity (Pa·s) |
|---|---|---|---|---|---|
| Native | 3.14 ± 0.10 a | 2.96 ± 0.01 a | Native | 3.14 ± 0.10 a | 2.96 ± 0.01 a |
| US5-40 | 2.69 ± 0.08 b | 2.93 ± 0.00 b | CL30:70 (0) | 0.77 ± 0.00 c | 0.84 ± 0.02 c |
| US15-40 | 2.65 ± 0.04 b | 2.77 ± 0.00 e | CL30:70 (2.5) | 0.14 ± 0.00 d | 0.25 ± 0.00 d |
| US25-40 | 2.56 ± 0.09 b | 2.85 ± 0.00 c | CL30:70 (5) | 0.04 ± 0.00 g | 0.04 ± 0.02 g |
| US35-40 | 2.47 ± 0.09 b | 2.50 ± 0.00 k | CL60:40 (0) | 1.77 ± 0.04 b | 1.54 ± 0.01 b |
| US5-50 | 2.56 ± 0.09 b | 2.79 ± 0.00 d | CL60:40 (2.5) | 0.04 ± 0.00 g | 0.05 ± 0.01 g |
| US15-50 | 2.51 ± 0.08 b | 2.73 ± 0.00 f | CL60:40 (5) | 0.03 ± 0.00 h | 0.05 ± 0.02 g |
| US25-50 | 2.48 ± 0.09 b | 2.65 ± 0.00 g | CL90:10 (0) | 0.08 ± 0.00 e | 0.14 ± 0.00 e |
| US35-50 | 2.48 ± 0.09 b | 2.63 ± 0.00 i | CL90:10 (2.5) | 0.06 ± 0.00 f | 0.14 ± 0.00 e |
| US5-60 | 2.40 ± 0.00 b | 2.64 ± 0.00 h | CL90:10 (5) | 0.04 ± 0.00 g | 0.10 ± 0.00 f |
| US15-60 | 2.42 ± 0.08 b | 2.77 ± 0.00 e | |||
| US25-60 | 2.50 ± 0.10 b | 2.73 ± 0.00 f | |||
| US35-60 | 2.48 ± 0.09 b | 2.58 ± 0.00 j |
| US-Treated Sample | Amylose Content (%) | CL-Treated Sample | Amylose Content (%) |
|---|---|---|---|
| Native starch | 24.81 ± 0.23 f | Native | 24.81 ± 0.23 a |
| US5-40 | 27.58 ± 2.15 ef | CL30:70 (0) | 14.95 ± 0.73 b |
| US15-40 | 28.10 ± 0.53 e | CL30:70 (2.5) | 2.32 ± 0.00 d |
| US25-40 | 31.21 ± 0.53 cd | CL30:70 (5) | 2.66 ± 0.48 d |
| US35-40 | 34.15 ± 0.23 abc | CL60:40 (0) | 10.97 ± 0.00 b |
| US5-50 | 26.71 ± 0.05 ef | CL60:40 (2.5) | 7.51 ± 2.93 c |
| US15-50 | 27.40 ± 0.53 ef | CL60:40 (5) | 10.10 ± 0.24 c |
| US25-50 | 31.56 ± 0.05 bcd | CL90:10 (0) | 9.41 ± 0.24 c |
| US35-50 | 34.33 ± 0.05 ab | CL90:10 (2.5) | 9.07 ± 0.24 c |
| US5-60 | 26.37 ± 0.05 ef | CL90:10 (5) | 7.34 ± 1.22 c |
| US15-60 | 29.13 ± 0.05 d | ||
| US25-60 | 31.73 ± 2.15 bcd | ||
| US35-60 | 37.26 ± 0.23 a |
| US-Treated Sample | aw | CL-Treated Sample | aw |
|---|---|---|---|
| Native | 0.430 ± 0.010 a | Native | 0.430 ± 0.010 a |
| US5-40 | 0.272 ± 0.003 b | CL30:70 (0) | 0.493 ± 0.000 a |
| US15-40 | 0.285 ± 0.004 b | CL30:70 (2.5) | 0.385 ± 0.001 c |
| US25-40 | 0.280 ± 0.002 b | CL30:70 (5) | 0.312 ± 0.001 d |
| US35-40 | 0.297 ± 0.001 b | CL60:40 (0) | 0.422 ± 0.001 bc |
| US5-50 | 0.295 ± 0.006 b | CL60:40 (2.5) | 0.300 ± 0.001 d |
| US15-50 | 0.312 ± 0.069 b | CL60:40 (5) | 0.446 ± 0.003 ab |
| US25-50 | 0.312 ± 0.004 b | CL90:10 (0) | 0.428 ± 0.000 bc |
| US35-50 | 0.314 ± 0.003 b | CL90:10 (2.5) | 0.304 ± 0.000 d |
| US5-60 | 0.297 ± 0.000 b | CL90:10 (5) | 0.393 ± 0.000 c |
| US15-60 | 0.270 ± 0.002 b | ||
| US25-60 | 0.301 ± 0.000 bc | ||
| US35-60 | 0.301 ± 0.000 bc |
| US-Treated Sample | DV50 (µm) | Span | CL-Treated Sample | DV50 (µm) | Span |
|---|---|---|---|---|---|
