Tomato Pomace as a Valuable Resource: Characterization of Sunflower and Rapeseed Oil Extracts from Ox Heart Tomato (Lycopersicon esculentum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The Extraction of Bioactive Compounds from Tomato Peels and Seeds by Maceration in Sunflower and Rapeseed Oil
2.2.2. Color Determination
2.2.3. The Antioxidant Activity
2.2.4. Determining the Refractive Index (RI) and the Peroxide Value (PV)
2.2.5. Individual Determination of Carotenoids
2.2.6. Evaluation of the Quality of Two Types of Oils Extracted from Tomatoes with Pomace by FTIR Spectroscopy
2.2.7. DSC (Differential Scanning Calorimetry) Analysis
3. Results and Discussion
3.1. Influence of Temperature on Colour in CIE L*a*b** Coordinates
3.2. Antioxidant Activity (DPPH) Assay and Total Lycopene Quantification
3.3. Analysis of Refractive Index and Peroxide Value
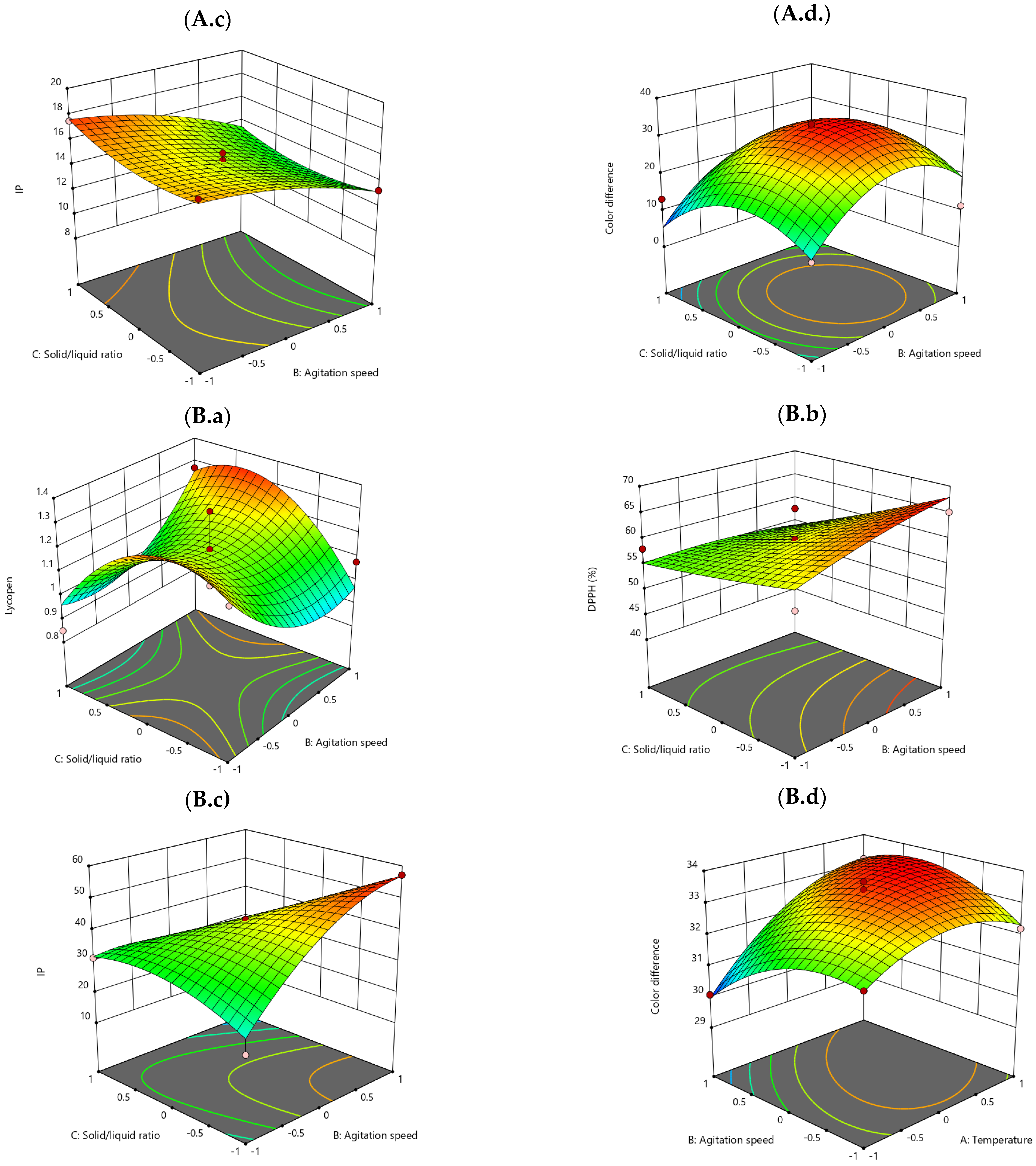
3.4. Model Fitting of Antioxidant Activity, Oxidative Stability, and Color Parameters
Surface Response 3D Plots
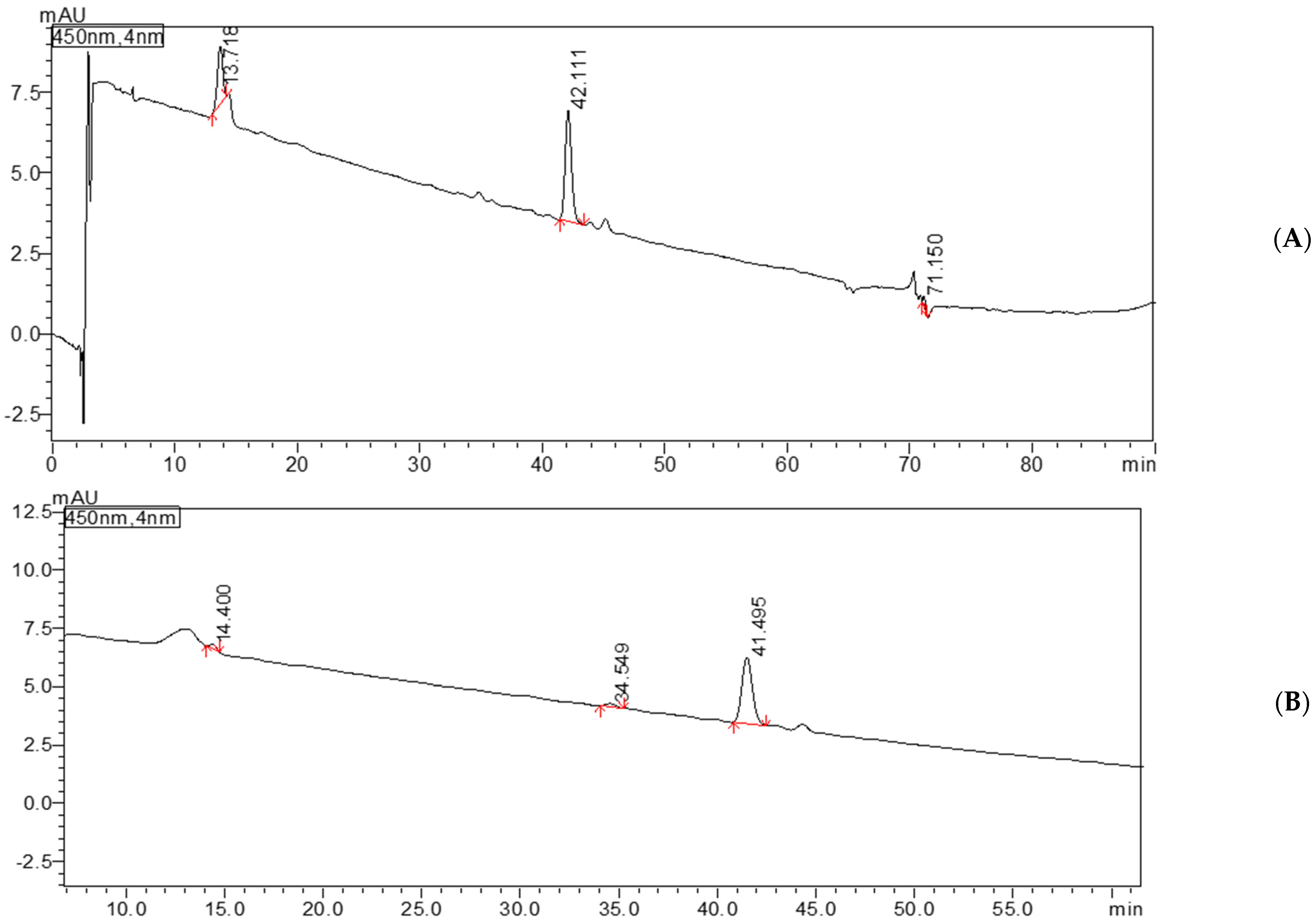
3.5. Quantification of Carotenoids in Oil Extracts Using HPLC-DAD Analysis
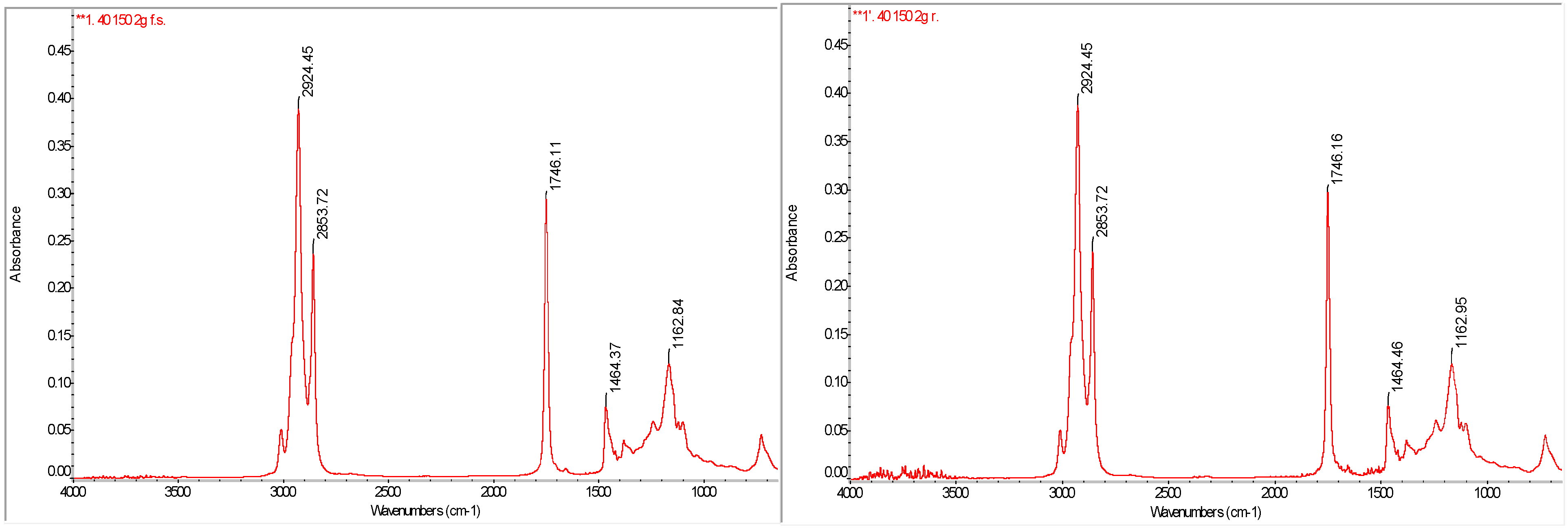
3.6. FTIR Analysis of Functional Groups in Oil Extracts
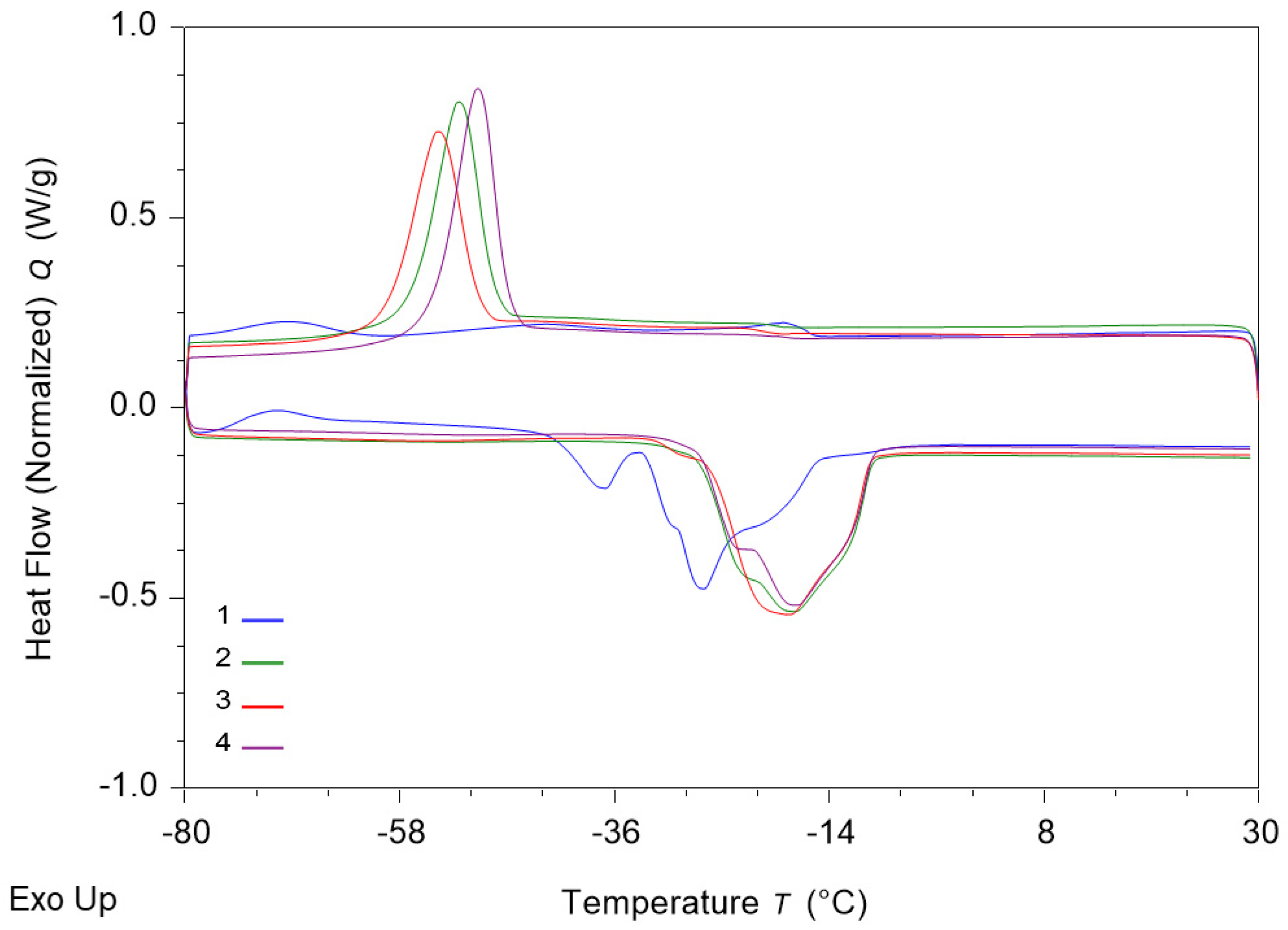
3.7. Results of DSC Analysis of Sunflower Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boccia, F.; Di Donato, P.; Covino, D.; Poli, A. Food Waste and Bio-Economy: A Scenario for the Italian Tomato Market. J. Clean. Prod. 2019, 227, 424–433. [Google Scholar] [CrossRef]
- Popescu, G.C. Some considerations regarding the romanian vegetable sector after accession to the european union. Curr. Trends Nat. Sci. 2017, 6, 209–219. [Google Scholar]
- Bologa, M.; Jitareanu, C.; Marta, A.; Slabu, C. Chlorophyll content index and leaf area of some tomato local cultivars from n-e romania, under salt stress. Ion Ionescu Brad Iaşi. 2012, 55, 69–74. [Google Scholar]
