Effects of Coconut Exocarp Flavonoid and EDTA-2Na on Aldehyde Generation During Pan-Frying Processing of Squid (Dsidicus gigas)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Lipid Extraction from Squid
2.4. Determination of Peroxide Value (PV)
2.5. Determination of Thiobarbituric Acid-Reactive Substances (TBARS)
2.6. Determination of Total Oxidation Value (TOTOX)
2.7. Fatty Acid Composition Analysis
2.8. Aldehyde Content Analysis
2.9. Statistical Analysis
3. Results and Discussion
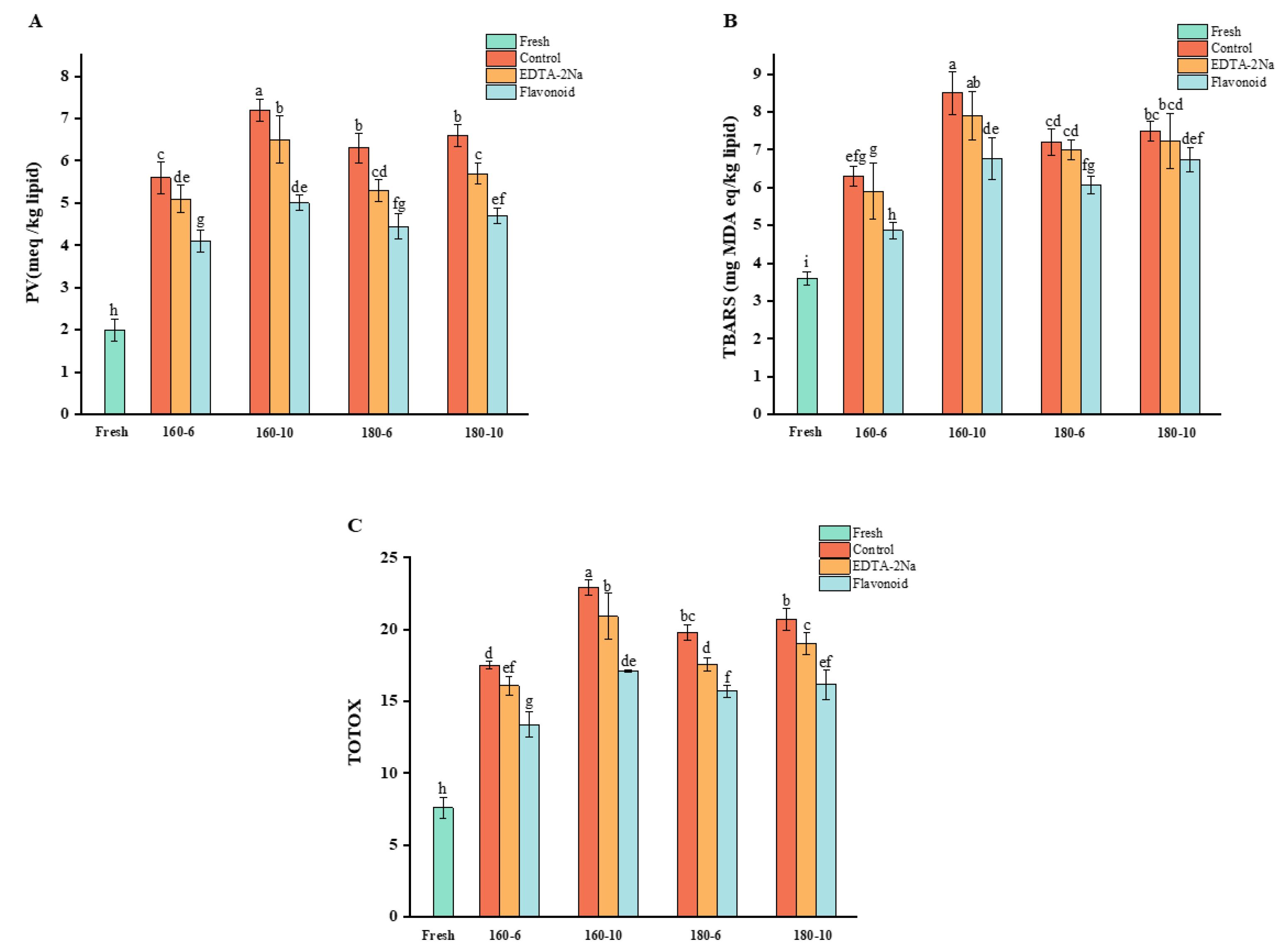
3.1. Changes in Oxidation Indices
3.1.1. Change in Peroxide Value
3.1.2. Change in Thiobarbituric Acid-Reactive Substances Value
3.1.3. Change in Total Oxidation Value
3.2. Changes in Fatty Acid Composition
3.3. Changes in Aldehyde Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFA | polyunsaturated fatty acids |
| EDTA-2Na | disodium ethylenediaminetetraacetate |
| n-3 PUFA | omega-3 PUFA |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| HNE | 4-hydroxy-nonenal |
| CEF | flavonoids extracted from coconut exocarp |
| DNPH | 2,4-dinitrophenylhydrazine |
| PV | peroxide value |
| TBARS | thiobarbituric acid-reactive substances |
| MDA | malondialdehyde |
| TOTOX | total oxidation |
| GC-MS | gas chromatography–mass spectrometry |
| HPLC | high-performance liquid chromatography |
| SD | standard deviation |
| HHE | pentanal, 4-hydroxy-hexenal |
| DDE | trans, trans-2,4-decadienal |
References
- Walchuk, C.; Wang, Y.; Suh, M. The impact of EPA and DHA on ceramide lipotoxicity in the metabolic syndrome. Brit J. Nutr. 2021, 125, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Letondor, A.; Buaud, B.; Vaysse, C.; Richard, E.; Laye, S.; Pallet, V.; Alfos, S. EPA/DHA and Vitamin A Supplementation Improves Spatial Memory and Alleviates the Age-related Decrease in Hippocampal RXRγ and Kinase Expression in Rats. Front. Aging Neurosci. 2016, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Kwon, O. Dietary intake of n-3 and n-6 polyunsaturated fatty acids in Korean toddlers 12–24 months of age with comparison to the dietary recommendations. Nutr. Res. Pract. 2019, 13, 344–351. [Google Scholar] [CrossRef]
- Toth, P.P.; Chapman, M.J.; Parhofer, K.G.; Nelson, J.R. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. Am. Heart J. Plus 2022, 17, 100148. [Google Scholar] [CrossRef]
- Aldhafiri, F.K. Investigating the Role of EPA and DHA on Cellular Oxidative Stress; Profiling Antidiabetic and Antihypertensive Potential. J. Pharm. Bioallied Sci. 2022, 14, 178–185. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Cai, W.; Guo, Y.; Xue, C.; Wang, J. DHA/EPA-Enriched Phosphatidylcholine Suppresses Tumor Growth and Metastasis via Activating Peroxisome Proliferator-Activated Receptor γ in Lewis Lung Cancer Mice. J. Agric. Food Chem. 2021, 69, 676–685. [Google Scholar] [CrossRef]
- van der Burg, K.P.; Cribb, L.; Firth, J.; Karmacoska, D.; Mischoulon, D.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; Oliver, G.; et al. EPA and DHA as markers of nutraceutical treatment response in major depressive disorder. Eur. J. Nutr. 2020, 59, 2439–2447. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Z.; Zhang, W.; Xia, G.; Li, C.; Rakariyatham, K.; Zhou, D. Advance in aldehydes derived from lipid oxidation: A review of the formation mechanism, attributable food thermal processing technology, analytical method and toxicological effect. Food Res. Int. 2025, 203, 115811. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef]
