Characterization of Biocalcium Microparticles from Saltwater Crocodile (Crocodylus porosus) Bone and Their Potential for Enhancing Fish Bologna Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation and Characterization of SC Biocal
2.2.1. Preparation of SC Biocal with Different Treatments
- (1)
- High-pressure heating process: Frozen minced SC bone samples were thawed under running water for about 1 h until they reached a temperature of 20 °C. Subsequently, the samples were autoclaved using an autoclave (Model No. 1925x All American, Miami, FL, USA) at 121 °C and 15 psi for 20 min.
- (2)
- Alkaline-soaked process: Autoclaved bone was subjected to high-pressure heating in a 10:1 (v/w) ratio and immersed in a 1.0 M KOH solution at 70 °C for 30 min while being stirred at 500 rpm with an overhead stirrer (Scilogex Os20-s LED Digital Overhead Stirrer, Pacific Star Corporation, Houston, TX, USA). Following drainage of the KOH solution, the treated bone was rinsed with running water at ten times the original volume for 5 min, with continuous stirring until the rinse water had a neutral pH. The resulting bones were then dried at 50 °C for 2 h in a cabinet tray dryer (Binder FED115, Tuttlingen, Germany) and then ground to a size of 3–4 mm.
- (3)
- Ethanol immersion process: The alkaline-soaked bone was immersed in 95% (v/v) ethanol at a ratio of 1:10 (w/v) at 25 °C and stirred for 30 min. Following this, the ethanol was drained, and the bone was washed with three volumes of ethanol for an additional 30 min. After stirring and draining, the resulting product was subjected to a bleaching and grinding step.
- (4)
- Bleaching and grinding process: The ethanol-immersed bone was soaked in a 2% (v/v) hydrogen peroxide (H2O2) solution at a bone-to-H2O2 ratio of 1:10 (w/v) at room temperature for 30 min, while being continuously stirred. After treatment, the sample was rinsed with ten volumes of running water. Subsequently, the sample was dried in a cabinet tray dryer at 50 °C for 5 h. Finally, the dried samples were crushed into a powder using a pin mill (Bonny YPT-302S, Yor Yong Hah Heng Ltd., Bangkok, Thailand), resulting in a product known as Biocal powder. The resulting Biocal was subjected to characterization.
2.2.2. Yield of Biocal
2.2.3. Color Measurement
2.2.4. Water Activity (aw)
2.2.5. Chemical Composition
