1. Introduction
Reference materials (RMs), characterized by adequate homogeneity and stability, are essential metrological tools for ensuring comparable, reliable, and high-quality analytical results. They play a critical role in calibration, method validation, and quality control across various scientific and industrial fields. However, the current availability of RMs is limited in terms of matrix types and target concentrations, which often fail to meet the diverse demands of real-world testing scenarios. This limitation highlights the necessity to expand the variety of matrix types and concentration gradients of RMs, particularly in areas such as food safety, where accurate and precise measurements are paramount.
Vegetable oils, particularly blended oils, are essential components of a balanced diet due to their high content of unsaturated fatty acids and other bioactive compounds [
1]. Peanut oil, for instance, is widely consumed for its pleasant aroma, digestibility, and nutritional benefits [
2]. However, peanuts are highly susceptible to contamination by Aspergillus species, which produce aflatoxins, particularly aflatoxin B1 (AFTB1) [
3]. Studies have shown that AFTB1 is a potent carcinogen, and its presence in peanut oil poses significant health risks [
4,
5]. Similarly, corn oil, derived from corn germ, is valued for its cholesterol-free composition and high levels of unsaturated fatty acids (80–85%), which contribute to the prevention of age-related diseases such as arteriosclerosis and diabetes [
6]. Despite its nutritional benefits, corn oil is prone to contamination by aflatoxins and zearalenone (ZEN), primarily due to inadequate monitoring of raw materials during production [
3,
7]. Recent research has highlighted the advantages of blended vegetable oils over single-source oils, as blending can improve oxidative stability and nutritional profiles [
1]. However, the risk of mycotoxin contamination remains a significant concern, as these toxins are not eliminated during the oil extraction process [
3]. This has led to increased scrutiny of mycotoxin levels in edible oils and the need for robust analytical methods to ensure food safety.
In response to the health risks posed by mycotoxins, many countries have established regulatory limits for AFTB1, total aflatoxins, and ZEN in edible oils. For example, the European Union has set maximum limits of 2 µg/kg for AFTB1 and 4 µg/kg for total aflatoxins in vegetable oils [
8]. Similarly, the U.S. Food and Drug Administration (FDA) has established action levels for aflatoxins in food products, including edible oils [
9]. These regulations underscore the importance of accurate and reliable analytical methods for mycotoxin detection.
However, the analysis of mycotoxins in vegetable oils presents several challenges [
10,
11]. The complex matrix of oils can interfere with the detection of low-concentration mycotoxins, leading to potential inaccuracies. Additionally, the lack of standardized methods and reference materials for mycotoxin analysis in oil matrices complicates method validation and quality assurance. These challenges highlight the need for matrix-specific certified reference materials (CRMs) to improve the accuracy and comparability of analytical results. Despite the growing demand for reliable mycotoxin analysis, the availability of CRMs for vegetable oil matrices remains limited. Most existing CRMs focus on single mycotoxins or simpler matrices, such as cereals or nuts, which do not reflect the complexity of vegetable oils [
4,
12,
13,
14]. For instance, CRMs for AFTB1 in peanut butter or ZEN in cornmeal are commercially available, but their applicability to oil matrices is limited due to differences in matrix effects and extraction efficiency. Furthermore, there is a notable lack of CRMs for the aflatoxin G group and ZEN in vegetable oils, which are critical for comprehensive mycotoxin analysis. Recent studies have emphasized the importance of matrix-matched CRMs for accurate mycotoxin quantification. For example, Zhao et al. [
15] developed a CRM for AFTB1 in peanut oil, demonstrating the feasibility of producing stable and homogeneous reference materials for complex oil matrices. However, their study was limited to a single mycotoxin and did not address the need for multi-mycotoxin CRMs. Similarly, our previous work (2018) [
16] initially ensured that the AFTB1 has long-term stability and homogeneity in oil-based CRMs.
This study aims to address these gaps by developing a matrix CRM for multiple mycotoxins, including aflatoxins and zearalenone, in blended vegetable oil. The research will focus on assessing the homogeneity and stability (both short- and long-term) of the CRM to ensure its reliability for analytical applications. By providing a robust metrological tool, this work seeks to enhance the accuracy and comparability of mycotoxin analysis in vegetable oils, thereby supporting food safety initiatives and regulatory efforts.
