Special Characterization and Excellent Antioxidant Capabilities of Zinc Chelated Squid Protein Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
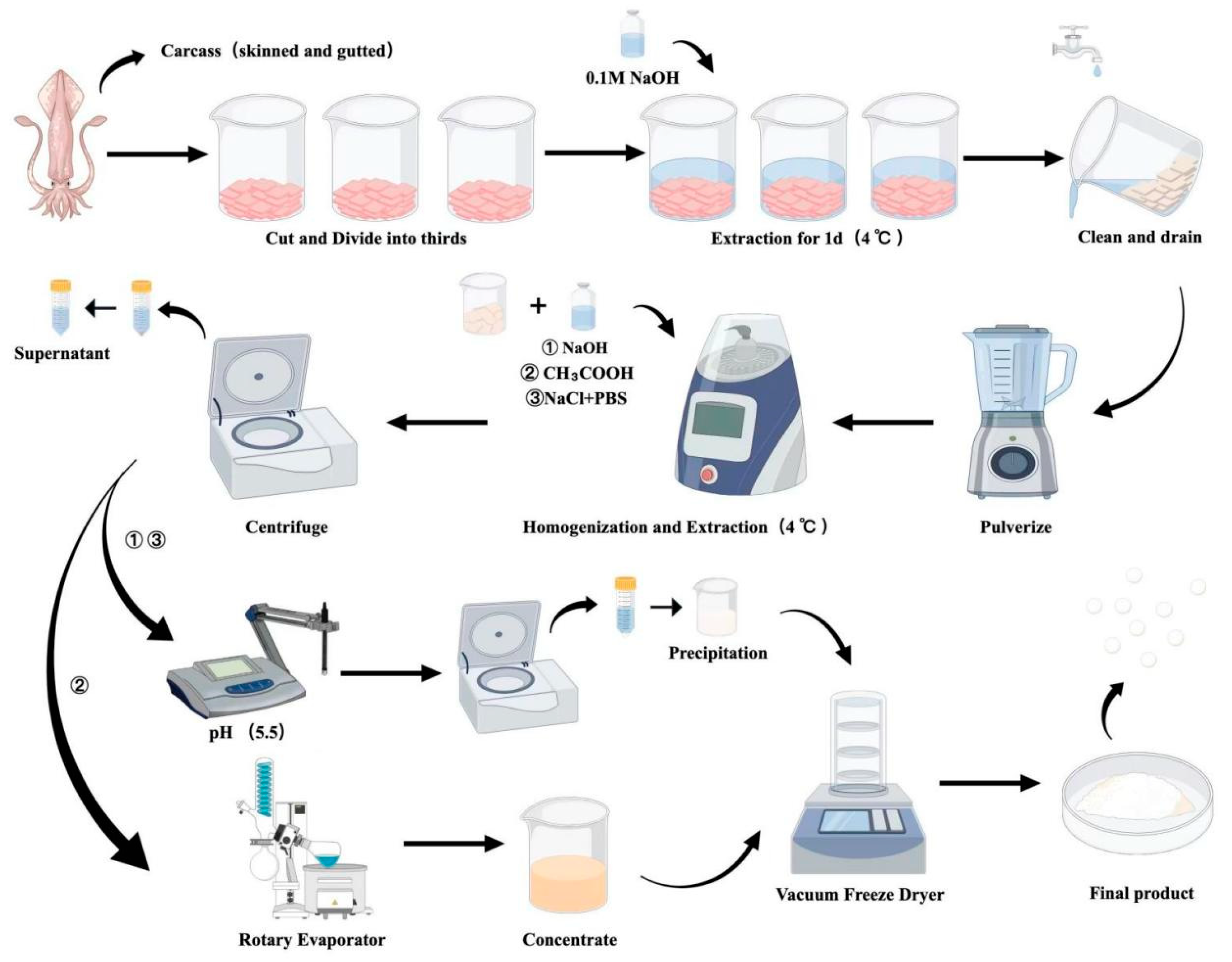
2.2. Preparation of Argentine Squid Protein (ASP)
2.2.1. Amino Acid Composition
2.2.2. Protein Extraction Rate (PER) and Protein Purity (PP)
2.3. Preparation of Argentine Squid Protein Peptides (ASPP)
2.3.1. Selection of Preparation Conditions for ASPP
2.3.2. Response Surface Methodology (RSM)
2.3.3. Degree of Hydrolysis (DH)
2.4. Preparation of Zinc-Chelated Argentine Squid Protein (Zn-ASPP)
Measurement and Calculation of Zinc Content and Zinc Chelation Rate
2.5. Structural Characterization
2.5.1. SDS-PAGE
2.5.2. Scanning Electron Microscope (SEM)
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Evaluation of Physical Property
2.6.1. Particle Size (PS)
2.6.2. Viscosity
2.7. Evaluation of Functional Properties
2.7.1. Solubility
2.7.2. Antioxidant Capacity of Zn-ASPP
2.7.3. Foamability and Foam Stability
2.7.4. Emulsibility and Emulsion Stability
2.8. Statistical Analysis
3. Results
3.1. Amino Acid Composition of ASP
3.2. Protein Extraction Rate (PER) and Protein Purity (PP)
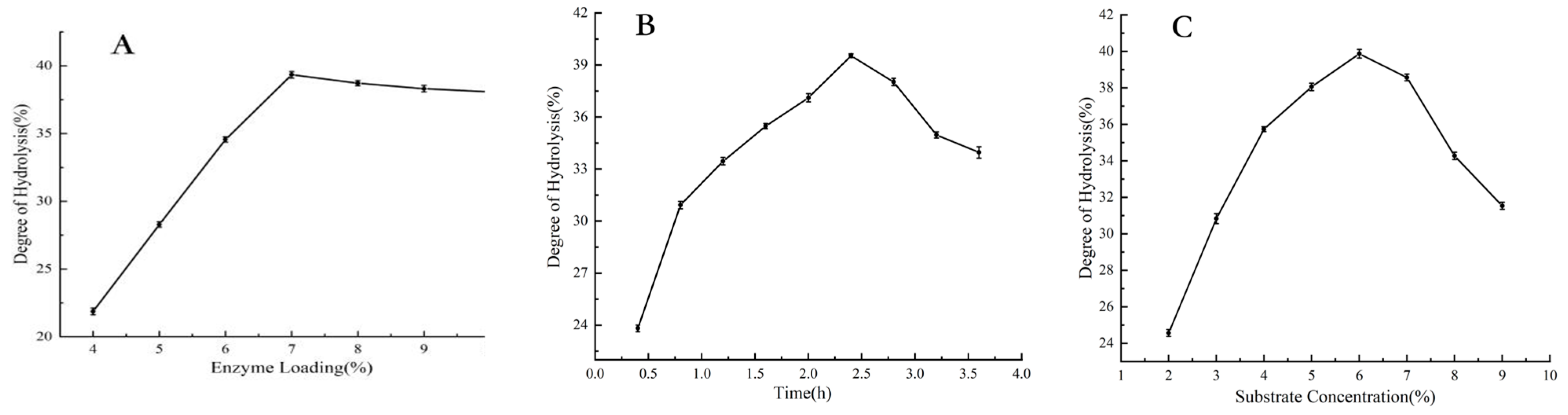
3.3. Preliminary Hydrolysis Condition
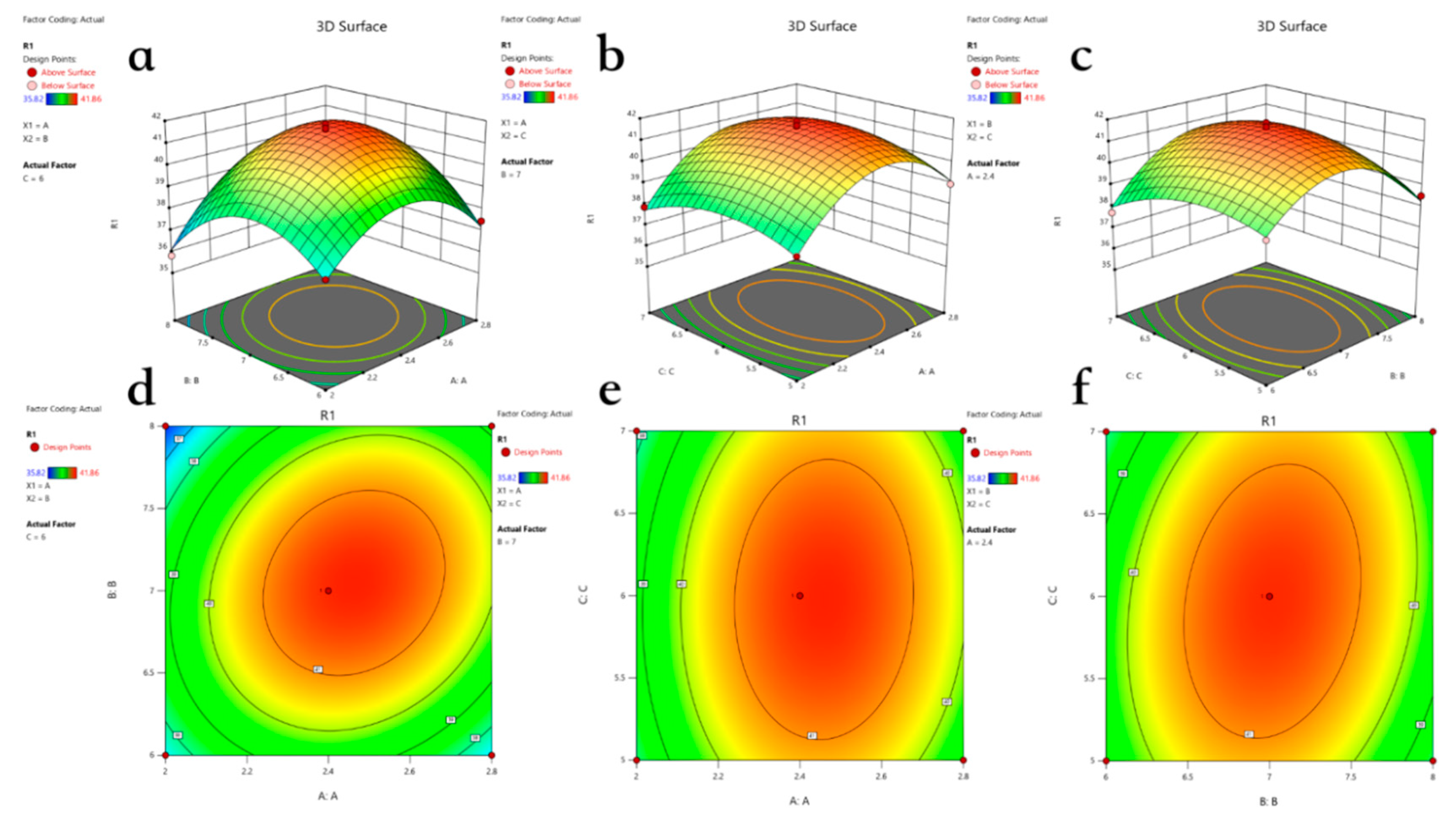
3.4. Optimal Hydrolysis Conditions Under RSM
3.5. Zinc Content and Zinc Chelation Rate
3.6. Structural Characterization
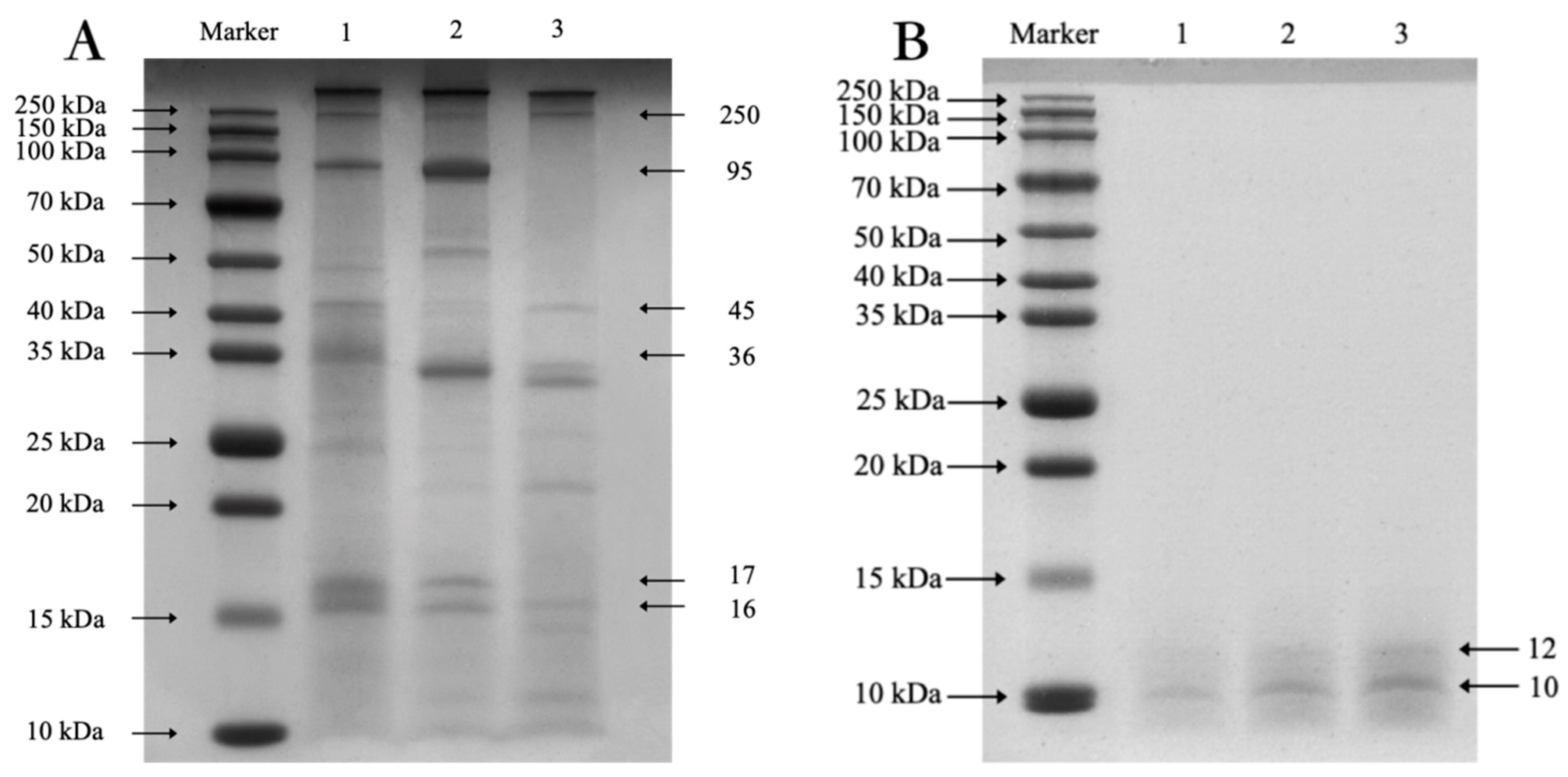
3.6.1. SDS-PAGE
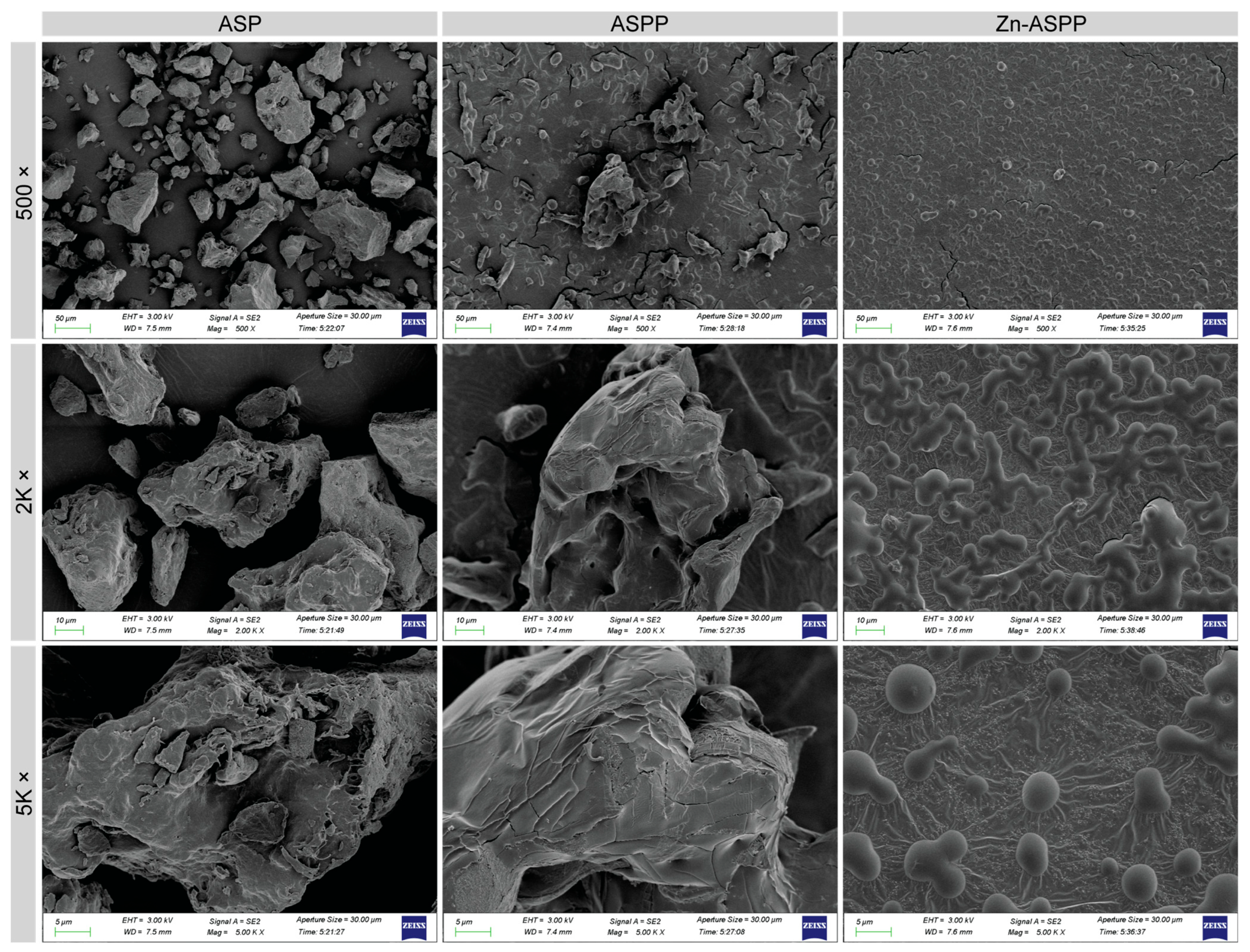
3.6.2. Microscopic Morphology of ASP, ASPP, and Zn-ASPP
3.6.3. Thermal Stability of ASP, ASPP, and Zn-ASPP
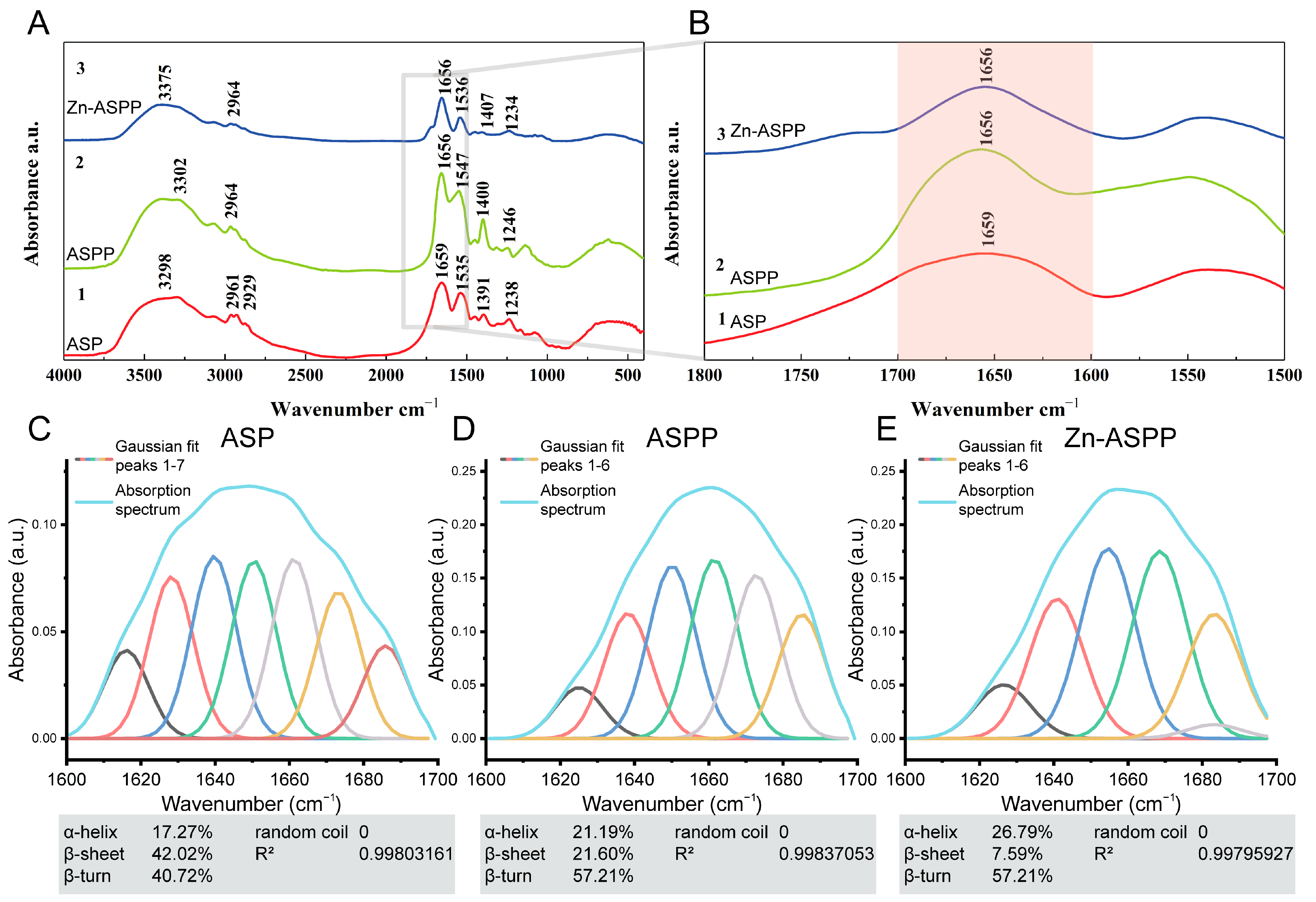
3.6.4. Structural Changes of ASP, ASPP, and Zn-ASPP Based on FTIR
3.7. Physical Property
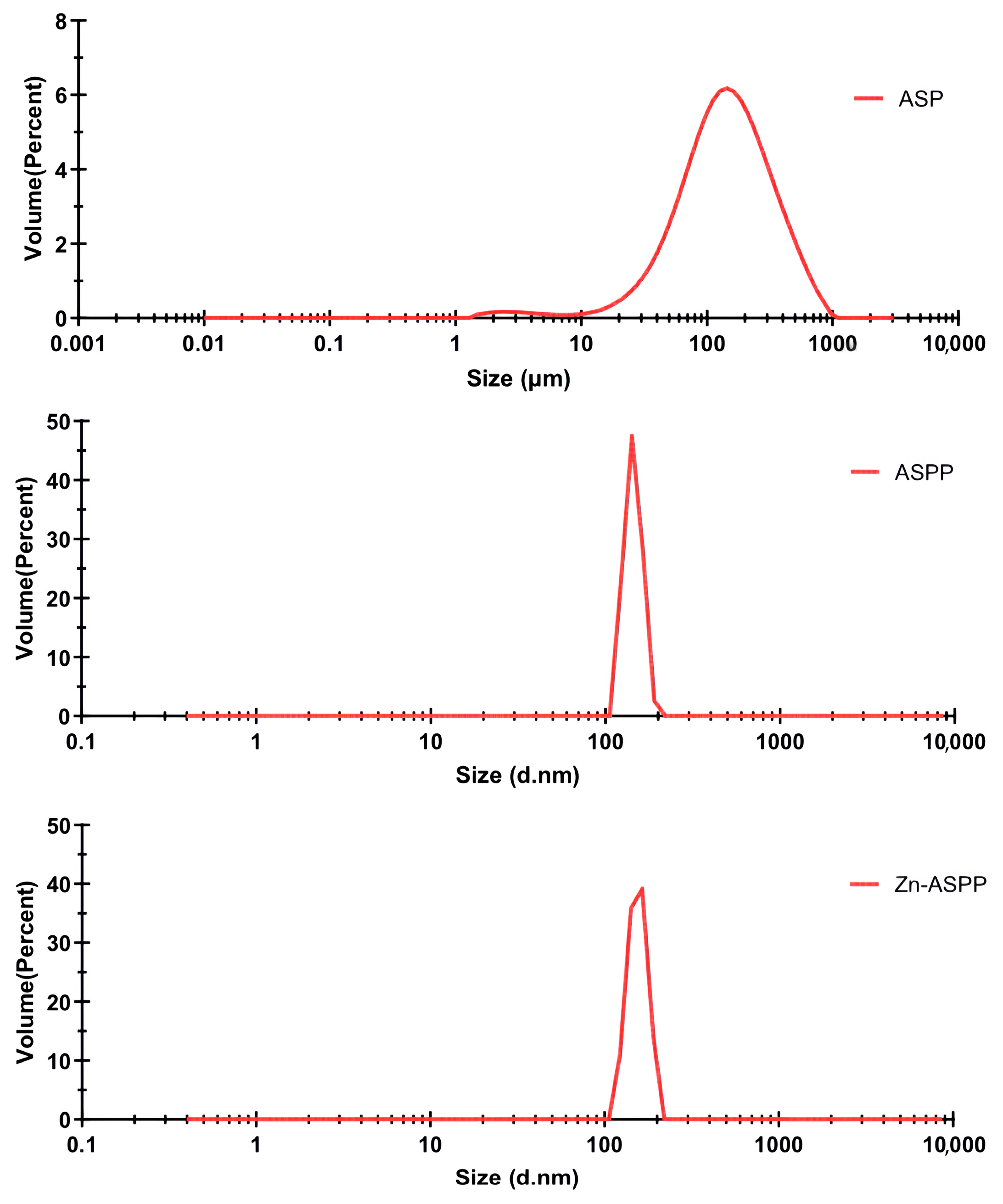
3.7.1. Particle Size of ASP, ASPP, and Zn-ASPP
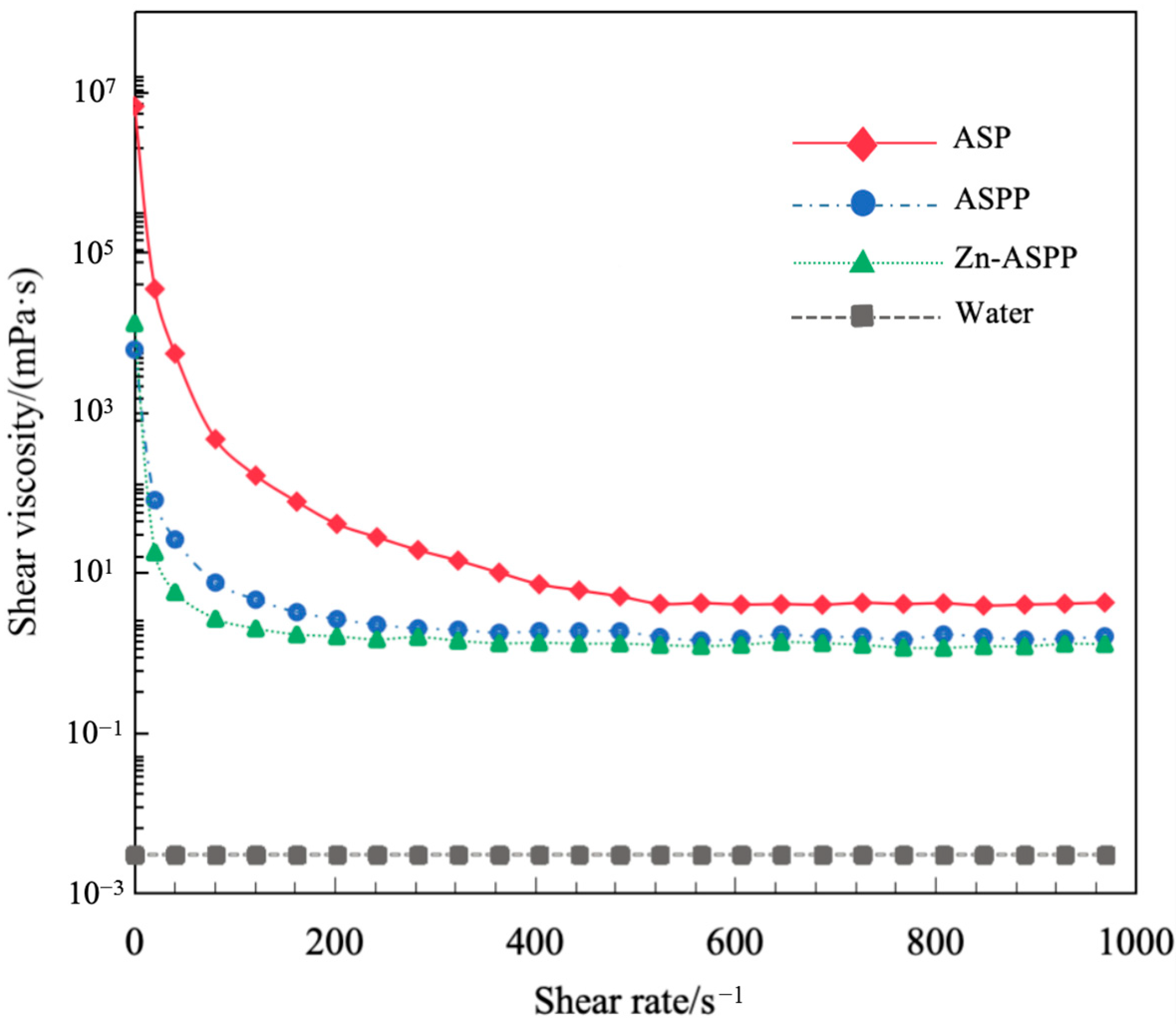
3.7.2. Viscosity of ASP, ASPP, and Zn-ASPP
3.8. Functional Properties
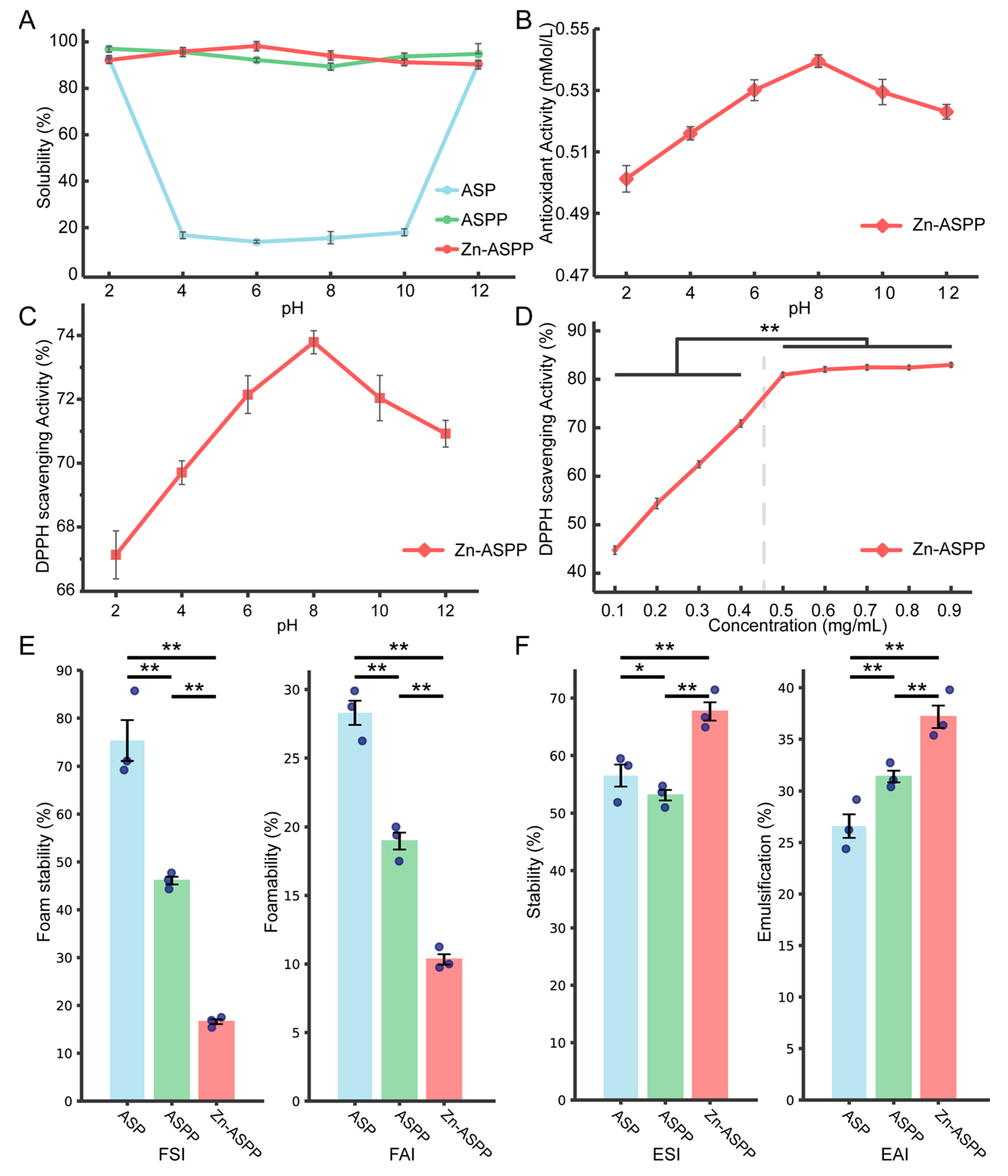
3.8.1. Solubility
3.8.2. Antioxidant Properties of Zn-ASPP
3.8.3. Foaming and Foam Stability
3.8.4. Emulsification and Emulsion Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Dong, E.; Zhang, X.; Huang, H.; Chen, Y.; Shi, S. Research progress in nutrition composition, preservation and utilization of squid. Jiangxi Fish. Sci. Technol. 2020, 5, 44–49. [Google Scholar]
- Shui, S.-S.; Yao, H.; Jiang, Z.-D.; Benjakul, S.; Aubourg, S.P.; Zhang, B. The differences of muscle proteins between neon flying squid (Ommastrephes bartramii) and jumbo squid (Dosidicus gigas) mantles via physicochemical and proteomic analyses. Food Chem. 2021, 364, 130374. [Google Scholar] [CrossRef]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Nutrition and functions of amino acids in aquatic crustaceans. Adv. Exp. Med. Biol. 2021, 1285, 169–198. [Google Scholar]
