Abstract
Protein content is a key quality indicator in nuts, influencing their color, taste, storage, and processing properties. Traditional methods for protein quantification, such as the Kjeldahl nitrogen method, are time-consuming and destructive, highlighting the need for rapid, convenient alternatives. This study explores the feasibility of using portable near-infrared spectroscopy (NIRS) for the quantitative prediction of protein content in Torreya grandis (T. grandis) kernels by comparing different sample states (with shell, without shell, and granules). Spectral data were acquired using a portable NIR spectrometer, and the protein content was determined via the Kjeldahl nitrogen method as a reference. Outlier detection was performed using principal component analysis combined with Mahalanobis distance (PCA-MD) and concentration residual analysis. Various spectral preprocessing techniques and partial least squares regression (PLSR) were applied to develop protein prediction models. The results demonstrated that portable NIRS could effectively predict protein content in T. grandis kernels, with the best performance being achieved using granulated samples. The optimized model (1Der-SNV-PLSR-G) significantly outperformed models based on whole kernels (with or without shell), with determination coefficients for the calibration set () and prediction set () of 0.92 and 0.86, respectively, indicating that the sample state critically influenced prediction accuracy. This study confirmed the potential of portable NIRS as a rapid and convenient tool for protein quantification in nuts, offering a practical alternative to conventional methods. The findings also suggested its broader applicability for quality assessment in other nuts and food products, contributing to advancements in food science and agricultural technology.
1. Introduction
With the improvement in people’s living standards, there is an increasing concern about the health and safety of food [1,2]. T. grandis kernels are a kind of high-quality nut with both medicinal and edible properties [3]. They are oval or oblate in shape, with a brown outer shell; are covered by a thin, hard, dark brown or black pseudotesta; have a white or pale yellow inner kernel; and have a soft texture and sweet flavor [4]. The main cultivation zones for T. grandis kernels are predominantly found in several mountainous regions of eastern and southern China, including Zhejiang’s Kuaiji and Tianmu ranges, Anhui’s Huangshan district, Fujian’s southern highlands, northeastern Jiangxi, and the western mountain areas of Hunan Province [3,5]. Protein is a major nutrient in nuts, and its content significantly influences their color, taste, storage, and processing, which makes it a crucial indicator for evaluating the quality of nuts [6,7]. Several analytical techniques are routinely used to measure protein content, such as the Kjeldahl nitrogen analysis method, the biuret colorimetric assay, the dye-binding technique, and ultraviolet (UV) absorption spectroscopy [8]. However, these traditional chemical detection methods are time-consuming, costly, and environmentally polluting, requiring complex sample preparation that involves destructive crushing and the use of potentially hazardous reagents [9,10]. Additionally, chemical analysis is only suitable for sampling and is not applicable for large-scale testing in food trading. Therefore, it is necessary to develop an efficient, rapid method for determining the protein content in T. grandis kernels.
Currently, a variety of advanced detection technologies are being applied for the analysis of food components and quality, such as NIRS [11,12], hyperspectral imaging technology [13,14,15], laser-induced breakdown spectroscopy [16,17], and Raman spectroscopy [18]. Simultaneously, these detection technologies are being integrated with deep learning techniques to achieve rapid and accurate determination of detection targets [19,20,21]. Some studies have focused on the relationship between food production and the sustainable development of the environment and humanity [22,23,24]. NIRS technology utilizes the optical response characteristics of organic compounds within the near-infrared spectral range for analysis. It offers numerous advantages, including rapid analysis, no pollution, and no need for complex preprocessing [25]. Additionally, it enables analysis without damaging the structure of the sample itself [26,27,28]. This technology can be applied to determine the protein content in various types of nuts [7]. Qiu et al. employed NIRS technology to quantitatively detect the protein content in northeastern pine nuts. They established quantitative analysis models for both pine kernels with and without shells using the partial least squares (PLS) method. The determination coefficients of the calibration sets () for the protein models of pine kernels with and without shells were 0.91 and 0.94, respectively, while the root mean square errors (RMSEs) were 0.67 and 0.58, respectively, indicating the good predictive performance of the models [29]. Similarly, Hu et al. collected NIR spectrum data for chestnuts both with and without shells, developing predictive models for their protein content. The results showed that the determination coefficients () of the models established from the spectrum data of both states were above 0.87. The model based on the spectrum data of chestnut kernels (shelled chestnuts) demonstrated better predictive performance, with and values of 0.91 and 0.80, respectively [30]. Yi et al. developed NIRS prediction models to determine the moisture, protein, and fat contents in walnuts. The results demonstrated that the determination coefficients for these three components were all above 0.96 [31]. Shi et al. investigated the capability of NIRS for quantitatively detecting the amino acid and crude protein content in soybeans, exploring the effects of grinding particle size and fat content on the predictive performance of the NIRS models. The results showed that the determination coefficients for the NIRS models for crude protein and amino acids met the required standards ( = 0.81–0.95). Additionally, grinding and lipid extraction were found to improve the accuracy of the prediction models [32]. Tang et al. established NIRS models for predicting protein and fat content in hickory nuts. The value of the protein model was greater than 0.85, and the root mean square error of the validation set was less than 0.05 [33]. Previous studies have successfully demonstrated the application of NIRS for protein quantification in various types of nuts. These studies have also established the theoretical foundation for exploring the NIRS quantitative prediction models of nuts in different states (in-shell, shelled, and kernels). However, these analyses were exclusively conducted using benchtop NIR spectrometers, which require (1) controlled laboratory conditions, (2) complex sample preparation, including the adjustment of sample physical state (moisture and temperature conditioning), the control of sample chemical stability (homogenization and particle size control), special cleaning methods, and (3) professional operation. These limitations fundamentally restrict their potential for field applications or rapid quality assessment in production settings. Building upon these foundational studies, but addressing their practical limitations, our study introduced two key methodological innovations. First, we employed portable NIRS technology that enabled on-site spectral acquisition without laboratory constraints. Second, we systematically investigated the portable NIR spectrum acquisition from T. grandis kernels in three distinct physical states (with/without shell and granules). Then, we explored the feasibility of developing protein prediction models using spectra obtained from each processing state, which represented a novel approach in nut quality analysis.
