Fermentation Preparation of Umami Sauce and Peptides from Kelp Scraps by Natural Microbial Flora
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Microbial Fermentation
2.3. 16s rRNA Sequencing
2.4. Analysis of Polysaccharide Lyase and Protease Activities
2.5. Isolation and Purification of Umami Peptides
2.6. UPLC–MS
2.7. Taste Analysis of Kelp Sauce and Umami Peptides via Electronic Tongue
2.8. Molecular Docking of Umami Peptides and Receptors TAS1R1–TAS1R3
2.9. Statistical Analysis
3. Results
3.1. Fermentation of Kelp Scraps into Kelp Sauces by Common Microbes
3.2. Natural Fermentation of Kelp Scraps into Kelp Sauces by Endotrophic or Epiphytic Microbiome
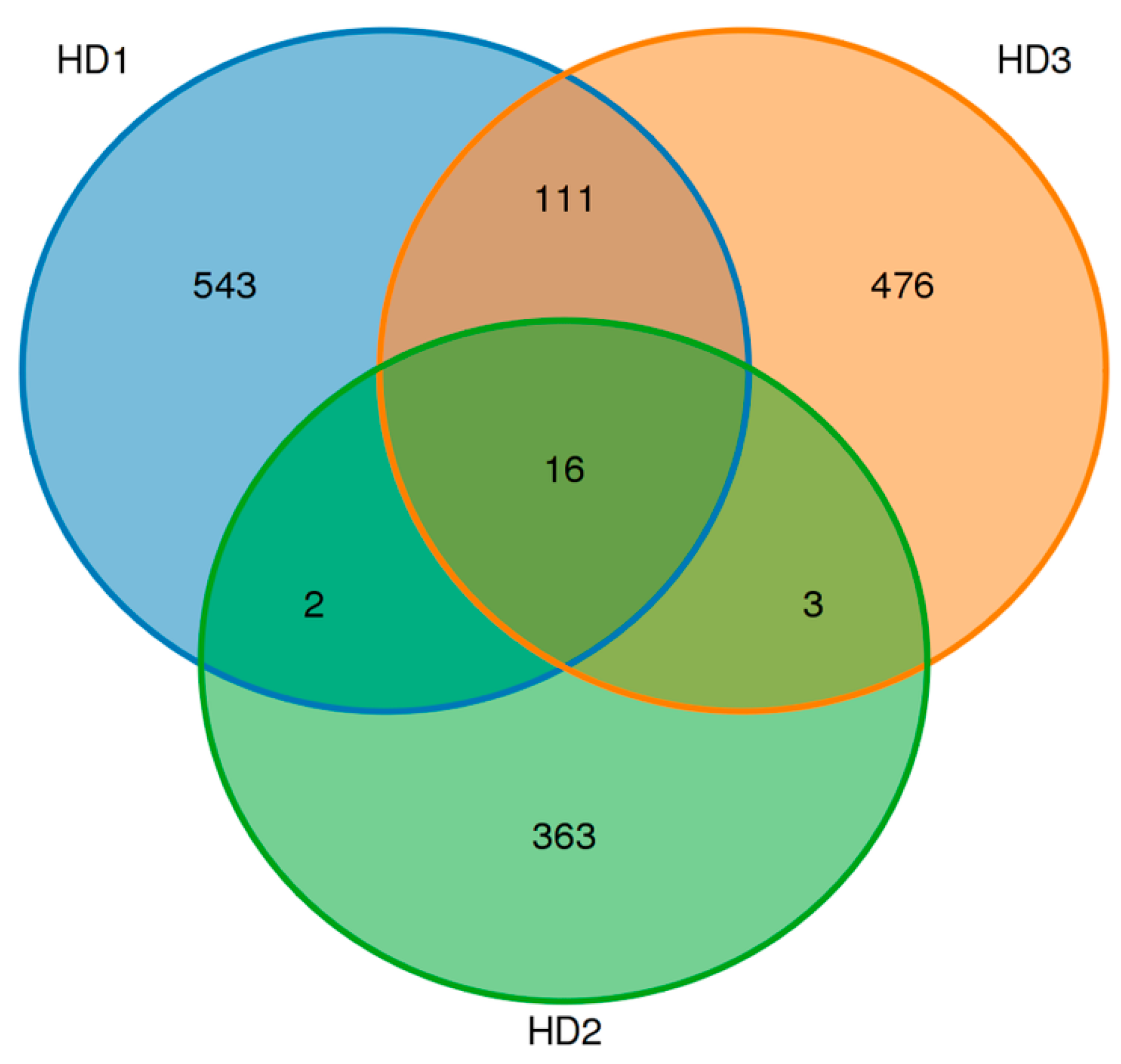
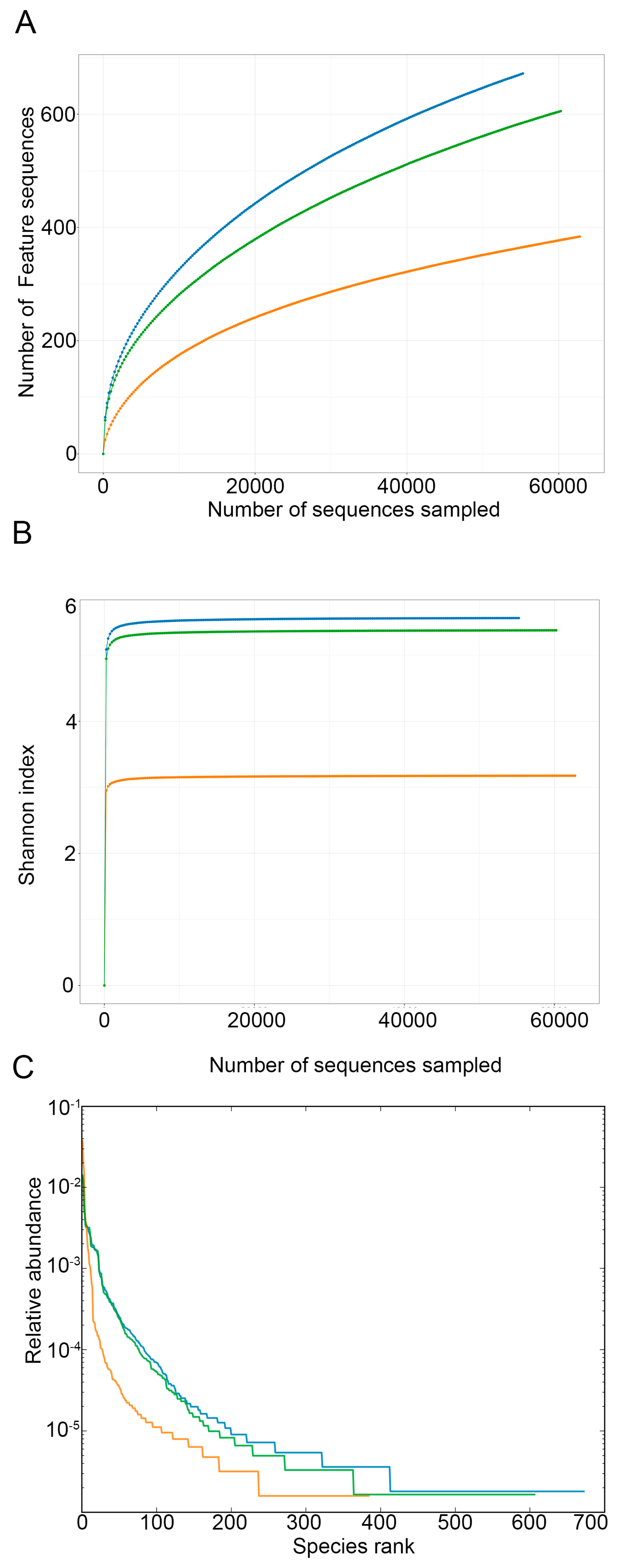
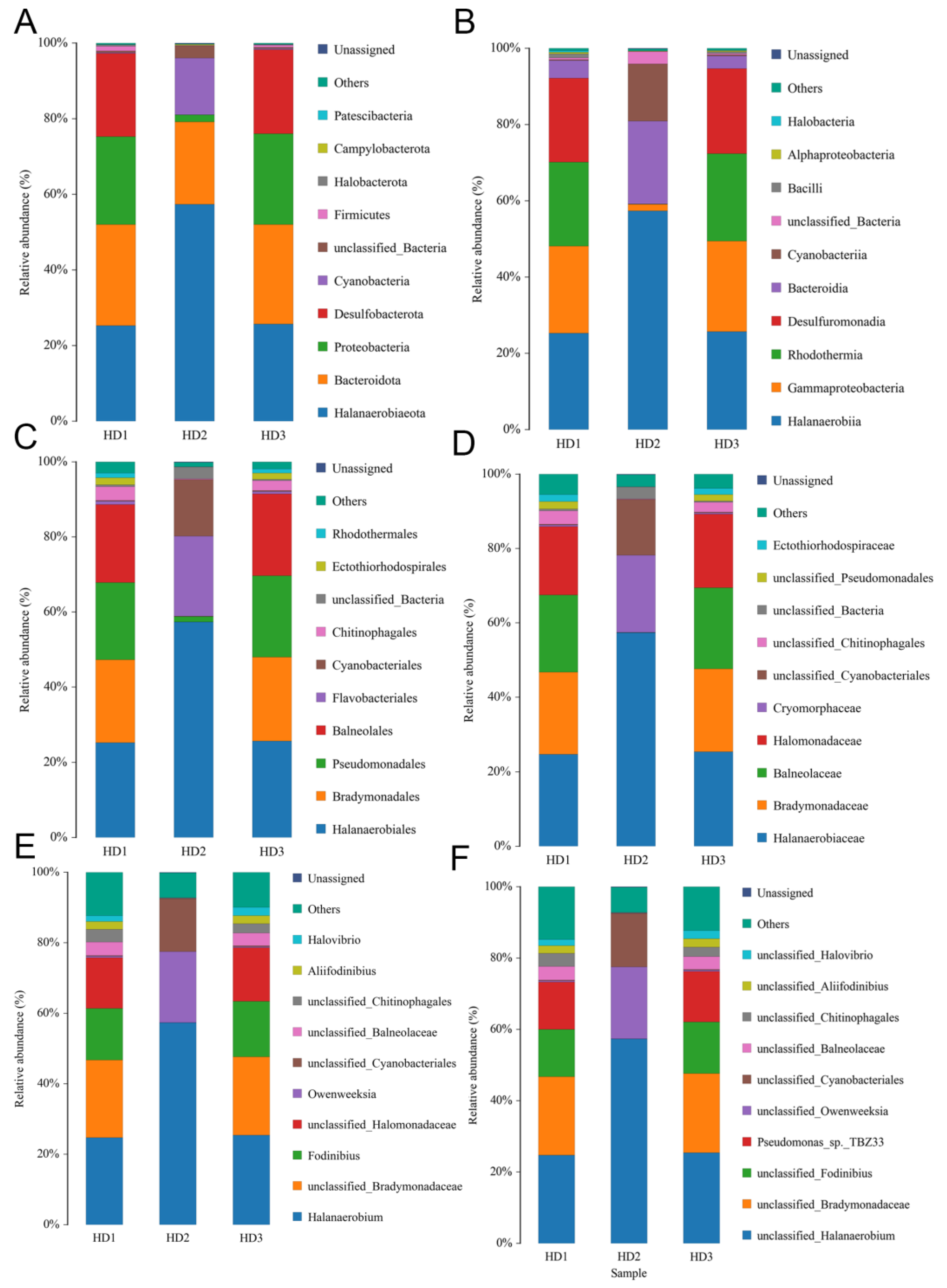
3.3. Sequencing Analysis of Microbiome of Kelp Sauces
3.4. Analysis of Active Enzymes During Fermentation of Kelp Sauces
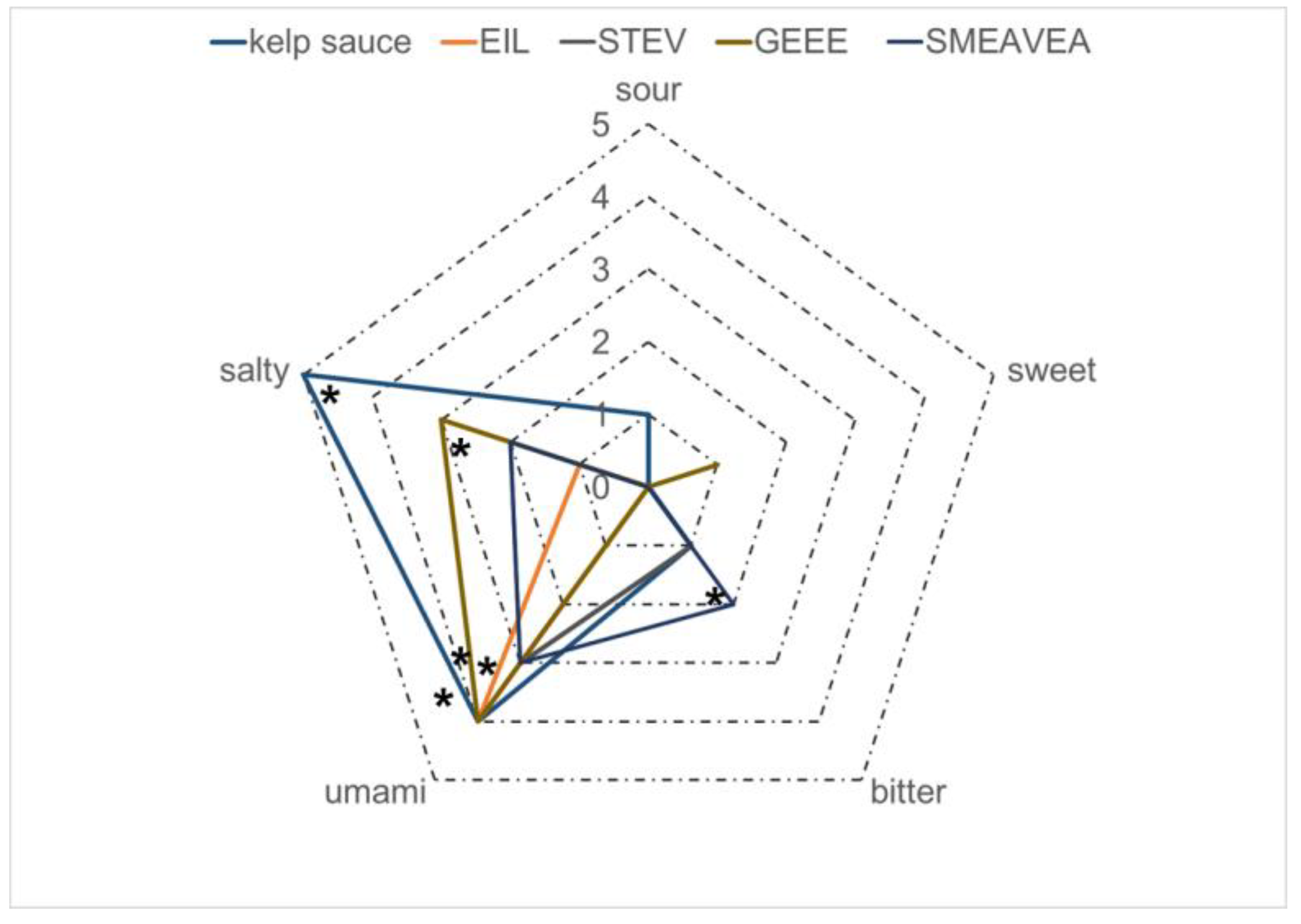
3.5. Taste Analysis of Kelp Sauce
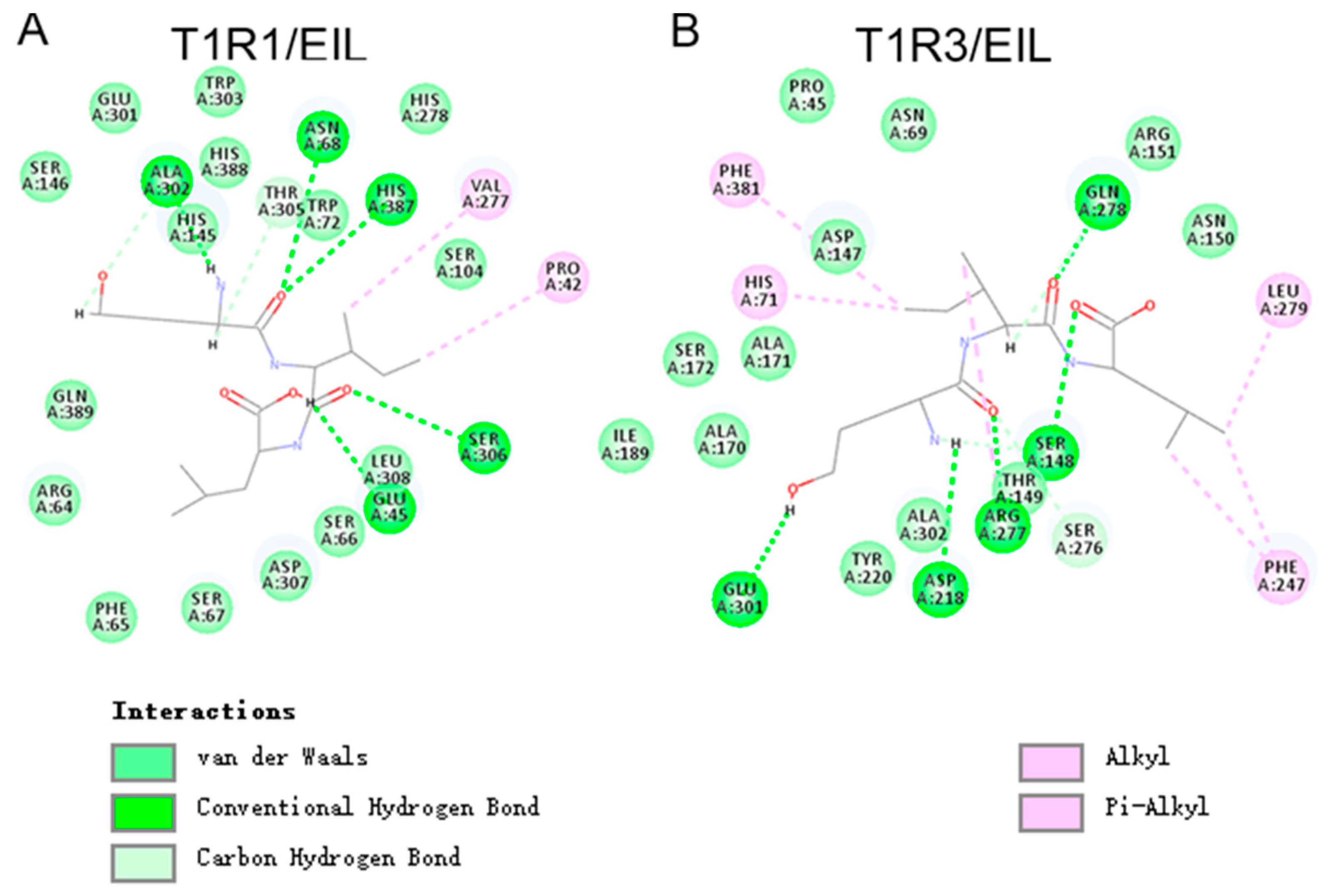
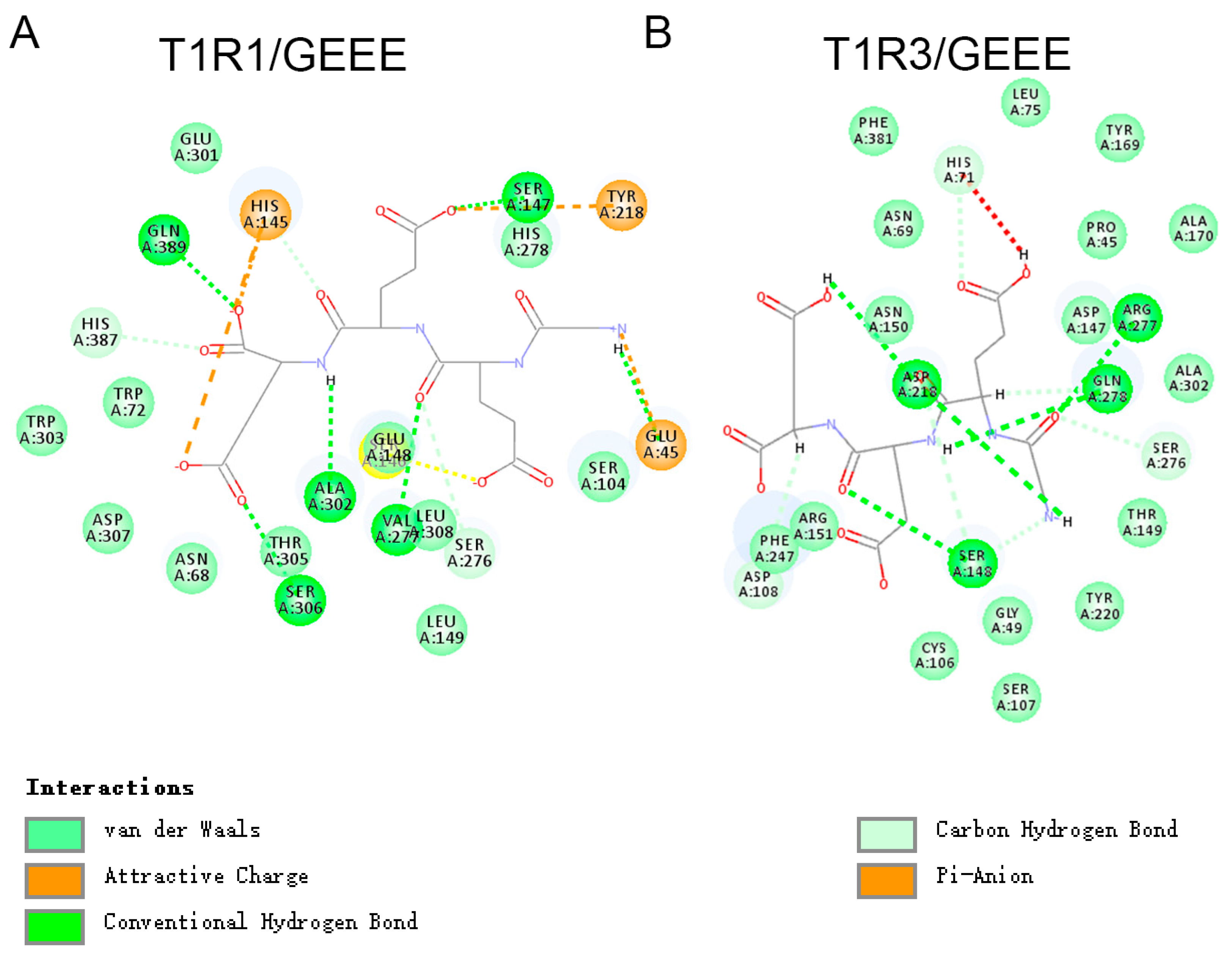
3.6. Molecular Docking of Umami Peptides and Receptors
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| FAO | Food and Agricultural Organization |
| ACE-1 | Angiotensin-1-Converting Enzyme 1 |
| MSG | Monosodium glutamate |
| LC–MS | Liquid chromatography–mass spectrometry |
| PolyM | Polymannuronic acid |
| PloyG | Polyguluronic acid |
| UPLC–MS | Ultra-performance liquid chromatography–mass spectrometry |
| SDs | Standard deviations |
| OTUs | Operational taxonomic units |
References