- Choi, J.H.; Kim, K.T.; Kim, S.M. Optimization and biochemical characteristics of an enzymatic squid hydrolysate for manufacture of a squid complex seasoning. Food Sci. Biotechnol. 2014, 23, 417–423. [Google Scholar] [CrossRef]
- Uren, R.C.; Bothma, A. Concentrations and relative compositions of metallic elements differ between predatory squid and filter-feeding sardine from the Indian and South Atlantic oceans. Reg. Stud. Mar. Sci. 2020, 35, 101137. [Google Scholar] [CrossRef]
- Garcia Barcia, L.; Argiro, J.; Babcock, E.A.; Cai, Y.; Shea, S.K.H.; Chapman, D.D. Mercury and arsenic in processed fins from nine of the most traded shark species in the Hong Kong and China dried seafood markets: The potential health risks of shark fin soup. Mar. Pollut. Bull. 2020, 157, 111281. [Google Scholar] [CrossRef]
- Ordiano-Flores, A.; Rosíles-Martínez, R.; Galván-Magaña, F. Biomagnification of mercury and its antagonistic interaction with selenium in yellowfin tuna Thunnus albacares in the trophic web of Baja California Sur, Mexico. Ecotoxicol. Environ. Saf. 2012, 86, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, S.P.; Trigo, M.; Prego, R.; Cobelo-García, A.; Medina, I. Nutritional and healthy value of chemical constituents obtained from Patagonian squid (Doryteuthis gahi) by-products captured at different seasons. Foods 2021, 10, 2144. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Akita, M.; Ogawa, M.; Goto, T. Evaluation of taurine biosynthesis in the livers of the spear squid Heterololigo bleekeri and the swordtip squid Uroteuthis edulis. Fish. Sci. 2021, 87, 717–725. [Google Scholar] [CrossRef]
- Fang, Q.; Liu, J.; Chen, L.; Chen, Q.; Wang, Y.; Li, Z.; Fu, W.; Liu, Y. Taurine supplementation improves hippocampal metabolism in immature rats with intrauterine growth restriction (IUGR) through protecting neurons and reducing gliosis. Metab. Brain Dis. 2022, 37, 2077–2088. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, R.; Fan, Z.; Luo, D.; Cai, G.; Li, X.; Han, J.; Zhuo, L.; Zhang, L.; Zhang, H. Taurine alleviates chronic social defeat stress-induced depression by protecting cortical neurons from dendritic spine loss. Cell. Mol. Neurobiol. 2023, 43, 827–840. [Google Scholar] [CrossRef]
- Owumi, S.E.; Popoola, O.; Otunla, M.T.; Okuu, U.A.; Najophe, E.S. Benzo-a-pyrene-induced reproductive toxicity was abated in rats co-treated with taurine. Toxin Rev. 2022, 41, 846–859. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and mechanisms of taurine as a therapeutic agent. Biomol. Ther. 2018, 26, 225. [Google Scholar] [CrossRef] [PubMed]