2.2.6. Bioavailability of Calcium in In Vitro Simulated Gastrointestinal Tract Model System
2.2.7. Microbial Analysis
2.2.8. Amino Acid Composition Analysis
2.2.9. Visual Appearance and Scanning Electron Microscopy (SEM) Analysis
2.2.10. X-Ray Diffraction (XRD)
2.3. Study on the Impact of SC Biocal Powder on the Characteristic and Properties of Bologna from Salmon Cuts/Surimi Mixed Gel
2.3.1. Preparation of Fish Meat
2.3.2. Bologna Preparation
2.3.3. Visual Appearance
2.3.4. Textural Properties
2.3.5. Expressible Moisture Content (EMC)
2.3.6. Whiteness
2.3.7. Protein Pattern
2.3.8. Sensory Evaluation
2.3.9. SEM Images
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of SC Biocal
3.1.1. Yield, Color, and Water Activity (aw)
3.1.2. Chemical Composition
3.1.3. Ca Bioavailability (%)
3.1.4. Microbial Assessment
3.1.5. Amino Acid Composition
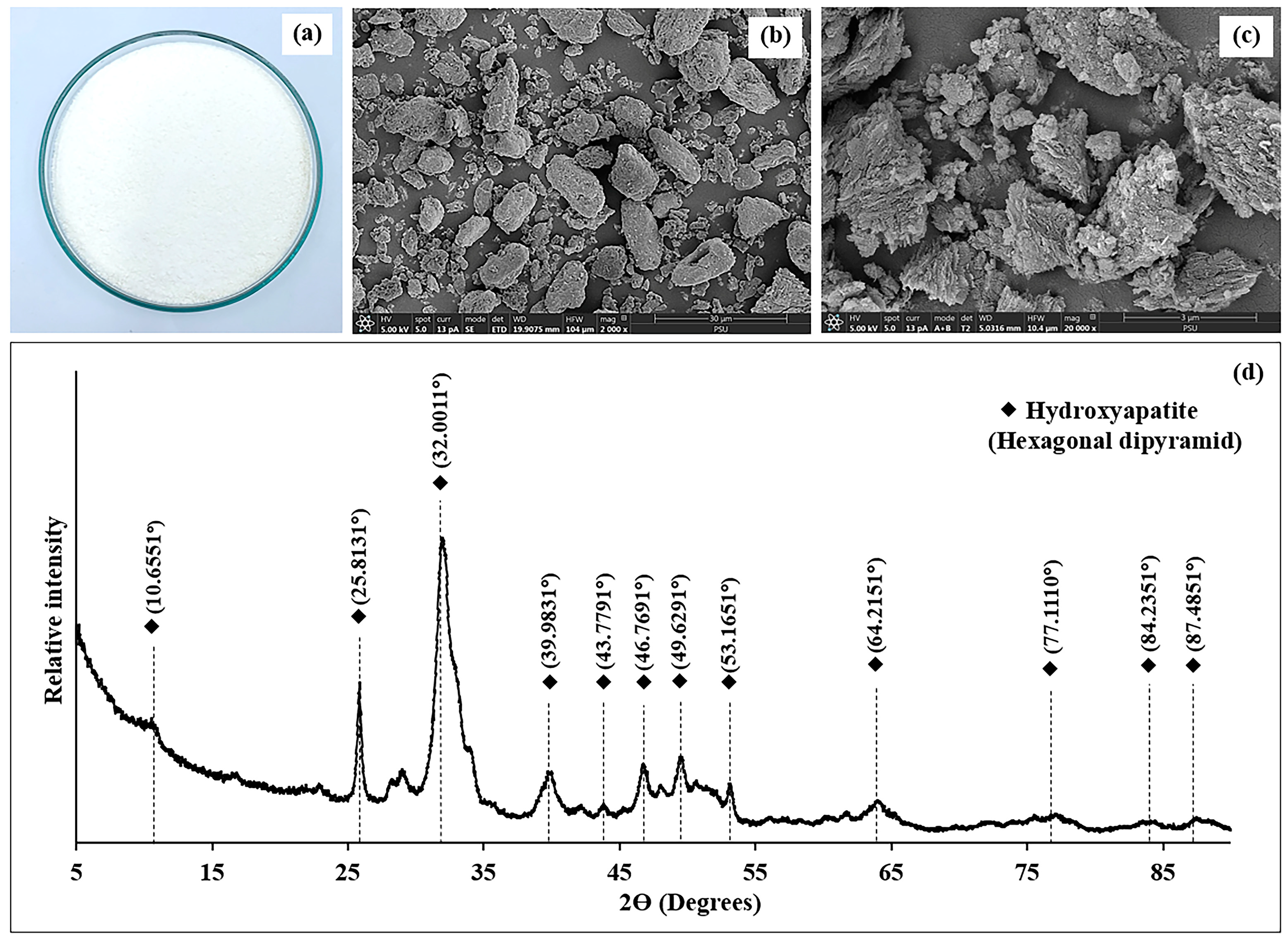
3.1.6. Visual Appearance and SEM Microstructure
3.1.7. X-Ray Diffraction (XRD) Patterns
3.2. The Potential Uses of Biocal in Bologna Made from Salmon Cuts/Surimi Mixed Gel
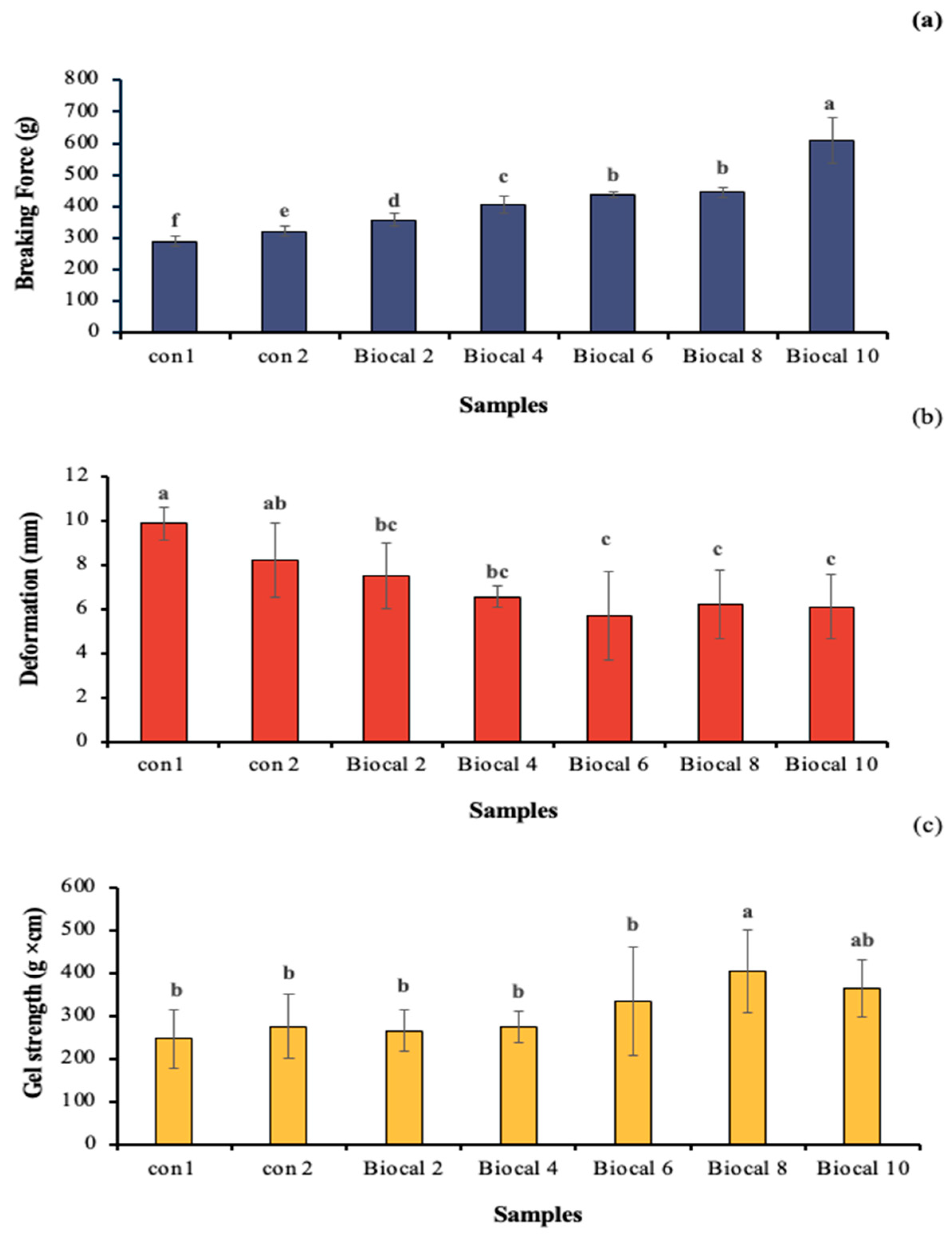
3.2.1. Textural Properties
Gel Penetration
TPA and Shear Force
3.2.2. EMC, Visual Appearance, and Color
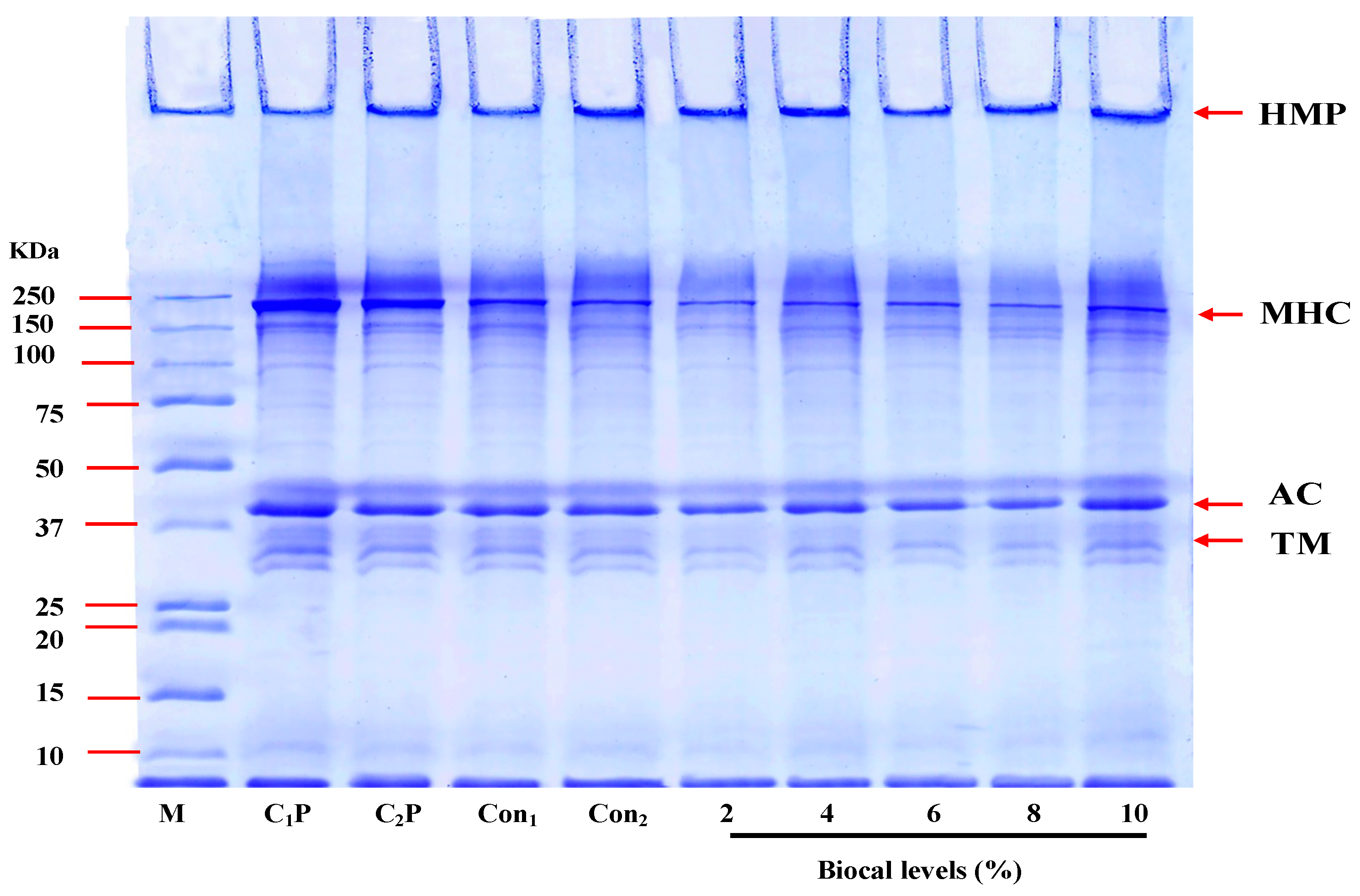
3.2.3. Protein Pattern
3.2.4. Sensory Evaluation
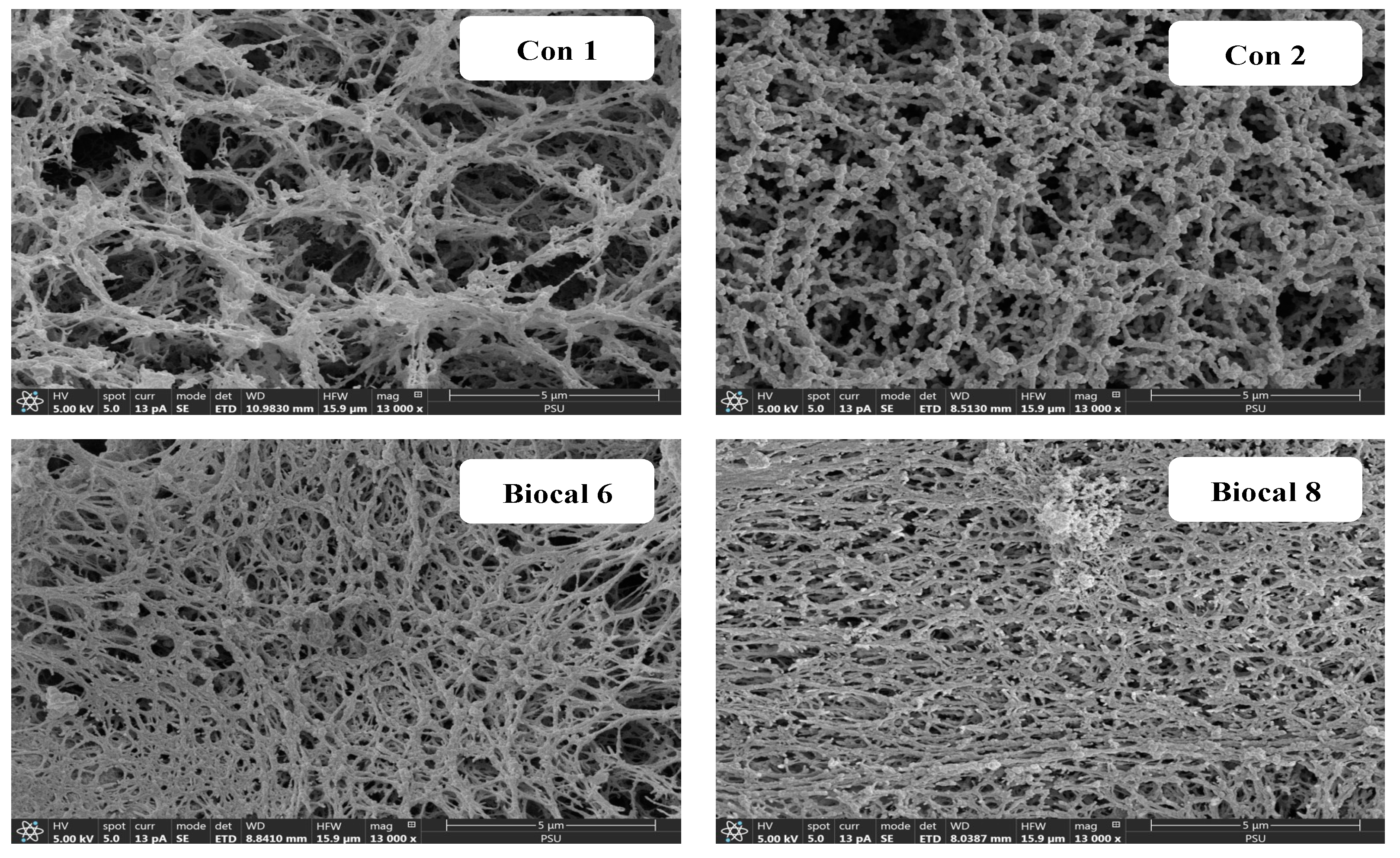
3.2.5. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sriket, C.; Niwet, J.; Kuimalee, S.; Benjakul, S.; Yarnpakdee, S.; Karnjanapratum, S.; Senphan, T. A comprehensive study of diverse techniques for enhanced physicochemical and structural properties of bio-calcium from hybrid catfish bone. Food Biosci. 2023, 56, 103398. [Google Scholar] [CrossRef]
- Zhao, Y.; Martin, B.R.; Weaver, C.M. Calcium bioavailability of calcium carbonate fortified soymilk is equivalent to cow’s milk in young women. J. Nutrit. 2005, 135, 2379–2382. [Google Scholar] [CrossRef]
- Hansen, M.; Thilsted, S.H.; Sandström, B.; Kongsbak, K.; Larsen, T.; Jensen, M.; Sørensen, S.S. Calcium Absorption from Small Soft-boned Fish. J. Trace Elem. Med. Biol. 1998, 12, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Nilsuwan, K.; Benjakul, S. Functional Ingredients from Seafood Processing Wastes: Protein Hydrolysate and Biocalcium. Turk. J. Fish. Aquat. Sci. 2024, 24, 25347. [Google Scholar] [CrossRef]