Based on the aforementioned information, there is limited research on NIRS for protein prediction in T. grandis kernels, and no studies have systematically evaluated the impact of kernel state (with/without shells and granules) on model performance. This gap hinders the development of reliable online detection systems for industrial applications. Therefore, this study aimed to (1) investigate the feasibility of using portable NIRS for rapid protein quantification in T. grandis kernels; (2) evaluate how different kernel states affect spectral data and model accuracy; and (3) develop optimized chemometric models to enable non-contact, high-throughput protein analysis. The findings could provide methodological guidance for real-time quality monitoring in nut processing, supporting the advancement of smart agriculture and precision food industry technologies.
2. Materials and Methods
2.1. Experimental Materials
The T. grandis kernels used in this study were sourced from smallholder farms in Zhanao Village (Jidong Town, Zhuji City, Zhejiang Province, China) and were harvested in September 2021. All samples were derived from fully mature T. grandis kernels with a growth cycle exceeding 30 months (from flowering to harvest). To explore the protein content of T. grandis kernels, portable NIR spectrum data were collected from three different states: (a) T. grandis kernels with shells, (b) without shells, and (c) kernel granules (as shown in Figure 1). We aim to explore the differences between the protein prediction models built based on portable NIR spectra from three different states and to determine whether the protein content of T. grandis kernels could be rapidly determined directly from the spectrum information of the shell in a non-destructive way. The same kernels were measured in their shelled form, their deshelled form, and then ground into granules. A sequential preparation protocol was implemented for comparative spectral analysis. Firstly, T. grandis kernels with shells were initially scanned using the portable NIR spectrometer. Secondly, each T. grandis kernel was carefully cracked using a laboratory hammer to preserve kernel integrity in relation to the pseudotesta (the dark protective layer surrounding the kernel, as shown in Figure 1b). T. grandis kernels without shells but with pseudotesta were scanned using the same portable NIR spectrometer. Lastly, the same kernel was ground using an agate mortar and then sieved through a 50-mesh sieve (particle size < 300 μm) to ensure uniformity. T. grandis kernel granules were scanned using the same portable NIR spectrometer. This paired-sample design ensured direct comparability of spectral data across all three states (T. grandis kernels with/without shells and granules) from identical biological samples. Particle size standardization was rigorously maintained through controlled grinding duration (15 min), uniform sieving protocol, and microscopic verification of granule morphology. The Kjeldahl nitrogen method measurements were performed on aliquots from the same homogenized granulated samples to maintain methodological consistency.

Figure 1.
Three states of the T. grandis kernel. (a) T. grandis kernel with shell; (b) T. grandis kernel without shell; (c) T. grandis kernel granules.
2.2. Portable NIR Spectrum Collection of T. grandis Kernels
The portable NIR spectra were acquired using a Smart Eye 1700 portable spectrometer (Huoyanjinjing Co., Ltd., Hangzhou, China) operated in the 1000–1650 nm spectral range with 1 nm resolution. The instrument was equipped with a dual-integrated vacuum tungsten lamp (NVC Lighting Co., Ltd., Guangzhou, Guangdong province, China) and a 128-element InGaAs array detector. All spectral measurements were conducted under controlled environmental conditions (25 ± 1 °C and 55% relative humidity). Prior to data collection, the spectrometer underwent a 30 min warm-up period to stabilize the system. For spectral acquisition, diffuse reflectance measurements were performed using a Spectralon white reference standard for background correction. The measurement parameters were set as follows: 50 accumulated scans per spectrum, 12.7 ms integration time, and 8 cm−1 spectral resolution [5]. Spectra were collected in the order of T. grandis kernels with shells, without shells, and granules. Each sample was placed in the sampling window area of the spectrometer, ensuring that the light source vertically illuminated the sample. After scanning, the T. grandis kernel granules were promptly placed in sample bags and the air was extracted from the bags, before being labeled with serial numbers and stored in a refrigerator for subsequent physicochemical analysis.
2.3. Determination of Protein Content in T. grandis Kernels
The Kjeldahl nitrogen method was selected as the reference method for protein content determination in T. grandis kernels because it is a well-established, standardized, and internationally recognized technique for total protein quantification in food and agricultural products. The specific operation steps were carried out in accordance with the Chinese National Food Safety Standard GB 5009.5-2016 “Determination of Protein content in Foods” [34]. The experimental instruments used include the KDN-04A Kjeldahl Nitrogen Analyzer (Lvbo Instruments Co., Ltd., Hangzhou, Zhejiang province, China) and the KDN-08C Digestion Furnace (Lvbo Instruments Co., Ltd., Zhejiang province, Hangzhou, China). The digestion reagents included copper sulfate (CuSO4), potassium sulfate (K2SO4), and sulfuric acid (H2SO4). The reagents used were 2% boric acid (H3BO3) solution (m/v), 40% sodium hydroxide (NaOH) solution (m/v), and 0.05 mol/L hydrochloric acid (HCl) standard solution. The mixed indicator solution was prepared by dissolving methyl red in ethanol to form a 0.1% ethanol solution, as well as by dissolving bromocresol green in ethanol to form a 0.5% ethanol solution, followed by mixing the two solutions in equal volumes. All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China) and were of analytical grade. The formula for calculating protein content is as follows:
where W is the mass fraction of protein content (%); v1 is the standard liquid consumption for blank titration (mL); v2 is the standard liquid volume consumed by reagent titration (mL); N is the concentration of hydrochloric acid standard solution (M); 0.014 is the millimolar mass of nitrogen; F is the protein coefficient; and m is the sample mass (g).
2.4. Principal Component Analysis (PCA) of T. grandis Kernels’ Portable NIR Spectra
The original portable NIR spectrum contains the complete spectral information of the samples, typically comprising thousands of wavelength points, resulting in high dimensionality. The high dimensionality of the spectral data can affect the speed of data analysis and the accuracy of model construction, so it is often necessary to reduce the dimensions of high-dimensional data [35]. Principal component analysis (PCA) is a commonly used method for the dimensionality reduction of high-dimensional data. By selecting principal components with the maximum variance, it is possible to filter out variables that best represent the characteristics of the original data, thereby eliminating redundancy and noise in the spectral data [36].