- Arise, R.O.; Adetiwa, O.M.; Adeoye, R.I.; Malomo, S.O. Synergistic enhancement of rat intestinal alkaline phosphatase activity by taurine and sodium butyrate protects against endotoxin-induced bowel inflammation. J. Food Biochem. 2022, 46, e14123. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, H.; Sila, A.; Bougatef, A.; Martínez-Alvarez, O. Protein Hydrolysis as a way to Valorise Squid-Processing byproducts: Obtaining and identification of ACE, DPP-IV and PEP inhibitory peptides. Mar. Drugs 2024, 22, 156. [Google Scholar] [CrossRef]
- Hua, P.; Xiong, Y.; Yu, Z.; Liu, B.; Zhao, L. Effect of Chlorella pyrenoidosa protein hydrolysate-calcium chelate on calcium absorption metabolism and gut microbiota composition in low-calcium diet-fed rats. Mar. Drugs 2019, 17, 348. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, L.; Lin, Y.; Cai, X.; Wang, S. The preservative potential of Octopus scraps peptides—Zinc chelate against Staphylococcus aureus: Its fabrication, antibacterial activity and action mode. Food Control 2019, 98, 24–33. [Google Scholar] [CrossRef]
- Tako, E. Essential minerals: Nutritional requirements, dietary sources, and deficiencies. In Nutrition Guide for Physicians and Related Healthcare Professions; Humana Press: Totowa, NJ, USA, 2022; pp. 365–376. [Google Scholar]
- Gherasim, A.; Arhire, L.I.; Niță, O.; Popa, A.D.; Graur, M.; Mihalache, L. The relationship between lifestyle components and dietary patterns. Proc. Nutr. Soc. 2020, 79, 311–323. [Google Scholar] [CrossRef]
- Guo, H.; Hong, Z.; Yi, R. Core-shell collagen peptide chelated calcium/calcium alginate nanoparticles from fish scales for calcium supplementation. J. Food Sci. 2015, 80, N1595–N1601. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; O’Keeffe, M.B.; Zhang, L.; Li, B.; Hou, H.; FitzGerald, R.J. Fractionation and identification of Alaska pollock skin collagen-derived mineral chelating peptides. Food Chem. 2015, 173, 536–542. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; Zhang, L.; Li, B.; Zhang, Z.; Hou, H.; Zhao, X.; FitzGerald, R.J. In vitro assessment of the multifunctional bioactive potential of Alaska pollock skin collagen following simulated gastrointestinal digestion. J. Sci. Food Agric. 2015, 95, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.M.; Hernandez-Espinosa, D.R.; Aizenman, E. Neurodevelopmental consequences of dietary zinc deficiency: A status report. Biol. Trace Elem. Res. 2023, 201, 5616–5639. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, L.L.; Sun, Y.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Determination of the free amino acid, organic acid, and nucleotide in commercial vinegars. J. Food Sci. 2017, 82, 1116–1123. [Google Scholar] [CrossRef]