- Testa, G.; Staurenghi, E.; Giannelli, S.; Sottero, B.; Gargiulo, S.; Poli, G.; Gamba, P.; Leonarduzzi, G. Up-regulation of PCSK6 by lipid oxidation products: A possible role in atherosclerosis. Biochimie 2021, 181, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.; Saimanen, I.; Koskela, R.; Holopainen, A.; Selander, T.; Eskelinen, M. Plasma Concentration of the Lipid Peroxidation (LP) Biomarker 4-Etaydroxynonenal (4-HNE) in Benign and Cancer Patients. In Vivo 2022, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Aleman, R.; Alviz-Amador, A.; Galindo-Murillo, R.; Perez-Gonzalez, H.; Rodriguez-Cavallo, E.; Mendez-Cuadro, D. Cysteine carbonylation with reactive carbonyl species from lipid peroxidation induce local structural changes on thioredoxin active site. J. Mol. Graph. Model. 2023, 124, 108533. [Google Scholar] [CrossRef]
- Ghonimi, N.A.M.; Elsharkawi, K.A.; Khyal, D.S.M.; Abdelghani, A.A. Serum malondialdehyde as a lipid peroxidation marker in multiple sclerosis patients and its relation to disease characteristics. Mult. Scler. Relat. Dis. 2021, 51, 102941. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, Y.; Liu, Z.; Xie, H.; Zhang, M.; Zheng, R.; Qin, L.; Yin, F.; Zhou, D. Simultaneous quantification of 24 aldehydes and ketones in oysters (Crassostrea gigas) with different thermal processing procedures by HPLC-electrospray tandem mass spectrometry. Food Res. Int. 2021, 147, 110559. [Google Scholar] [CrossRef]
- Leung, K.S.; Galano, J.; Durand, T.; Lee, J.C. Profiling of Omega-Polyunsaturated Fatty Acids and Their Oxidized Products in Salmon after Different Cooking Methods. Antioxidants 2018, 7, 96. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, D.; Li, A.; Zhao, M.; Hu, Y.; Li, D.; Xie, H.; Zhao, Q.; Hu, X.; Zhang, J.; et al. Effects of temperature and heating time on the formation of aldehydes during the frying process of clam assessed by an HPLC-MS/MS method. Food Chem. 2020, 308, 125650. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, Y.; Huang, Y.; Yang, W.; Shi, H.; Wang, J.; Liu, Z.; Zhang, X.; Li, C.; Xia, G.; et al. Effects of phenolic acid grafted-chitosan hydrocolloids on the aldehyde contents from lipid oxidation in golden pompano (Trachinotus blochii) fillets during pan-frying. Food Chem. 2025, 463, 141270. [Google Scholar] [CrossRef]
- Rajesh, T.P.; Basheer, V.A.; Packirisamy, A.S.B.; Ravi, S.N.; Vallinayagam, S. Effective Inhibition of Enzymatic Browning and Carcinogenic Acrylamide in Fried Food by Polyphenols. Top. Catal. 2024, 67, 300–312. [Google Scholar] [CrossRef]
- Rutkowska, J.; Antoniewska, A.; Martinez-Pineda, M.; Nawirska-Olszanska, A.; Zbikowska, A.; Baranowski, D. Black Chokeberry Fruit Polyphenols: A Valuable Addition to Reduce Lipid Oxidation of Muffins Containing Xylitol. Antioxidants 2020, 9, 394. [Google Scholar] [CrossRef]
