Sodium Alginate/Cuprous Oxide Composite Materials with Antibacterial Properties: A Preliminary Study Revealing the Counteracting Effects of Oligosaccharides in the Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of SA-Based Hydrogel Beads
2.3. Material Characterization
2.3.1. UV-Vis Spectroscopy
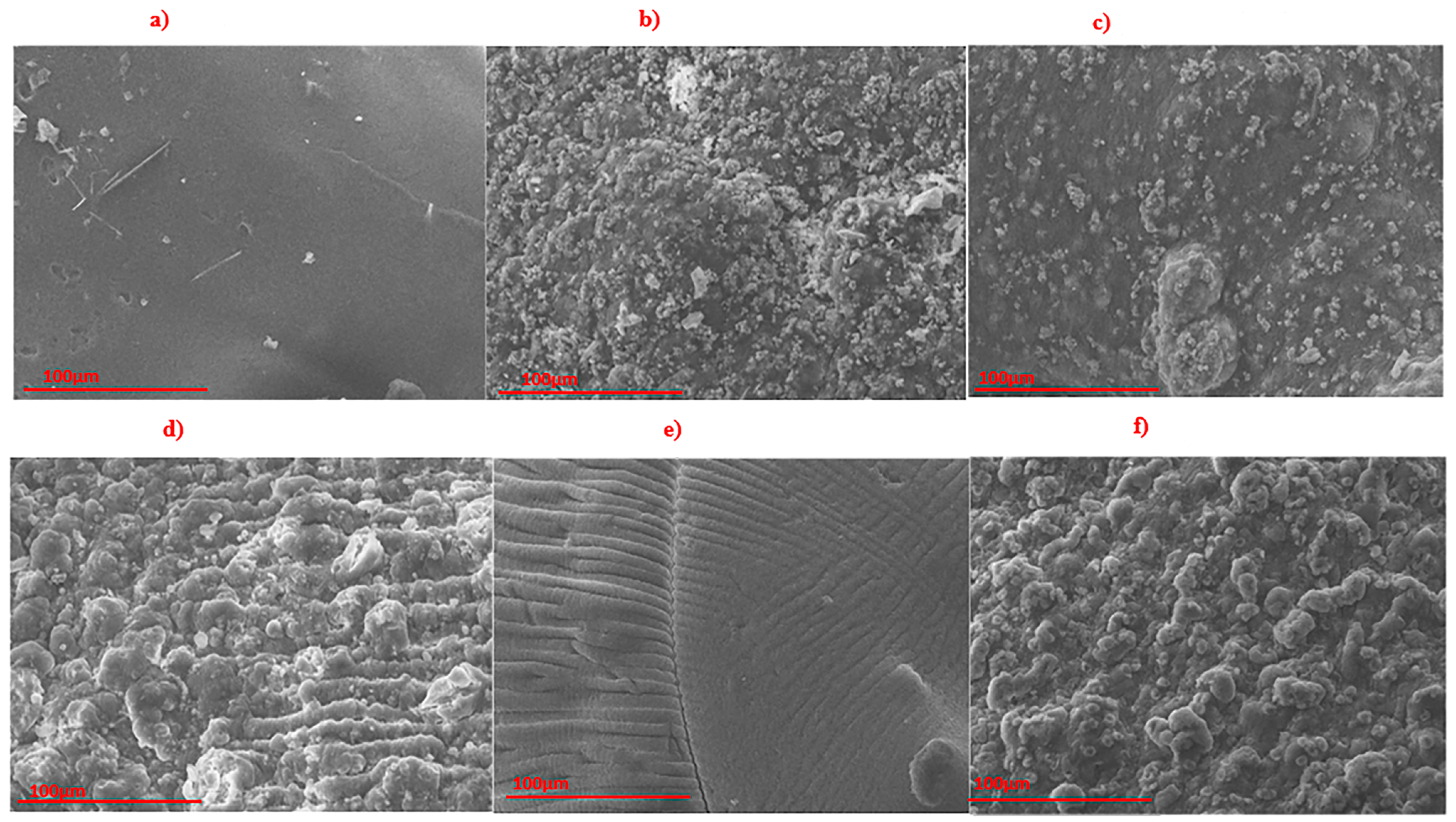
2.3.2. Morphological Analysis
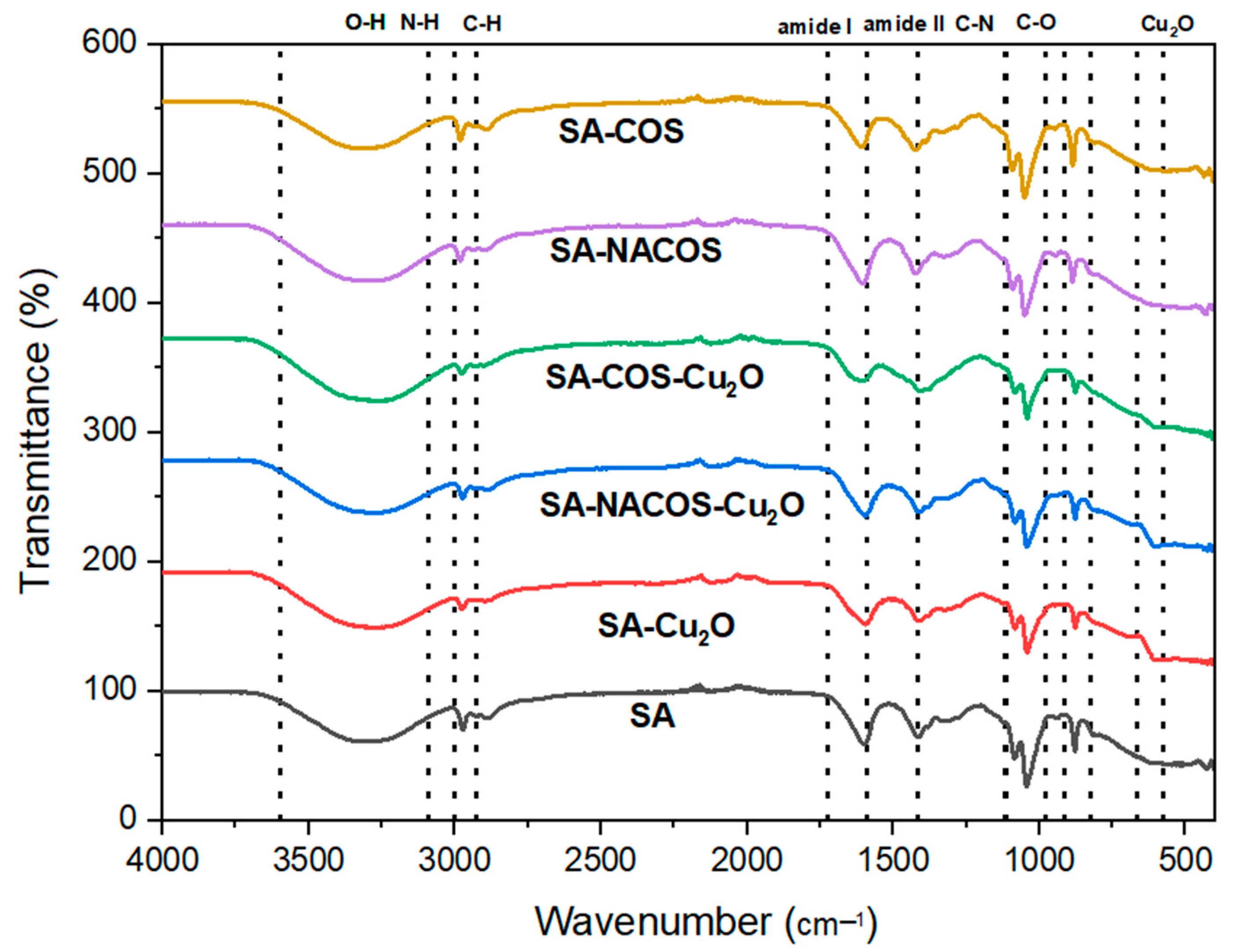
2.3.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.4. Swelling Assay
2.3.5. Water Contact Angle (WCA) Analysis
2.3.6. Antibacterial Assay
2.3.7. Microbial Activity Under Different Carbon Sources
3. Result and Discussion
3.1. Morphology and Composition Characterization
3.2. Swelling Behavior
3.3. Surface Hydrophilicity
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COS | chitosan oligosaccharide |
| NACOS | chitin oligosaccharides |
| SA | sodium alginate |
| WCA | water contact angle |
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Xie, F. Biopolymer-Based Multilayer Films and Coatings for Food Preservation: An Update of the Recent Development. Curr. Food Sci. Technol. Rep. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Zhao, S.; Liu, P.; Xie, F.; Zhang, B. Biofunctional chitosan–biopolymer composites for biomedical applications. Mater. Sci. Eng. R Rep. 2024, 159, 100775. [Google Scholar] [CrossRef]
- Xie, F. Natural polymer starch-based materials for flexible electronic sensor development: A review of recent progress. Carbohydr. Polym. 2024, 337, 122116. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Zhao, S.; Zhang, B.; Xie, F. Advanced functional chitosan-based nanocomposite materials for performance-demanding applications. Prog. Polym. Sci. 2024, 157, 101872. [Google Scholar] [CrossRef]
- Xie, F.; Gao, C.; Avérous, L. Alginate-based materials: Enhancing properties through multiphase formulation design and processing innovation. Mater. Sci. Eng. R Rep. 2024, 159, 100799. [Google Scholar] [CrossRef]