- Chang, S.K.; Zhang, Y. Protein analysis. In Food Analysis; Springer: Cham, Switzerland, 2017; pp. 315–331. [Google Scholar]
- Liu, X.J.; Zhang, Y.T.; Wang, X.D.; Wu, Y.; Li, K.; Xu, H. Effects of Different Proteases on the Enzymatic Hydrolysis and Structure of Wheat Gluten Protein. Sci. Technol. Food Ind. 2025. [Google Scholar] [CrossRef]
- Wei, M.; Chen, P.; Zheng, P.; Tao, X.; Yu, X.; Wu, D. Purification and characterization of aspartic protease from Aspergillus niger and its efficient hydrolysis applications in soy protein degradation. Microb. Cell Factories 2023, 22, 42. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Qi, J.; Tian, Y.; Liu, H.; Zhang, F.; Liu, S. Preparation and Antibacterial Activity of Antimicrobial Peptide From Grass Carp Scale. Mod. Food 2025, 2, 110–114. [Google Scholar]
- Gu, C.T. Preparation, Purification, and Application of Antimicrobial Peptides from Crucian Carp Scales in Fruit and Vegetable Preservation. Master’s Thesis, Zhejiang Gongshang University, Hangzhou, China, 2019. [Google Scholar]
- Perez-Velazquez, M.; Maldonado-Othón, C.A.; González-Félix, M.L. Molecular Weights and Optimum Temperature and pH for Pepsin Activity of Three Sciaenid Finfish Species From the Gulf of California. Arch. Biol. Sci. 2024, 76, 83–90. [Google Scholar] [CrossRef]
- Rutherfurd, S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Downey, B.; Udenigwe, C.C. Influence of structural and surface properties of whey-derived peptides on zinc-chelating capacity, and in vitro gastric stability and bioaccessibility of the zinc-peptide complexes. Food Chem. 2018, 240, 1227–1232. [Google Scholar] [CrossRef]
- Wortmann, A.; Rossi, F.; Lelais, G.; Zenobi, R. Determination of zinc to beta-peptide binding constants with electrospray ionization mass spectrometry. J. Mass Spectrom. 2005, 40, 777–784. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Ao, J. Separation and identification of zinc-chelating peptides from sesame protein hydrolysate using IMAC-Zn2+ and LC–MS/MS. Food Chem. 2012, 134, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Tao, R.; Li, R.; Wang, P.; Ma, A.; Zhang, H. Comparative studies on the stabilization of pea protein dispersions by using various polysaccharides. Food Hydrocoll. 2020, 98, 105233. [Google Scholar] [CrossRef]
- Sanchez-Alonso, I.; Solas, M.T.; Borderías, A.J. Technological implications of addition of wheat dietary fibre to giant squid (Dosidicus gigas) surimi gels. J. Food Eng. 2007, 81, 404–411. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zheng, C.; Wang, D.; Zhan, H. Effect of temperature on foaming ability and foam stability of typical surfactants used for foaming agent. J. Surfactants Deterg. 2017, 20, 615–622. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Xiccato, G.; Trocino, A. Energy and protein metabolism and requirements. In Nutrition of the Rabbit; CABI: Wallingford, UK, 2010; pp. 83–118. [Google Scholar]