- Leung, K.S.; Leung, H.H.; Wu, C.Y.; Galano, J.; Durand, T.; Lee, J.C. Limited Antioxidant Effect of Rosemary in Lipid Oxidation of Pan-Fried Salmon. Biomolecules 2019, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhao, M.; Wang, X.; Wang, J.; Li, C.; Dong, X.; Liu, Z.; Zhou, D. Plasma-activated water in combination with coconut exocarp flavonoids emerge as promising preservation technique for golden pompano: Impact of the treatment sequence. Food Chem. 2024, 447, 138981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lv, H.; Lu, Y.; Lu, Y.; Lv, L. Trapping of Acrolein by Curcumin and the Synergistic Inhibition Effect of Curcumin Combined with Quercetin. J. Agric. Food Chem. 2021, 69, 294–301. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Wang, X.; Li, C.; Wang, J.; Liu, Z.; Shen, X.; Zhou, D. Response surface methodology-optimized extraction of flavonoids with antioxidant and antimicrobial activities from the exocarp of three genera of coconut and characterization by HPLC-IT-TOF-MS/MS. Food Chem. 2022, 391, 132966. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- AOCS. Official method Cd 8b-90. Sampling and Analysis of Commercial Fats and Oils: Peroxide Value, Acetic Acid-Isooctane Method; American Oil Chemists’ Society: Champaign-Urbana, IL, USA, 2003. [Google Scholar]
- Hu, Y.; Zhao, G.; Yin, F.; Liu, Z.; Wang, J.; Qin, L.; Zhou, D. Effects of roasting temperature and time on aldehyde formation derived from lipid oxidation in scallop (Patinopecten yessoensis) and the deterrent effect by antioxidants of bamboo leaves. Food Chem. 2022, 369, 130936. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Storage stability of microencapsulated seal blubber oil. J. Food Lipids 1995, 2, 73–86. [Google Scholar] [CrossRef]
- Yan, X.; Li, L.; Liu, P.; Xu, J.; Wang, Z.; Ding, L.; Yang, L. Targeted metabolomics profiles serum fatty acids by HFD induced non-alcoholic fatty liver in mice based on GC-MS. J. Pharmaceut Biomed. 2022, 211, 114620. [Google Scholar] [CrossRef]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Bejaoui, S.; Chetoui, I.; Ghribi, F.; Soudani, N.; Cafsi, M.E. Different frying processes stimulate lipid peroxidation and promote changes in the composition of cholesterol, free fatty acids and triglycerides in the commercial clam’s tissues Venerupis decussata. Int. J. Food Eng. 2022, 18, 87–103. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R. Influence of food type, oil type and frying frequency on the formation of trans-fatty acids during repetitive deep-frying. Food Control 2023, 147, 109557. [Google Scholar] [CrossRef]
- Andreo, A.I.; Doval, M.M.; Romero, A.M.; Judis, M.A. Influence of heating time and oxygen availability on lipid oxidation in meat emulsions. Eur. J. Lipid Sci. Technol. 2003, 105, 207–213. [Google Scholar] [CrossRef]
- de Oliveira, V.S.; Viana, D.S.B.; Keller, L.M.; de Melo, M.T.T.; Mulandeza, O.F.; Barbosa, M.I.M.J.; Junior, J.L.B.; Saldanha, T. Impact of air frying on food lipids: Oxidative evidence, current research, and insights into domestic mitigation by natural antioxidants. Trends Food Sci. Technol. 2024, 147, 104465. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Lin, D.; Shen, Q.; Saleh, A.S.M. The changes in the volatile aldehydes formed during the deep-fat frying process. J. Food Sci. Tech. Mys 2015, 52, 7683–7696. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Lee, M.; Tirtawijaya, G.; Cho, W.; Sohn, J.; Kim, J.; Choi, J. Application of Deep, Vacuum, and Air Frying Methods to Fry Chub Mackerel (Scomber japonicus). Processes 2021, 9, 1225. [Google Scholar] [CrossRef]