- Du, X.; Xiao, S.; Luo, Q.; Liu, X.; Liu, J. Laminaria japonica cyclic peptides exert anti-colorectal carcinoma effects through apoptosis induction in vitro and in vivo. J. Pept. Sci. 2022, 28, e3385. [Google Scholar] [CrossRef]
- Shirosaki, M.; Koyama, T. Laminaria japonica as a food for the prevention of obesity and diabetes. Adv. Food Nutr. Res. 2011, 64, 199–212. [Google Scholar] [CrossRef]
- Zhao, S.; Pan, Z.; Azarakhsh, N.; Ramaswamy, H.S.; Duan, H.; Wang, C. Effects of high-pressure processing on the physicochemical and adsorption properties, structural characteristics, and dietary fiber content of kelp (Laminaria japonica). Curr. Res. Food Sci. 2024, 8, 100671. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Rogel-Castillo, C.; Latorre-Castaneda, M.; Munoz-Munoz, C.; Agurto-Munoz, C. Seaweeds in Food: Current Trends. Plants 2023, 12, 2287. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.Y.; Oh, Y.R.; Park, J.M. Production of biodiesel from carbon sources of macroalgae, Laminaria japonica. Bioresour. Technol. 2014, 169, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yi, Y.L.; Guo, S.; Zhang, F.; Yan, H.; Zhan, Z.L.; Zhu, Y.; Duan, J.A. Isolation, structural characterization and bioactivities of polysaccharides from Laminaria japonica: A review. Food Chem. 2022, 370, 131010. [Google Scholar] [CrossRef]
- Luan, F.; Zou, J.; Rao, Z.; Ji, Y.; Lei, Z.; Peng, L.; Yang, Y.; He, X.; Zeng, N. Polysaccharides from Laminaria japonica: An insight into the current research on structural features and biological properties. Food Funct. 2021, 12, 4254–4283. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Guo, W.; Liu, J.; Lao, W.; Hu, P.; Lin, Y.; Chen, H. Laminaria japonica Peptides Suppress Liver Cancer by Inducing Apoptosis: Possible Signaling Pathways and Mechanism. Mar. Drugs 2022, 20, 704. [Google Scholar] [CrossRef]