- Peres, H.; Oliva-Teles, A. Effect of the dietary essential to non-essential amino acid ratio on growth, feed utilization and nitrogen metabolism of European sea bass (Dicentrarchus labrax). Aquaculture 2006, 256, 395–402. [Google Scholar] [CrossRef]
- Deng, L.; Li, H.; Jiang, J. Nutrition evaluation of Norway salmon. Sci. Technol. Food Ind. 2012, 33, 377–379. [Google Scholar]
- Wei, C.; Wang, X.; Jiang, X.; Cao, L. Preparation of quinoa bran dietary fiber-based zinc complex and investigation of its antioxidant capacity in vitro. Front. Nutr. 2023, 10, 1183501. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, D.; Bao, Z.; Chen, Y.; Lin, S. Mechanism of targeted uric acid reduction by soybean protein peptide SHECN in hyperuricemia mice and improvement of liver injury. Food Biosci. 2024, 62, 105545. [Google Scholar] [CrossRef]
- Raman, M.; Mathew, S. Study of chemical properties and evaluation of collagen in mantle, epidermal connective tissue and tentacle of Indian Squid, Loligo duvauceli Orbigny. J. Food Sci. Technol. 2014, 51, 1509–1516. [Google Scholar] [CrossRef]
- Highashihara, M.; Frado, L.; Craig, R.; Ikebe, M. Inhibition of conformational change in smooth muscle myosin by a monoclonal antibody against the 17-kDa light chain. J. Biol. Chem. 1989, 264, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Vylegzhanina, A.V.; Kogan, A.E.; Katrukha, I.A.; Koshkina, E.V.; Bereznikova, A.V.; Filatov, V.L.; Bloshchitsyna, M.N.; Bogomolova, A.P.; Katrukha, A.G. Full-size and partially truncated cardiac troponin complexes in the blood of patients with acute myocardial infarction. Clin. Chem. 2019, 65, 882–892. [Google Scholar] [CrossRef]
- Blanco-Pascual, N.; Fernández-Martín, F.; Montero, P. Jumbo squid (Dosidicus gigas) myofibrillar protein concentrate for edible packaging films and storage stability. LWT-Food Sci. Technol. 2014, 55, 543–550. [Google Scholar] [CrossRef]
- Chen, T.-T.; Cui, Z.-H.; Bao, H.-R.; Guo, Q.-Y. Study on thermal processing characteristics of two kinds of squid carcass meat. Food Ferment. Ind. 2023, 49, 51–59. [Google Scholar]
- Niu, Y.; Chen, J.; Fan, Y.; Kou, T. Effect of flavonoids from Lycium barbarum leaves on the oxidation of myofibrillar proteins in minced mutton during chilled storage. J. Food Sci. 2021, 86, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Kang, H.; Zou, L.; Zhang, H.; Cai, C.; Wang, B.; Ke, H. Effect of high temperature treatment on chemical forces of beef proteins and structure of myofibrillar protein. Food Sci. 2018, 39, 80–86. [Google Scholar]
