The Role of Diet and Nutrition in Cancer Development and Management: From Molecular Mechanisms to Personalized Interventions
Abstract
1. Introduction
2. Interrelation of Intracellular Mechanisms of Oncogenesis and Nutrition
2.1. Inflammation
2.2. Oxidative Stress
2.3. Insulin and IGF-1 Signaling
2.4. Cell Cycle Regulation
2.5. Apoptosis
3. Current Dietary Strategies
3.1. Starvation and Caloric Restriction
3.2. The Ketogenic Diet
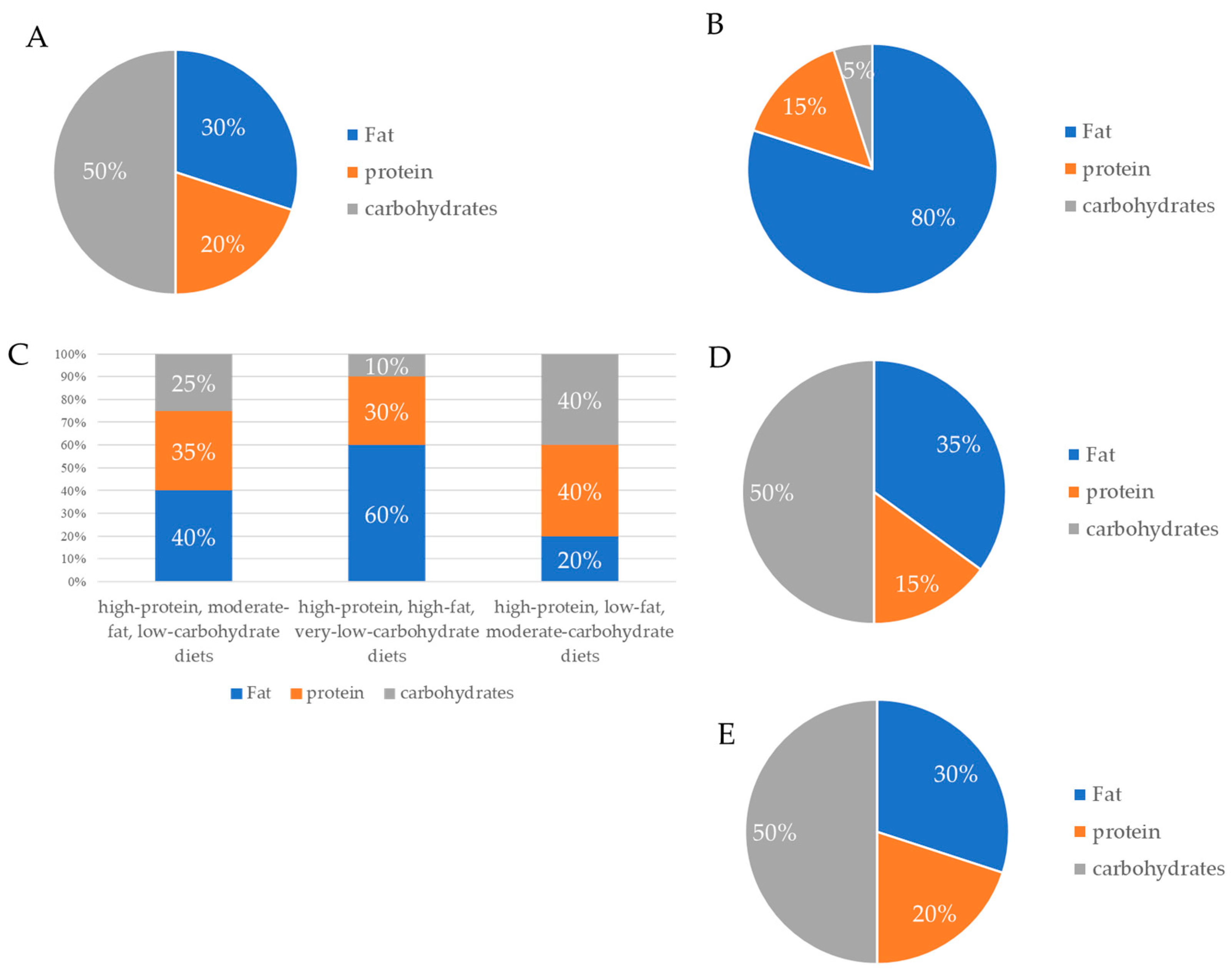
3.3. High-Protein Diet
3.4. The Mediterranean Diet
3.5. Vegetarian and Vegan Diets
4. Diet and the Gut Microbiota
5. Conclusions
- Targeting Tumor Metabolism: Dietary interventions such as caloric restriction and ketogenic diets can exploit the metabolic vulnerabilities of cancer cells, potentially enhancing the efficacy of conventional therapies.