- Purcell, D.; Packer, M.A.; Hayes, M. Identification of Bioactive Peptides from a Laminaria digitata Protein Hydrolysate Using In Silico and In Vitro Methods to Identify Angiotensin-1-Converting Enzyme (ACE-1) Inhibitory Peptides. Mar. Drugs 2023, 21, 90. [Google Scholar] [CrossRef]
- Pinela, J.; Pereira, C.; Dias, M.I.; Barros, L. Editorial: Functional foods processing and preservation. Food Biosci. 2023, 53, 102664. [Google Scholar] [CrossRef]
- Perumal, P.K.; Huang, C.Y.; Chen, C.W.; Anisha, G.S.; Singhania, R.R.; Dong, C.D.; Patel, A.K. Advances in oligosaccharides production from brown seaweeds: Extraction, characterization, antimetabolic syndrome, and other potential applications. Bioengineered 2023, 14, 2252659. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohydr. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Guo, Z.; Tang, T.; Zhu, B. Alginate degrading enzymes: An updated comprehensive review of the structure, catalytic mechanism, modification method and applications of alginate lyases. Crit. Rev. Biotechnol. 2021, 41, 953–968. [Google Scholar] [CrossRef]
- Du, Y.; Shim, S.M.; Wang, L.; Gao, X.; Fu, X. Impact of Monascus purpureus combined with Lactobacillus plantarum and Saccharomyces cerevisiae fermentation on nutritional and flavor characteristics of Pyropia yezoensis. Food Chem. 2025, 472, 142973. