Effects of Glutathione-Enriched Inactive Dry Yeast on the Flavor Profile of Kiwi Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
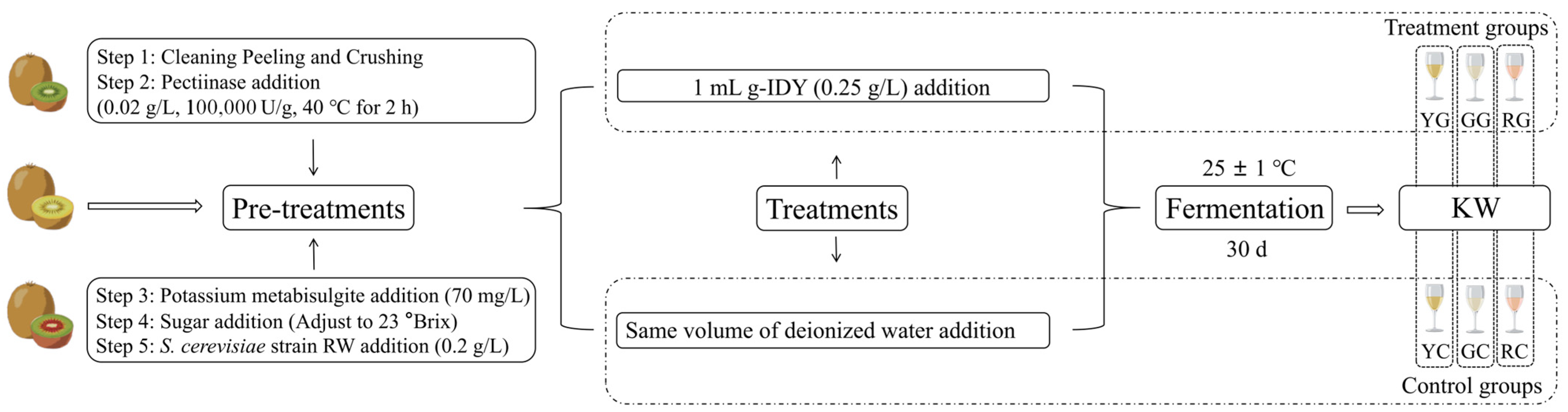
2.2. KW Samples
2.3. GC-IMS Analysis
2.4. Relative Odor Activity Value
2.5. 1H-NMR Analysis
2.6. E-Tongue
2.7. Statistical Analysis
3. Results and Discussion
3.1. Key Aroma Compounds in KW
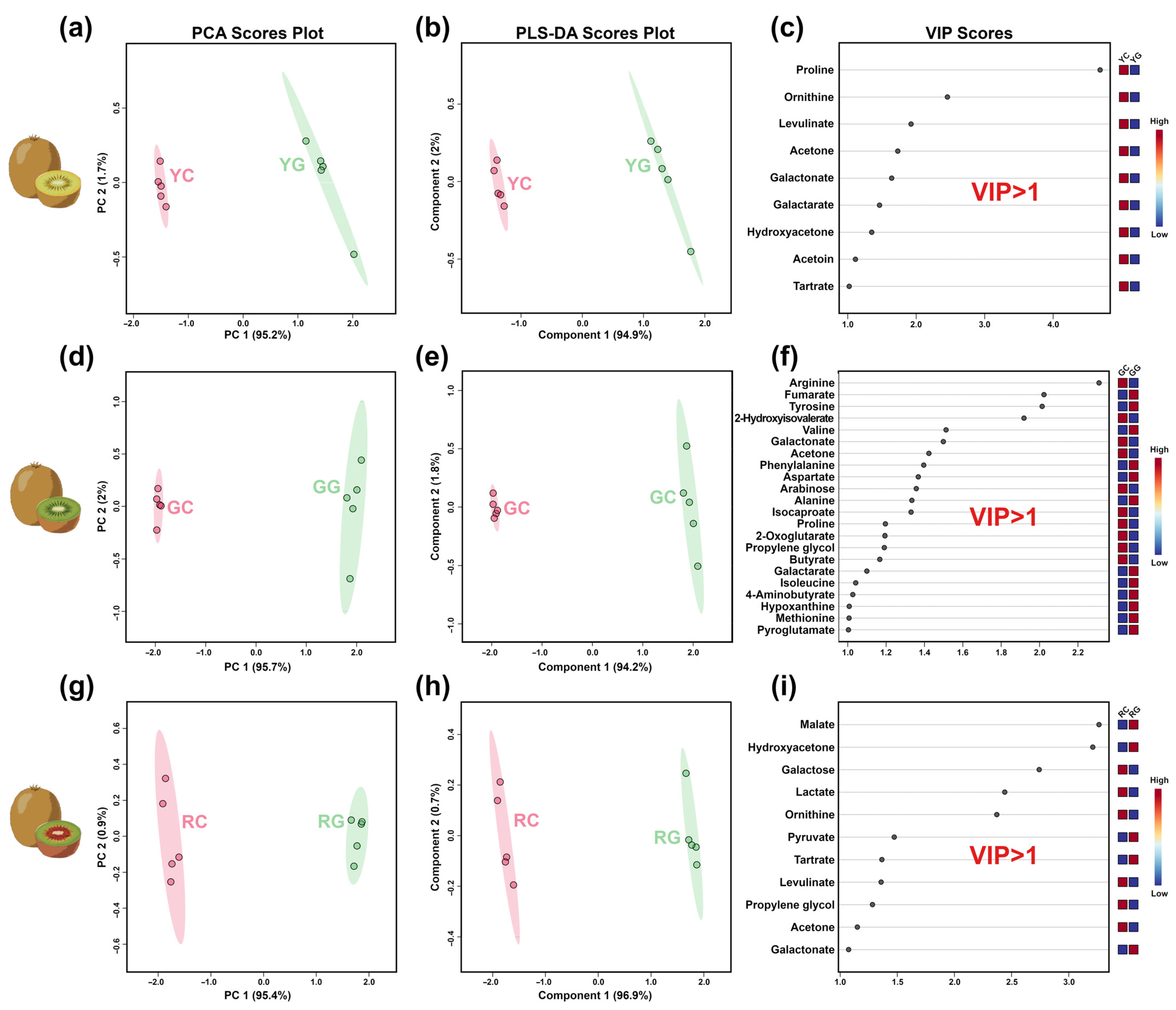
3.2. Key Taste Components in KW
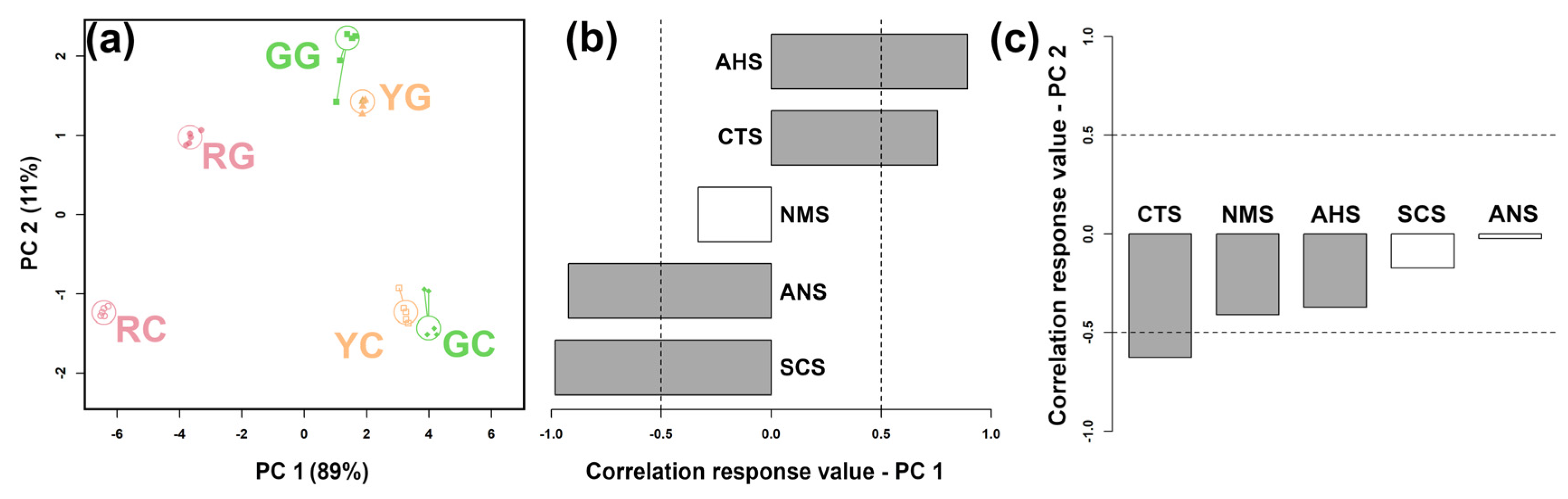
3.3. E-Tongue Analysis
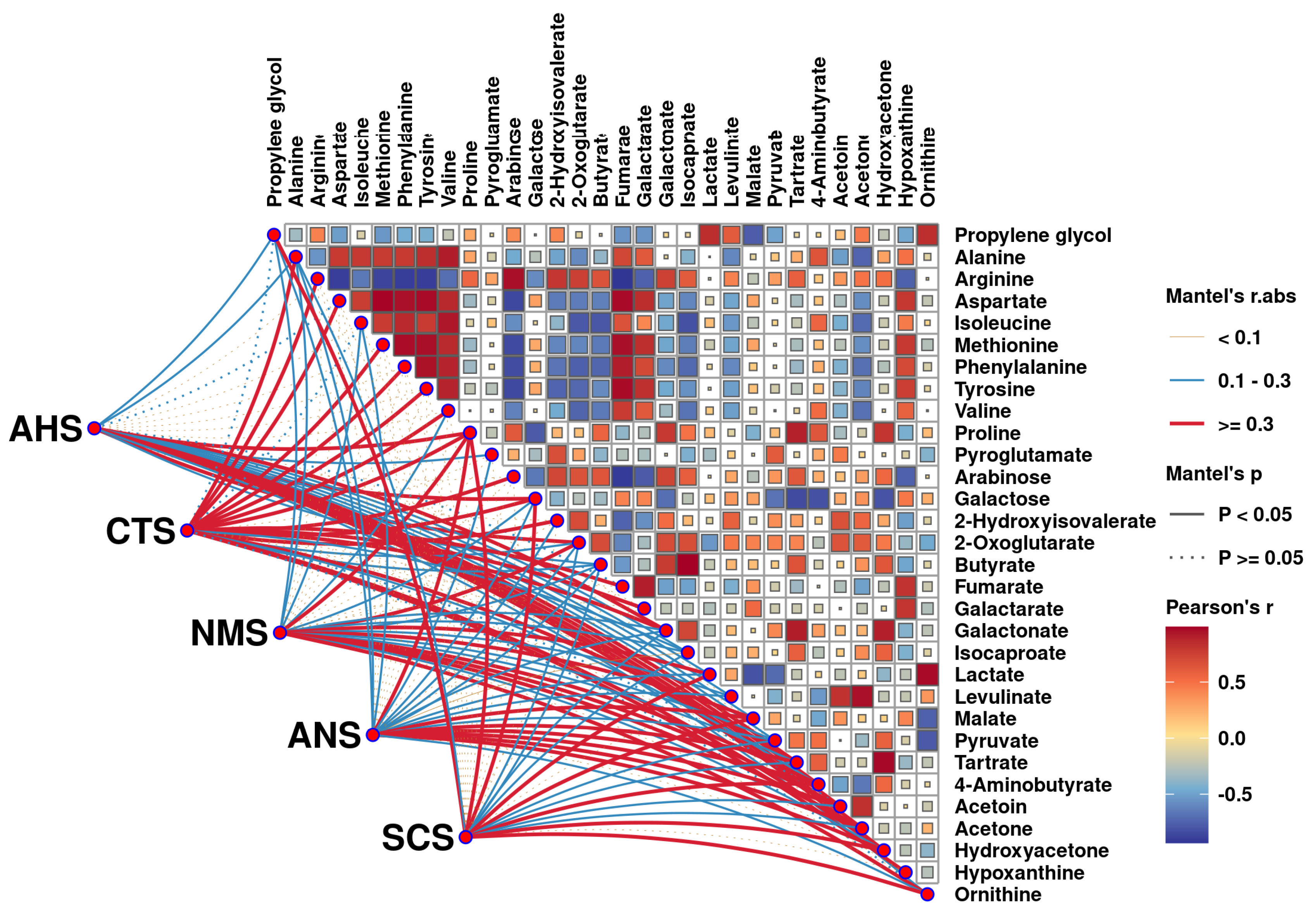
3.4. Correlation Between E-Tongue and Key Taste Compounds
3.5. Correlation Between Key Aroma and Taste Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Con sent Statement
Data Availability Statement
Conflicts of Interest
References
- Sonni, F.; Clark, A.C.; Prenzler, P.D.; Riponi, C.; Scollary, G.R. Antioxidant Action of Glutathione and the Ascorbic Acid/Glutathione Pair in a Model White Wine. J. Agric. Food Chem. 2011, 59, 3940–3949. [Google Scholar] [CrossRef] [PubMed]
- Tomašević, M.; Gracin, L.; Ćurko, N.; Ganić, K.K. Impact of Pre-Fermentative Maceration and Yeast Strain Along with Glutathione and SO2 Additions on the Aroma of Vitis vinifera L. Pošip Wine and Its Evaluation During Bottle Aging. LWT 2017, 81, 67–76. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Zhang, J.; Liu, M.; Wei, X.; Fan, M. Effect of Reduced Glutathione on the Quality Characteristics of Apple Wine During Alcoholic Fermentation. Food Chem. 2019, 300, 125130. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qi, Y.; Zhao, N.; Cao, Y.; Xu, J.; Fan, M. Multivariate Analysis Reveals Effect of Glutathione-Enriched Inactive Dry Yeast on Amino Acids and Volatile Components of Kiwi Wine. Food Chem. 2020, 329, 127086. [Google Scholar] [CrossRef] [PubMed]
- Kritzinger, E.C.; Stander, M.A.; Du Toit, W.J. Assessment of Glutathione Levels in Model Solution and Grape Ferments Supplemented with Glutathione-Enriched Inactive Dry Yeast Preparations Using a Novel UPLC-MS/MS Method. Food Addit. Contam. Part A 2013, 30, 80–92. [Google Scholar] [CrossRef]
- Xie, L.; Yu, K.; Xiao, X.; Wei, Z.; Xiong, R.; Du, Y.; Li, Y.; Ma, Y. Study on the Kinetic Model of Mixed Fermentation by Adding Glutathione-Enriched Inactive Dry Yeast. Fermentation 2024, 10, 329. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Characterization of Different Non-Saccharomyces Yeasts via Mono-Fermentation to Produce Polyphenol-Enriched and Fragrant Kiwi Wine. Food Microbiol. 2022, 103, 103867. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Wan, F.; Cai, Z.; Zeng, R.; Tang, J.; Nie, X.; Jiang, X.; Zhu, C.; Laghi, L. Comprehensive Comparison of Flavor and Metabolomic Profiles in Kiwi Wine Fermented by Kiwifruit Flesh with Different Colors. LWT 2024, 208, 116719. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.-Q.; Xu, Y.-H.; Li, A.-H.; Tao, Y.-S. Increased Glycosidase Activities Improved the Production of Wine Varietal Odorants in Mixed Fermentation of P. fermentans and High Antagonistic S. cerevisiae. Food Chem. 2020, 332, 127426. [Google Scholar] [CrossRef]
- Ling, M.; Bai, X.; Cui, D.; Shi, Y.; Duan, C.; Lan, Y. An Efficient Methodology for Modeling to Predict Wine Aroma Expression Based on Quantitative Data of Volatile Compounds: A Case Study of Oak Barrel-Aged Red Wines. Food Res. Int. 2023, 164, 112440. [Google Scholar] [CrossRef]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces Cerevisiae and Non-Saccharomyces Strains on Alcoholic Fermentation Behavior and Aroma Profile of Yellow-Fleshed Peach Wine. LWT 2022, 155, 112993. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Z.; Lu, X.; Yi, Y.; Tian, Q.; Deng, J.; Jiang, D.; Tang, J.; Laghi, L. Effects of Saccharomyces Cerevisiae Strains on the Metabolomic Profiles of Guangan Honey Pear Cider. LWT 2023, 182, 114816. [Google Scholar] [CrossRef]
- Yang, Y.; Ai, L.; Mu, Z.; Liu, H.; Yan, X.; Ni, L.; Zhang, H.; Xia, Y. Flavor Compounds with High Odor Activity Values (OAV > 1) Dominate the Aroma of Aged Chinese Rice Wine (Huangjiu) by Molecular Association. Food Chem. 2022, 383, 132370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, J.; Yang, Y.; Deng, J.; Zhu, K.; Yi, Y.; Tang, J.; Jiang, X.; Zhu, C.; Laghi, L. Effects of S. cerevisiae Strains on the Sensory Characteristics and Flavor Profile of Kiwi Wine Based on E-Tongue, GC-IMS and 1H-NMR. LWT 2023, 185, 115193. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, X.; Lan, Q.; Wan, F.; Yang, Z.; Nie, X.; Cai, Z.; Hu, B.; Tang, J.; Zhu, C.; et al. Comparison of Aroma and Taste Profiles of Kiwi Wine Fermented with/without Peel by Combining Intelligent Sensory, Gas Chromatography-Mass Spectrometry, and Proton Nuclear Magnetic Resonance. Foods 2024, 13, 1729. [Google Scholar] [CrossRef]
- Yang, Z.; Lan, Q.; Liu, X.; Cai, Z.; Zeng, R.; Tang, J.; Jiang, X.; Zhu, C.; Hu, B.; Laghi, L. Effects of Pretreatment Methods on the Flavor Profile and Sensory Characteristics of Kiwi Wine Based on 1H NMR, GC-IMS and E-tongue. LWT 2024, 203, 116375. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Laghi, L.; Deng, J.; Dao, X.; Tang, J.; Ji, L.; Zhu, C.; Picone, G. Characterization of Flavor Profile of “Nanx Wudl” Sour Meat Fermented from Goose and Pork Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS) Combined with Electronic Nose and Tongue. Foods 2023, 12, 2194. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B Stat. Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Zhao, P.; Ma, Z.; Zhang, J.; Ma, W.; Wang, X. Investigating the Effect of Three Phenolic Fractions on the Volatility of Floral, Fruity, and Aged Aromas by HS-SPME-GC-MS and NMR in Model Wine. Food Chem. X 2022, 13, 100281. [Google Scholar] [CrossRef]
- Zang, X.; Du, Q.; Qu, R.; Ye, D.; Lu, Y.; Liu, Y. Analysis of Volatile Aroma Compounds and Sensory Characteristics Contributing to Regional Style of Red Wines from Hexi Corridor Based on Sixteen Grape Varieties/Clones. Fermentation 2022, 8, 501. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Ren, L.; Su, Z.; Bian, X.; Fan, J.; Wang, Y.; Han, B.; Zhang, N. HS-GC-IMS and PCA to Characterize the Volatile Flavor Compounds in Three Sweet Cherry Cultivars and Their Wines in China. Molecules 2022, 27, 9056. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Zheng, H.; Lin, J.; Nie, G.; Fan, X.; García-Martín, J.F. The State-of-the-Art Research of the Application of Ultrasound to Winemaking: A Critical Review. Ultrason. Sonochem. 2023, 95, 106384. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Wang, J.; Yuan, Q.; Lei, Y.; Peng, W.; Zhang, M.; Li, X.; Sun, X.; Ma, T. Evaluation of the Color and Aroma Characteristics of Commercially Available Chinese Kiwi Wines via Intelligent Sensory Technologies and Gas Chromatography-Mass Spectrometry. Food Chem. X 2022, 15, 100427. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, G.; Hasan, K.M.F.; Kang, Y.; Wu, Y.; Li, H.; Zhou, S. Cultivar Difference Characterization of Kiwifruit Wines on Phenolic Profiles, Volatiles and Antioxidant Activity. Food Chem. X 2023, 18, 100691. [Google Scholar] [CrossRef]
- Russo, P.; Tufariello, M.; Renna, R.; Tristezza, M.; Taurino, M.; Palombi, L.; Capozzi, V.; Rizzello, C.G.; Grieco, F. New Insights into the Oenological Significance of Candida zemplinina: Impact of Selected Autochthonous Strains on the Volatile Profile of Apulian Wines. Microorganisms 2020, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Lv, J.; Ma, Y.; Guan, X. Changes in the Physicochemical Components, Polyphenol Profile, and Flavor of Persimmon Wine During Spontaneous and Inoculated Fermentation. Food Sci. Nutr. 2020, 8, 2728–2738. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Guo, Y.; Liu, N.; Wang, K.; Gong, P.; Zhao, Y.; Cai, L. Effect of In Vitro Digestion and Fermentation of Kiwifruit Pomace Polysaccharides on Structural Characteristics and Human Gut Microbiota. Int. J. Biol. Macromol. 2023, 253, 127141. [Google Scholar] [CrossRef]