- Modulating Inflammation and Oxidative Stress: Diets rich in antioxidants, polyphenols, and omega-3 fatty acids can mitigate chronic inflammation and oxidative stress, key drivers of tumorigenesis.
- Gut Microbiota as a Therapeutic Target: The gut microbiota plays a critical role in shaping cancer risk and treatment responses. Dietary patterns that promote microbial diversity, such as the Mediterranean and plant-based diets, offer promising avenues for cancer prevention and management.
- Personalized Nutrition: The integration of dietary strategies tailored to the molecular and clinical characteristics of individual patients holds immense potential for improving outcomes and quality of life.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bizuayehu, H.M.; Dadi, A.F.; Ahmed, K.Y.; Tegegne, T.K.; Hassen, T.A.; Kibret, G.D.; Ketema, D.B.; Bore, M.G.; Thapa, S.; Odo, D.B.; et al. Burden of 30 cancers among men: Global statistics in 2022 and projections for 2050 using population-based estimates. Cancer 2024, 130, 3708–3723. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence Update on the Relationship Between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef]
- Toward personalized cancer management: Role of precision nutrition–diet interventions. J. Funct. Foods 2024, 123, 106584. [CrossRef]
- Xiao, Y.-L.; Gong, Y.; Qi, Y.-J.; Shao, Z.-M.; Jiang, Y.-Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Sig. Transduct. Target Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- Mercier, B.D.; Tizpa, E.; Philip, E.J.; Feng, Q.; Huang, Z.; Thomas, R.M.; Pal, S.K.; Dorff, T.B.; Li, Y.R. Dietary Interventions in Cancer Treatment and Response: A Comprehensive Review. Cancers 2022, 14, 5149. [Google Scholar] [CrossRef]
- Gray, A.; Dang, B.N.; Moore, T.B.; Clemens, R.; Pressman, P. A review of nutrition and dietary interventions in oncology. SAGE Open Med. 2020, 8, 2050312120926877. [Google Scholar] [CrossRef]
- Golonko, A.; Pienkowski, T.; Swislocka, R.; Orzechowska, S.; Marszalek, K.; Szczerbinski, L.; Swiergiel, A.H.; Lewandowski, W. Dietary factors and their influence on immunotherapy strategies in oncology: A comprehensive review. Cell Death Dis. 2024, 15, 254. [Google Scholar] [CrossRef]
- Locasale, J.W. Diet and Exercise in Cancer Metabolism. Cancer Discov. 2022, 12, 2249–2257. [Google Scholar] [CrossRef]
- Lampe, J.W.; Navarro, S.L.; Hullar, M.A.J.; Shojaie, A. Inter-individual differences in response to dietary intervention: Integrating omics platforms toward personalised dietary recommendations. Proc. Nutr. Soc. 2013, 72, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Allen, A.E.; Locasale, J.W. The Molecular Link from Diet to Cancer Cell Metabolism. Mol. Cell. 2020, 78, 1034–1044. [Google Scholar] [CrossRef]
- Tajan, M.; Vousden, K.H. Dietary Approaches to Cancer Therapy. Cancer Cell 2020, 37, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Bultman, S.; D’Adamo, C.; Daniel, C.R.; Debelius, J.; Ho, E.; Eliassen, H.; Lemanne, D.; Mukherjee, P.; Seyfried, T.N.; et al. Personalized Nutrition in Disrupting Cancer—Proceedings From the 2017 American College of Nutrition Annual Meeting. J. Am. Coll. Nutr. 2019, 38, 1–14. [Google Scholar] [CrossRef]