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Y.R.; Oh, S.; Paik, M.J.; Je, J.G.; Heo, J.H.; Lee, T.K.; Fu, X.; Xu, J.; Gao, X.; et al. Arsenic removal from the popular edible seaweed Sargassum fusiforme by sequential processing involving hot water, citric acid, and fermentation. Chemosphere 2022, 292, 133409. [Google Scholar] [CrossRef]
- Qi, L.; Gao, X.; Pan, D.; Sun, Y.; Cai, Z.; Xiong, Y.; Dang, Y. Research progress in the screening and evaluation of umami peptides. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1462–1490. [Google Scholar] [CrossRef]
- Sano, C. History of glutamate production. Am. J. Clin. Nutr. 2009, 90, 728s–732s. [Google Scholar] [CrossRef]
- Schilling, B.M.; Goodrick, J.C.; Wan, N.C. Scale-up of a high cell density continuous culture with Pichia pastoris X-33 for the constitutive expression of rh-chitinase. Biotechnol. Prog. 2001, 17, 629–633. [Google Scholar] [CrossRef]
- Masuo, N.; Ito, K.; Yoshimune, K.; Hoshino, M.; Matsushima, K.; Koyama, Y.; Moriguchi, M. Molecular cloning, overexpression, and purification of Micrococcus luteus K-3-type glutaminase from Aspergillus oryzae RIB40. Protein Expr. Purif. 2004, 38, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhang, Y.Q.; Zhang, X.R.; Zhang, X.D.; Sun, X.M.; Wang, X.F.; Sun, X.H.; Song, X.Y.; Zhang, Y.Z.; Wang, N.; et al. High-Level Extracellular Production of a Trisaccharide-Producing Alginate Lyase AlyC7 in and Its Agricultural Application. Mar. Drugs 2024, 22, 230. [Google Scholar] [CrossRef]
- Cao, S.S.; Li, L.; Li, Q.; Jiang, L.; Zhu, B.W.; Yao, Z. A novel alginate lyase and its domain functions for the preparation of unsaturated monosaccharides. Appl. Microbiol. Biotechnol. 2023, 107, 1737–1749. [Google Scholar] [CrossRef]