2.5. Elimination of Outlier Samples
In portable NIR spectroscopy, outlier detection was critical for ensuring model robustness. Outliers were classified as (1) spectral outliers (caused by instrumental errors or improper sampling techniques) and (2) chemical outliers (resulting from atypical protein content or reference method variability) [5,37]. Our outlier screening protocol employed a two-stage process. Spectral outliers were identified using PCA combined with Mahalanobis distance (PCA-MD). MD is a metric used to measure the similarity or difference between two samples. The MD of the samples was calculated and samples with excessively large MD were removed, thereby improving the accuracy of the model. The threshold of MD was empirically determined as 3× the median absolute deviation from the spectral PCA score cluster centroid [38]. Concentration residuals referred to the absolute error between the actual values determined by physicochemical experiments and the predicted values from the portable NIR spectrum quantitative model. Under normal conditions, concentration residuals were close to zero, indicating that the model’s predictions align with the true values. The concentration residuals method selected different thresholds based on this principle to eliminate samples with excessively large concentration residuals. In this study, samples were flagged as chemical outliers when the absolute difference between predicted and measured protein content exceeded 0.4%.
2.6. Sample Set Division of T. grandis Kernels’ Portable NIR Spectra
After removing outlier samples, the samples were divided into a calibration set and a prediction set at a ratio of 3:1 according to the X-Y distance metric (SPXY method). Here, the variable X represented the three states of portable NIR spectra of T. grandis kernel, and the variable Y represented the measured chemical data. By simultaneously calculating the distances between samples based on both X and Y variables, the SPXY method maximized the characterization of interactions and correlations within the sample distribution.
2.7. Establishment of a Quantitative Prediction Model for Determining Protein Content in T. grandis Kernels
When establishing a quantitative prediction model for determining protein content in T. grandis kernels, the baseline drift and light scattering noise of the original portable NIR spectrum can cause discrepancies between measured values and true values; therefore, it is essential to employ preprocessing methods to eliminate redundancy and noise in the spectrum [7]. The preprocessing methods adopted in this study include Savitzky–Golay smoothing (SG), normalization (Normalize), multiplicative scatter correction (MSC), standard normal variate (SNV), first derivative (1Der), second derivative (2Der), and a combination of two preprocessing methods. Among these, SG Smoothing reduced high-frequency noise (e.g., instrument noise, sample heterogeneity) while preserving spectral peak shapes. Normalize compensated for intensity variations caused by differences in sample concentration, thickness, or path length, ensuring spectra were on a comparable scale. MSC corrected for multiplicative scatter effects caused by particle size differences and surface scattering, common in powdered or irregularly shaped samples. SNV served a comparable purpose to MSC, but did not require a reference spectrum and processed each spectrum independently. Derivative removed baseline offsets and enhanced the resolution of overlapping peaks. These methods were screened to determine the most suitable preprocessing method for improving the accuracy of the model. Partial least squares regression (PLSR) is a statistical method used for regression analysis, and the model built by PLSR can explain the relationship between dependent variables and multiple independent variables. We used the “one standard error rule” to determine optimal latent variables (LVs) by identifying the LV number minimizing root mean square error (RMSE) and selecting the simplest model (fewest LVs) within one standard error of this minimum. PLSR combines the characteristics of PCA and multiple linear regression, making it particularly suitable for handling situations where independent variables exhibit high collinearity. The PLSR models were implemented using a full-spectrum approach that inherently incorporated all latent variables without manual selection. This method leveraged the entire spectral dataset to preserve covariance structures between predictors and responses, thereby eliminating the need for traditional latent variable optimization steps.
2.8. Evaluation of the Quantitative Prediction Model for Determining Protein Content in T. grandis Kernels
The accuracy of the quantitative prediction model for determining protein content in T. grandis kernels is evaluated by the determination coefficient () and root mean square error (RMSEC) of the calibration set. The value, which describes the degree of model fitting to the sample data, is calculated using Equation (2), while the RMSEC is calculated using Equation (3).
where is the measured protein content of sample i; is the predicted protein content of sample i; is the mean measured protein content; and is the sample number of the calibration set.
The predictive ability of the model is assessed using the determination coefficient () and root mean square error (RMSEP) of the prediction set. Here, the value is used to test the model’s predictive performance, and is calculated using Equation (4), while the RMSEP is calculated using Equation (5).
where is the measured protein content of sample j; is the predicted protein content of sample j; and is the sample number of the prediction set. The higher the and values, and the lower the RMSEC and RMSEP values, the better the regression fitting effect of the model [39].
The ratio of performance to deviation (RPD) and the ratio of error range (RER) are employed to evaluate the quantitative prediction model. The RPD is calculated using Equation (6), while the RER is calculated using Equation (7).
where is the mean predicted protein content of the prediction set.
3. Results and Discussion
3.1. Determination Results of Protein Content in T. grandis Kernels
The protein content in T. grandis kernels, determined using the Kjeldahl nitrogen method, is shown in Table 1. The analysis of protein content in T. grandis kernel samples revealed a broad distribution that encompassed and extended beyond the typical range (7.70–11.50%) reported in previous studies [40]. This wide variability in protein content in our samples enhanced the statistical robustness of our dataset, as it represented the natural diversity encountered in practical applications. Such comprehensive coverage of protein content ensured that the developed model would have greater predictive reliability. The substantial sample variation supported the model’s potential for accurate protein content prediction in real-world scenarios.

Table 1.
Statistical analysis of protein content in T. grandis kernels.
3.2. Portable NIR Spectrum Analysis of T. grandis Kernels
Figure 2 shows the original portable NIR spectra of T. grandis kernels under different states— T. grandis kernels with shells, T. grandis kernels without shells, and T. grandis kernel granules. According to previous studies, the characteristic functional groups of proteins mainly include –NH2, –NH, and –COOH in the wavelength range of 1000–1650 nm, and the peaks of protein absorption are primarily located at 1000–1200 nm and 1420–1520 nm [41]. As shown in Figure 2, there were two distinct absorption peaks in the portable NIR spectra of T. grandis kernels at 1200 nm and 1450 nm. The peak at 1200 nm corresponded to the stretching vibration of the carbon–hydrogen bond (C–H) in proteins, while the peak at 1450 nm corresponded to the stretching vibration of the amide bond (N–H) in proteins. Overall, the portable NIR spectrum trends in T. grandis kernels under three different states were consistent. However, the absorbance of T. grandis kernels without shells was higher than that of those with shells, while the absorbance of T. grandis kernel granules was higher than that of kernels without shells. In conclusion, the portable NIR spectrum absorption peaks of T. grandis kernels under the three states (with shells, without shells, and in the form of granules) aligned well with the wavelength ranges of the protein absorption peaks reported in the literature, and the collected spectra provided the necessary information for modeling.