- Tang, C.-Q.; Lin, K.; Zhou, X.-G.; Liu, S.-L. In situ detection of Amide A Bands of proteins in water by Raman Ratio Spectrum. Chin. J. Chem. Phys. 2016, 29, 129–134. [Google Scholar] [CrossRef]
- Jin, Y.; Su, Z. Water diffusion in polycarbonate film studied by 2D FTIR spectroscopy. Chin. J. Appl. Chem. 2011, 28, 16–21. [Google Scholar] [CrossRef]
- Singh, B.R.; DeOliveira, D.B.; Fu, F.-N.; Fuller, M.P. Fourier transform infrared analysis of amide III bands of proteins for the secondary structure estimation. In Proceedings of the Biomolecular Spectroscopy III, Angeles, CA, USA, 1 May 1993; pp. 47–55. [Google Scholar]
- Doyle, B.B.; Bendit, E.; Blout, E.R. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolym. Orig. Res. Biomol. 1975, 14, 937–957. [Google Scholar] [CrossRef]
- Maiti, K.S. Ultrafast N–H vibrational dynamics of hydrogen-bonded cyclic amide reveal by 2DIR spectroscopy. Chem. Phys. 2018, 515, 509–512. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Al Sagheer, F.; Bumajdad, A. Aramid-zirconia nanocomposite coating with excellent corrosion protection of stainless steel in saline media. Front. Chem. 2020, 8, 391. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Tong, P.; Mao, X. Zinc-binding capacity of yak casein hydrolysate and the zinc-releasing characteristics of casein hydrolysate-zinc complexes. J. Dairy Sci. 2011, 94, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- De Meutter, J.; Goormaghtigh, E. Amino acid side chain contribution to protein FTIR spectra: Impact on secondary structure evaluation. Eur. Biophys. J. 2021, 50, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Modi, T.; Campitelli, P.; Kazan, I.C.; Ozkan, S.B. Protein folding stability and binding interactions through the lens of evolution: A dynamical perspective. Curr. Opin. Struct. Biol. 2021, 66, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, J. A review on particle size effect in metal-catalyzed heterogeneous reactions. Chin. J. Chem. 2020, 38, 1422–1444. [Google Scholar] [CrossRef]
- Guo, H.; Yu, Y.; Hong, Z.; Zhang, Y.; Xie, Q.; Chen, H. Effect of collagen peptide-chelated zinc nanoparticles from pufferfish skin on zinc bioavailability in rats. J. Med. Food 2021, 24, 987–996. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Li, Z.; Yi, R.; Shi, S.; Wu, K.; Li, Y.; Wu, S. Physicochemical and functional properties of type I collagens in red stingray (Dasyatis akajei) skin. Mar. Drugs 2019, 17, 558. [Google Scholar] [CrossRef]
- Gbogouri, G.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- De La Fuente-Betancourt, G.; García-Carreño, F.; Navarrete Del Toro, M.; Córdova-Murueta, J.H.; Lugo-Sánchez, M.E. Protein solubility and production of gels from jumbo squid. J. Food Biochem. 2009, 33, 273–290. [Google Scholar] [CrossRef]