- Xie, F. Alginate-based nanocomposites for food preservation: Recent progress showcasing heightened material properties and functionalities. Adv. Nanocompos. 2024, 1, 248–274. [Google Scholar] [CrossRef]
- Yang, J.-M.; Panda, P.K.; Jie, C.J.; Dash, P.; Chang, Y.-H. Poly (vinyl alcohol)/chitosan/sodium alginate composite blended membrane: Preparation, characterization, and water-induced shape memory phenomenon. Polym. Eng. Sci. 2022, 62, 1526–1537. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.-H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Taran, M. Optimized synthesis, characterization, and antibacterial activity of an alginate–cupric oxide bionanocomposite. J. Appl. Polym. Sci. 2018, 135, 45682. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, J.; Li, R.; Zhou, J.; Wei, J.; Jiao, S.; Wang, Z.A.; Du, Y. Chitosan Oligosaccharides Coupling Inhibits Bacterial Biofilm-Related Antibiotic Resistance against Florfenicol. Molecules 2020, 25, 6043. [Google Scholar] [CrossRef]
- Liu, X.; Xia, W.; Jiang, Q.; Yu, P.; Yue, L. Chitosan oligosaccharide-N-chlorokojic acid mannich base polymer as a potential antibacterial material. Carbohydr. Polym. 2018, 182, 225–234. [Google Scholar] [CrossRef]
- Wang, N.; Tian, J.; Wang, L.; Song, C.; Wen, C.; Fu, Y.; Song, S. Fabrication, characterization, and antibacterial properties of sodium alginate/chito-oligosaccharide gel beads. Food Hydrocoll. 2024, 156, 110286. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Mai, D.X.N.; Doan, U.T.T.; Nguyen, T.T.; Dang, Y.T.; Ta, H.K.T.; Phan, T.B.; Pham, N.K. The chitosan/ZnO bio-nanocomposites with selective antibacterial efficiency. J. Mater. Res. 2021, 36, 508–517. [Google Scholar] [CrossRef]