- Akkız, H.; Şimşek, H.; Balcı, D.; Ülger, Y.; Onan, E.; Akçaer, N.; Delik, A. Inflammation and cancer: Molecular mechanisms and clinical consequences. Front. Oncol. 2025, 15, 1564572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Sig. Transduct. Target Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Laviano, A.; Rianda, S.; Molfino, A.; Rossi Fanelli, F. Omega-3 fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 156–161. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, S.; Ning, C.; Huo, Q. Omega-3 Fatty Acids Supplementation Improve Nutritional Status and Inflammatory Response in Patients with Lung Cancer: A Randomized Clinical Trial. Front. Nutr. 2021, 8, 686752. [Google Scholar] [CrossRef]
- Charles-Messance, H.; Mitchelson, K.A.; Castro, E.D.M.; Sheedy, F.J.; Roche, H.M. Regulating metabolic inflammation by nutritional modulation. J. Allergy Clin. Immunol. 2020, 146, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Saleh, E.A.M.; Al-Dolaimy, F.; Baymakov, S.; Ullah, M.I.; Khlewee, I.H.; Bisht, Y.S.; Alsaalamy, A.H. Oxidative stress affects the beginning of the growth of cancer cells through a variety of routes. Pathol. Res. Pract. 2023, 249, 154664. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Dong, R.; Wang, J.; Guan, R.; Sun, J.; Jin, P.; Shen, J. Role of Oxidative Stress in the Occurrence, Development, and Treatment of Breast Cancer. Antioxidants 2025, 14, 104. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Abdal Dayem, A.; Cho, S. Correlation Between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, S.K.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Minireview: IGF, Insulin, and Cancer. Endocrinology 2011, 152, 2546–2551. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Schmitz, G. Over-stimulation of insulin/IGF-1 signaling by Western diet may promote diseases of civilization: Lessons learnt from Laron syndrome. Nutr. Metab. 2011, 8, 41. [Google Scholar] [CrossRef]
- Klement, R.J.; Fink, M.K. Dietary and pharmacological modification of the insulin/IGF-1 system: Exploiting the full repertoire against cancer. Oncogenesis 2016, 5, e193. [Google Scholar] [CrossRef]
- Cancemi, G.; Cicero, N.; Allegra, A.; Gangemi, S. Effect of Diet and Oxidative Stress in the Pathogenesis of Lymphoproliferative Disorders. Antioxidants 2023, 12, 1674. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Dunger, D.B.; Giovannucci, E.L. Insulin, Insulin-Like Growth Factor-I (IGF-I), IGF Binding Proteins, Their Biologic Interactions, and Colorectal Cancer. J. Natl. Cancer Inst. 2002, 94, 972–980. [Google Scholar] [CrossRef]
- Bowers, L.W.; Rossi, E.L.; O’Flanagan, C.H.; deGraffenried, L.A.; Hursting, S.D. The Role of the Insulin/IGF System in Cancer: Lessons Learned from Clinical Trials and the Energy Balance-Cancer Link. Front. Endocrinol. 2015, 6, 77. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Abdelhamid, A.M.; Saber, S.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; Yahya, G.; Salama, M.M. Cell cycle machinery in oncology: A comprehensive review of therapeutic targets. FASEB J. 2024, 38, e23734. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G. The pathophysiology of the cell cycle in cancer and treatment strategies using various cell cycle checkpoint inhibitors. Pathol. Res. Pract. 2023, 251, 154854. [Google Scholar] [CrossRef]