- Katragadda, H.R.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, A.A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010, 120, 59–65. [Google Scholar] [CrossRef]
- Peng, B.; Li, J.; Yu, C.; Hu, M.; Zhong, B.; Shi, S.; Tu, Z. Lipidomics profile and volatile compounds of squids (Illex argentinus, Ommastrephes bartram and Dosidicus gigas) in the Argentine, North Pacific Ocean, Equator and Peru by UPLC-triple TOF-MS and HS-SPME-GC-O-MS. Food Res. Int. 2024, 189, 114559. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Chen, X.; Yu, W. Trophic Niche and Diversity of a Pelagic Squid (Dosidicus gigas): A Comparative Study Using Stable Isotope, Fatty Acid, and Feeding Apparatuses Morphology. Front. Mar. Sci. 2020, 7, 642. [Google Scholar] [CrossRef]
- Li, J.; Tu, Z.; Sha, X.; Zhang, L.; Lin, D.; Zeng, K.; Wang, H.; Pang, J.; Tang, P. Effect of Frying on Fatty Acid Profile, Free Amino Acids and Volatile Compounds of Grass Carp (Ctenopharyngodon idellus) Fillets. J. Food Process. Preserv. 2017, 41, e13088. [Google Scholar] [CrossRef]
- Mekonnen, M.F.; Desta, D.T.; Alemayehu, F.R.; Kelikay, G.N.; Daba, A.K. Evaluation of fatty acid-related nutritional quality indices in fried and raw nile tilapia, (Oreochromis niloticus), fish muscles. Food Sci. Nutr. 2020, 8, 4814–4821. [Google Scholar] [CrossRef]
- Chaula, D.; Laswai, H.; Chove, B.; Dalsgaard, A.; Mdegela, R.; Hyldig, G. Fatty Acid Profiles and Lipid Oxidation Status of Sun Dried, Deep Fried, and Smoked Sardine (Rastrineobola argentea) from Lake Victoria, Tanzania. J. Aquat. Food Prod. Technol. 2019, 28, 165–176. [Google Scholar] [CrossRef]
- Di Bella, G.; Litrenta, F.; Pino, S.; Tropea, A.; Potorti, A.G.; Nava, V.; Turco, V.L. Variations in fatty acid composition of Mediterranean anchovies (Engraulis encrasicolus) after different cooking methods. Eur. Food Res. Technol. 2022, 248, 2285–2290. [Google Scholar] [CrossRef]
- Biandolino, F.; Parlapiano, I.; Denti, G.; Di Nardo, V.; Prato, E. Effect of Different Cooking Methods on Lipid Content and Fatty Acid Profiles of Mytilus galloprovincialis. Foods 2021, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Abedi, E.; Mohammadzadeh, B.; Afsharnaderi, A. Effect of frying in different culinary fats on the fatty acid composition of silver carp. Food Sci. Nutr. 2013, 1, 292–297. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Li, S.; Yan, J.; Qin, W.; Saleh, A.S.; Zhang, Q. Plant phenolic extracts for the quality protection of frying oil during deep frying: Sources, effects, and mechanisms. Grain Oil Sci. Technol. 2023, 6, 148–161. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Lee, W.J.; Huang, F.; Wang, Y.; Li, Y. Comparison between synthetic and rosemary-based antioxidants for the deep frying of French fries in refined soybean oils evaluated by chemical and non-destructive rapid methods. Food Chem. 2021, 335, 127638. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Dar, M.M. Improving oxidative stability of soyabean oil by apple pomace extract during deep frying of french fries. Food Biosci. 2022, 49, 101874. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC-MS. Int. J. Food Prop. 2018, 20, S2926–S2938. [Google Scholar] [CrossRef]