- Jo, Y.; Bang, W.S.; Kim, M.K. Changes of Physiochemical and Enzymatic Activities of doenjang Prepared with Different Amount of Rice koji during 30 Days of Fermentation. Foods 2021, 10, 372. [Google Scholar] [CrossRef]
- Amin, M.N.G.; Kusnadi, J.; Hsu, J.L.; Doerksen, R.J.; Huang, T.C. Identification of a novel umami peptide in tempeh (Indonesian fermented soybean) and its binding mechanism to the umami receptor T1R. Food Chem. 2020, 333, 127411. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jiang, H.; Guo, R.; Yang, B.; You, G.; Zhao, M.; Liu, X. Taste, umami-enhance effect and amino acid sequence of peptides separated from silkworm pupa hydrolysate. Food Res. Int. 2018, 108, 144–150. [Google Scholar] [CrossRef]
- Li, X.; Meng, H.; Zhu, Y.; Shu, S.; Bao, Y.; Jiang, S.; Liu, Y. Correlation analysis on sensory characteristics and physicochemical indices of bone broth under different processing methods. Food Chem. Adv. 2022, 1, 100036. [Google Scholar] [CrossRef]
- Dang, Y.; Gao, X.; Xie, A.; Wu, X.; Ma, F. Interaction between umami peptide and taste receptor T1R1/T1R3. Cell Biochem. Biophys. 2014, 70, 1841–1848. [Google Scholar] [CrossRef]
- Yue, H.; Sun, Y.; Jing, H.; Zeng, S.; Ouyang, H. The Analysis of Laminaria japonica Industry and International Trade Situation in China. In Proceedings of the Selected Articles of 2013 World Agricultural Outlook Conference; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–51. [Google Scholar]