- Kumar, M.; Chandran, D.; Tomar, M.; Bhuyan, D.J.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Radha; Dhumal, S.; Singh, S.; et al. Valorization Potential of Tomato (Solanum lycopersicum L.) Seed: Nutraceutical Quality, Food Properties, Safety Aspects, and Application as a Health-Promoting Ingredient in Foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Al-Wandawi, H.; Abdul-Rahman, M.; Al-Shaikhly, K. Tomato Processing Wastes as Essential Raw Materials Source. J. Agric. Food Chem. 1985, 33, 804–807. [Google Scholar] [CrossRef]
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G. A Comprehensive Overview of Tomato Processing By-Product Valorization by Conventional Methods versus Emerging Technologies. Foods 2023, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-Pressure Homogenization Treatment to Recover Bioactive Compounds from Tomato Peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Knoblich, M.; Anderson, B.; Latshaw, D. Analyses of Tomato Peel and Seed Byproducts and Their Use as a Source of Carotenoids. J. Sci. Food Agric. 2005, 85, 1166–1170. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of Lycopene from Industrially Derived Tomato Processing By-Products by Pulsed Electric Fields-Assisted Extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable Valorisation of Tomato Pomace: A Comprehensive Review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial Effects and Potential Risks of Tomato Consumption for Human Health: An Overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Zuorro, A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayana, R.; Baskaran, V. Influence of Olive Oil on the Bioavailability of Carotenoids. Eur. J. Lipid Sci. Technol. 2013, 115, 1085–1093. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef]
- Broszeit, G.; Diepenbrock, F.; Gräf, O.; Hecht, D.; Heinze, J.; Martin, H.D.; Mayer, B.; Schaper, K.; Smie, A.; Strehblow, H.H. Vinylogous β-Carotenes: Generation, Storage, and Delocalization of Charge in Carotenoids. Liebigs Ann. 1997, 1997, 2205–2213. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 293–334. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, X.; Qiang, S.; Su, J.; Li, J. Anti-Oxidation and Anti-Inflammatory Potency Evaluation of Ferulic Acid Derivatives Obtained through Virtual Screening. Int. J. Mol. Sci. 2021, 22, 11305. [Google Scholar] [CrossRef]