- Huang, T.C.; Ho, C.T.; Fu, H.Y. Inhibition of lipid oxidation in pork bundles processing by superheated steam frying. J. Agric. Food Chem. 2004, 52, 2924–2928. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Q.; Sheng, C.; You, J. Gas Purge Microextraction Coupled with Stable Isotope Labeling-Liquid Chromatography/Mass Spectrometry for the Analysis of Bromophenols in Aquatic Products. J. Agric. Food Chem. 2016, 64, 9452–9458. [Google Scholar] [CrossRef]
- Xu, L.; Ji, X.; Wu, G.; Karrar, E.; Yao, L.; Wang, X. Influence of Oil Types and Prolonged Frying Time on the Volatile Compounds and Sensory Properties of French Fries. J. Oleo Sci. 2021, 70, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Dong, J.; He, X.; Wang, J.; Li, C.; Dong, L.; Zhang, Y.; Zhou, X.; Wang, H.; Yi, Y.; et al. Impact of Heating Temperature and Fatty Acid Type on the Formation of Lipid Oxidation Products During Thermal Processing. Front. Nutr. 2022, 9, 913297. [Google Scholar] [CrossRef] [PubMed]
- Bastos, L.C.S.; de Almeida Costa, E.A.; Pereira, P.A.P. Development, validation and application of an UFLC-DAD-ESI-MS method for determination of carbonyl compounds in soybean oil during continuous heating. Food Chem. 2017, 218, 518–524. [Google Scholar] [CrossRef]
- Meinert, L.; Andersen, L.T.; Bredie, W.L.; Bjergegaard, C.; Aaslyng, M.D. Chemical and sensory characterisation of pan-fried pork flavour: Interactions between raw meat quality, ageing and frying temperature. Meat Sci. 2007, 75, 229–242. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Banerjee, A.; Ghosh, S.; Ghosh, M. Anti-oxidative effect of turmeric on frying characteristics of soybean oil. J. Food Sci. Technol. 2015, 52, 1760–1765. [Google Scholar] [CrossRef]
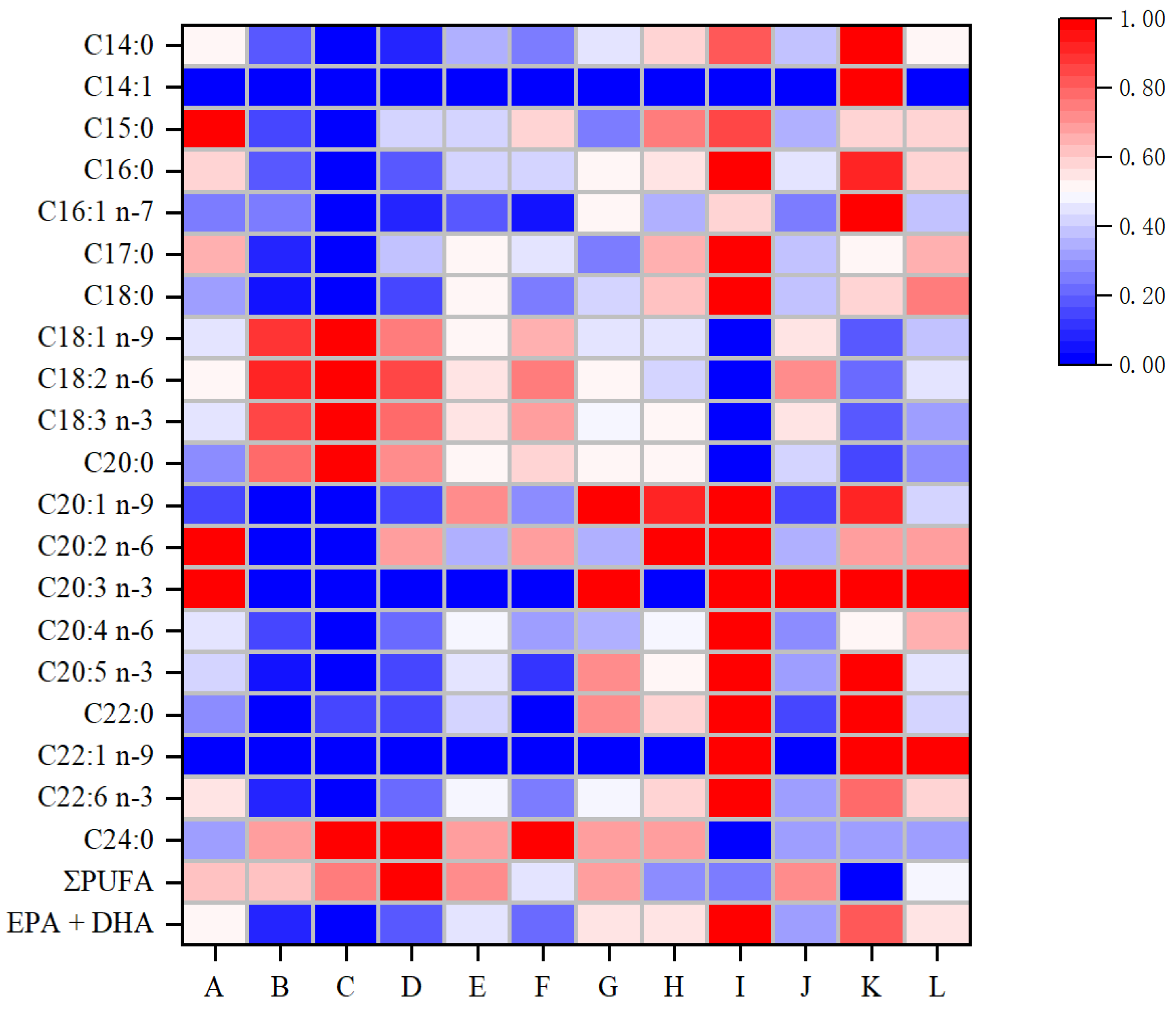

| Fatty Acid | Fresh | Control-6 | Control-10 | EDTA-2Na-6 | EDTA-2Na-10 | CEF-6 | CEF-10 |
|---|---|---|---|---|---|---|---|
| C12:0 | 0.03 ± 0.01 a | 0.01 ± 0.00 bc | 0.01 ± 0.00 c | 0.01 ± 0.00 bc | 0.01 ± 0.00 c | 0.01 ± 0.00 b | 0.01 ± 0.00 bc |
| C13:0 | 0.02 ± 0.00 | nd | nd | nd | nd | nd | nd |
| C14:0 | 2.32 ± 0.56 a | 0.77 ± 0.02 c | 0.60 ± 0.01 d | 0.69 ± 0.07 cd | 0.64 ± 0.0 d | 0.92 ± 0.05 b | 0.71 ± 0.02 cd |
| C14:1 n-5 | 0.03 ± 0.01 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| C15:0 | 0.75 ± 0.02 a | 0.22 ± 0.01 b | 0.12 ± 0.00 d | 0.15 ± 0.02 bcd | 0.17 ± 0.01 bcd | 0.20 ± 0.00 bc | 0.14 ± 0.00 cd |
| C16:0 | 33.28 ± 1.61 a | 16.64 ± 0.70 bc | 15.18 ± 0.10 c | 16.04 ± 1.34 c | 16.06 ± 0.52 c | 18.14 ± 0.25 b | 16.13 ± 0.77 c |
| C16:1 n-7 | 0.58 ± 0.03 a | 0.23 ± 0.01 c | 0.23 ± 0.00 c | 0.22 ± 0.03 c | 0.19 ± 0.00 c | 0.30 ± 0.01 b | 0.23 ± 0.01 c |
| C17:0 | 1.46 ± 0.02 a | 0.40 ± 0.01 b | 0.29 ± 0.01 b | 0.37 ± 0.05 b | 0.36 ± 0.01 b | 0.47 ± 0.01 b | 0.35 ± 0.02 b |
| C18:0 | 10.63 ± 0.60 a | 3.69 ± 0.16 b | 3.41 ± 0.05 b | 3.93 ± 0.42 b | 3.64 ± 0.14 b | 4.45 ± 0.16 b | 3.79 ± 0.21 b |
| C18:1 n-9 | 2.60 ± 0.25 d | 20.02 ± 1.31 b | 22.10 ± 0.38 a | 20.37 ± 1.98 b | 20.99 ± 0.72 ab | 17.69 ± 0.90 c | 20.51 ± 1.18 b |
| C18:2 n-6 | 0.25 ± 0.03 c | 45.14 ± 2.88 a | 48.30 ± 0.99 a | 45.56 ± 3.78 a | 47.02 ± 1.55 a | 41.26 ± 2.03 b | 46.74 ± 2.54 a |