- Tagliapietra, B.L.; Clerici, M. Brown algae and their multiple applications as functional ingredient in food production. Food Res. Int. 2023, 167, 112655. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Wang, X.-F.; Sun, X.-M.; Sun, M.-L.; Zhang, X.-Y.; Zhang, Y.-Q.; Xu, F. Cost-effective production of alginate oligosaccharides from Laminaria japonica roots by Pseudoalteromonas agarivorans A3. Microb. Cell Fact. 2023, 22, 179. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nunez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G.M. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef]
- Sorensen, J.S.; Madsen, S.K.; Bang-Berthelsen, C.H.; Hansen, L.T. Quality and safety aspects in fermentation of winged kelp (Alaria esculenta) and sugar kelp (Saccharina latissima) by the natural microbiota with or without addition of a Lactiplantibacillus plantarum starter culture. Food Res. Int. 2021, 150, 110800. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, B.; Zhong, F.; Chen, J.; Zhang, T. Effect of Microbial Fermentation on the Fishy-Odor Compounds in Kelp (Laminaria japonica). Foods 2021, 10, 2532. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; Ni, H.; Wang, Y. The Volatile Compounds Change during Fermentation of Saccharina japonica Seedling. Foods 2024, 13, 1992. [Google Scholar] [CrossRef]
- Udomsil, N.; Pongjanla, S.; Rodtong, S.; Tanasupawat, S.; Yongsawatdigul, J. Extremely halophilic strains of Halobacterium salinarum as a potential starter culture for fish sauce fermentation. J. Food Sci. 2022, 87, 5375–5389. [Google Scholar] [CrossRef]