- Shukla, S.; Meeran, S.M.; Katiyar, S.K. Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention. Cancer Lett. 2014, 355, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Yang, W.; Dong, J.; Li, L. Regulation of dietary polyphenols on cancer cell pyroptosis and the tumor immune microenvironment. Front. Nutr. 2022, 9, 974896. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer Chemoprevention by Dietary Polyphenols: Promising Role for Epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.; Al-Foheidi, M.H.; Al-Mansour, M.M. Energy and caloric restriction, and fasting and cancer: A narrative review. Support Care Cancer 2021, 29, 2299–2304. [Google Scholar] [CrossRef]
- Pomatto-Watson, L.C.D.; Bodogai, M.; Bosompra, O.; Kato, J.; Wong, S.; Carpenter, M.; Duregon, E.; Chowdhury, D.; Krishna, P.; Ng, S.; et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat. Commun. 2021, 12, 6201. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef]
- Meeran, S.M.; Ahmed, A.; Tollefsbol, T.O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenet. 2010, 1, 101–116. [Google Scholar] [CrossRef]
- Cozzo, A.J.; Coleman, M.F.; Pearce, J.B.; Pfeil, A.J.; Etigunta, S.K.; Hursting, S.D. Dietary Energy Modulation and Autophagy: Exploiting Metabolic Vulnerabilities to Starve Cancer. Front. Cell Dev. Biol. 2020, 8, 590192. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Dysregulation of Apoptosis in Cancer. J. Clin. Oncol. 1999, 17, 2941. [Google Scholar] [CrossRef]
- Papaliagkas, V.; Anogianaki, A.; Anogianakis, G.; Ilonidis, G. The proteins and the mechanisms of apoptosis: A mini-review of the fundamentals. Hippokratia 2007, 11, 108–113. [Google Scholar]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Khan, N.; Adhami, V.M.; Mukhtar, H. Apoptosis by dietary agents for prevention and treatment of cancer. Biochem. Pharmacol. 2008, 76, 1333–1339. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Mukhtar, H. Apoptosis by dietary factors: The suicide solution for delaying cancer growth. Carcinogenesis 2007, 28, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Watts, E.L.; Moore, S.C.; Gunter, M.J.; Chatterjee, N. Adiposity and cancer: Meta-analysis, mechanisms, and future perspectives. medRxiv 2024. [Google Scholar] [CrossRef]
- Mahamat-saleh, Y.; Aune, D.; Freisling, H.; Hardikar, S.; Jaafar, R.; Rinaldi, S.; Gunter, M.J.; Dossus, L. Association of metabolic obesity phenotypes with risk of overall and site-specific cancers: A systematic review and meta-analysis of cohort studies. Br. J. Cancer 2024, 131, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Ilerhunmwuwa, N.P.; Abdul Khader, A.H.S.; Smith, C.; Cliff, E.R.S.; Booth, C.M.; Hottel, E.; Aziz, M.; Lee-Smith, W.; Goodman, A.; Chakraborty, R.; et al. Dietary interventions in cancer: A systematic review of all randomized controlled trials. J. Natl. Cancer Inst. 2024, 116, 1026–1034. [Google Scholar] [CrossRef]
- Carlson, D.A.; True, C.; Wilson, C.G. Oxidative stress and food as medicine. Front. Nutr. 2024, 11, 1394632. [Google Scholar] [CrossRef] [PubMed]
- Meynet, O.; Ricci, J.-E. Caloric restriction and cancer: Molecular mechanisms and clinical implications. Trends Mol. Med. 2014, 20, 419–427. [Google Scholar] [CrossRef]
- Xie, L.; Wang, W. Weight control and cancer preventive mechanisms: Role of IGF-1-mediated signaling pathways. Exp. Biol. Med. 2013, 238, 127–132. [Google Scholar] [CrossRef]
- Galet, C.; Gray, A.; Said, J.W.; Castor, B.; Wan, J.; Beltran, P.J.; Calzone, F.J.; Elashoff, D.; Cohen, P.; Aronson, W.J. Effects of Calorie Restriction and IGF-1 Receptor Blockade on the Progression of 22Rv1 Prostate Cancer Xenografts. Int. J. Mol. Sci. 2013, 14, 13782–13795. [Google Scholar] [CrossRef]