| C18:3 n-3 | 0.13 ± 0.02 d | 0.62 ± 0.03 b | 0.73 ± 0.02 a | 0.65 ± 0.08 b | 0.68 ± 0.02 ab | 0.50 ± 0.03 c | 0.65 ± 0.04 b |
| C20:0 | 0.16 ± 0.02 f | 0.33 ± 0.03 c | 0.40 ± 0.00 a | 0.36 ± 0.04 bc | 0.37 ± 0.01 ab | 0.29 ± 0.02 d | 0.35 ± 0.02 bc |
| C20:1 n-9 | 1.59 ± 0.19 a | 0.54 ± 0.02 b | 0.52 ± 0.02 b | 0.62 ± 0.07 b | 0.56 ± 0.02 b | 0.66 ± 0.01 b | 0.54 ± 0.03 b |
| C20:2 n-6 | 0.28 ± 0.01 a | 0.09 ± 0.00 b | 0.06 ± 0.00 b | 0.07 ± 0.01 b | 0.08 ± 0.00 b | 0.09 ± 0.00 b | 0.07 ± 0.00 b |
| C20:3 n-3 | 0.12 ± 0.02 a | 0.03 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b |
| C20:4 n-6 | 7.70 ± 0.54 a | 1.72 ± 0.05 b | 1.23 ± 0.05 b | 1.77 ± 0.19 b | 1.51 ± 0.05 b | 2.58 ± 0.01 b | 1.46 ± 0.08 b |
| C20:5 n-3 | 8.93 ± 0.01 a | 2.17 ± 0.08 bc | 1.64 ± 0.03 c | 2.19 ± 0.24 bc | 1.72 ± 0.06 c | 3.02 ± 0.05 b | 2.01 ± 0.12 bc |
| C22:0 | 0.65 ± 0.02 a | 0.23 ± 0.01 bc | 0.21 ± 0.00 c | 0.24 ± 0.03 bc | 0.21 ± 0.01 c | 0.28 ± 0.00 b | 0.22 ± 0.01 bc |
| C22:1 n-9 | nd | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.04 ± 0.00 a | 0.03 ± 0.00 b |
| C22:6 n-3 | 28.37 ± 1.28 a | 7.02 ± 0.24 c | 4.80 ± 0.15 d | 6.60 ± 0.76 cd | 5.62 ± 0.20 cd | 8.99 ± 0.11 b | 5.93 ± 0.43 cd |
| C24:0 | 0.12 ± 0.02 a | 0.09 ± 0.00 cd | 0.10 ± 0.00 bc | 0.10 ± 0.00 bc | 0.11 ± 0.00 ab | 0.08 ± 0.00 d | 0.09 ± 0.01 cd |
| ΣPUFA | 45.78 ± 0.57 b | 56.79 ± 0.13 a | 56.78 ± 0.06 a | 56.86 ± 0.32 a | 56.65 ± 0.09 a | 56.47 ± 0.04 a | 56.87 ± 0.11 a |
| EPA + DHA | 37.30 ± 0.41 a | 9.19 ± 0.16 c | 6.44 ± 0.10 f | 8.79 ± 0.58 c | 7.34 ± 0.16 e | 12.01 ± 0.10 b | 7.94 ± 0.30 d |
| Fatty Acid | Fresh | Control-6 | Control-10 | EDTA-2Na-6 | EDTA-2Na-10 | CEF-6 | CEF-10 |
|---|---|---|---|---|---|---|---|
| C12:0 | 0.03 ± 0.01 a | 0.01 ± 0.00 c | 0.01 ± 0.00 c | 0.01 ± 0.00 bc | 0.01 ± 0.00 bc | 0.01 ± 0.00 b | 0.01 ± 0.00 bc |
| C13:0 | 0.02 ± 0.00 | nd | nd | nd | nd | nd | nd |
| C14:0 | 2.32 ± 0.56 a | 0.51 ± 0.01 c | 0.55 ± 0.01 c | 0.74 ± 0.00 bc | 0.80 ± 0.01 bc | 1.02 ± 0.00 b | 0.78 ± 0.03 bc |
| C14:1 n-5 | 0.03 ± 0.01 a | 0.01 ± 0.00 c | 0.01 ± 0.00 c | 0.01 ± 0.00 bc | 0.01 ± 0.00 bc | 0.02 ± 0.00 b | 0.01 ± 0.00 bc |
| C15:0 | 0.75 ± 0.02 a | 0.10 ± 0.00 f | 0.15 ± 0.00 d | 0.13 ± 0.00 e | 0.19 ± 0.00 b | 0.17 ± 0.00 c | 0.17 ± 0.01 cd |
| C16:0 | 33.28 ± 1.61 a | 14.57 ± 0.02 d | 15.28 ± 0.04 d | 16.42 ± 0.06 c | 16.51 ± 0.04 c | 17.79 ± 0.15 b | 16.66 ± 0.21 c |
| C16:1 n-7 | 0.58 ± 0.03 a | 0.18 ± 0.01 e | 0.20 ± 0.00 e | 0.29 ± 0.01 c | 0.25 ± 0.00 d | 0.39 ± 0.01 b | 0.26 ± 0.01 d |
| C17:0 | 1.46 ± 0.02 a | 0.27 ± 0.00 f | 0.35 ± 0.00 d | 0.32 ± 0.00 e | 0.40 ± 0.00 b | 0.37 ± 0.00 c | 0.40 ± 0.02 b |
| C18:0 | 10.63 ± 0.60 a | 3.36 ± 0.04 d | 3.53 ± 0.04 cd | 3.82 ± 0.04 bc | 4.02 ± 0.03 b | 3.99 ± 0.03 b | 4.17 ± 0.13 b |
| C18:1 n-9 | 2.60 ± 0.25 f | 22.77 ± 0.20 a | 21.51 ± 0.10 b | 20.01 ± 0.13 c | 19.92 ± 0.04 c | 18.62 ± 0.12 e | 19.56 ± 0.28 d |
| C18:2 n-6 | 0.25 ± 0.03 f | 49.01 ± 0.37 a | 47.81 ± 0.13 b | 45.24 ± 0.27 c | 44.58 ± 0.25 d | 42.83 ± 0.20 e | 44.64 ± 0.70 d |
| C18:3 n-3 | 0.13 ± 0.02 f | 0.77 ± 0.02 a | 0.71 ± 0.01 b | 0.63 ± 0.00 c | 0.64 ± 0.01 c | 0.55 ± 0.01 e | 0.59 ± 0.01 d |
| C20:0 | 0.16 ± 0.02 f | 0.43 ± 0.01 a | 0.39 ± 0.01 b | 0.36 ± 0.01 c | 0.36 ± 0.01 c | 0.31 ± 0.00 e | 0.33 ± 0.01 d |