- Mei, L.; Xu, Z.; Shi, Y.; Lin, C.; Jiao, S.; Zhang, L.; Li, P. Multivalent and synergistic chitosan oligosaccharide-Ag nanocomposites for therapy of bacterial infection. Sci. Rep. 2020, 10, 10011. [Google Scholar] [CrossRef]
- Shivakumara, L.R.; Demappa, T. Synthesis and swelling behavior of sodium alginate/poly(vinyl alcohol) hydrogels. Tirkish J. Pharm. Sci. 2019, 16, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Dubern, J.-F.; Romero, M.; Mai-Prochnow, A.; Messina, M.; Trampari, E.; Gijzel, H.N.-v.; Chan, K.-G.; Carabelli, A.M.; Barraud, N.; Lazenby, J.; et al. ToxR is a c-di-GMP binding protein that modulates surface-associated behaviour in Pseudomonas aeruginosa. NPJ Biofilms Microbiomes 2022, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Ithakisiou, G.-N.; van Putten, J.P.M.; Pieters, R.J.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides Against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605. [Google Scholar] [CrossRef]
- Abidin, M.Z.; Kourmentza, C.; Karatzas, A.K.; Niranjan, K. Enzymatic hydrolysis of thermally pre-treated chitin and antimicrobial activity of N,N′-diacetylchitobiose. J. Chem. Technol. Biotechnol. 2019, 94, 2529–2536. [Google Scholar] [CrossRef]
- Zainal Abidin, M.; Kourmentza, K.; Niranjan, K. Chitin Oligosaccharide N,N′-Diacetylchitobiose (GlcNAc2) as Antimicrobial Coating against Listeria monocytogenes on Ready-to-Eat Shrimp. Sustainability 2023, 15, 10099. [Google Scholar] [CrossRef]
- Álvarez, K.; Alvarez, V.A.; Gutiérrez, T.J. Biopolymer Composite Materials with Antimicrobial Effects Applied to the Food Industry. In Functional Biopolymers; Thakur, V.K., Thakur, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 57–96. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]





| Code | Composition |
|---|---|
| SA | SA |
| SA-Cu2O | SA/Cu2O = 1:2 (w/w) |
| SA-NACOS-Cu2O | SA/chitin oligosaccharide/Cu2O = 1:1:2 (w/w) |