- Liu, M.; Yang, K.; Qi, Y.; Zhang, J.; Fan, M.; Wei, X. Fermentation Temperature and the Phenolic and Aroma Profile of Persimmon Wine. J. Inst. Brew. 2018, 124, 269–275. [Google Scholar] [CrossRef]
- Šuklje, K.; Antalick, G.; Buica, A.; Coetzee, Z.A.; Brand, J.; Schmidtke, L.M.; Vivier, M.A. Inactive Dry Yeast Application on Grapes Modify Sauvignon Blanc Wine Aroma. Food Chem. 2016, 197, 1073–1084. [Google Scholar] [CrossRef]
- Valero, E.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Concentration of Amino Acids in Wine After the End of Fermentation by Saccharomyces cerevisiae Strains. J. Sci. Food Agric. 2003, 83, 830–835. [Google Scholar] [CrossRef]
- Bueno, M.; Carrascón, V.; Ferreira, V. Release and Formation of Oxidation-Related Aldehydes during Wine Oxidation. J. Agric. Food Chem. 2016, 64, 608–617. [Google Scholar] [CrossRef]
- Procopio, S.; Krause, D.; Hofmann, T.; Becker, T. Significant Amino Acids in Aroma Compound Profiling During Yeast Fermentation Analyzed by PLS Regression. LWT Food Sci. Technol. 2013, 51, 423–432. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Ren, Y.; Wang, X.; Li, H.; Liu, Z.; Yue, T.; Gao, Z. Effect of Inoculation Method on the Quality and Nutritional Characteristics of Low-Alcohol Kiwi Wine. LWT 2022, 156, 113049. [Google Scholar] [CrossRef]
- Sereni, A.; Phan, Q.; Osborne, J.; Tomasino, E. Impact of the Timing and Temperature of Malolactic Fermentation on the Aroma Composition and Mouthfeel Properties of Chardonnay Wine. Foods 2020, 9, 802. [Google Scholar] [CrossRef]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Francis, I.L.; Henschke, P.A. Comparison of Inorganic and Organic Nitrogen Supplementation of Grape Juice—Effect on Volatile Composition and Aroma Profile of a Chardonnay Wine Fermented with Saccharomyces Cerevisiae Yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Zhao, Z.; Xu, W.; Wang, L.; Lin, X.; Hu, X.; Li, C. Flavor Characteristics of Shanlan Rice Wines Fermented for Different Time Based on HS-SPME-GC-MS-O, HS-GC-IMS, and Electronic Sensory Analyses. Food Chem. 2024, 432, 137150. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, D.; Lomolino, G.; Sato, R.; Vincenzi, S.; De Iseppi, A. Umami in Wine: Impact of Glutamate Concentration and Contact with Lees on the Sensory Profile of Italian White Wines. Beverages 2023, 9, 52. [Google Scholar] [CrossRef]
- Gao, D.; Wang, C.; Shi, H.; Liang, H. Widely Targeted Metabonomic Analysis to Study Effect of GSH on Metabolites of Chardonnay Wine during Simulated Oxidation. Fermentation 2023, 9, 815. [Google Scholar] [CrossRef]