| C20:1 n-9 | 1.59 ± 0.19 a | 0.52 ± 0.01 d | 0.54 ± 0.01 cd | 0.66 ± 0.01 b | 0.65 ± 0.01 bc | 0.65 ± 0.01 bc | 0.58 ± 0.02 bcd |
| C20:2 n-6 | 0.28 ± 0.01 a | 0.06 ± 0.00 d | 0.08 ± 0.00 b | 0.07 ± 0.00 c | 0.09 ± 0.00 b | 0.08 ± 0.00 b | 0.08 ± 0.00 b |
| C20:3 n-3 | 0.12 ± 0.02 a | 0.02 ± 0.00 d | 0.02 ± 0.00 cd | 0.03 ± 0.00 bcd | 0.02 ± 0.00 cd | 0.03 ± 0.00 b | 0.03 ± 0.00 bc |
| C20:4 n-6 | 7.70 ± 0.54 a | 1.02 ± 0.02 e | 1.34 ± 0.02 de | 1.56 ± 0.02 cd | 1.79 ± 0.03 bc | 1.81 ± 0.02 bc | 2.04 ± 0.11 b |
| C20:5 n-3 | 8.93 ± 0.01 a | 1.55 ± 0.03 g | 1.75 ± 0.04 f | 2.59 ± 0.06 c | 2.33 ± 0.04 d | 2.99 ± 0.02 b | 2.23 ± 0.11 e |
| C22:0 | 0.65 ± 0.02 a | 0.22 ± 0.00 e | 0.22 ± 0.00 e | 0.26 ± 0.00 c | 0.25 ± 0.00 d | 0.28 ± 0.00 b | 0.24 ± 0.01 d |
| C22:1 n-9 | nd | 0.03 ± 0.00 d | 0.03 ± 0.00 bc | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.04 ± 0.00 a | 0.04 ± 0.00 ab |
| C22:6 n-3 | 28.37 ± 1.28 a | 4.48 ± 0.06 e | 5.41 ± 0.10 d | 6.71 ± 0.20 c | 7.04 ± 0.18 c | 7.95 ± 0.12 b | 7.08 ± 0.30 c |
| C24:0 | 0.12 ± 0.02 a | 0.11 ± 0.00 bc | 0.11 ± 0.00 bc | 0.10 ± 0.00 bc | 0.10 ± 0.00 b | 0.09 ± 0.01 d | 0.09 ± 0.00 cd |
| ΣPUFA | 45.78 ± 0.57 b | 56.91 ± 0.31 a | 57.12 ± 0.09 a | 56.83 ± 0.21 a | 56.49 ± 0.18 a | 56.24 ± 0.17 a | 56.66 ± 0.56 a |
| EPA + DHA | 37.30 ± 0.41 a | 6.03 ± 0.05 e | 7.16 ± 0.08 d | 9.30 ± 0.16 c | 9.37 ± 0.14 c | 10.94 ± 0.08 b | 9.31 ± 0.23 c |
| Compounds | A | B | C | D | E | F | G | H | I |
|---|---|---|---|---|---|---|---|---|---|
| Acrolein | 0.04 ± 0.01 cd | 0.06 ± 0.01 bc | 0.13 ± 0.01 a | 0.02 ± 0.00 de | 0.03 ± 0.02 de | 0.06 ± 0.01 b | 0.01 ± 0.00 e | 0.02 ± 0.01 de | 0.05 ± 0.02 bc |
| Propanal | 23.17 ± 0.67 a | 22.32 ± 0.26 abc | 21.11 ± 0.43 de | 22.21 ± 0.54 abcd | 22.68 ± 0.58 ab | 22.62 ± 0.51 ab | 19.96 ± 0.21 e | 21.82 ± 0.41 bcd | 21.25 ± 1.59 cd |
| Butanal | 12.09 ± 0.69 c | 14.62 ± 0.94 b | 19.53 ± 3.27 a | 11.67 ± 0.39 c | 12.70 ± 1.16 bc | 13.40 ± 0.74 bc | 8.77 ± 1.02 d | 11.67 ± 0.91 c | 13.63 ± 0.72 bc |
| Pentanal | 18.84 ± 4.39 c | 20.99 ± 3.77 c | 59.37 ± 3.98 a | 19.51 ± 6.17 c | 19.76 ± 6.39 c | 31.38 ± 5.31 b | 14.59 ± 0.89 c | 15.62 ± 1.03 c | 38.89 ± 6.96 b |
| Trans-2-hexenal | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Hexanal | 4.03 ± 0.19 c | 4.04 ± 0.05 c | 7.03 ± 0.76 a | 3.77 ± 0.57 c | 3.35 ± 0.27 c | 5.05 ± 0.44 b | 3.98 ± 0.45 c | 3.33 ± 1.02 c | 3.86 ± 0.49 c |
| HHE | 73.43 ± 2.29 b | 77.06 ± 3.10 b | 90.06 ± 3.92 a | 78.72 ± 9.64 b | 77.94 ± 1.84 b | 72.00 ± 5.04 b | 74.67 ± 2.71 b | 80.65 ± 7.43 ab | 77.41 ± 3.79 b |
| Trans, trans-2,4-heptadienal | nd | nd | 0.01 ± 0.00 a | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | nd | 0.01 ± 0.00 a |
| Octanal | 1.70 ± 0.16 bc | 1.72 ± 0.06 bc | 3.39 ± 0.53 a | 1.57 ± 0.36 bc | 1.76 ± 0.39 bc | 2.91 ± 0.30 a | 1.49 ± 0.30 c | 1.56 ± 0.49 bc | 2.05 ± 0.18 b |
| Nonanal | 22.62 ± 2.71 a | 18.34 ± 4.09 ab | 20.31 ± 5.4 a | 8.76 ± 0.63 c | 8.11 ± 1.26 c | 9.22 ± 1.33 c | 15.65 ± 1.79 b | 9.03 ± 0.73 c | 10.76 ± 1.91 c |
| DDE | 0.21 ± 0.07 abc | 0.27 ± 0.05 ab | 0.33 ± 0.20 a | 0.10 ± 0.68 c | 0.16 ± 0.59 bc | 0.33 ± 0.46 a | 0.13 ± 0.32 bc | 0.15 ± 0.12 bc | 0.21 ± 0.55 abc |