- Jung, W.K.; Kim, S.K. Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius belengerii) frame. Eur. Food Res. Technol. 2007, 224, 763–767. [Google Scholar] [CrossRef]
- Arkanit, K.; Senphan, T.; Issapap, N.; Mungmueang, N.; Sriket, P.; Benjakul, S.; Sriket, C. Physicochemical properties and bioavailability of bio-calcium products from tilapia bone: A comparative study with synthetic hydroxyapatite. J. Agric. Food Res. 2025, 19, 101708. [Google Scholar] [CrossRef]
- Loan Program to Enhance Liquidity for Crocodile Farmers and Related Business Operators. Available online: https://www4.fisheries.go.th/local/index.php/main/site/crocodile-softloan (accessed on 16 November 2024).
- Wood, A.; Ogawa, M.; Portier, R.J.; Schexnayder, M.; Shirley, M.; Losso, J.N. Biochemical properties of alligator (Alligator mississippiensis) bone collagen. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 246–249. [Google Scholar] [CrossRef]
- Lewis, K.; Boonyang, U.; Evans, L.; Siripaisarnpipat, S.; Ben-Nissan, B. A comparative study of Thai and Australian crocodile bone for use as a potential biomaterial. Key Eng. Mater. 2006, 309, 15–18. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Haq, M.; Ali, M.S.; Park, J.S.; Kim, J.W.; Zhang, W.; Chun, B.S. Atlantic salmon (Salmo salar) waste as a unique source of biofunctional protein hydrolysates: Emerging productions, promising applications, and challenges mitigation. Food Chem. 2025, 462, 141017. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis: Association of Official Analytical Chemists; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Feist, B.; Mikula, B. Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem. 2014, 147, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Bergman, I.; Loxley, R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Benjakul, S.; Mad-Ali, S.; Senphan, T.; Sookchoo, P. Characteristics of biocalcium from pre-cooked skipjack tuna bone as affected by different treatments. Waste Biomass Valorization 2018, 9, 1369–1377. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Santana, P.; Huda, N.; Yang, T.A. Physicochemical properties and sensory characteristics of sausage formulated with surimi powder. J. Food Sci. Technol. 2015, 52, 1507–1515. [Google Scholar] [CrossRef]