- Liu, P.-T.; Yu, K.-J.; Li, Y.-T.; Duan, C.-Q.; Yan, G.-L. The Content of Linoleic Acid in Grape Must Influences the Aromatic Effect of Branched-Chain Amino Acids Addition on Red Wine. Food Res. Int. 2018, 114, 214–222. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces Cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]


| Compounds | CAS | Peak Intensity | p Value | Trend | Peak Intensity | p Value | Trend | Peak Intensity | p Value | Trend | Odor Threshold Value (mg/Kg) | ROAV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GG | GC | RG | RC | YG | YC | GG | GC | RG | RC | YG | YC | |||||||||
| Ethyl hexanoate | 123-66-0 | 8.98 × 102 ± 80.8 | 2.54 × 103 ± 1.75 × 102 | 0.013 | ↑ | 2.81 × 103 ± 1.26 × 102 | 3.20 × 103 ± 3.46 × 102 | 0.085 | = | 2.46 × 103 ± 92.6 | 1.97 × 103 ± 29.1 | 0.060 | = | 0.005 | 9.70 | 41.40 | 34.83 | 49.45 | 36.49 | 35.91 |
| Hexyl acetate-D | 142-92-7 | 1.29 × 102 ± 9.22 | 7.28 × 102 ± 25.3 | 0.001 | ↑ | 1.13 × 102 ± 17.5 | 1.07 × 102 ± 31.2 | 0.577 | = | 2.63 × 102 ± 7.65 | 5.85 × 102 ± 1.16 × 102 | 0.005 | ↑ | 0.002 | 3.48 | 29.75 | 3.48 | 4.04 | 9.75 | 26.66 |
| Hexyl acetate-M | 142-92-7 | 2.73 × 102 ± 1.32 × 10 | 1.43 × 103 ± 14.8 | 0.001 | ↑ | 2.86 × 102 ± 31.4 | 1.71 × 102 ± 16.5 | 0.157 | ↓ | 8.53 × 102 ± 15.5 | 1.11 × 103 ± 1.15 × 102 | 0.007 | ↑ | 0.002 | 7.38 | 58.24 | 8.90 | 6.74 | 31.67 | 50.63 |
| Isoamyl acetate-D | 123-92-2 | 1.32 × 102 ± 8.26 | 1.95 × 103 ± 1.15 × 102 | 0.001 | ↑ | 2.41 × 103 ± 6.01 × 102 | 8.43 × 102 ± 3.59 × 102 | 0.152 | = | 2.87 × 103 ± 41.9 | 1.86 × 103 ± 50.4 | 0.010 | ↓ | 0.088 | 0.08 | 1.80 | 1.72 | 0.77 | 2.42 | 1.92 |
| 1-Hexanol-M | 111-27-3 | 1.18 × 104 ± 2.02 × 102 | 5.53 × 103 ± 4.21 × 102 | 0.013 | ↓ | 4.12 × 103 ± 6.82 × 102 | 4.26 × 103 ± 5.18 × 102 | 0.260 | = | 5.28 × 103 ± 1.53 × 102 | 3.85 × 103 ± 1.42 × 102 | 0.040 | ↓ | 0.0056 | 83.59 | 80.33 | 45.19 | 58.78 | 69.96 | 62.44 |
| Pentanal | 110-62-3 | 7.36 × 103 ± 6.06 × 102 | 1.56 × 103 ± 77.3 | 0.002 | ↓ | 2.68 × 102 ± 1.93 | 2.62 × 102 ± 83.1 | 0.924 | = | 6.72 × 102 ± 24.3 | 3.95 × 102 ± 14.2 | 0.023 | ↓ | 0.022 | 18.04 | 5.79 | 0.75 | 0.90 | 2.26 | 1.63 |
| GC | GG | p Value | Trend | RC | RG | p Value | Trend | YC | YG | p Value | Trend | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols and polyols | ||||||||||||
| Propylene glycol | 1.47 × 10−3 ± 6.39 × 10−5 | 3.75 × 10−4 ± 1.24 × 10−5 | <0.01 | ↓ | 4.52 × 10−3 ± 1.04 × 10−4 | 1.23 × 10−3 ± 5.53 × 10−5 | <0.01 | ↓ | 2.31 × 10−3 ± 1.29 × 10−4 | 1.32 × 10−3 ± 8.65 × 10−5 | <0.01 | ↓ |