2. Materials and Methods
2.1. Chemicals and Reagents
Pure aflatoxin B1 (AFTB1, 2.97 ± 0.09 µg/mL, product no. ERM-AC057), aflatoxin B2 (AFTB2, 2.98 ± 0.06 µg/mL, product no. ERM-AC058), aflatoxin G1 (AFTG1, 3.78 ± 0.13 µg/mL, product no. ERM-AC059), aflatoxin G2 (AFTG2, 2.98 ± 0.06 µg/mL, product no. ERM-AC060), and zearalenone (ZEN, 9.95 ± 0.30 µg/mL, product no. ERM-AC699) were obtained from the Institute for Reference Materials and Measurements (IRMM). The isotopes 13C-AFTB1, 13C-AFTB2, 13C-AFTG1, 13C-AFTG2, and 13C-ZEN in acetonitrile were purchased from Pribolab Pte. Ltd. (Qingdao, China). Certificated reference materials GBW(E)100386 (Aflatoxin B1 in maize) and GBW(E)100383 (zearalenone and deoxynivalenol in maize) were purchased from National Food and Strategic Reserves Administration (Beijing, China).
Methanol, acetonitrile, and formic acid were HPLC grade and purchased from Merck (Merck, Germany). Phosphate-buffer salts were AR grade and purchased from Pribolab Pte.Ltd.(Qingdao, China).
2.2. Selection of Candidate Reference Material
Vegetable oil samples were collected from retail markets throughout China, and comprehensive analyses were conducted to determine the presence of aflatoxins and zearalenone. These analyses aimed to identify naturally contaminated materials that could serve as raw reference materials.
Following established regulatory limits [
17] and food safety risk monitoring data from 2013 to 2022 in China, the AFTB1 and ZEN concentrations in the CRM were approximately defined as 10 µg/kg and 30 µg/kg, respectively. It plans to develop 500 packages of this reference material, each containing 40 grams. Firstly, the collected oil samples are filtered to remove obvious residue sediments and impurities, so as to avoid interfering substances. Subsequently, the concentrations of AFTB1, B2, G1, G2, and ZEN were measured in the collected peanut oil and corn oil samples. Initial results showed that the peanut oil contained AFTB1 at 75 µg/kg, AFTB2 at 12 µg/kg, AFTG1 at 0.88 µg/kg, AFTG2 at 0.22 µg/kg, and the corn oil contained ZEN at 160 µg/kg. Next, to create the blended vegetable oil CRM, precise quantities of the collected oils were combined. A total of 4 kg of aflatoxin-contaminated peanut oil, 5 kg of zearalenone-contaminated corn oil, 6 kg of aflatoxin-free peanut oil, and 5 kg of zearalenone-free corn oil were accurately weighed and mixed in a 1:1 ratio. This formulation resulted in a blended oil containing AFTB1, AFTB2, AFTG1, AFTG2, and ZEN as the target analytes. However, the naturally low concentrations of AFTG1 and AFTG2 in the collected oils were insufficient to meet the sensitivity requirements of various detection methods. Therefore, in this study, 1 mg each of AFTG1 and AFTG2 (purity >99%, purchased from Fermentek Ltd., Jerusalem, Israel) was weighed and dissolved in chloroform to prepare stock solutions with concentrations of 100 µg/mL. Subsequently, 0.8 mL of AFTG1 and 0.4 mL of AFTG2 stock solution were added to 20 kg of the blended vegetable oil. This fortification process achieved final concentrations of approximately 4 µg/kg for AFTG1 and 2 µg/kg for AFTG2 in CRM. The fortified blended oil was stirred at low speed for 72 h to ensure homogeneity and then packaged into brown glass bottles, sealed with aluminum foil, and stored at room temperature away from light. This packaging and storage protocol was designed to maintain the stability and integrity of the CRM during its shelf life.