- Calligaris, S.; Arrighetti, G.; Barba, L.; Nicoli, M.C. Phase Transition of Sunflower Oil as Affected by the Oxidation Level. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 591–598. [Google Scholar] [CrossRef]
- Calligaris, S.; Manzocco, L.; Conte, L.S.; Nicoli, M.C. Application of a Modified Arrhenius Equation for the Evaluation of Oxidation Rate of Sunflower Oil at Subzero Temperatures. J. Food Sci. 2004, 69, E361–E366. [Google Scholar] [CrossRef]
- Maran, J.P.; Prakash, K.A. Process Variables Influence on Microwave Assisted Extraction of Pectin from Waste Carcia papaya L. Peel. Int. J. Biol. Macromol. 2015, 73, 202–206. [Google Scholar] [CrossRef]
- Stinco, C.M.; Rodríguez-Pulido, F.J.; Escudero-Gilete, M.L.; Gordillo, B.; Vicario, I.M.; Meléndez-Martínez, A.J. Lycopene Isomers in Fresh and Processed Tomato Products: Correlations with Instrumental Color Measurements by Digital Image Analysis and Spectroradiometry. Food Res. Int. 2013, 50, 111–120. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Jing, Y.; Chi, Y.; Koch, K.; Villarreal-lozoya, J.E.; Lombardini, L.; Cisneros-zevallos, L.; New, I.; Lattanzio, V.; Cardinali, A.; National, I.; et al. Impact of Extrusion on Physical Characteristics, Composition, and Radical Scavenging Activity of Pecan Nut Shell (Carya illinoinensis). Food Chem. 2019, 306, 1–17. [Google Scholar]
- Yovcheva, T.; Nikolova, K.; Viraneva, A.; Bodurov, I.; Eftimov, T. Characterization of Extra Virgin Olive Oils Adulterated with Sunflower Oil Using Different Physical Methods. Bulg. Chem. Commun. 2014, 46, 16–19. [Google Scholar]
- Romanić, R.; Lužaić, T.; Pezo, L.; Radić, B.; Kravić, S. Omega 3 Blends of Sunflower and Flaxseed Oil—Modeling Chemical Quality and Sensory Acceptability. Foods 2024, 13, 3722. [Google Scholar] [CrossRef]
- Giacomelli, L.M.; Mattea, M.; Ceballos, C.D. Analysis and Characterization of Edible Oils by Chemometric Methods. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 303–308. [Google Scholar] [CrossRef]
- Shikanga, E.A.; Combrinck, S.; Regnier, T. South African Lippia Herbal Infusions: Total Phenolic Content, Antioxidant and Antibacterial Activities. S. Afr. J. Bot. 2010, 76, 567–571. [Google Scholar] [CrossRef]