| Amino acids, peptides, and analogs | ||||||||||||
| Ornithine | 7.18 × 10−4 ± 1.46 × 10−4 | 5.57 × 10−4 ± 7.69 × 10−5 | 0.073 | = | 3.09 × 10−3 ± 2.69 × 10−4 | 2.67 × 10−4 ± 7.71 × 10−5 | <0.01 | ↓ | 2.14 × 10−4 ± 6.60 × 10−5 | 2.75 × 10−5 ± 1.59 × 10−5 | <0.01 | ↓ |
| Alanine | 4.18 × 10−4 ± 2.09 × 10−5 | 1.96 × 10−3 ± 2.33 × 10−5 | <0.01 | ↑ | 1.04 × 10−3 ± 9.56 × 10−6 | 1.43 × 10−3 ± 9.31 × 10−5 | <0.01 | ↑ | 8.94 × 10−4 ± 6.04 × 10−5 | 1.04 × 10−3 ± 7.36 × 10−5 | <0.01 | ↑ |
| 4-Aminobutyrate | 4.24 × 10−4 ± 4.11 × 10−5 | 1.39 × 10−3 ± 1.93 × 10−5 | <0.01 | ↑ | 1.43 × 10−3 ± 2.57 × 10−5 | 1.95 × 10−3 ± 7.75 × 10−5 | <0.01 | ↑ | 1.19 × 10−3 ± 6.13 × 10−5 | 1.69 × 10−3 ± 1.27 × 10−4 | <0.01 | ↑ |
| Arginine | 2.80 × 10−2 ± 1.27 × 10−3 | 1.97 × 10−3 ± 2.05 × 10−4 | <0.01 | ↓ | 2.59 × 10−2 ± 9.24 × 10−4 | 3.29 × 10−2 ± 2.23 × 10−3 | 0.115 | = | 3.17 × 10−2 ± 2.27 × 10−3 | 2.48 × 10−2 ± 1.42 × 10−3 | <0.01 | ↓ |
| Aspartate | 1.13 × 10−4 ± 2.92 × 10−5 | 5.38 × 10−4 ± 5.57 × 10−6 | <0.01 | ↑ | 1.64 × 10−4 ± 1.59 × 10−5 | 1.77 × 10−4 ± 2.39 × 10−5 | 0.060 | = | 1.76 × 10−4 ± 1.22 × 10−5 | 2.35 × 10−4 ± 1.80 × 10−5 | <0.01 | ↑ |
| Isoleucine | 3.86 × 10−5 ± 5.65 × 10−6 | 1.28 × 10−4 ± 6.48 × 10−6 | <0.01 | ↑ | 8.88 × 10−5 ± 5.62 × 10−6 | 7.45 × 10−5 ± 6.19 × 10−6 | <0.01 | ↓ | 6.83 × 10−5 ± 7.25 × 10−6 | 1.03 × 10−4 ± 9.79 × 10−6 | <0.01 | ↑ |
| Methionine | 6.95 × 10−5 ± 4.79 × 10−6 | 2.22 × 10−4 ± 2.50 × 10−6 | <0.01 | ↑ | 8.52 × 10−5 ± 5.19 × 10−6 | 9.31 × 10−5 ± 5.70 × 10−6 | 0.046 | ↑ | 9.88 × 10−5 ± 7.84 × 10−6 | 1.23 × 10−4 ± 7.70 × 10−6 | <0.01 | ↑ |
| Phenylalanine | 8.26 × 10−5 ± 1.45 × 10−5 | 4.10 × 10−4 ± 6.76 × 10−6 | <0.01 | ↑ | 1.58 × 10−4 ± 7.19 × 10−6 | 1.44 × 10−4 ± 8.66 × 10−6 | <0.01 | ↓ | 1.24 × 10−4 ± 1.87 × 10−5 | 2.35 × 10−4 ± 1.63 × 10−5 | <0.01 | ↑ |
| Proline | 8.09 × 10−5 ± 6.52 × 10−6 | 2.16 × 10−5 ± 8.56 × 10−6 | <0.01 | ↓ | 5.79 × 10−4 ± 3.04 × 10−5 | 1.16 × 10−3 ± 4.45 × 10−5 | <0.01 | ↑ | 3.00 × 10−4 ± 2.42 × 10−5 | 6.23 × 10−6 ± 2.72 × 10−6 | <0.01 | ↓ |
| Pyroglutamate | 1.20 × 10−4 ± 2.17 × 10−5 | 3.90 × 10−4 ± 9.76 × 10−5 | <0.01 | ↑ | 5.31 × 10−4 ± 1.71 × 10−5 | 5.87 × 10−4 ± 5.55 × 10−5 | 0.097 | = | 2.43 × 10−3 ± 1.39 × 10−4 | 2.73 × 10−3 ± 2.12 × 10−4 | <0.01 | ↑ |
| Tyrosine | 4.40 × 10−5 ± 6.97 × 10−6 | 4.46 × 10−4 ± 4.98 × 10−6 | <0.01 | ↑ | 1.27 × 10−4 ± 8.64 × 10−6 | 1.29 × 10−4 ± 9.17 × 10−6 | 0.010 | ↑ | 8.45 × 10−5 ± 9.40 × 10−6 | 1.34 × 10−4 ± 5.94 × 10−6 | <0.01 | ↑ |
| Valine | 4.47 × 10−5 ± 1.18 × 10−5 | 2.51 × 10−4 ± 5.27 × 10−6 | <0.01 | ↓ | 1.41 × 10−4 ± 7.09 × 10−6 | 1.37 × 10−4 ± 1.55 × 10−5 | <0.01 | ↓ | 1.13 × 10−4 ± 1.16 × 10−5 | 1.45 × 10−4 ± 1.29 × 10−5 | <0.01 | ↑ |