- Migdał, W.; Różycki, M.; Mucha, A.; Tyra, M.; Natonek-Wiśniewska, M.; Walczycka, M.; Kulawik, P.; Węsierska, E.; Zając, M.; Tkaczewska, J. Meat texture profile and cutting strength analyses of pork depending on breed and age. Ann. Anim. Resour. Sci. 2020, 20, 677–692. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Combination effect of high pressure treatment and ethanolic extract from coconut husk on gel properties of sardine surimi. LWT 2018, 91, 361–367. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Wijayanti, I.; Singh, A.; Benjakul, S.; Sookchoo, P. Textural, sensory, and chemical characteristic of threadfin bream (Nemipterus sp.) surimi gel fortified with bio-calcium from bone of Asian sea bass (Lates calcarifer). Foods 2021, 10, 976. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; McGraw Hill, Inc.: Dubuque, IA, USA, 1997. [Google Scholar]
- Jindapon, N.; Klinmalai, P.; Surayot, U.; Tanadchangsaeng, N.; Pichaiaukrit, W.; Phimolsiripol, Y.; Vichasilp, C.; Wangtueai, S. Preparation, characterization, and biological properties of hydroxyapatite from bigeye snapper (Priancanthus tayenus) bone. Int. J. Mol. Sci. 2023, 24, 2776. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Irshad, S.; Hammad, H.; Liu, J.; Shahbaz, H.M.; Ahmed, W.; Regenstein, J.M. Improved effect of autoclave processing on size reduction, chemical structure, nutritional, mechanical and in vitro digestibility properties of fish bone powder. Adv. Powder Technol. 2020, 31, 2513–2520. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sae-leaw, T.; Suzuki, N.; Kitani, Y.; Sookchoo, P. Effect of alkaline treatment on characteristics of bio-calcium and hydroxyapatite powders derived from salmon bone. Appl. Sci. 2020, 10, 4141. [Google Scholar] [CrossRef]
- Kumar, S.A.; Khare, P.; Kumar Nagar, H.; Raghuwanshi, N.; Srivastava, R. Hydroxyproline: A potential biochemical marker and its role in the pathogenesis of different diseases. Curr. Protein Pept. Sci. 2016, 17, 596–602. [Google Scholar] [CrossRef]
- Benjakul, S.; Mad-Ali, S.; Sookchoo, P. Characteristics of biocalcium powders from pre-cooked tongol (Thunnus tonggol) and yellowfin (Thunnus albacores) tuna bones. Food Biophys. 2017, 12, 412–421. [Google Scholar] [CrossRef]
- Wagner, A.M.; Gran, M.P.; Peppas, N.A. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm. Sin. B 2018, 8, 147–164. [Google Scholar] [CrossRef]