- Jhaumeer Laulloo, S.S.; Gupta Bhowon, M.; Hoolash, A. Influence Of Chemical Refining Processes On The Total Phenolics And Antioxidant Activity Of Sunflower Oil. Int. J. Nutr. 2015, 1, 38–47. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Physicochemical Composition of Tomato Seed Oil for an Edible Use: The Effect of Cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical Methods for Determining the Peroxide Value of Edible Oils: A Mini-Review. Food Chem 2021, 358, 129834. [Google Scholar] [CrossRef]
- Kumar, S.; Venkatachalapathy, N.; Rawson, A.; Vignesh, S.; Ck, S. Blending of Tomato Seed Oil and Sunflower Oil: Its Characteristics and Quality Evaluation. Pharma Innov. J. 2022, 11, 1449–1453. [Google Scholar]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.K.; Nolasco, S.M.; Tomás, M.C. Characterization of Chia Seed Oils Obtained by Pressing and Solvent Extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Codex Alimentarius Norma Del Codex Para Aceites Vegetales Especificados. Codex Stan 1999, 210.
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Physicochemical Characterization of Chia Seed Oil from Yucatán, México. Agric. Sci. 2014, 5, 220–226. [Google Scholar]
- Kehili, M.; Sayadi, S.; Frikha, F.; Zammel, A.; Allouche, N. Optimization of Lycopene Extraction from Tomato Peels Industrial By-Product Using Maceration in Refined Olive Oil. Food Bioprod. Process. 2019, 117, 321–328. [Google Scholar] [CrossRef]
- Wiguna, B.; Kahfi, J.; Aji, G.K.; Rachman, D.; Nasori, A.S.; Muhamaludin; Maryana, E.; Atmaji, P.; Taniwiryono, D.; Purwanto, W.; et al. The Influence of Virgin Red Palm Oil on the Physical and Textural Properties of Various Vegetable Full-Fat Mayonnaise. Sains Malays. 2023, 52, 2843–2854. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Sunsandee, N.; Chutvirasakul, B.; Ramakul, P. Extraction of Lycopene from Tomato with Environmentally Benign Solvents: Box-Behnken Design and Optimization. Chem. Eng. Commun. 2020, 207, 574–583. [Google Scholar] [CrossRef]