| Carbohydrates and carbohydrate conjugates | ||||||||||||
| Arabinose | 6.93 × 10−3 ± 7.90 × 10−4 | 1.49 × 10−3 ± 3.60 × 10−4 | <0.01 | ↓ | 6.91 × 10−3 ± 6.48 × 10−4 | 8.89 × 10−3 ± 7.75 × 10−4 | 0.223 | = | 7.28 × 10−3 ± 5.00 × 10−4 | 6.99 × 10−3 ± 1.57 × 10−4 | 0.05 | = |
| Galactose | 5.79 × 10−3 ± 1.82 × 10−4 | 6.26 × 10−3 ± 3.93 × 10−4 | 0.053 | = | 4.16 × 10−3 ± 3.65 × 10−4 | 2.41 × 10−4 ± 7.12 × 10−5 | <0.01 | ↓ | 3.32 × 10−3 ± 2.99 × 10−4 | 3.06 × 10−3 ± 1.76 × 10−4 | 0.631 | = |
| Organic acids and derivatives | ||||||||||||
| 2-Hydroxyisovalerate | 5.29 × 10−5 ± 8.91 × 10−6 | 6.28 × 10−6 ± 2.81 × 10−6 | <0.01 | ↓ | 5.86 × 10−5 ± 7.28 × 10−6 | 5.93 × 10−5 ± 1.34 × 10−5 | 0.115 | = | 1.06 × 10−4 ± 4.68 × 10−6 | 7.11 × 10−5 ± 7.01 × 10−6 | <0.01 | ↓ |
| 2-Oxoglutarate | 7.92 × 10−4 ± 5.69 × 10−5 | 2.02 × 10−4 ± 2.68 × 10−5 | <0.01 | ↓ | 2.55 × 10−4 ± 1.29 × 10−5 | 6.44 × 10−4 ± 3.89 × 10−5 | <0.01 | ↑ | 8.19 × 10−4 ± 5.95 × 10−5 | 4.92 × 10−4 ± 3.13 × 10−5 | <0.01 | ↓ |
| Butyrate | 2.91 × 10−4 ± 2.13 × 10−5 | 7.62 × 10−5 ± 1.81 × 10−6 | <0.01 | ↓ | 1.33 × 10−4 ± 8.19 × 10−6 | 2.88 × 10−4 ± 2.41 × 10−5 | <0.01 | ↑ | 1.60 × 10−4 ± 1.13 × 10−5 | 8.30 × 10−5 ± 5.85 × 10−6 | <0.01 | ↓ |
| Fumarate | 3.92 × 10−5 ± 5.47 × 10−6 | 4.03 × 10−4 ± 5.56 × 10−6 | <0.01 | ↑ | 3.83 × 10−5 ± 5.97 × 10−6 | 5.19 × 10−5 ± 3.35 × 10−6 | 0.146 | = | 4.77 × 10−5 ± 4.74 × 10−6 | 7.33 × 10−5 ± 8.67 × 10−6 | <0.01 | ↑ |
| Galactarate | 1.12 × 10−4 ± 7.06 × 10−6 | 4.00 × 10−4 ± 1.30 × 10−5 | <0.01 | ↑ | 4.81 × 10−5 ± 3.60 × 10−6 | 1.09 × 10−4 ± 6.68 × 10−6 | <0.01 | ↑ | 1.49 × 10−4 ± 9.70 × 10−6 | 3.86 × 10−5 ± 6.30 × 10−6 | <0.01 | ↓ |
| Galactonate | 5.13 × 10−4 ± 7.87 × 10−5 | 9.49 × 10−5 ± 2.99 × 10−5 | <0.01 | ↓ | 3.33 × 10−4 ± 2.71 × 10−5 | 1.16 × 10−3 ± 1.14 × 10−4 | <0.01 | ↑ | 7.94 × 10−4 ± 5.10 × 10−5 | 1.76 × 10−4 ± 1.01 × 10−5 | <0.01 | ↓ |
| Isocaproate | 1.49 × 10−4 ± 6.91 × 10−6 | 3.24 × 10−5 ± 1.79 × 10−6 | <0.01 | ↓ | 5.62 × 10−5 ± 4.49 × 10−6 | 1.34 × 10−4 ± 1.31 × 10−5 | <0.01 | ↑ | 7.16 × 10−5 ± 7.43 × 10−6 | 3.00 × 10−5 ± 3.15 × 10−7 | <0.01 | ↓ |
| Lactate | 2.26 × 10−3 ± 1.17 × 10−4 | 3.95 × 10−3 ± 2.38 × 10−4 | <0.01 | ↑ | 2.18 × 10−2 ± 1.56 × 10−3 | 1.70 × 10−3 ± 1.77 × 10−4 | <0.01 | ↓ | 1.39 × 10−3 ± 1.18 × 10−4 | 1.27 × 10−3 ± 9.28 × 10−5 | 0.320 | = |
| Levulinate | 2.77 × 10−5 ± 1.80 × 10−6 | 9.28 × 10−6 ± 1.46 × 10−6 | <0.01 | ↓ | 2.71 × 10−5 ± 1.05 × 10−6 | 6.85 × 10−6 ± 8.91 × 10−7 | <0.01 | ↓ | 3.58 × 10−5 ± 2.60 × 10−6 | 6.43 × 10−6 ± 1.65 × 10−6 | <0.01 | ↓ |