- Shabkhizan, R.; Haiaty, S.; Moslehian, M.S.; Bazmani, A.; Sadeghsoltani, F.; Bagheri, H.S.; Rahbarghazi, R.; Sakhinia, E. The Beneficial and Adverse Effects of Autophagic Response to Caloric Restriction and Fasting. Adv. Nutr. 2023, 14, 1211–1225. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Icard, P.; Ollivier, L.; Forgez, P.; Otz, J.; Alifano, M.; Fournel, L.; Loi, M.; Thariat, J. Perspective: Do Fasting, Caloric Restriction, and Diets Increase Sensitivity to Radiotherapy? A Literature Review. Adv. Nutr. 2020, 11, 1089–1101. [Google Scholar] [CrossRef]
- Kalam, F.; James, D.L.; Li, Y.R.; Coleman, M.F.; Kiesel, V.A.; Cespedes Feliciano, E.M.; Hursting, S.D.; Sears, D.D.; Kleckner, A.S. Intermittent fasting interventions to leverage metabolic and circadian mechanisms for cancer treatment and supportive care outcomes. JNCI Monogr. 2023, 2023, 84–103. [Google Scholar] [CrossRef] [PubMed]
- De Gruil, N.; Pijl, H.; van der Burg, S.H.; Kroep, J.R. Short-Term Fasting Synergizes with Solid Cancer Therapy by Boosting Antitumor Immunity. Cancers 2022, 14, 1390. [Google Scholar] [CrossRef] [PubMed]
- Di Tano, M.; Raucci, F.; Vernieri, C.; Caffa, I.; Buono, R.; Fanti, M.; Brandhorst, S.; Curigliano, G.; Nencioni, A.; de Braud, F.; et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 2020, 11, 2332. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, H.; Liu, Z.; Liang, Z.; Zhu, J.; Zhang, R.; Li, Y. Fasting as an Adjuvant Therapy for Cancer: Mechanism of Action and Clinical Practice. Biomolecules 2024, 14, 1437. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminazdeh-Gohari, S.; Kofler, B. Ketogenic diet in cancer therapy. Aging 2018, 10, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Tan-Shalaby, J. Ketogenic Diets and Cancer: Emerging Evidence. Fed. Pract. 2017, 34, 37S–42S. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahmod, A.I.; Kamal, A.; Rashid, H.M.; Alashqar, A.M.D.; Khater, S.; Jamal, D.; Waly, M. Ketogenic Diet in Cancer Prevention and Therapy: Molecular Targets and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2021, 43, 558–589. [Google Scholar] [CrossRef]
- Woolf, E.C.; Curley, K.L.; Liu, Q.; Turner, G.H.; Charlton, J.A.; Preul, M.C.; Scheck, A.C. The Ketogenic Diet Alters the Hypoxic Response and Affects Expression of Proteins Associated with Angiogenesis, Invasive Potential and Vascular Permeability in a Mouse Glioma Model. PLoS ONE 2015, 10, e0130357. [Google Scholar] [CrossRef]
- Su, Z.; Liu, Y.; Xia, Z.; Rustgi, A.K.; Gu, W. An unexpected role for the ketogenic diet in triggering tumor metastasis by modulating BACH1-mediated transcription. Sci. Adv. 2024, 10, eadm9481. [Google Scholar] [CrossRef]
- Chaudhary, R. Ketogenic diet as a treatment and prevention strategy for cancer: A therapeutic alternative. Nutrition 2024, 124, 112427. [Google Scholar] [CrossRef]
- Allen, B.G.; Bhatia, S.K.; Anderson, C.M.; Eichenberger-Gilmore, J.M.; Sibenaller, Z.A.; Mapuskar, K.A.; Schoenfeld, J.D.; Buatti, J.M.; Spitz, D.R.; Fath, M.A. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014, 2, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; TeSlaa, T.; Ng, S.; Nofal, M.; Wang, L.; Lan, T.; Zeng, X.; Cowan, A.; McBride, M.; Lu, W.; et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med 2022, 3, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, L.S.; Santos-Veloso, M.A.O. Ketogenic diet and metastasis: A critical review of the literature and possible mechanisms. Clin. Nutr. ESPEN 2023, 57, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.-M.; van Walree, N.; Schramel, F.; Soud, M.Y.-E.; Baltruškevičienė, E.; Lybaert, W.; Veldhorst, M.; van den Berg, C.A.; Kaasa, S. High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study. Nutrients 2023, 15, 5030. [Google Scholar] [CrossRef] [PubMed]