- Corbu, A.R.; Rotaru, A.; Nour, V. Edible Vegetable Oils Enriched with Carotenoids Extracted from By-Products of Sea Buckthorn (Hippophae rhamnoides ssp. sinensis): The Investigation of Some Characteristic Properties, Oxidative Stability and the Effect on Thermal Behaviour. J. Therm. Anal. Calorim. 2020, 142, 735–747. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Mousdis, G.A.; Georgiou, C.A.; Alexa, E.; Moigradean, D.; Cocan, I. Detection of Thermal Processing Impact on Olive and Sunflower Oil Quality by FTIR Spectroscopy. J. Agroaliment. Process. Technol. 2013, 19, 48–56. [Google Scholar]
- Ropciuc, S.; Dranca, F.; Oroian, M.A.; Leahu, A.; Prisacaru, A.E.; Spinei, M.; Codină, G.G. Characterization of Beeswax and Rice Bran Wax Oleogels Based on Different Types of Vegetable Oils and Their Impact on Wheat Flour Dough Technological Behavior during Bun Making. Gels 2024, 10, 194. [Google Scholar] [CrossRef]
- Van Wetten, I.A.; Van Herwaarden, A.W.; Splinter, R.; VanRuth, S.M. Oil Analysis by Fast DSC. Procedia Eng. 2014, 87, 280–283. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Nagamizu, H.; Ogawa, T.; Adachi, S. Phase Behavior of a Binary Mixture of Rapeseed and Soybean Oils. Food Sci. Technol. Res. 2021, 27, 43–48. [Google Scholar] [CrossRef]

| Independent Variables | Coded Values | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Solvent/liquid ratio (g/mL)—X1 | 2 | 4 | 6 |
| Temperature (°C)—X2 | 40 | 60 | 80 |
| Stirring speed (rpm) | 150 | 200 | 250 |
| No. | Samples Sunflowers Oil | L* | a* | b* | C* | ΔE* | ||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Agitation Speed (rpm) | Quantity—Tomato Pomace (g) | ||||||
| 1. | 40 | 150 | 2 | 94.41 | −5.83 | 53.39 | 59.48 | 5.10 |
| 2. | 40 | 250 | 2 | 94.38 | −5.44 | 62.05 | 62.29 | 21.86 |
| 3. | 40 | 200 | 1 | 97.01 | −5.44 | 33.53 | 33.97 | 12.94 |
| 4. | 40 | 200 | 3 | 92.93 | −3.91 | 75.50 | 75.60 | 27.64 |
| 5. | 60 | 150 | 1 | 96.45 | −5.95 | 39.89 | 40.33 | 8.77 |
| 6. | 60 | 150 | 3 | 92.07 | −3.35 | 83.63 | 83.70 | 30.44 |
| 7. | 80 | 150 | 2 | 95.24 | −5.83 | 53.07 | 53.39 | 17.62 |
| 8. | 60 | 200 | 2 | 93.93 | −5.37 | 66.08 | 66.29 | 23.73 |
| 9. | 60 | 200 | 2 | 93.86 | −5.43 | 66.86 | 67.08 | 24.00 |
| 10. | 60 | 200 | 2 | 93.88 | −5.44 | 66.51 | 66.73 | 23.92 |
| 11. | 80 | 250 | 2 | 93.67 | −5.58 | 68.37 | 68.60 | 24.69 |
| 12. | 60 | 150 | 1 | 96.45 | −6.05 | 41.68 | 42.11 | 8.71 |