- Sriket, C.; Niwet, J.; Pui, L.P.; Yarnpakdee, S.; Senphan, T. Effects of different processes on characteristics and properties of bio-calcium from hybrid catfish (Pangasianodon gigas × Pangasianodon hypophthalmus). Int. J. Food Sci. Technol. 2023, 58, 2161–2169. [Google Scholar] [CrossRef]
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci Technol. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. Proline precursors and collagen synthesis: Biochemical challenges of nutrient supplementation and wound healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef]
- Bender, D.A. Amino Acid Metabolism, 3rd ed.; John Wiley & Sons: New York, NJ, USA, 2012. [Google Scholar]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomater 2020, 10, 1627. [Google Scholar] [CrossRef]
- Muñoz, F.; Haidar, Z.S.; Puigdollers, A.; Guerra, I.; Padilla, M.C.; Ortega, N.; Balcells, M.; García, M.J. Efficient hydroxyapatite extraction from salmon bone waste: An improved lab-scaled physico-chemico-biological process. Molecules 2024, 29, 4002. [Google Scholar] [CrossRef] [PubMed]
- Chanarat, S.; Benjakul, S.; H-Kittikun, A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012, 92, 844–852. [Google Scholar] [CrossRef]
- Petcharat, T.; Benjakul, S. Effect of gellan and calcium chloride on properties of surimi gel with low and high setting phenomena. RSC Adv. 2017, 7, 52423–52434. [Google Scholar] [CrossRef]
- Pudtikajorn, K.; Sae-leaw, T.; Buamard, N.; Zhou, A.; Ma, L.; Benjakul, S. Characterisation of fish tofu fortified with skipjack tuna (Katsuwonus pelamis) eyeball scleral cartilage biocalcium. Int. J. Food Sci. Tech. 2022, 57, 6711–6721. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Rosenthal, A. Human oral processing and texture profile analysis parameters: Bridging the gap between the sensory evaluation and the instrumental measurements. J. Texture Stud. 2019, 50, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Wijayanti, I.; Benjakul, S.; Chantakun, K.; Prodpran, T.; Sookchoo, P. Effect of Asian sea bass bio-calcium on textural, rheological, sensorial properties and nutritive value of Indian mackerel fish spread at different levels of potato starch. Int. J. Food Sci. Technol. 2022, 57, 3181–3195. [Google Scholar] [CrossRef]
- Yin, T.; Park, J.W.; Xiong, S. Effects of micron fish bone with different particle size on the properties of silver carp (Hypophthalmichthys molitrix) surimi gels. J. Food Qual. 2017, 2017, 8078062. [Google Scholar] [CrossRef]