- Boutière, M.; Cottet-Rousselle, C.; Coppard, C.; Couturier, K.; Féart, C.; Couchet, M.; Corne, C.; Moinard, C.; Breuillard, C. Protein intake in cancer: Does it improve nutritional status and/or modify tumour response to chemotherapy? J. Cachexia Sarcopenia Muscle 2023, 14, 2003–2015. [Google Scholar] [CrossRef]
- Orsso, C.E.; Caretero, A.; Poltronieri, T.S.; Arends, J.; de van der Schueren, M.A.; Kiss, N.; Laviano, A.; Prado, C.M. Effects of high-protein supplementation during cancer therapy: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2024, 120, 1311–1324. [Google Scholar] [CrossRef]
- Feasibility of two levels of protein intake in patients with colorectal cancer: Findings from the Protein Recommendation to Increase Muscle (PRIMe) randomized controlled pilot trial. ESMO Open 2024, 9, 103604. [CrossRef]
- Ford, K.L.; Arends, J.; Atherton, P.J.; Engelen, M.P.; Gonçalves, T.J.; Laviano, A.; Lobo, D.N.; Phillips, S.M.; Ravasco, P.; Deutz, N.E.; et al. The importance of protein sources to support muscle anabolism in cancer: An expert group opinion. Clin. Nutr. 2022, 41, 192–201. [Google Scholar] [CrossRef]
- Schalla, J.; Frommelt, S.; Geisler, S.; Isenmann, E. Is there a beneficial effect of a high-protein diet on body composition and strength capacity in physical active middle-aged individuals?—An eight-week randomized controlled trial. Front. Sports Act. Living. 2024, 6, 1346637. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.-W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Jahromi, M.K.; Ahmadirad, H.; Farhadnejad, H.; Norouzzadeh, M.; Mokhtari, E.; Teymoori, F.; Saber, N.; Heidari, Z.; Mirmiran, P.; Rashidkhani, B. High-protein diet scores, macronutrient substitution, and breast cancer risk: Insights from substitution analysis. BMC Women’s Health 2024, 24, 121. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Monllor-Tormos, A.; García-Vigara, A.; Morgan, O.; García-Pérez, M.Á.; Mendoza, N.; Tarín, J.J.; Cano, A. Mediterranean diet for cancer prevention and survivorship. Maturitas 2023, 178, 107841. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Braakhuis, A.J.; Bishop, K.S. The Mediterranean Diet and Breast Cancer: A Personalised Approach. Healthcare 2019, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Mastrantoni, L.; Terranova, R.; Colloca, G.F.; Zuccalà, G.; Landi, F. The role of Mediterranean diet in cancer incidence and mortality in the older adults. npj Aging 2024, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Aguilera-Buenosvinos, I.; Morales Berstein, F.; González-Gil, E.M.; Dossus, L.; Gunter, M.J.; Biessy, C.; Masala, G.; Santucci De Magistris, M.; Laouali, N.; Shah, S.; et al. Adherence to the Mediterranean Diet and Obesity-Linked Cancer Risk in EPIC. JAMA Netw. Open 2025, 8, e2461031. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef]
- Su, Y.; Cochrane, B.B.; Reding, K.; Herting, J.R.; Tinker, L.F.; Zaslavsky, O. Mediterranean Diet and Fatigue Among Community-Dwelling Postmenopausal Women. J. Nutr. Gerontol. Geriatr. 2022, 41, 22–45. [Google Scholar] [CrossRef]