| 13. | 60 | 250 | 3 | 91.75 | −2.48 | 87.56 | 87.59 | 31.48 |
| 14. | 80 | 200 | 1 | 96.19 | −6.35 | 42.83 | 43.29 | 11.05 |
| 15. | 80 | 200 | 3 | 91.34 | −2.36 | 88.82 | 88.85 | 32.66 |
| No. | Samples Rapeseed Oil | L* | a* | b* | C* | ΔE* | ||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Agitation Speed (rpm) | Quantity—Tomato Pomace (g) | ||||||
| 1. | 40 | 150 | 2 | 89.92 | 0.52 | 132.79 | 132.79 | 31.99 |
| 2. | 40 | 250 | 2 | 89.91 | 0.83 | 132.95 | 132.95 | 36.48 |
| 3. | 40 | 200 | 1 | 91.19 | −2.69 | 131.29 | 131.31 | 33.05 |
| 4. | 40 | 200 | 3 | 89.14 | 2.57 | 133.54 | 133.56 | 38.24 |
| 5. | 60 | 150 | 1 | 91.30 | −3.00 | 131.17 | 131.20 | 32.72 |
| 6. | 60 | 150 | 3 | 88.61 | 3.27 | 133.39 | 133.43 | 39.40 |
| 7. | 80 | 150 | 2 | 90.40 | −0.57 | 132.42 | 132.42 | 35.25 |
| 8. | 60 | 200 | 2 | 89.76 | 0.80 | 132.77 | 132.77 | 36.84 |
| 9. | 60 | 200 | 2 | 89.70 | 0.95 | 132.88 | 132.88 | 36.99 |
| 10. | 60 | 200 | 2 | 89.77 | 0.80 | 132.92 | 132.92 | 36.82 |
| 11. | 80 | 250 | 2 | 89.56 | 0.93 | 132.45 | 132.45 | 37.32 |
| 12. | 60 | 150 | 1 | 91.07 | −2.17 | 131.62 | 131.64 | 33.41 |
| 13. | 60 | 250 | 3 | 88.35 | 4.13 | 133.60 | 133.66 | 39.90 |
| 14. | 80 | 200 | 1 | 91.13 | −2.49 | 131.02 | 131.04 | 33.23 |
| 15. | 80 | 200 | 3 | 87.90 | 4.18 | 133.04 | 133.11 | 40.88 |
| No. crt | Lycopene mg/100 g | DPPH (%) | ||
|---|---|---|---|---|
| Sunflower Oil | Rapeseed Oil | Sunflower Oil | Rapeseed Oil | |
| 1 | 1.67 | 1.21 | 63.58 | 50.35 |
| 2 | 1.65 | 1.35 | 50.66 | 52.30 |
| 3 | 1.46 | 1.14 | 62.66 | 50.66 |
| 4 | 1.48 | 1.19 | 51.79 | 57.02 |
| 5 | 1.61 | 0.88 | 50.76 | 55.79 |
| 6 | 1.58 | 0.82 | 57.43 | 67.38 |
| 7 | 1.71 | 0.83 | 70.46 | 51.28 |
| 8 | 1.69 | 1.07 | 51.28 | 40.41 |
| 9 | 1.71 | 1.23 | 58.05 | 56.71 |
| 10 | 1.6 | 1.08 | 61.53 | 65.12 |
| 11 | 2.01 | 0.85 | 50.66 | 58.05 |
| 12 | 2.14 | 1.27 | 64.61 | 57.74 |
| 13 | 1.64 | 1.32 | 51.89 | 57.94 |
| 14 | 2 | 0.9 | 62.97 | 61.23 |
| 15 | 1.62 | 1.01 | 53.33 | 60.61 |
| No. crt. | Temperature (°C) | Agitation Speed (rpm) | Amount—Tomatoes Pomace (g) | Refractive Index | Peroxide Value meq O2/kg |
|---|---|---|---|---|---|
| 1. | 40 | 150 | 2 | 1.4705 | 0.027 |
| 2. | 40 | 250 | 2 | 1.4706 | 0.039 |