- Capanoglu, E.; Kamiloglu, S.; Demirci Cekic, S.; Sozgen Baskan, K.; Avan, A.N.; Uzunboy, S.; Apak, R. Antioxidant activity and capacity measurement. In Plant Antioxidants and Health; Springer: Cham, Switzerland, 2020; pp. 1–66. [Google Scholar]
- Zhang, F.; Yang, A.; Ma, S.; Shang, H.; Wang, Z. Antioxidant activity of extracts from Uncaria scandens. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 330, p. 042059. [Google Scholar]
- Li, P.; Lu, Y.; Long, G.; Li, S.; Li, K.; Jiang, B.; Wu, W. Structural Characterization of Acid DES-Modified Alkaline Lignin and Evaluation of Antioxidant Properties. Forests 2023, 14, 550. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Zhang, J.; Yan, W.; Mao, X. Food emulsions stabilized by proteins and emulsifiers: A review of the mechanistic explorations. Int. J. Biol. Macromol. 2024, 261, 129795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hou, Y.; Liao, A.; Chen, X.; Wang, Z.; Zhao, P.; Pan, L.; Huang, J. Preparation of wheat protein peptides-calcium chelate by the ultrasound method: Structural characteristics and bioactivity. Food Biosci. 2024, 61, 104848. [Google Scholar] [CrossRef]
- Matsubara, M.; Muraki, Y.; Suzuki, H.; Hatano, N.; Muraki, K. Critical amino acid residues regulating TRPA1 Zn2+ response: A comparative study across species. J. Biol. Chem. 2024, 300, 107302. [Google Scholar] [CrossRef]


| Protease | pH | Temperature/°C |
|---|---|---|
| Pepsin [26] | 2.5 | 37 |
| Acid Protease [27] | 4.8 | 50 |
| Papain [28] | 6.0 | 50 |
| Neutral Protease [29] | 7.0 | 50 |
| Trypsin [30] | 8.0 | 37 |
| Level | A (Enzyme Dosage/%) | B (Enzymolysis Time/h) | C (Substrate Concentration/%) |
|---|---|---|---|
| −1 | 6 | 2.0 | 5 |
| 0 | 7 | 2.4 | 6 |
| 1 | 8 | 2.8 | 7 |
| Amino Acid (%) | ASP (g/100 g) | Ommastrephes Bartramii (g/100 g) | Pacific Squid (g/100 g) | Salmon (g/100 g) |
|---|---|---|---|---|
| Aspartic acid | 6.97 | 7.45 | 7.69 | 3.03 |
| Threonine | 3.57 | 3.62 | 3.64 | 1.52 |
| Serine | 3.40 | 3.57 | 3.62 | 1.20 |
| Glutamic acid | 8.03 | 8.72 | 9.27 | 4.29 |
| Glycine | 3.41 | 3.60 | 3.11 | 1.40 |
| Alanine | 4.69 | 4.86 | 5.04 | 1.94 |
| Valine | 3.42 | 3.35 | 3.25 | 1.62 |
| Methionine | 2.34 | 2.25 | 2.12 | 1.00 |
| Isoleucine | 4.00 | 3.97 | 3.94 | 1.49 |
| Leucine | 6.99 | 7.26 | 7.32 | 2.55 |
| Tyrosine | 2.84 | 2.80 | 2.60 | 1.14 |
| Phenylalanine | 3.96 | 3.89 | 3.40 | 1.48 |
| Lysine | 7.07 | 7.56 | 7.25 | 2.81 |
| Histidine | 1.91 | 2.09 | 1.92 | 0.90 |
| Arginine | 6.96 | 7.30 | 7.50 | 1.90 |
| Proline | 3.20 | 3.45 | 2.84 | 0.90 |
| Tryptophan | 0.72 | 0.67 | 0.65 | 0.47 |
| Total amino acids | 73.48 | 76.41 | 75.16 | 29.95 |
| EAA/TAA | 43.64 | 42.63 | 42 | 43.17 |
| EAA/NEAA | 77.45 | 74.29 | 72.42 | 75.97 |