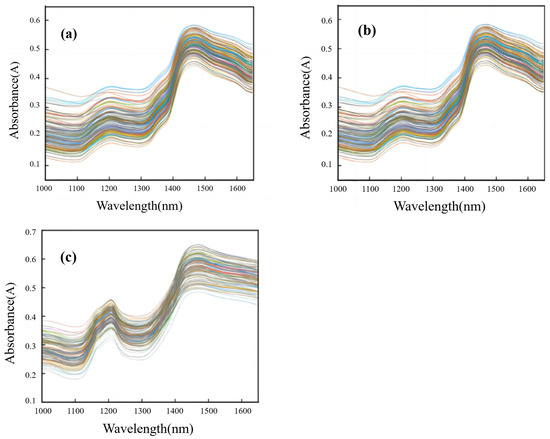
Figure 2.
Portable NIR spectra of T. grandis kernels under different states. (a) T. grandis kernels with shells, (b) T. grandis kernels without shells, and (c) T. grandis kernel granules.
3.3. Portable NIR Spectrum PCA of T. grandis Kernels
PCA was applied to reduce the dimensionality of the spectral data under different states; the results are shown in Figure 3. For T. grandis kernels with shells, the first principal component (PC1) after “Normalize” preprocessing accounted for 88.64% of the variance, and the cumulative contribution of the first four principal components reached 99.64%. Based on the clustering effect of the principal components, the first four principal component scores after “Normalize” preprocessing were selected for Mahalanobis distance calculation. For T. grandis kernels without shells, the cumulative contribution of the principal components was highest after “MSC” preprocessing, with the first four principal components accounting for 99.84% of the variance; therefore, the first four principal component scores after “MSC” preprocessing were selected for Mahalanobis distance calculation. For T. grandis kernel granules, the first principal component after “SNV” preprocessing accounted for 95.14% of the variance, and the cumulative contribution of the first four principal components reached 99.97%; thus, the first four principal component scores after “SNV” preprocessing were selected for Mahalanobis distance calculation.
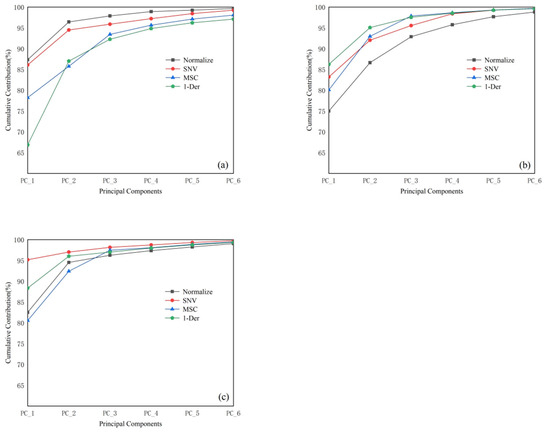
Figure 3.
Cumulative contribution of the first six principal components of different preprocessing methods of portable NIR spectra. (a) T. grandis kernels with shells, (b) T. grandis kernels without shells, and (c) T. grandis kernel granules.
3.4. Removal of Outlier Samples with Abnormal Protein Contents in T. grandis Kernels
Based on the PCA results of the portable NIR spectra of T. grandis kernels under different states, the selected principal component score matrix was used to calculate the Mahalanobis distance. The distribution of MD is shown in Figure 4 for T. grandis kernels with shells, T. grandis kernels without shells, and T. grandis kernel granules. In the figures, samples with excessively large MD (marked in red) were identified as outlier samples. From Figure 4a, it can be observed that samples numbered 24, 45, 83, 101, and 115 had excessively large Mahalanobis distance values in the MD distribution of T. grandis kernels with shells, resulting in a total of five samples being identified as outlier samples. From Figure 4b, it can be seen that samples numbered 7, 30, and 82 had excessively large MD values in the MD distribution of T. grandis kernels without shells, leading to a total of three samples being identified as outlier samples. From Figure 4c, it was evident that samples numbered 66, 75, 91, and 111 had excessively large MD values in the Mahalanobis distance distribution of T. grandis kernel granules, resulting in a total of four samples being identified as outlier samples. These outlier samples indicated anomalies in the samples, such as measurement errors, contamination, or other irregularities.
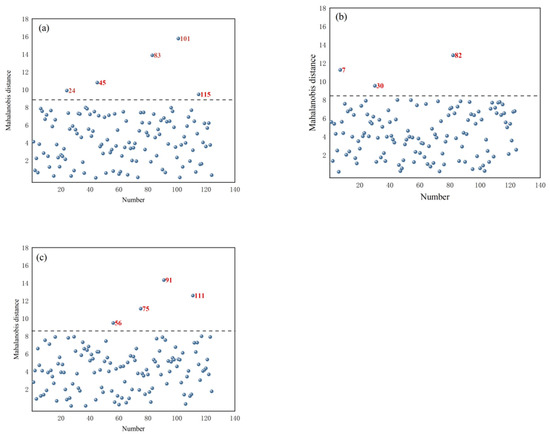
Figure 4.
Map of Mahalanobis distance of (a) T. grandis kernels with shells, (b) T. grandis kernels without shells, and (c) T. grandis kernel granules.
Figure 5 shows the concentration residual distribution for T. grandis kernels with shells, T. grandis kernels without shells, and T. grandis kernel granules. In the figures, samples marked in red were identified as outlier samples. For T. grandis kernels with shells, samples numbered 14, 73, 95, 105, 113, and 119 (a total of six samples) were removed as outlier samples. For T. grandis kernels without shells, samples numbered 9, 17, 34, 59, 77, 92, 106, 111, and 117 (a total of nine samples) were removed as outlier samples. For T. grandis kernel granules, samples numbered 5, 17, 28, 47, 59, 62, and 117 (a total of seven samples) were removed as outlier samples. These outliers, identified based on their excessively large absolute concentration residuals (the absolute difference between predicted and measured protein contents exceeded 0.4%), helped to eliminate potential errors caused by measurement inaccuracies, sample contamination, or other anomalies, thereby improving the quality of the data and the robustness of the subsequent modeling.