2.3. Analytical Methods
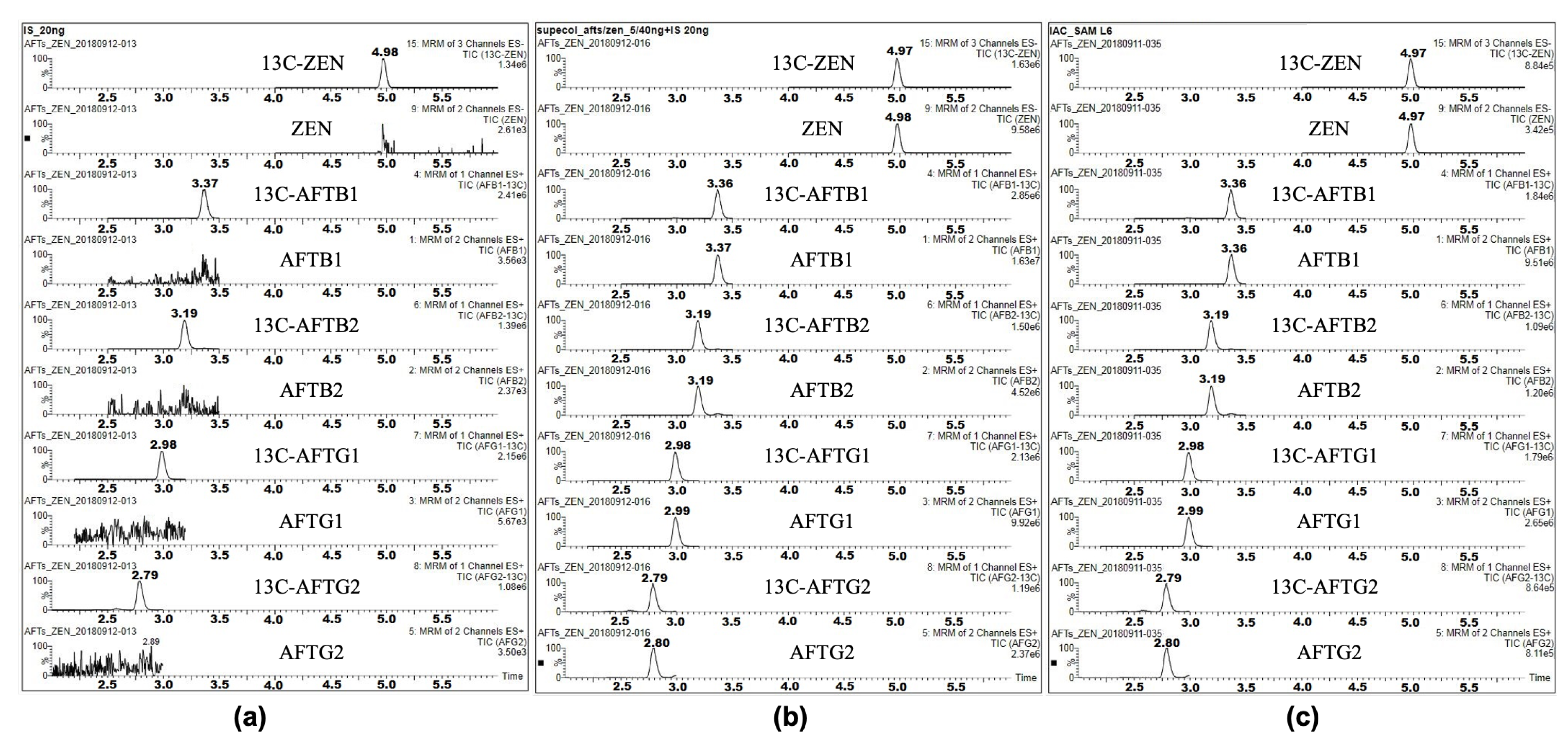
The dilute-and-shoot method was employed in the preliminary experiments and certification. This method is widely recognized for its simplicity and efficiency in sample preparation [
18]. The detailed steps are as follows: Accurately weigh 2 g of the sample (to an accuracy of 0.001 g) and place it in a 50 mL centrifuge tube. Add 8 mL of an acetonitrile–water solution (70:30, volume ratio) and mix for 1 min using a vortex mixer. Place the tube on a rotary shaker and extract by shaking for 30 min. Then, centrifuge at 8500 rpm for 10 min. Accurately transfer 0.5 mL of the supernatant to another 1.5 mL centrifuge tube. Add 1 mL of water, mix by vortex, and then centrifuge at 10,000 rpm for 10 min at 4 ℃. Aspirate the supernatant through a 0.22 μm filter membrane. Aspirate 180 μL of the processed sample filtrate into a 300 μL insert tube. Add 20 μL of the stable isotope mixed standard solution, mix, and prepare for LC-MS/MS analysis.