| Number | A (Enzyme Dosage/%) | B (Enzymolysis Time/h) | C (Substrate Concentration/%) | Y(DH/%) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 37.45 |
| 2 | 1 | −1 | 0 | 37.46 |
| 3 | −1 | 1 | 0 | 35.82 |
| 4 | 1 | 1 | 0 | 38.70 |
| 5 | −1 | 0 | −1 | 38.15 |
| 6 | 1 | 0 | −1 | 38.98 |
| 7 | −1 | 0 | 1 | 37.89 |
| 8 | 1 | 0 | 1 | 39.25 |
| 9 | 0 | −1 | −1 | 38.95 |
| 10 | 0 | 1 | −1 | 38.52 |
| 11 | 0 | −1 | 1 | 37.73 |
| 12 | 0 | 1 | 1 | 39.24 |
| 13 | 0 | 0 | 0 | 41.62 |
| 14 | 0 | 0 | 0 | 41.86 |
| 15 | 0 | 0 | 0 | 41.68 |
| 16 | 0 | 0 | 0 | 41.67 |
| 17 | 0 | 0 | 0 | 41.14 |
| Variation | Square Sum | Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | −1 | −1 | 0 | 37.45 | ||
| A | 1 | −1 | 0 | 37.46 | <0.0001 ** | significant |
| B | −1 | 1 | 0 | 35.82 | 0.0006 ** | |
| C | 1 | 1 | 0 | 38.70 | 0.4512 | |
| 5 | −1 | 0 | −1 | 38.15 | 0.5887 | |
| 6 | 1 | 0 | −1 | 38.98 | 0.0022 ** | |
| 7 | −1 | 0 | 1 | 37.89 | 0.4149 | |
| 8 | 1 | 0 | 1 | 39.25 | 0.0157 * | |
| 9 | 0 | −1 | −1 | 38.95 | <0.0001 ** | |
| 10 | 0 | 1 | −1 | 38.52 | <0.0001 ** | |
| 11 | 0 | −1 | 1 | 37.73 | ||
| 12 | 0 | 1 | 1 | 39.24 | 0.3095 | not significant |
| 13 | 0 | 0 | 0 | 41.62 | ||
| 14 | 0 | 0 | 0 | 41.86 | ||
| 15 | 0 | 0 | 0 | 41.68 | ||
| 16 | 0 | 0 | 0 | 41.67 | ||
| 17 | 0 | 0 | 0 | 41.14 |
| Shore | Absorption Peak (cm−1) | Peak Assignment | ||
|---|---|---|---|---|
| ASP | ASPP | Zn-ASPP | ||
| Amide A | 3298 | 3302 | 3375 | N-H stretching [51] |
| Amide B | 2961, 2929 | 2964 | 2964 | C-H Stretch [52] |
| Amide I | 1659 | 1656 | 1656 | C=O Stretch [53] |
| Amide II | 1535 | 1547 | 1536 | N-H bending and C-N stretching [54] |
| Amide III | 1238 | 1246 | 1234 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wang, T.; Liu, L.; Kong, Y.; Liu, Y.; Wu, W.; Diao, X. Special Characterization and Excellent Antioxidant Capabilities of Zinc Chelated Squid Protein Nanoparticles. Foods 2025, 14, 1789. https://doi.org/10.3390/foods14101789
Zhou Q, Wang T, Liu L, Kong Y, Liu Y, Wu W, Diao X. Special Characterization and Excellent Antioxidant Capabilities of Zinc Chelated Squid Protein Nanoparticles. Foods. 2025; 14(10):1789. https://doi.org/10.3390/foods14101789
Chicago/Turabian StyleZhou, Qiyi, Tianming Wang, Lixin Liu, Yaqi Kong, Yifan Liu, Wenhui Wu, and Xiaozhen Diao. 2025. "Special Characterization and Excellent Antioxidant Capabilities of Zinc Chelated Squid Protein Nanoparticles" Foods 14, no. 10: 1789. https://doi.org/10.3390/foods14101789
APA StyleZhou, Q., Wang, T., Liu, L., Kong, Y., Liu, Y., Wu, W., & Diao, X. (2025). Special Characterization and Excellent Antioxidant Capabilities of Zinc Chelated Squid Protein Nanoparticles. Foods, 14(10), 1789. https://doi.org/10.3390/foods14101789