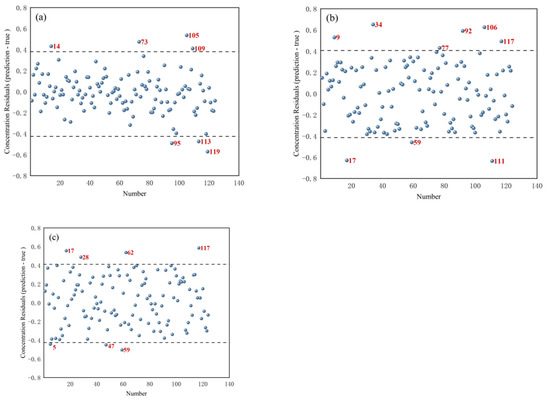
Figure 5.
Concentration residual distribution of (a) T. grandis kernels with shells, (b) T. grandis kernels without shells, and (c) T. grandis kernel granules.
The outlier samples removed using the PCA-MD and concentration residual methods for the portable NIR spectra of T. grandis kernels under different states are listed in Table 2.

Table 2.
Elimination results of outlier samples for the portable NIR spectra of T. grandis kernels under different states.
3.5. Results of Sample Division of T. grandis Kernel Samples
The sample division results for the T. grandis kernels after removing outlier samples are shown in Table 3. Due to different outliers being removed under the three different states of T. grandis kernels (with shells, without shells, and in the form of granules), the sample division results varied according to state. Overall, the protein content distribution range of the calibration set samples (6.46–12.44%) was wider than that of the prediction set samples (6.52–12.03%) across all three states. This indicates that the sample division was well executed, effectively preventing issues related to model adaptability that could arise if prediction set samples had fallen outside the range of the calibration set.

Table 3.
Calibration sets and prediction sets for determining protein contents in T. grandis kernels.
3.6. Establishment and Analysis of a Quantitative Model for Determining Protein Content in T. grandis Kernels
The samples were strategically partitioned into a calibration set (75%) and a prediction set (25%) using the SPXY algorithm after removing outlier samples. This approach optimizes sample selection by maintaining comparable distributions of protein contents in both sets while maximizing spectral variability, thereby ensuring robust model development and reliable performance evaluation. The 3:1 ratio provided sufficient calibration samples for model training while retaining an adequate independent set for prediction. The established quantitative prediction models for determining protein contents in T. grandis kernels based on portable NIR spectra under three different states are shown in Table 4. The and values of the protein content quantitative prediction model based on the original portable NIR spectra of T. grandis kernels with shells were 0.60 and 0.59, respectively. This model’s predictive performance for determining protein content was relatively poor. The established quantitative prediction models for determining protein content in T. grandis kernels exhibited varying degrees of reduced accuracy after preprocessing methods such as 1Der, 2Der, SG, Baseline, and MSC were applied. This was because these preprocessing methods led to the loss of spectral data corresponding to the protein-related bands while reducing noise, resulting in a decline in model accuracy. This highlighted the importance of selecting proper preprocessing methods to preserve essential spectral features while minimizing noise. In contrast, the models for predicting protein content in T. grandis kernels established after spectral preprocessing with Normalize, SNV, 1Der+SNV, 2Der+SNV, and SG+SNV showed improved prediction accuracy. This indicated that the preprocessing methods were reasonable and could still enhance the performance of the prediction model. Among these, the model based on PLSR after the combination of the 2Der and SNV (2Der-SNV-PLSR-S) preprocessing methods had the best performance for the portable NIR spectra of T. grandis kernels with shells, with and values of 0.69 and 0.67, respectively. This suggests that the high-precision prediction of protein content in T. grandis kernels could not be achieved with the shell state.

Table 4.
Modeling results after applying different preprocessing methods.
The and values of the protein content quantitative prediction model based on the original portable NIR spectra of T. grandis kernels without shells were 0.70 and 0.68, respectively. Compared to the protein quantitative prediction model based on T. grandis kernels with shells, the predictive performance of the model based on the portable NIR spectra without shells was significantly improved. Among these, the model based on PLSR after combined preprocessing using 1Der and SNV (1Der-SNV-PLSR-WS) had the best performance, with and values of 0.84 and 0.74, respectively (Figure 6). Compared to the optimal prediction protein model in the shelled state, the and values increased by 21.17% and 10.74%, respectively. It was concluded that the predictive performance of the optimal protein model preprocessed using 1Der-SNV-PLSR-WS, based on deshelled T. grandis kernels, was superior to that of the optimal model preprocessed using 2Der-SNV-PLSR-S for kernels in the shelled state. After deshelling, the near-infrared light could directly penetrate the pseudotesta of the kernel, allowing the spectral information of the kernel to be better captured. As a result, the spectral information in the protein-related bands was more complete, leading to a higher accuracy of the established model.
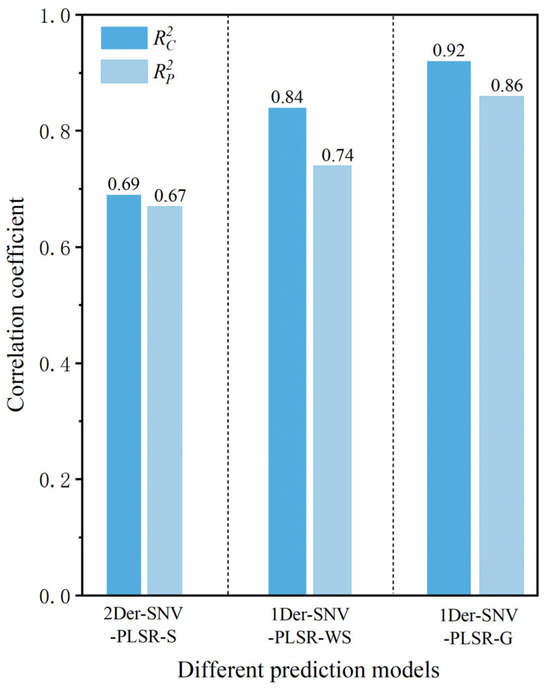
Figure 6.
Comparison of different models for predicting protein content in T. grandis kernels.