Sample analysis was performed on a Waters Xevo TQ-S (Manchester, UK) LC-MS/MS, which was equipped with a Z-spray electrospray ionization (ESI) interface. Chromatographic separation was performed with an Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm) at 40 ℃ column temperature. A flow rate of 0.3 mL/min was used, and the injection volume was set at 10 μL. The mobile phase consisted of solvent A (acetonitrile) and solvent B (water). The gradient elution program is listed as follows: 0 min∼1.5min, 15% A; 1.5 min∼6.5 min, 15%∼95% A; 6.5 min∼7.5 min, 95%A; 7.5 min∼8.0 min, 15% A. The mass spectrometer was operated with a source temperature of 150 ℃, a nebulizing gas of 150 L/h, a desolvation gas of 800 L/h, and a collision gas of 0.15mL/min. The detailed multiple reaction monitoring (MRM) parameters of the analytes are listed in
Table 1.
Quantification of the target analytes (aflatoxins and zearalenone) was achieved using the stable isotope-labeled internal standards for calibration. The internal standards corrected for matrix effects and ensured accurate quantification. Data analysis was performed using Waters MassLynx software (V4.2), and the results were validated against established quality control criteria.
2.4. Comparative HPLC-FLD Analysis
Given that high-performance liquid chromatography with fluorescence detection (HPLC-FLD) is globally recognized as the benchmark method for aflatoxin analysis in regulatory settings, this study incorporated an immunoaffinity column (IAC) cleanup HPLC-FLD protocol as a supplementary approach. The implementation of this orthogonal methodology aimed to (i) verify the metrological traceability and cross-platform comparability of certified reference material (CRM) values and (ii) demonstrate the CRM’s applicability to routine analytical workflows widely adopted in food testing laboratories.
Chromatographic conditions: An ACQUITY UPLC I-Class system (Waters, Singapore) equipped with a large-volume flow-cell fluorescence detector was employed. Separation was achieved on an Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm) maintained at 40°C, with a 10 μL injection volume. The mobile phase comprised methanol/acetonitrile (6:4, v/v; eluent A) and 0.1% formic acid (eluent B), with the following gradient profile at 0.3 mL/min: 0–1.5 min: 25% A; 1.5–6.5 min: 25–95% A; 6.5–7.5 min: 95% A; 7.5–8.0 min: 25% A. Fluorescence detection parameters were optimized at λex/λem = 274 nm/460 nm for ZEN and 365 nm/460 nm for aflatoxins.
Sample preparation: 2 g of the sample (to an accuracy of 0.001 g) were extracted with 8 mL acetonitrile/water (70:30, volume ratio) via 1 min vortex mixing followed by 30 min rotary shaking. After centrifugation (8500 rpm, 10 min), 2.0 mL supernatant was diluted with 25 mL PBS buffer (pH 7.2~7.4) to prepare the loading solution. Purification was performed using an Afla-ZEN 2-in-1 IAC (Pribonlab, Qingdao, China) with sequential PBS washing (5 mL), ultrapure water rinsing (5 mL), and methanol elution (3 mL). The eluate was nitrogen-evaporated (40°C), reconstituted in the initial mobile phase, and filtered prior to analysis.
2.5. Homogeneity and Stability Studies
Homogeneity and stability studies are critical steps to ensure the CRM’s reliability over its shelf life. To ensure the uniformity of the CRM, a random sampling method was employed. The experimental design for homogeneity testing is as follows: 500 aliquots of the candidate CRM were prepared, with each package containing approximately 40 g of the blended vegetable oil. Twenty bags were randomly selected from the 500 packages for homogeneity testing. Each selected bag was assigned a unique random number ranging from 1 to 20. Each bag was measured three times to assess intra-package variability. For each measurement, a minimum sample size of 2 g (accurate to 0.001 g) was taken from the bag.