- Yang, S.; Tu, Z.C.; Wang, H.; Hu, Y.M. Effects of coagulant promoter on the physical properties and microstructure of the mixed system of ultrafine fishbone and surimi. LWT 2020, 131, 109792. [Google Scholar] [CrossRef]
- Kaewudom, P.; Benjakul, S.; Kijroongrojana, K. Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase. Food Biosci. 2013, 1, 39–47. [Google Scholar] [CrossRef]
- Hemung, B.O.; Yongsawatdigul, J.; Chin, K.B.; Limphirat, W.; Siritapetawee, J. Silver carp bone powder as natural calcium for fish sausage. J. Aquat. Food Prod. Technol. 2018, 27, 305–315. [Google Scholar] [CrossRef]
- Yin, T.; Park, J.W. Effects of nano-scaled fish bone on the gelation properties of Alaska pollock surimi. Food Chem. 2014, 150, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Park, J.W. Textural and rheological properties of Pacific whiting surimi as affected by nano-scaled fish bone and heating rates. Food Chem. 2015, 180, 42–47. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Content (g) |
|---|---|
| Surimi: Salmon cut (70:30) | 76.8 |
| Cold water | 10.0 |
| Tapioca flour | 5.0 |
| Shortening | 2.5 |
| Sugar | 2.0 |
| Salt | 2.0 |
| Ground spices (pepper: ginger: garlic; 2:1:1) | 1.0 |
| Carrageenan | 0.5 |
| Monosodium glutamate (MSG) | 0.2 |
| MTGase (0.05% of fish meat) | 0.038 |
| Coloring agent (orange) | 0.01 |
| Parameter | Contents |
|---|---|
| Yield (%) | 16.83 ± 0.29 |
| Color | |
| L* | 90.28 ± 0.02 |
| a* | −0.32 ± 0.06 |
| b* | 8.25 ± 0.25 |
| Water activity (aw) | 0.51 ± 0.01 |
| Proximate composition (%) | |
| Moisture | 4.87 ± 0.06 |
| Protein | 10.38 ± 0.32 |
| Lipid | 2.80 ± 0.09 |
| Ash | 69.71 ± 0.14 |
| Mineral component | |
| Ca (% wt) | 26.25 |
| P (% wt) | 13.72 |
| Hg (μg/kg) | <5 |
| As (mg/kg) | ND |
| Pb (mg/kg) | ND |
| Cd (mg/kg) | ND |
| Hydroxyproline content (mg/g sample) | 2.85 ± 0.26 |
| Crystallinity (%) | 69.92 |
| Ca bioavailability (%) | 16.10 ± 0.37 |
| Microbial assessment | |
| Total plate count (CFU/g) | ND |
| Yeasts and molds (CFU/g) | ND |
| Salmonella spp. (in 25 g) | ND |
| Escherichia coli (MPN/g) | <3 |
| Staphylococcus aureus (CFU/g) | ND |
| Amino Acid Composition (g/100 g) | Contents |
|---|---|
| Aspartic acid | 0.94 |
| Threonine | 0.49 |
| Serine | 0.81 |
| Glutamic acid | 1.67 |