- Chuprom, J.; Bovornreungroj, P.; Ahmad, M.; Kantachote, D.; Dueramae, S. Approach toward enhancement of halophilic protease production by Halobacterium sp. strain LBU50301 using statistical design response surface methodology. Biotechnol. Rep. 2016, 10, 17–28. [Google Scholar] [CrossRef]
- del Campo, M.M.; Camacho, R.M.; Mateos-Díaz, J.C.; Müller-Santos, M.; Córdova, J.; Rodríguez, J.A. Solid-state fermentation as a potential technique for esterase/lipase production by halophilic archaea. Extremophiles 2015, 19, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Akolkar, A.V.; Durai, D.; Desai, A.J. Halobacterium sp. SP1(1) as a starter culture for accelerating fish sauce fermentation. J. Appl. Microbiol. 2010, 109, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef] [PubMed]
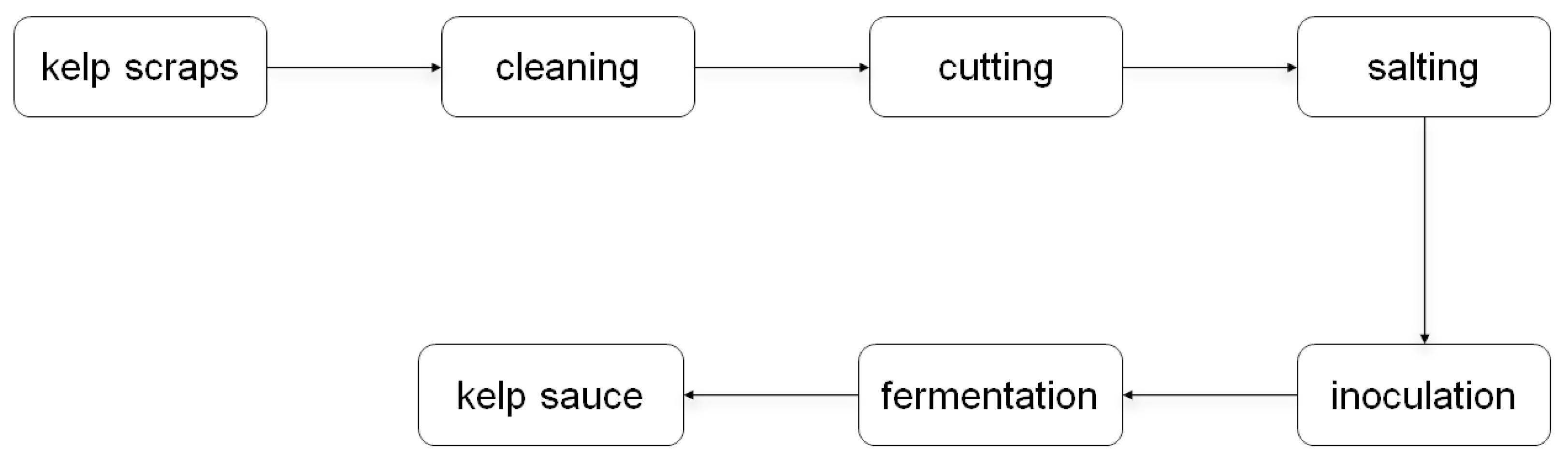


| Strains | Substance | Fermentation Temperature |
|---|---|---|
| L. plantarum m506 | sterilized kelp scraps | 30 |
| S. cerevisiae mj1003 | sterilized kelp scraps | 30 |
| P. pastoris X33 | sterilized kelp scraps | 30 |
| Bacillus sp. K102 | sterilized kelp scraps | 30 |
| A. oryzae RIB40 | sterilized kelp scraps | 30 |
| S. cerevisiae mj1003/L. plantarum m506 | sterilized kelp scraps | 30 |
| A. oryzae RIB40/L. plantarum m506 | sterilized kelp scraps | 30 |
| Natural microbial flora | cleaned kelp scraps | RT |
| No. | Sequence | Length | Mass | Peptide No. | Score |
|---|---|---|---|---|---|
| 1 | SMEAVEA | 7 | 735.1 | 21 | 150.0 |
| 2 | IVSLAPEVL | 9 | 939.6 | 10 | 148.2 |
| 3 | EIL | 3 | 373.5 | 18 | 89.8 |
| 4 | ISFLNK | 6 | 720.4 | 12 | 87.8 |
| 5 | SVEEIK | 6 | 703.4 | 11 | 84.0 |
| 6 | LENAIR | 6 | 714.4 | 14 | 69.1 |