To assess the short-term and long-term stability of the CRM, a split-group storage and testing approach was implemented. The experimental design for stability testing is as follows: Thirty bags of the prepared CRM were divided into 10 groups, with 3 bags in each group. Five groups were stored at 25 °C to simulate typical storage conditions for long-term stability. Testing was conducted on the day after sample preparation (Day 0) and after 1, 3, 6, and 12 months. The remaining five groups were stored at 65 °C to accelerate degradation and assess stability under stress conditions for short-term stability. Testing was conducted on the day after sample preparation (Day 0) and after 1, 3, 7, and 14 days. At each testing time point, one group was randomly selected from the stored groups. From each bag in the selected group, two samples were taken, and each sample was weighed at 2 g (accurate to 0.001 g).
2.6. Collaborative Value Assignment
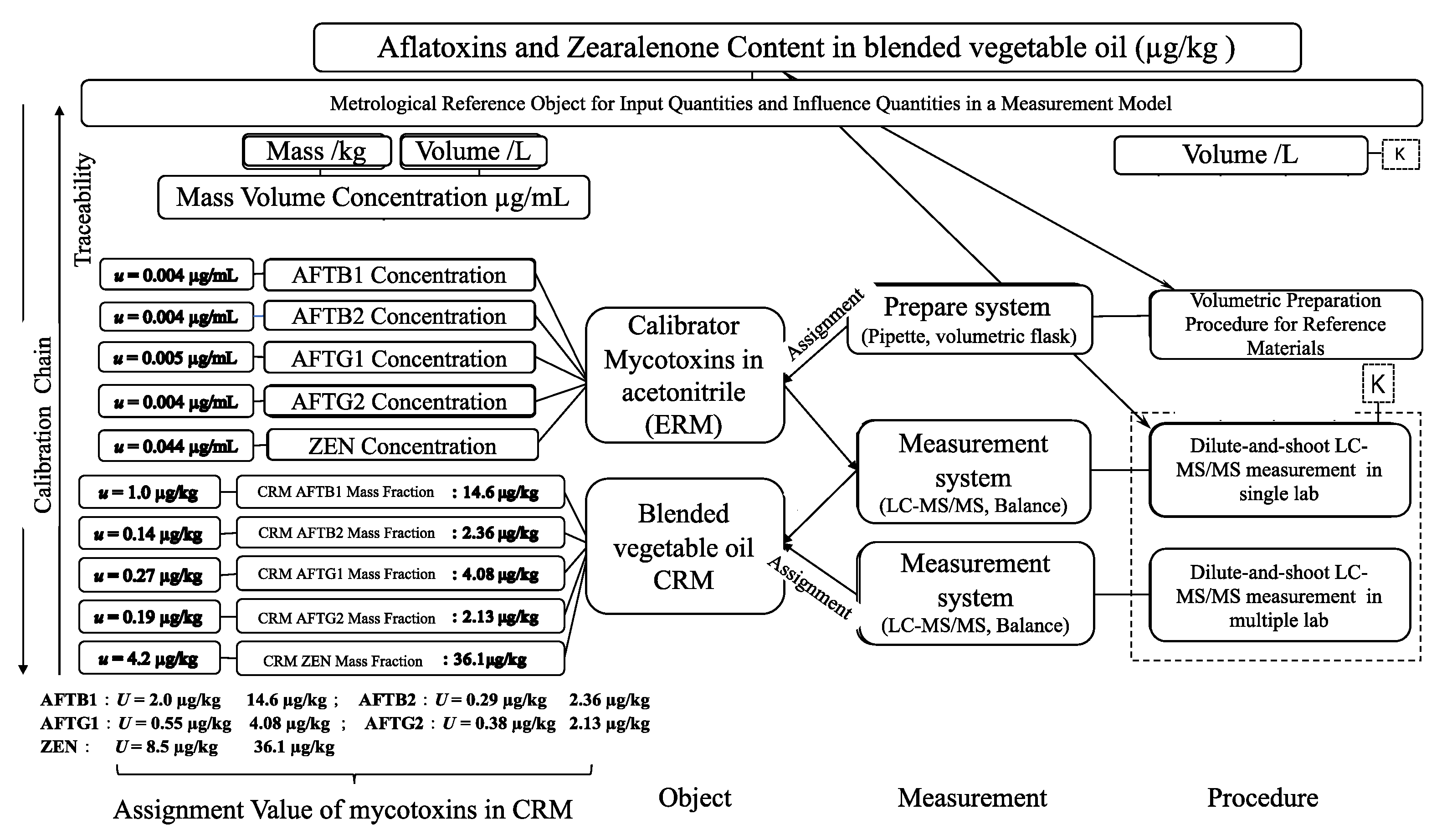
To ensure the accuracy, reliability, and traceability of the CRM, a multi-laboratory collaborative value assignment approach was adopted. This approach involved the participation of six authoritative domestic testing institutions, each representing high-level detection capabilities in China. These laboratories must have extensive experience in the detection and analysis of mycotoxins in food, particularly aflatoxins and zearalenone. Laboratories were selected to represent diverse functional institutions, including disease control centers, quality inspection agencies, and customs laboratories, to ensure broad applicability of the CRM in regulatory and supervisory contexts. Possessing different brands of LC-MS/MS instruments, laboratories were randomly assigned two CRM samples, with each sample measured at least six times, yielding a minimum of 12 data points per laboratory. Participants used the same or similar validated methods, ensuring consistency while allowing methodological diversity. For quality control, ERM-series certified reference materials and maize powder CRMs (GBW(E)100386/GBW(E)100383) were used, alongside spike recovery validation to assess method accuracy.
2.7. Statistical Analysis