| SA-COS-Cu2O | SA/chitosan oligosaccharide/Cu2O = 1:1:2 (w/w) |
| SA-NACOS | SA/chitin oligosaccharide = 1:1 (w/w) |
| SA-COS | SA/chitosan oligosaccharide = 1:1 (w/w) |
| Sample | Swelling Ratio (%) at 6 h | Contact Angle (°) | Antibacterial Activity (Inhibition % Against MRSA) |
|---|---|---|---|
| SA | 160.4 ± 12.2 | 56.6 ± 1.6 | – |
| SA-Cu2O | 18.6 ± 6.0 | 72.7 ± 8.8 | Highest (67.4 ± 11.9%) |
| SA-NACOS | 203.9 ± 17.3 | 33.3 ± 9.2 | – |
| SA-NACOS-Cu2O | 90.1 ± 4.9 | 72.4 ± 6.8 | High (51.6 ± 7.7%) |
| SA-COS | 116.1 ± 10.3 | N/A a | – |
| SA-COS-Cu2O | 16.1 ± 3.9 | N/A a | Low (10 ± 16.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, R.; Wang, F.; Suginta, W.; Chang, C.-Y.; Xie, F. Sodium Alginate/Cuprous Oxide Composite Materials with Antibacterial Properties: A Preliminary Study Revealing the Counteracting Effects of Oligosaccharides in the Matrix. Foods 2025, 14, 1666. https://doi.org/10.3390/foods14101666
Thomas R, Wang F, Suginta W, Chang C-Y, Xie F. Sodium Alginate/Cuprous Oxide Composite Materials with Antibacterial Properties: A Preliminary Study Revealing the Counteracting Effects of Oligosaccharides in the Matrix. Foods. 2025; 14(10):1666. https://doi.org/10.3390/foods14101666
Chicago/Turabian StyleThomas, Reeba, Fengyi Wang, Wipa Suginta, Chien-Yi Chang, and Fengwei Xie. 2025. "Sodium Alginate/Cuprous Oxide Composite Materials with Antibacterial Properties: A Preliminary Study Revealing the Counteracting Effects of Oligosaccharides in the Matrix" Foods 14, no. 10: 1666. https://doi.org/10.3390/foods14101666
APA StyleThomas, R., Wang, F., Suginta, W., Chang, C.-Y., & Xie, F. (2025). Sodium Alginate/Cuprous Oxide Composite Materials with Antibacterial Properties: A Preliminary Study Revealing the Counteracting Effects of Oligosaccharides in the Matrix. Foods, 14(10), 1666. https://doi.org/10.3390/foods14101666