- Capodici, A.; Mocciaro, G.; Gori, D.; Landry, M.J.; Masini, A.; Sanmarchi, F.; Fiore, M.; Coa, A.A.; Castagna, G.; Gardner, C.D.; et al. Cardiovascular health and cancer risk associated with plant based diets: An umbrella review. PLoS ONE 2024, 19, e0300711. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Salamanca-Fernández, E.; Garcia-Villanova, B.; Sánchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef]
- Wang, T.; Masedunskas, A.; Willett, W.C.; Fontana, L. Vegetarian and vegan diets: Benefits and drawbacks. Eur. Heart J. 2023, 44, 3423–3439. [Google Scholar] [CrossRef]
- Lanou, A.J.; Svenson, B. Reduced cancer risk in vegetarians: An analysis of recent reports. Cancer Manag. Res. 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Hardt, L.; Mahamat-Saleh, Y.; Aune, D.; Schlesinger, S. Plant-Based Diets and Cancer Prognosis: A Review of Recent Research. Curr. Nutr. Rep. 2022, 11, 695–716. [Google Scholar] [CrossRef]
- Godos, J.; Bella, F.; Sciacca, S.; Galvano, F.; Grosso, G. Vegetarianism and breast, colorectal and prostate cancer risk: An overview and meta-analysis of cohort studies. J. Hum. Nutr. Diet 2017, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Batista, K.S.; Cintra, V.M.; Lucena, P.A.F.; Manhães-de-Castro, R.; Toscano, A.E.; Costa, L.P.; Queiroz, M.E.B.S.; de Andrade, S.M.; Guzman-Quevedo, O.; Aquino, J.d.S. The role of vitamin B12 in viral infections: A comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 2022, 80, 561–578. [Google Scholar] [CrossRef]
- Nakatsu, G.; Andreeva, N.; MacDonald, M.H.; Garrett, W.S. Interactions between diet and gut microbiota in cancer. Nat. Microbiol. 2024, 9, 1644–1654. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T. Diet, Gut Microbiota, and Colorectal Cancer Prevention: A Review of Potential Mechanisms and Promising Targets for Future Research. Curr. Color. Cancer Rep. 2017, 13, 429–439. [Google Scholar] [CrossRef]
- Nguyen, N.-T.A.; Jiang, Y.; McQuade, J.L. Eating away cancer: The potential of diet and the microbiome for shaping immunotherapy outcome. Front. Immunol. 2024, 15, 1409414. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Sun, J. Microbiome and diet in colon cancer development and treatment. Cancer J. 2023, 29, 89–97. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.V.; de Camargo, M.R.; Russo, E.; Amedei, A. Role of diet and gut microbiota on colorectal cancer immunomodulation. World J. Gastroenterol. 2019, 25, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Greathouse, K.L.; Wyatt, M.; Johnson, A.J.; Toy, E.P.; Khan, J.M.; Dunn, K.; Clegg, D.J.; Reddy, S. Diet-microbiome interactions in cancer treatment: Opportunities and challenges for precision nutrition in cancer. Neoplasia 2022, 29, 100800. [Google Scholar] [CrossRef]
- Szczyrek, M.; Bitkowska, P.; Chunowski, P.; Czuchryta, P.; Krawczyk, P.; Milanowski, J. Diet, Microbiome, and Cancer Immunotherapy—A Comprehensive Review. Nutrients 2021, 13, 2217. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer. Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef]
- Zackular, J.P.; Rogers, M.A.M.; Ruffin, M.T.; Schloss, P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014, 7, 1112–1121. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
| Mechanism | Description | Nutritional Factors Involved |
|---|---|---|
| Inflammation | Chronic inflammation promotes tumor development and progression in various types of cancer. | - Omega-3 fatty acids (anti-inflammatory) - Refined carbohydrates and saturated fats (pro-inflammatory) |