| Malate | 3.77 × 10−2 ± 9.64 × 10−3 | 3.82 × 10−2 ± 2.63 × 10−4 | 0.428 | = | 6.06 × 10−4 ± 2.05 × 10−4 | 2.18 × 10−2 ± 1.79 × 10−3 | <0.01 | ↑ | 2.97 × 10−2 ± 1.84 × 10−3 | 2.41 × 10−2 ± 2.06 × 10−3 | <0.01 | ↓ |
| Pyruvate | 7.72 × 10−4 ± 1.15 × 10−4 | 8.81 × 10−4 ± 4.93 × 10−5 | 0.103 | = | 2.95 × 10−4 ± 1.24 × 10−5 | 1.59 × 10−3 ± 1.75 × 10−4 | <0.01 | ↑ | 1.26 × 10−3 ± 1.82 × 10−4 | 1.82 × 10−3 ± 3.21 × 10−4 | <0.01 | ↑ |
| Tartrate | 7.73 × 10−6 ± 1.89 × 10−6 | 3.91 × 10−6 ± 4.84 × 10−7 | 0.008 | ↓ | 9.08 × 10−6 ± 1.53 × 10−6 | 4.33 × 10−5 ± 6.21 × 10−6 | <0.01 | ↑ | 1.67 × 10−5 ± 1.59 × 10−6 | 6.17 × 10−6 ± 4.86 × 10−7 | <0.01 | ↓ |
| Others | ||||||||||||
| Acetoin | 1.63 × 10−4 ± 9.18 × 10−6 | 8.63 × 10−5 ± 6.64 × 10−6 | <0.01 | ↓ | 8.74 × 10−5 ± 1.03 × 10−5 | 4.58 × 10−5 ± 6.11 × 10−6 | <0.01 | ↓ | 2.65 × 10−4 ± 1.87 × 10−5 | 9.12 × 10−5 ± 8.76 × 10−6 | <0.01 | ↓ |
| Acetone | 4.28 × 10−4 ± 3.83 × 10−5 | 8.31 × 10−5 ± 2.87 × 10−6 | <0.01 | ↓ | 2.87 × 10−4 ± 1.58 × 10−5 | 9.05 × 10−5 ± 7.00 × 10−6 | <0.01 | ↓ | 4.33 × 10−4 ± 3.54 × 10−5 | 8.97 × 10−5 ± 9.50 × 10−6 | <0.01 | ↓ |
| Hydroxyacetone | 2.68 × 10−5 ± 3.71 × 10−6 | 2.14 × 10−5 ± 5.89 × 10−6 | 0.121 | ↓ | 5.27 × 10−6 ± 2.29 × 10−6 | 1.71 × 10−4 ± 2.61 × 10−5 | <0.01 | ↑ | 6.68 × 10−5 ± 3.95 × 10−6 | 1.90 × 10−5 ± 2.12 × 10−6 | <0.01 | ↓ |
| Hypoxanthine | 3.01 × 10−5 ± 8.31 × 10−6 | 1.02 × 10−4 ± 3.76 × 10−5 | 0.011 | ↑ | 2.15 × 10−5 ± 5.28 × 10−6 | 2.21 × 10−5 ± 5.27 × 10−6 | 0.291 | = | 4.29 × 10−5 ± 5.93 × 10−6 | 3.38 × 10−5 ± 1.19 × 10−5 | 0.572 | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Song, C.; Lan, Q.; Hu, B.; Ao, Z.; Zhang, S.; Tang, J.; Du, X.; Zhu, C.; Laghi, L. Effects of Glutathione-Enriched Inactive Dry Yeast on the Flavor Profile of Kiwi Wine. Foods 2025, 14, 1760. https://doi.org/10.3390/foods14101760
Yang Z, Song C, Lan Q, Hu B, Ao Z, Zhang S, Tang J, Du X, Zhu C, Laghi L. Effects of Glutathione-Enriched Inactive Dry Yeast on the Flavor Profile of Kiwi Wine. Foods. 2025; 14(10):1760. https://doi.org/10.3390/foods14101760
Chicago/Turabian StyleYang, Zhibo, Chuan Song, Qiuyu Lan, Bin Hu, Zonghua Ao, Suyi Zhang, Junni Tang, Xin Du, Chenglin Zhu, and Luca Laghi. 2025. "Effects of Glutathione-Enriched Inactive Dry Yeast on the Flavor Profile of Kiwi Wine" Foods 14, no. 10: 1760. https://doi.org/10.3390/foods14101760
APA StyleYang, Z., Song, C., Lan, Q., Hu, B., Ao, Z., Zhang, S., Tang, J., Du, X., Zhu, C., & Laghi, L. (2025). Effects of Glutathione-Enriched Inactive Dry Yeast on the Flavor Profile of Kiwi Wine. Foods, 14(10), 1760. https://doi.org/10.3390/foods14101760