The and values of the protein content quantitative prediction model based on the original portable NIR spectra of T. grandis kernel granules were 0.80 and 0.79, respectively. The model’s predictive performance for protein content was the highest among the three states of T. grandis kernels. The models established after spectral preprocessing showed an improved prediction accuracy. Among these, the model based on PLSR after combined preprocessing using 1Der and SNV (1Der-SNV-PLSR-G) had the best performance, with and values of 0.92 and 0.86, respectively (Figure 6). Compared to the optimal protein prediction model that was preprocessed using 2Der-SNV-PLSR-S in relation to kernels in the shelled state, the and values increased by 33.43% and 27.98%, respectively. Compared to the optimal protein prediction model that was preprocessed using 1Der-SNV-PLSR-WS in relation to kernels in the unshelled state, the and values increased by 10.11% and 15.56%, respectively. After the T. grandis kernels were ground into granules, the portable NIR spectra could capture complete protein-related information. As a result, the predictive accuracy of the model established under this state was the highest.
The optimized protein content quantitative prediction model (1Der-SNV-PLSR-G) based on T. grandis kernel granules, with and of 0.92 and 0.86, respectively, indicated that the sample form critically influenced prediction accuracy. However, it required destructive sampling—grinding T. grandis kernels into a fine powder, which limited the application of the model for non-destructive detection. This method was ideal for high-precision scenarios such as laboratory research or medical-grade analysis. In contrast, the and of the optimized protein content quantitative prediction model (2Der-SNV-PLSR-S) with shells reached values of 0.69 and 0.67. The RPD and RER were 3.71 (higher than 2.5) and 24.7 (higher than 15), respectively. These data also indicated that the predictive ability of the 2Der-SNV-PLSR-S model had good performance. This model is a non-destructive, suitable, and viable option for large-scale screening and applications where moderate accuracy is acceptable. Its ability to preserve sample integrity made it ideal for rapid field assessments, preliminary quality checks, and high-throughput industrial sorting.
Aulia et al. determined the concentrations of amino acids and crude protein in soybeans using NIRS. They found that sample particle size and the type of spectroscopic instrument significantly influenced the predictive models. Optimizing the grinding and extraction processes increased the value from 0.605 to 0.952 [42]. Similarly, Zhu et al. utilized NIRS combined with PLSR to rapidly and reliably determine the protein content in coffee beans from different regions. The results showed that the OSC-PLSR model performed best for protein content prediction, with an value of 0.982, demonstrating excellent performance metrics [41]. Magwaza et al. developed a rapid and accurate model using NIRS to determine the protein content in sweet potatoes. They established a calibration model using PLSR and achieved the best model performance after 2Der preprocessing. The model demonstrated excellent performance, with an value of 0.98 [43]. The results indicated that NIRS was capable of quickly and accurately predicting the protein content in sweet potatoes. This study confirmed that NIRS combined with PLSR could serve as an alternative method for determining the protein content in nuts. These findings highlight the effectiveness of NIRS and PLSR in accurately predicting biochemical components in agricultural products.
3.7. The Predictive Performance of the Optimal Quantitative Model Preprocessed with 1Der-SNV-PLSR-G for Protein Content in T. grandis Kernels
As indicated above, the optimal quantitative model for determining protein content in T. grandis kernels was established using the portable NIR spectra collected from T. grandis kernel granules (1Der-SNV-PLSR-G). The samples in the prediction set were input into the optimal model, which was preprocessed using 1Der-SNV-PLSR-G, for protein content prediction; the results are shown in Figure 7. The figure shows that scatter points are distributed on both sides of the fitted line, with no significant outliers, indicating that the model’s predictions were accurate and consistent with the actual measured value. This confirmed the model’s predictive capability. For the optimal quantitative model preprocessed using 1Der-SNV-PLSR-G, the value of the prediction set was 0.86, while the slope and the bias of the model were 0.99 and 0.27, respectively. This demonstrated that the systematic errors were in the controllable range and that the quantitative analysis model established using portable NIR spectra could achieve the precise prediction of protein content in T. grandis kernels. The model’s predictive performance met the required standards for practical application. The regression plots reveal a systematic bias (0.27) in the optimal 1Der-SNV-PLSR-G model, particularly at higher protein content ranges. This bias might stem from the uneven distribution of protein in T. grandis kernels and the residual matrix effects between the calibration set and the prediction set. Although the current model remains acceptable for screening purposes ( = 0.86), the bias can impact absolute quantification in precision-demanding practical applications. The direction of the bias requires incorporating additional spectral preprocessing and expanding the calibration set to better capture the high variability in protein content.
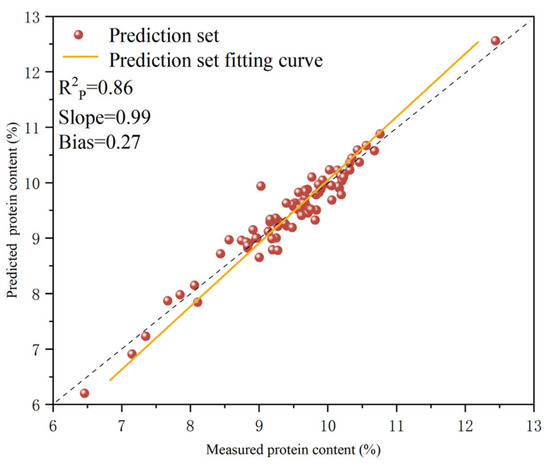
Figure 7.
Correlations between measured and predicted protein contents in T. grandis kernels.
4. Conclusions
This study successfully established portable NIRS models for the rapid prediction of protein content in T. grandis kernels across three processing states (with shells, shelled, and in the form of granules). The results demonstrated that the optimal model for quantitative protein prediction (1Der-SNV-PLSR-G) based on the portable NIR spectra of T. grandis kernel granules was established using PLSR combined with 1Der and SNV preprocessing, with and values of 0.92 and 0.86, respectively. This high precision meets the requirements for practical quality control applications in nut processing. These findings established NIRS as a viable alternative to traditional protein analysis methods for T. grandis quality control. The limitations of this study included potential geographical bias due to the exclusive use of samples from the Kuaiji Mountain region and the sole focus on protein content without considering other quality parameters. Future research should expand sample diversity across multiple growing regions and develop multi-parameter prediction models. The methodology provides a foundation for developing rapid, on-site protein detection systems during nut processing and contributes to advancing precision agriculture technologies for specialty crops.