Statistical analyses were conducted using Microsoft Excel. Results were expressed as means ± standard deviation (SD) to provide a measure of central tendency and variability. One-way analysis of variance (ANOVA) was used to assess intra- and inter-package variability. This parametric test is robust for comparing means across multiple groups. The F-test within ANOVA determined whether observed variations were statistically significant, with a p-value < 0.05 indicating significance. For long-term and short-term stability data, regression analysis was performed to evaluate trends over time. A p-value < 0.05 in regression analysis indicated significant changes in analyte concentrations, suggesting instability. Data from multiple laboratories were combined to calculate consensus values and their associated uncertainties. Grubbs’ test was applied to detect and remove outliers, ensuring the reliability of the dataset. The mean, SD, and confidence intervals were calculated to provide a comprehensive summary of the collaborative study results.
4. Discussion
The development and certification of a CRM for aflatoxins (AFTB1, AFTB2, AFTG1, AFTG2) and ZEN in blended corn/peanut vegetable oil represent a significant advancement in the field of food safety and mycotoxin analysis. This study not only addresses the critical need for matrix-specific CRMs but also provides a comprehensive evaluation of the analytical methods, stability, and collaborative value assignment, ensuring the reliability and traceability of the CRM for use in food safety monitoring and regulatory compliance.
The method, based on high-performance liquid chromatography isotope-dilution tandem mass spectrometry, was optimized and validated for accurate mycotoxin quantification in the blended vegetable oil. The dilute-and-shoot approach, combined with isotope-labeled internal standards, was chosen for its simplicity and efficiency in sample preparation [
15]. The recovery rates for the target mycotoxins ranged from 90.3% to 97.3%, which is within the acceptable range for regulatory purposes, demonstrating the method’s robustness and suitability for complex oil matrices. The evaluation of ME revealed minimal interference, with ME values close to 1, indicating neither significant enhancement nor suppression of the target analytes. This finding is crucial, as matrix effects are a common challenge in mycotoxin analysis, particularly in complex matrices like vegetable oils [
11]. The use of isotope-labeled internal standards further mitigated matrix effects, ensuring accurate quantification and method reliability. The timing of isotope internal standard addition was also investigated, and the results showed no significant difference between adding the internal standard at the onset of extraction or prior to injection, simplifying the sample preparation process without compromising accuracy.