| 7 | GEEE | 4 | 462.4 | 26 | 63.2 |
| 8 | IDHIYRDR | 8 | 1086.6 | 5 | 9.4 |
| 9 | RYQMGYIK | 8 | 1057.5 | 9 | 9.2 |
| 10 | STEV | 4 | 434.4 | 17 | 5.9 |
| 11 | EIPPATYNFRLYSGKMLELYGR | 22 | 2617.3 | 3 | 2.8 |
| 12 | SRVWKYQMGQMPISQLSK | 18 | 2166.1 | 2 | 1.0 |
| 13 | YKKDHDDDDPVDILVDVDFDK | 21 | 2505.2 | 3 | 0.5 |
| 14 | DRFCFCAEALYKAQAETGEIK | 21 | 2506.2 | 2 | 0.5 |
| 15 | FFRSKLNICEQCGYHLK | 17 | 2199.1 | 2 | 0.2 |
| 16 | IWICNWNKNDNAT | 13 | 1647.7 | 2 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wu, R.; Wu, Y.; Liang, F.; Chen, Y.; Yang, F.; Zheng, H.; Wang, Z.; Xu, H.; Chen, S.; et al. Fermentation Preparation of Umami Sauce and Peptides from Kelp Scraps by Natural Microbial Flora. Foods 2025, 14, 1751. https://doi.org/10.3390/foods14101751
Huang J, Wu R, Wu Y, Liang F, Chen Y, Yang F, Zheng H, Wang Z, Xu H, Chen S, et al. Fermentation Preparation of Umami Sauce and Peptides from Kelp Scraps by Natural Microbial Flora. Foods. 2025; 14(10):1751. https://doi.org/10.3390/foods14101751
Chicago/Turabian StyleHuang, Jizi, Ruimei Wu, Yijing Wu, Feiyang Liang, Yiming Chen, Fujia Yang, Huawei Zheng, Zonghua Wang, Huibin Xu, Songbiao Chen, and et al. 2025. "Fermentation Preparation of Umami Sauce and Peptides from Kelp Scraps by Natural Microbial Flora" Foods 14, no. 10: 1751. https://doi.org/10.3390/foods14101751
APA StyleHuang, J., Wu, R., Wu, Y., Liang, F., Chen, Y., Yang, F., Zheng, H., Wang, Z., Xu, H., Chen, S., & Yao, G. (2025). Fermentation Preparation of Umami Sauce and Peptides from Kelp Scraps by Natural Microbial Flora. Foods, 14(10), 1751. https://doi.org/10.3390/foods14101751