| HNE | 8.27 ± 1.08 bc | 9.22 ± 0.50 b | 12.06 ± 1.12 a | 7.60 ± 1.09 bc | 9.11 ± 0.82 bc | 11.97 ± 2.64 a | 7.23 ± 0.08 c | 7.33 ± 0.53 bc | 8.79 ± 0.15 bc |
| Compounds | A | B | C | D | E | F | G | H | I |
|---|---|---|---|---|---|---|---|---|---|
| Acrolein | 0.08 ± 0.01 c | 0.12 ± 0.01 b | 0.15 ± 0.00 a | nd | 0.04 ± 0.01 d | 0.14 ± 0.01 ab | 0.04 ± 0.01 d | 0.08 ± 0.02 c | 0.08 ± 0.01 c |
| Propanal | 25.86 ± 0.42 cd | 27.49 ± 1.13 bcd | 32.68 ± 1.22 a | 27.97 ± 1.44 bcd | 29.70 ± 0.54 b | 30.09 ± 3.79 ab | 25.60 ± 0.36 d | 26.63 ± 1.47 cd | 28.39 ± 0.49 bc |
| Butanal | 15.39 ± 1.86 de | 20.71 ± 3.76 ab | 22.30 ± 1.10 a | 15.31 ± 0.47 de | 16.61 ± 0.95 cd | 19.45 ± 2.80 abc | 12.08 ± 0.58 f | 13.06 ± 0.78 ef | 17.99 ± 0.64 bcd |
| Pentanal | 43.44 ± 8.06 d | 53.58 ± 7.54 c | 78.36 ± 2.32 a | 32.18 ± 2.74 e | 46.57 ± 3.87 cd | 71.91 ± 7.00 ab | 30.08 ± 3.88 e | 41.51 ± 7.60 d | 63.46 ± 1.47 b |
| Trans-2-hexenal | 0.01 ± 0.00 b | nd | nd | 0.01 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | nd | nd | nd |
| Hexanal | 5.91 ± 0.99 b | 7.41 ± 1.65 a | 6.27 ± 0.87 ab | 3.46 ± 0.20 de | 4.62 ± 0.44 cd | 6.33 ± 0.57 ab | 2.84 ± 0.64 e | 3.43 ± 0.40 de | 5.43 ± 0.22 bc |
| HHE | 214.41 ± 17.60 bc | 225.05 ± 13.09 a | 203.17 ± 28.49 bc | 195.01 ± 20.75 b | 196.45 ± 15.07 b | 211.00 ± 8.04 bc | 146.02 ± 22.40 c | 150.24 ± 7.51 c | 163.08 ± 7.57 c |
| Trans, trans-2,4-heptadienal | 0.06 ± 0.01 b | 0.04 ± 0.00 d | 0.08 ± 0.00 a | 0.05 ± 0.01 bcd | 0.08 ± 0.02 a | 0.08 ± 0.01 a | 0.01 ± 0.01 e | 0.04 ± 0.01 cd | 0.05 ± 0.00 bc |
| Octanal | 2.61 ± 0.16 cd | 2.81 ± 0.23 bc | 3.27 ± 0.16 a | 2.29 ± 0.17 d | 2.62 ± 0.23 cd | 3.03 ± 0.18 ab | 1.55 ± 0.40 e | 1.76 ± 0.32 e | 2.82 ± 0.19 bc |
| Nonanal | 8.15 ± 2.12 a | 7.93 ± 2.14 ab | 8.23 ± 1.40 a | 3.69 ± 0.52 d | 5.77 ± 0.77 bcd | 5.77 ± 1.27 bcd | 6.49 ± 0.83 abc | 5.44 ± 0.94 cd | 8.22 ± 0.81 a |
| DDE | 0.73 ± 0.26 a | 0.81 ± 0.07 a | 0.62 ± 0.13 ab | 0.23 ± 0.13 c | 0.36 ± 0.11 c | 0.42 ± 0.11 bc | 0.22 ± 0.32 c | 0.36 ± 0.12 c | 0.40 ± 0.05 c |
| HNE | 17.30 ± 2.66 d | 20.94 ± 2.46 abc | 22.92 ± 2.17 a | 15.84 ± 1.55 de | 17.75 ± 1.04 cd | 22.54 ± 0.57 ab | 13.38 ± 0.72 e | 16.97 ± 2.92 de | 18.96 ± 3.17 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lin, H.; Zhao, M.; Lu, Y.; Xia, G.; Liu, Z. Effects of Coconut Exocarp Flavonoid and EDTA-2Na on Aldehyde Generation During Pan-Frying Processing of Squid (Dsidicus gigas). Foods 2025, 14, 1925. https://doi.org/10.3390/foods14111925
Wang X, Lin H, Zhao M, Lu Y, Xia G, Liu Z. Effects of Coconut Exocarp Flavonoid and EDTA-2Na on Aldehyde Generation During Pan-Frying Processing of Squid (Dsidicus gigas). Foods. 2025; 14(11):1925. https://doi.org/10.3390/foods14111925
Chicago/Turabian StyleWang, Xinwen, Hongping Lin, Mantong Zhao, Yuehan Lu, Guanghua Xia, and Zhongyuan Liu. 2025. "Effects of Coconut Exocarp Flavonoid and EDTA-2Na on Aldehyde Generation During Pan-Frying Processing of Squid (Dsidicus gigas)" Foods 14, no. 11: 1925. https://doi.org/10.3390/foods14111925
APA StyleWang, X., Lin, H., Zhao, M., Lu, Y., Xia, G., & Liu, Z. (2025). Effects of Coconut Exocarp Flavonoid and EDTA-2Na on Aldehyde Generation During Pan-Frying Processing of Squid (Dsidicus gigas). Foods, 14(11), 1925. https://doi.org/10.3390/foods14111925