| Proline | 2.95 |
| Glycine | 4.40 |
| L-Alanine | 2.32 |
| Cystine | ND |
| Methionine | 0.01 |
| Isoleucine | 0.61 |
| Leucine | 0.61 |
| Tyrosine | 0.09 |
| Phenylalanine | 0.52 |
| Histidine | ND |
| Lysine | 0.81 |
| L-Arginine | 1.59 |
| Tryptophan | ND |
| Valine | 0.43 |
| Hydroxylysine | 0.11 |
| Hydroxyproline | 2.35 |
| Essential amino acids | 4.30 |
| Non-essential amino acids | 17.14 |
| Parameters | Con 1 | Con 2 | Biocal 2 | Biocal 4 | Biocal 6 | Biocal 8 | Biocal 10 | |
|---|---|---|---|---|---|---|---|---|
| TPA | ||||||||
| Hardness (g) | 3600.2 ± 134.5 g | 4768.0 ± 226.0 bf | 5514.7 ± 175.2 e | 6800.7 ± 183.6 d | 7717.0 ± 244.8 c | 8172.1 ± 164.39 b | 9051.2 ± 286.07 a | |
| Adhesiveness (g × s) | −75.29 ± 1.06 a | −88.79 ± 1.83 b | −106.90 ± 1.78 c | −120.74 ± 4.52 d | −126.06 ± 2.42 e | −133.58 ± 2.22 f | −141.51 ± 3.14 g | |
| Springiness (cm) | 0.71 ± 0.02 d | 0.71 ±0.04 d | 0.72 ± 0.03 d | 0.76 ± 0.01 c | 0.80 ± 0.02 bc | 0.81 ± 0.00 ab | 0.84 ± 0.01 a | |
| Cohesiveness | 0.65 ± 0.13 b | 0.65 ± 0.03 b | 0.66 ± 0.04 b | 0.73 ± 0.03 ab | 0.74 ± 0.02 ab | 0.76 ± 0.02 ab | 0.78 ± 0.04 a | |
| Gumminess (g) | 2333.6 ± 393.5 f | 3119.7 ± 162.5 e | 3646.0 ± 307.6 e | 4976.8 ± 130.5 d | 5679.9 ± 309.8 c | 6241.5 ± 212.1 b | 7049.2 ± 7291.3 a | |
| Chewiness (kg × cm) | 161.69 ± 25.21 f | 219.05 ± 20.48 e | 258.27 ± 28.03 e | 372.97 ± 11.58 d | 443.16 ± 26.74 c | 498.01 ± 17.47 b | 581.48 ± 20.29 a | |
| Shear force (g) | 3671.75 ± 61.82e | 4604.04 ± 111.41 e | 5618.47 ± 218.94 d | 6737.47 ± 183.15 c | 7665.69 ± 263.51 b | 8112.12 ± 205.10 ab | 9168.43 ± 185.45 a | |
| EMC (%) | 2.70 ± 0.09a | 2.65 ± 0.05 a | 2.06 ± 0.11 b | 1.95 ± 0.18 bc | 1.74 ± 0.11 c | 1.70 ± 0.22 c | 1.68 ± 0.15 c | |
| Color | ||||||||
| L* | 63.55 ± 0.94 d | 64.71 ± 1.09 d | 66.37 ± 1.91 c | 66.57 ± 0.45 bc | 67.05 ± 0.58 b | 68.12 ±1.13 b | 71.60 ± 1.02 a | |
| a* | 2.11 ± 0.13 e | 2.87 ± 0.35 d | 3.10 ± 0.41 cd | 3.33 ± 0.44 bcd | 3.47 ± 0.42 bc | 3.87 ± 0.42 b | 5.48 ± 0.56 a | |
| b* | 11.19 ± 0.36 d | 11.98 ± 0.49 c | 11.83 ± 0.48 cd | 12.30 ± 0.25 c | 13.17 ± 0.61 b | 14.04 ± 0.81a | 14.64 ± 0.41a | |
| Whiteness (%) | 61.81 ± 0.97 d | 62.62 ± 1.08 cd | 64.21 ± 1.84 b | 64.22 ± 0.47 bc | 64.34 ± 0.59 bc | 64.94 ± 1.01 bc | 67.58 ± 1.14 a | |
| Likeness score | ||||||||