| 3. | 40 | 200 | 1 | 1.4706 | 0.001 |
| 4. | 40 | 200 | 3 | 1.4702 | 1.39 |
| 5. | 60 | 150 | 1 | 1.4703 | 1.05 |
| 6. | 60 | 150 | 3 | 1.4709 | 1.74 |
| 7. | 80 | 150 | 2 | 1.4710 | 1.81 |
| 8. | 60 | 200 | 2 | 1.4711 | 1.20 |
| 9. | 60 | 200 | 2 | 1.4703 | 1.69 |
| 10. | 60 | 200 | 2 | 1.4703 | 1.33 |
| 11. | 80 | 250 | 2 | 1.4703 | 1.75 |
| 12. | 60 | 150 | 1 | 1.4708 | 1.32 |
| 13. | 60 | 250 | 3 | 1.4704 | 1.45 |
| 14. | 80 | 200 | 1 | 1.4710 | 1.38 |
| 15. | 80 | 200 | 3 | 1.4703 | 1.56 |
| No. crt. | Temperature (°C) | Agitation Speed (rpm) | Amount—Tomatoes Pomace (g) | Refractive Index | Peroxide Value meq O2/kg |
|---|---|---|---|---|---|
| 1. | 40 | 150 | 2 | 1.4708 | 1.20 |
| 2. | 40 | 250 | 2 | 1.4706 | 4.13 |
| 3. | 40 | 200 | 1 | 1.4708 | 3.25 |
| 4. | 40 | 200 | 3 | 1.4703 | 2.43 |
| 5. | 60 | 150 | 1 | 1.4711 | 5.64 |
| 6. | 60 | 150 | 3 | 1.4703 | 2.55 |
| 7. | 80 | 150 | 2 | 1.4712 | 1.21 |
| 8. | 60 | 200 | 2 | 1.4708 | 2.41 |
| 9. | 60 | 200 | 2 | 1.4703 | 2.01 |
| 10. | 60 | 200 | 2 | 1.4708 | 5.72 |
| 11. | 80 | 250 | 2 | 1.4709 | 3.12 |
| 12. | 60 | 150 | 1 | 1.4712 | 1.80 |
| 13. | 60 | 250 | 3 | 1.4705 | 2.46 |
| 14. | 80 | 200 | 1 | 1.4711 | 4.45 |
| 15. | 80 | 200 | 3 | 1.4710 | 6.13 |
| Source | PV (meq O2/kg) | Lycopene (mg/100 g) | DPPH (%) | Color Difference (ΔE) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value p-Value | F-Value p-Value | F-Value p-Value | F-Value p-Value | F-Value p-Value | F-Value p-Value | F-Value p-Value | F-Value p-Value | |||||||||
| Sunflower Oil | Rapeseed Oil | Sunflower Oil | Rapeseed Oil | Sunflower Oil | Rapeseed Oil | Sunflower Oil | Rapeseed Oil | |||||||||
| Model | 43.49 | 0.0003 | 5.19 | 0.0422 | 2.94 | 0.12 | 2.01 | 0.0289 | 6.39 | 0.0275 | 3.22 | 0.0155 | 2.11 | 0.2120 | 11.35 | 0.0078 |
| X1 | 0.03 | 0.8540 | 0.01 | 0.9227 | 0.02 | 0.88 | 0.8702 | 0.3937 | 19.28 | 0.0071 | 0.57 | 0.4840 | 0.61 | 0.4670 | 34.81 | 0.0020 |
| X2 | 93.91 | 0.0002 | 1.61 | 0.2598 | 1.16 | 0.33 | 0.0102 | 0.9236 | 1.54 | 0.2701 | 1.21 | 0.3220 | 2.17 | 0.2011 | 3.32 | 0.1278 |
| X3 | 3.04 | 0.1416 | 11.71 | 0.0188 | 9.83 | 0.02 | 0.0006 | 0.9809 | 4.05 | 0.1005 | 9.86 | 0.0257 | 1.22 | 0.3192 | 2.27 | 0.1920 |