Author Contributions
Y.G.: writing—original draft, writing—review and editing, investigation, and data analysis. H.Z.: writing—original draft, investigation, data analysis, and methodology. J.W.: data analysis, methodology, and funding acquisition. K.L.: data analysis, methodology, and funding acquisition. Y.H.: writing—original draft, and investigation. H.F.: data analysis, methodology, and investigation. M.H.: data analysis, methodology, and writing—original draft. L.Y.: writing—original draft, writing—review and editing, and investigation. C.Z.: conceptualization, data curation, calibration, visualization, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the R&D Project of National Forest and Grass Machinery Sci-Tech Innovation Park (Grant No. 2023YG03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Bushueva, A.; Adeleye, T.; Roy, P. Socioeconomic and Environmental Prospects of the Food Industry. Agric. Rural. Stud. 2024, 2, 16. [Google Scholar] [CrossRef]
- Fan, S.; Zhu, Y.; Fang, X. Big Food Vision and Food Security in China. Agric. Rural. Stud. 2023, 1, 1. [Google Scholar] [CrossRef]
- Guan, S.; Shang, Y.; Zhao, C. Storage Time Detection of Torreya grandis Kernels Using Near Infrared Spectroscopy. Sustainability 2023, 15, 7757. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Y.; Shen, J.; Meng, X.; Suo, J.; Zhang, Z.; Song, L.; Wu, J. Acceleration of Aril Cracking by Ethylene in Torreya grandis During Nut Maturation. Front. Plant Sci. 2021, 12, 761139. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Y.; Guan, S.; Shang, Y.; Bao, L.; Yan, X.; Hassan, M.; Xu, L.; Zhao, C. A Sustainable Way to Determine the Water Content in Torreya grandis Kernels Based on Near-Infrared Spectroscopy. Sustainability 2023, 15, 12423. [Google Scholar] [CrossRef]
- Jian, F.; Zhang, Z.; Li, D.; Luo, F.; Wu, Q.; Lu, F.; Dai, Z.; Nie, M.; Xu, Y.; Feng, L.; et al. Evaluation of the digestibility and antioxidant activity of protein and lipid after mixing nuts based on in vitro and in vivo models. Food Chem. 2023, 414, 135706. [Google Scholar] [CrossRef] [PubMed]
- Golly, M.K.; Ma, H.; Duan, Y.Q.; Liu, D.D.; Quaisie, J.; Tuli, J.A.; Mintah, B.K.; Dzah, C.S.; Agordoh, P.D. Effect of multi-frequency countercurrent ultrasound treatment on extraction optimization, functional and structural properties of protein isolates from Walnut (Juglans regia L.) meal. J. Food Biochem. 2020, 44, 13210. [Google Scholar] [CrossRef]
- Jancewicz, L.J.; Swift, M.L.; Penner, G.B.; Beauchemin, K.A.; Koenig, K.M.; Chibisa, G.E.; He, M.L.; McKinnon, J.J.; Yang, W.; McAllister, T.A. Development of near-infrared spectroscopy calibrations to estimate fecal composition and nutrient digestibility in beef cattle. Can. J. Anim. Sci. 2016, 97, 51–64. [Google Scholar]
- Jiang, H.; Lin, H.; Lin, J.; Adade, S.Y.S.; Chen, Q.; Xue, Z.; Chan, C. Non-destructive detection of multi-component heavy metals in corn oil using nano-modified colorimetric sensor combined with near-infrared spectroscopy. Food Control. 2022, 133, 108640. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Li, J.; Chen, X.; Jiang, H. Classification of Tea Quality Levels Using Near-Infrared Spectroscopy Based on CLPSO-SVM. Foods 2022, 11, 1658. [Google Scholar] [CrossRef]
- Lin, H.; Jiang, H.; Lin, J.; Chen, Q.; Ali, S.; Teng, S.W.; Zuo, M. Rice Freshness Identification Based on Visible Near-Infrared Spectroscopy and Colorimetric Sensor Array. Food Anal. Meth. 2021, 14, 1305–1314. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zou, X.B.; Shen, T.T.; Shi, J.Y.; Mariod, A.A. Near-Infrared (NIR) Spectroscopy for Rapid Measurement of Antioxidant Properties and Discrimination of Sudanese Honeys from Different Botanical Origin. Food Anal. Meth. 2016, 9, 2631–2641. [Google Scholar] [CrossRef]
- Shang, Y.; Bao, L.; Bi, H.; Guan, S.; Xu, J.; Gu, Y.; Zhao, C. Authenticity Discrimination and Adulteration Level Detection of Camellia Seed Oil via Hyperspectral Imaging Technology. Food Anal. Meth. 2024, 17, 450–463. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, L.; Wu, J.; Hu, S.; Shang, Y.; Hassan, M.; Zhao, C. Quantitative Prediction of Acid Value of Camellia Seed Oil Based on Hyperspectral Imaging Technology Fusing Spectral and Image Features. Foods 2024, 13, 3249. [Google Scholar] [CrossRef]
- Kucha, C.; Samaranayaka, A.; Asavajaru, P.; Ngadi, M. High-throughput precision assessment of pea-derived protein products using near infrared hyperspectral imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 331, 125770. [Google Scholar] [CrossRef]
- Wang, W.; Kong, W.; Shen, T.; Man, Z.; Zhu, W.; He, Y.; Liu, F.; Liu, Y. Application of laser-induced breakdown spectroscopy in detection of cadmium content in rice stems. Front. Plant Sci. 2020, 11, 599616. [Google Scholar] [CrossRef]
- Liu, F.; Shen, T.; Kong, W.; Peng, J.; Zhang, C.; Song, K.; Wang, W.; Zhang, C.; He, Y. Quantitative analysis of cadmium in tobacco roots using laser-induced breakdown spectroscopy with variable index and chemometrics. Front. Plant Sci. 2018, 9, 1316. [Google Scholar] [CrossRef]
- Chen, R.; Liu, F.; Zhang, C.; Wang, W.; Yang, R.; Zhao, Y.; Peng, J.; Kong, W.; Huang, J. Trends in digital detection for the quality and safety of herbs using infrared and Raman spectroscopy. Front. Plant Sci. 2023, 14, 1128300. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.; Liu, C.; Zhou, L.; Tang, L.; Wei, H.; Fan, K.; Wang, A.; Tang, Y. Rapid and Non-Destructive Geographical Origin Identification of Chuanxiong Slices Using Near-Infrared Spectroscopy and Convolutional Neural Networks. Agriculture 2024, 14, 1281. [Google Scholar] [CrossRef]