| Appearance ns | 7.10 ± 1.04 | 7.17 ± 1.21 | 7.13 ± 1.36 | 6.70 ± 1.36 | 6.90 ± 1.34 | 6.83 ± 1.53 | 6.78 ± 1.51 | |
| Color | 8.08 ± 1.05 a | 7.47 ± 1.13 b | 6.93 ± 1.38 c | 7.07 ± 1.31 bc | 7.33 ± 1.20 bc | 7.33 ± 1.24 bc | 7.00 ± 1.34 bc | |
| Texture | 6.77 ± 1.33 bc | 6.57 ± 1.24 c | 6.78 ± 1.44 bc | 6.55 ± 1.47 c | 7.12 ± 1.22 ab | 7.33 ± 1.13 a | 6.28 ± 1.58 c | |
| Odor | 7.45 ± 1.13 a | 7.5 ± 1.14 a | 7.05 ± 1.29 ab | 6.6 ± 1.51 bc | 6.47 ± 1.61 c | 6.45 ± 1.56 c | 5.75 ± 1.78 d | |
| Flavor | 7.88 ± 0.92 a | 7.33 ± 1.16 b | 6.47 ± 1.66 cd | 6.32 ± 1.55 cd | 6.57 ± 1.44 c | 6.20 ± 1.63 cd | 5.92 ± 1.69 d | |
| Taste | 7.05 ± 1.33 ab | 7.17 ± 1.34 ab | 6.95 ± 1.41 ab | 6.8 ± 1.47 ab | 6.97 ± 0.95 ab | 7.25 ± 1.34 a | 6.68 ± 1.25 b | |
| Sandy mouth feel | 7.19 ± 0.91 a | 7.14 ± 1.17 a | 7.07 ± 1.26 a | 7.09 ± 1.18 a | 6.74 ± 1.11 a | 5.52 ± 1.50 b | 5.05 ± 1.57 b | |
| Overall | 7.73 ± 1.33 a | 7.48 ± 1.34 a | 7.40 ± 0.86 ab | 7.47 ± 1.18 a | 7.42 ± 0.95 ab | 7.13 ± 1.55 bc | 5.78 ± 1.61 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senphan, T.; Mungmueang, N.; Karnjanapratum, S.; Wangtueai, S.; Jongjareonrak, A.; Yarnpakdee, S. Characterization of Biocalcium Microparticles from Saltwater Crocodile (Crocodylus porosus) Bone and Their Potential for Enhancing Fish Bologna Quality. Foods 2025, 14, 1732. https://doi.org/10.3390/foods14101732
Senphan T, Mungmueang N, Karnjanapratum S, Wangtueai S, Jongjareonrak A, Yarnpakdee S. Characterization of Biocalcium Microparticles from Saltwater Crocodile (Crocodylus porosus) Bone and Their Potential for Enhancing Fish Bologna Quality. Foods. 2025; 14(10):1732. https://doi.org/10.3390/foods14101732
Chicago/Turabian StyleSenphan, Theeraphol, Natthapong Mungmueang, Supatra Karnjanapratum, Sutee Wangtueai, Akkasit Jongjareonrak, and Suthasinee Yarnpakdee. 2025. "Characterization of Biocalcium Microparticles from Saltwater Crocodile (Crocodylus porosus) Bone and Their Potential for Enhancing Fish Bologna Quality" Foods 14, no. 10: 1732. https://doi.org/10.3390/foods14101732
APA StyleSenphan, T., Mungmueang, N., Karnjanapratum, S., Wangtueai, S., Jongjareonrak, A., & Yarnpakdee, S. (2025). Characterization of Biocalcium Microparticles from Saltwater Crocodile (Crocodylus porosus) Bone and Their Potential for Enhancing Fish Bologna Quality. Foods, 14(10), 1732. https://doi.org/10.3390/foods14101732