| X12 | 114.27 | 0.0001 | 6.05 | 0.0573 | 0.02 | 0.87 | 0.1030 | 0.7613 | 0.12 | 0.7401 | 0.27 | 0.6241 | 0.10 | 0.7570 | 11.19 | 0.0204 |
| X13 | 141.07 | < 0.0001 | 7.91 | 0.0374 | 0.0018 | 0.96 | 1.14 | 0.3337 | 19.55 | 0.0069 | 7.06 | 0.0450 | 0.13 | 0.7328 | 9.86 | 0.0256 |
| X23 | 0.40 | 0.5506 | 10.88 | 0.0215 | 1.03 | 0.35 | 4.13 | 0.0978 | 0.27 | 0.6208 | 1.06 | 0.3495 | 0.01 | 0.9017 | 2.26 | 0.1932 |
| X12 | 23.31 | 0.0048 | 5.16 | 0.0722 | 5.25 | 0.07 | 1.06 | 0.3512 | 0.23 | 0.6461 | 8.77 | 0.0314 | 4.36 | 0.0912 | 11.76 | 0.0186 |
| X22 | 5.21 | 0.0713 | 3.04 | 0.1418 | 1.53 | 0.27 | 3.30 | 0.1291 | 6.31 | 0.0538 | 0.14 | 0.7233 | 7.18 | 0.0438 | 12.53 | 0.0166 |
| X32 | 8.39 | 0.0339 | 1.54 | 0.2703 | 6.63 | 0.04 | 6.78 | 0.0480 | 7.37 | 0.0420 | 0.01 | 0.8938 | 5.46 | 0.0667 | 19.88 | 0.0066 |
| R2 | 43.49 | 0.90 | 0.84 | 0.78 | 0.92 | 0.85 | 0.79 | 0.95 | ||||||||
| Adj-R2 | 0.96 | 0.72 | 0.55 | 0.39 | 0.77 | 0.58 | 0.41 | 0.86 | ||||||||
| CV% | 3.80 | 23.58 | 7.05 | 12.81 | 5.11 | 7.52 | 43.07 | 1.34 | ||||||||
| Adeq Pre | 22.07 | 7.63 | 5.20 | 4.42 | 9.21 | 6.77 | 4.39 | 9.93 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flaiş, D.; Spinei, M.; Oroian, M. Tomato Pomace as a Valuable Resource: Characterization of Sunflower and Rapeseed Oil Extracts from Ox Heart Tomato (Lycopersicon esculentum). Foods 2025, 14, 1662. https://doi.org/10.3390/foods14101662
Flaiş D, Spinei M, Oroian M. Tomato Pomace as a Valuable Resource: Characterization of Sunflower and Rapeseed Oil Extracts from Ox Heart Tomato (Lycopersicon esculentum). Foods. 2025; 14(10):1662. https://doi.org/10.3390/foods14101662
Chicago/Turabian StyleFlaiş, Dumitriţa, Mariana Spinei, and Mircea Oroian. 2025. "Tomato Pomace as a Valuable Resource: Characterization of Sunflower and Rapeseed Oil Extracts from Ox Heart Tomato (Lycopersicon esculentum)" Foods 14, no. 10: 1662. https://doi.org/10.3390/foods14101662
APA StyleFlaiş, D., Spinei, M., & Oroian, M. (2025). Tomato Pomace as a Valuable Resource: Characterization of Sunflower and Rapeseed Oil Extracts from Ox Heart Tomato (Lycopersicon esculentum). Foods, 14(10), 1662. https://doi.org/10.3390/foods14101662