The stability of the CRM was thoroughly evaluated under both short-term (65 °C for 14 days) and long-term (25 °C for 12 months) storage conditions. The results of the stability studies, analyzed using linear regression, showed no significant degradation of the target mycotoxins over time. The slopes of the regression lines for all analytes were not statistically significant (p > 0.05), confirming the stability of the CRM under the tested conditions. This stability is critical for ensuring the CRM’s reliability over its shelf life, particularly for its use in long-term analytical applications and quality control. The short-term stability test at 65 °C, which simulates accelerated degradation conditions, further validated the CRM’s robustness under stress conditions. The absence of significant degradation under these conditions suggests that the CRM can withstand potential temperature fluctuations during transportation and storage, which is essential for its practical application in laboratories worldwide.
The certification of the CRM involved a multi-laboratory collaborative study, with six authoritative domestic laboratories participating in the value assignment process. This collaborative approach ensured the accuracy, reliability, and traceability of the CRM’s certified values. Each laboratory performed at least six measurements per sample, yielding a minimum of 12 data points per laboratory. The use of validated methods and quality control measures, such as spike recovery validation and the use of certified reference materials, further enhanced the reliability of the results. Consensus values for aflatoxins and ZEN were calculated as the mean of laboratory means, with relative standard deviations (RSDs) ranging from 3.79% to 7.99%. These low RSDs indicate good agreement among the participating laboratories, confirming the CRM’s homogeneity and the robustness of the analytical methods used. The uncertainty associated with the certified values was evaluated through type A and type B uncertainty assessments, considering contributions from homogeneity, stability, and the characterization process. The combined standard uncertainty (uCRM) for the target mycotoxins ranged from 0.14 µg/kg to 4.2 µg/kg, with expanded uncertainties (UCRM) at a 95% confidence level (k = 2) ranging from 0.29 µg/kg to 8.5 µg/kg.
To further validate the applicability of the CRM across diverse analytical platforms, the accuracy and reliability of the certified values were cross-verified using the HPLC-FLD method, a widely adopted method in regulatory laboratories. As demonstrated in
Table 9, the results from IAC-HPLC-FLD exhibited excellent agreement with the certified values derived from isotope-dilution LC-MS/MS. The relative differences between methods were consistently below 5%, confirming the CRM’s robustness across analytical techniques. This dual-method validation underscores the CRM’s versatility in supporting laboratories utilizing either fluorescence-based or mass spectrometry-based workflows. By ensuring traceability and compatibility with both routine (HPLC-FLD) and advanced (LC-MS/MS) methods, this CRM addresses the critical need for matrix-matched reference materials capable of harmonizing multi-mycotoxin analysis in complex oil matrices.
The study builds on our previous work, which developed a reference material for AFTB1 in peanut oil. It goes further by including multiple mycotoxins (AFTB1, AFTB2, AFTG1, AFTG2, and ZEN) in a blended vegetable oil matrix, which more closely reflects real-world scenarios where multiple mycotoxins may co-occur. This multi-mycotoxin approach is particularly important given the increasing regulatory scrutiny of mycotoxin levels in edible oils and the need for comprehensive analytical methods. The inclusion of ZEN, a mycotoxin with estrogenic effects, further broadens the applicability of the CRM, as ZEN contamination in vegetable oils has been increasingly reported in recent years [
21,
22]. Moreover, the study addresses the limitations of existing CRMs, which are often limited to single mycotoxins or simpler matrices [
13,
23,
24,
25]. The development of a CRM for a blended vegetable oil matrix, which is more complex and representative of real-world food products, represents a significant step forward in mycotoxin analysis. The study also highlights the importance of matrix-matched CRMs for accurate mycotoxin quantification, as differences in matrix effects and extraction efficiency can significantly impact analytical results [
26].
Furthermore, this CRM enriches China’s national reference materials library, serving as a valuable resource for researchers, regulators, and the food industry. It also sets a precedent for the development of additional matrix-specific CRMs for other mycotoxins and food matrices, which will further enhance the robustness of mycotoxin analysis globally. The CRM’s certification by six authoritative domestic laboratories ensures its broad applicability in regulatory and supervisory contexts, making it a valuable tool for ensuring food safety and compliance with national and international standards. This is particularly important in light of the stringent regulatory limits for mycotoxins in edible oils set by organizations such as the European Union, the FDA, and China.