| Native | 11.10 ± 0.10 bcd | 11.25 ± 0.06 d | Native | 11.10 ± 1.66 f | 124.66 ± 1.53 a |
| US5-40 | 9.83. ± 0.55 de | 11.13 ± 0.57 d | CL30:70 (0) | 124.33 ± 2.88 a | 124.33 ± 0.57 b |
| US15-40 | 8.66 ± 1.04 efg | 10.99 ± 0.86 d | CL30:70 (2.5) | 75.83 ± 3.06 c | 165.66 ± 1.53 c |
| US25-40 | 8.80 ± 0.47 efg | 11.07 ± 0.54 d | CL30:70 (5) | 56.1 ± 2.19 e | 152.33 ± 0.58 c |
| US35-40 | 11.95 ± 1.00 bc | 10.72 ± 0.82 d | CL60:40 (0) | 88.66 ± 1.33 b | 190.00 ± 1.00 c |
| US5-50 | 9.17 ± 0.49 def | 14.41 ± 0.86 bc | CL60:40 (2.5) | 63.53 ± 0.20 d | 154.33 ± 1.53 c |
| US15-50 | 9.99 ± 0.59 cde | 16.54 ± 0.95 ab | CL60:40 (5) | 72.33 ± 2.30 c | 154.33 ± 1.58 c |
| US25-50 | 10.03 ± 0.40 cde | 12. 25 ± 0.49 cd | CL90:10 (0) | 86.3 ± 0.88 b | 184.00 ± 1.73 c |
| US35-50 | 12.73 ± 1.27 b | 12.19 ± 1.45 d | CL90:10 (2.5) | 56.83 ± 2.20 e | 149.33 ± 0.58 c |
| US5-60 | 5.40 ± 0.22 h | 17.27 ± 0.53 a | CL90:10 (5) | 60.3 ± 0.26 de | 156.00 ± 1.00 c |
| US15-60 | 7.44 ± 0.90 fgh | 14.76 ± 0.59 b | |||
| US25-60 | 6.99 ± 0.54 gh | 16.01 ± 0.67 ab | |||
| US35-60 | 15.64 ± 0.42 a | 11.55 ± 0.26 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cigarroa, A.S.; Rodríguez-Jimenes, G.d.C.; Aparicio-Saguilán, A.; Carpintero-Tepole, V.; García-Alvarado, M.Á.; Carrera, C.; Fernández Barbero, G.; Vázquez-Espinosa, M.; Vázquez-León, L.A. Effect of Ultrasound and Chemical Cross-Linking on the Structural and Physicochemical Properties of Malanga (Colocasia esculenta) Starch. Foods 2025, 14, 2609. https://doi.org/10.3390/foods14152609
Martínez-Cigarroa AS, Rodríguez-Jimenes GdC, Aparicio-Saguilán A, Carpintero-Tepole V, García-Alvarado MÁ, Carrera C, Fernández Barbero G, Vázquez-Espinosa M, Vázquez-León LA. Effect of Ultrasound and Chemical Cross-Linking on the Structural and Physicochemical Properties of Malanga (Colocasia esculenta) Starch. Foods. 2025; 14(15):2609. https://doi.org/10.3390/foods14152609
Chicago/Turabian StyleMartínez-Cigarroa, Ana Sofía, Guadalupe del Carmen Rodríguez-Jimenes, Alejandro Aparicio-Saguilán, Violeta Carpintero-Tepole, Miguel Ángel García-Alvarado, Ceferino Carrera, Gerardo Fernández Barbero, Mercedes Vázquez-Espinosa, and Lucio Abel Vázquez-León. 2025. "Effect of Ultrasound and Chemical Cross-Linking on the Structural and Physicochemical Properties of Malanga (Colocasia esculenta) Starch" Foods 14, no. 15: 2609. https://doi.org/10.3390/foods14152609
APA StyleMartínez-Cigarroa, A. S., Rodríguez-Jimenes, G. d. C., Aparicio-Saguilán, A., Carpintero-Tepole, V., García-Alvarado, M. Á., Carrera, C., Fernández Barbero, G., Vázquez-Espinosa, M., & Vázquez-León, L. A. (2025). Effect of Ultrasound and Chemical Cross-Linking on the Structural and Physicochemical Properties of Malanga (Colocasia esculenta) Starch. Foods, 14(15), 2609. https://doi.org/10.3390/foods14152609