- Jiang, H.; He, Y.; Chen, Q. Determination of acid value during edible oil storage using a portable NIR spectroscopy system combined with variable selection algorithms based on an MPA-based strategy. J. Sci. Food Agric. 2021, 101, 3328–3335. [Google Scholar] [CrossRef]
- Nasir, V.; Nourian, S.; Zhou, Z.; Rahimi, S.; Avramidis, S.; Cool, J. Classification and characterization of thermally modified timber using visible and near-infrared spectroscopy and artificial neural networks: A comparative study on the performance of different NDE methods and ANNs. Wood Sci. Technol. 2019, 53, 1093–1109. [Google Scholar] [CrossRef]
- Diallo, T.; Abay, C. Malian Farmers’ Perception of Sustainable Agriculture: A Case of Southern Mali Farmers. Agric. Rural. Stud. 2024, 2, 19. [Google Scholar] [CrossRef]
- Belletti, Â.; Schneider, S. The Relationship between Agri-Food Production and Macro-Economic Dynamics: A Study on Soybeans in Brazilian South and Chinese Mainland. Agric. Rural. Stud. 2023, 1, 9. [Google Scholar] [CrossRef]
- Zeng, G.; Zhao, Y.; Sun, S. Sustainable development mechanism of food culture’s translocal production based on authenticity. Sustainability 2014, 6, 7030–7047. [Google Scholar] [CrossRef]
- Chang, X.; Huang, X.; Xu, W.; Tian, X.; Wang, C.; Wang, L.; Yu, S. Monitoring of dough fermentation during Chinese steamed bread processing by near-infrared spectroscopy combined with spectra selection and supervised learning algorithm. J. Food Process Eng. 2021, 44, e13783. [Google Scholar] [CrossRef]
- Fan, S.; Li, J.; Xia, Y.; Tian, X.; Guo, Z.; Huang, W. Long-term evaluation of soluble solids content of apples with biological variability by using near-infrared spectroscopy and calibration transfer method. Postharvest Biol. Technol. 2019, 151, 79–87. [Google Scholar] [CrossRef]
- Fan, S.; Pan, T.; Li, G. Evaluation of the physicochemical content and solid-state fermentation stage of Zhenjiang aromatic vinegar using near-infrared spectroscopy. Int. J. Food Eng. 2020, 16, 20200127. [Google Scholar] [CrossRef]
- Sheng, R.; Cheng, W.; Li, H.; Ali, S.; Agyekum, A.A.; Chen, Q. Model development for soluble solids and lycopene contents of cherry tomato at different temperatures using near-infrared spectroscopy. Postharvest Biol. Technol. 2019, 156, 110952. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, J. Application of Band Optimization of Near-infrared Spectra for Quantitative Detection of Proteins in Northeastern Pine Nuts. Mod. Food Sci. Tech. 2016, 32, 303–309. [Google Scholar]
- Hu, J.; Ma, X.; Liu, L.; Wu, Y.; Ouyang, J. Rapid evaluation of the quality of chestnuts using near-infrared reflectance spectroscopy. Food Chem. 2017, 231, 141–147. [Google Scholar] [CrossRef]
- Yi, J.; Sun, Y.; Zhu, Z.; Liu, N.; Lu, J. Near-infrared reflectance spectroscopy for the prediction of chemical composition in walnut kernel. Int. J. Food Pr. 2017, 20, 1633–1642. [Google Scholar] [CrossRef]
- Shi, D.; Hang, J.; Neufeld, J.; Zhao, S.; House, J.D. Estimation of crude protein and amino acid contents in whole, ground and defatted ground soybeans by different types of near-infrared (NIR) reflectance spectroscopy. J. Food Compos. Anal. 2022, 111, 104601. [Google Scholar] [CrossRef]
- Tang, W.; Xu, J.; Hu, D.; Zhao, C. Determination of protein and fat content inpecan based on near infrared spectroscopy. Cereals Oils 2022, 12, 158–161. [Google Scholar]
- GB 5009.5-2016; Determination of Protein content in Foods. China National Food Safety Standard: Beijing China, 2016.
- Ma, J.; Sun, D.; Pu, H.; Wei, Q.; Wang, X. Protein content evaluation of processed pork meats based on a novel single shot (snapshot) hyperspectral imaging sensor. J. Food Eng. 2019, 240, 207–213. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, F.; Shi, M. Rapid Identification and Visualization of Jowl Meat Adulteration in Pork Using Hyperspectral Imaging. Foods 2020, 9, 154. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Zhang, G.; Wang, D.; Tilley, M.; Li, Y. Rapid determination of total phenolic content of whole wheat flour using near-infrared spectroscopy and chemometrics. Food Chem. 2021, 344, 128633. [Google Scholar] [CrossRef]
- Hayes, M. Measuring Protein Content in Food: An Overview of Methods. Foods 2020, 9, 1340. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, C.; Shao, Q.; Yang, Z.; Zhang, X.; Xu, X.; Hassan, M. Determination of water content in corn stover silage using near-infrared spectroscopy. Int. J. Agric. Biol. Eng. 2019, 12, 143–148. [Google Scholar] [CrossRef]
- Xie, L. Analysis of Protein Amino Acid Composition in Chinese Torreya Nuts. Food Res. Dev. 2003, 24, 106–107. [Google Scholar]
- Zhu, M.; Long, Y.; Chen, Y.; Huang, Y.; Tang, L.; Gan, B.; Yu, Q.; Xie, J. Fast determination of lipid and protein content in green coffee beans from different origins using NIR spectroscopy and chemometrics. J. Food Compos. Anal. 2021, 102, 104055. [Google Scholar] [CrossRef]
- Aulia, R.; Kim, Y.; Amanah, H.Z.; Andi, A.M.A.; Kim, H.; Kim, H.; Lee, W.; Kim, K.; Baek, J.; Cho, B. Non-destructive prediction of protein contents of soybean seeds using near-infrared hyperspectral imaging. Infrared Phys. Technol. 2022, 127, 104365. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Naidoo, S.I.M.; Laurie, S.M.; Laing, M.D.; Shimelis, H. Development of NIRS models for rapid quantification of protein content in sweetpotato [Ipomoea batatas (L.) LAM.]. LWT-Food Sci. Tech. 2016, 72, 63–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).