| Oxidative stress | Imbalance between pro-oxidants and antioxidants leads to DNA damage and mutations, contributing to carcinogenesis. | - Antioxidants (e.g., vitamins C and E, polyphenols) - Prooxidants (e.g., heterocyclic amines in charred meat) |
| Insulin and IGF-1 signaling | Overactivation of insulin and IGF-1 pathways promotes cell proliferation and survival in multiple cancer types. | - High glycemic load diets (increase insulin and IGF-1) - Calorie restriction (decrease insulin and IGF-1) |
| Cell cycle regulation | Dysregulation of cell cycle checkpoints leads to uncontrolled cell division, a hallmark of cancer. | - Folate and vitamin B12 (essential for DNA synthesis and repair) - Phytochemicals (e.g., curcumin, resveratrol) |
| Apoptosis | Evasion of programmed cell death allows cancer cells to survive and proliferate. | - Omega-3 fatty acids and flavonoids (induce apoptosis) - Saturated fats (inhibit apoptosis) |
| Diet Type | Definition |
|---|---|
| Calorie restriction | Reducing total calorie intake over an extended period, lasting from a few months to several years. |
| Starvation | Starvation refers to an acute shortage of calories over an extended period of time, resulting in exhaustion. Most fasting methods allow unrestricted access to water, which is why they are also called water-only fasting. |
| Intermittent fasting | Short-term weekly fasting of 24 h, once or twice a week. |
| Ketogenic diet | A diet high in fat, low in carbohydrates, and low or high in protein intake. |
| High-protein diet | A diet in which >20% of calories come from protein. |
| Mediterranean diet | A diet based on the traditional diet and habits of the Mediterranean countries of Portugal, Spain, Italy, and Greece. It includes a high consumption of fruits, vegetables, and legumes, and moderate consumption of unprocessed cereals, olive oil, fish, and dairy products, with occasional consumption of meat and wine. |
| Vegetarian and vegan diets | The diet includes a high intake of fruits, vegetables, legumes, and unprocessed grains, with or without dairy products (lactovegetarianism) (veganism). The diet excludes meat, seafood, and poultry. Some varieties of vegetarian diets also include eggs (ovo-lacto-vegetarianism), fish (pescetarianism), or both (ovo-pescetarianism). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruban, M.; Pozhidaeva, E.; Bolotina, L.; Kaprin, A. The Role of Diet and Nutrition in Cancer Development and Management: From Molecular Mechanisms to Personalized Interventions. Foods 2025, 14, 1788. https://doi.org/10.3390/foods14101788
Ruban M, Pozhidaeva E, Bolotina L, Kaprin A. The Role of Diet and Nutrition in Cancer Development and Management: From Molecular Mechanisms to Personalized Interventions. Foods. 2025; 14(10):1788. https://doi.org/10.3390/foods14101788
Chicago/Turabian StyleRuban, Maxim, Elizaveta Pozhidaeva, Larisa Bolotina, and Andrey Kaprin. 2025. "The Role of Diet and Nutrition in Cancer Development and Management: From Molecular Mechanisms to Personalized Interventions" Foods 14, no. 10: 1788. https://doi.org/10.3390/foods14101788
APA StyleRuban, M., Pozhidaeva, E., Bolotina, L., & Kaprin, A. (2025). The Role of Diet and Nutrition in Cancer Development and Management: From Molecular Mechanisms to Personalized Interventions. Foods, 14(10), 1788. https://doi.org/10.3390/foods14101788






