Effects of Spray-Drying Conditions on the Functional and Physicochemical Properties of Young Barley Grass Juice Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spray-Drying
2.3. Powder Characterization
2.3.1. Dry Matter Content
2.3.2. Water Activity
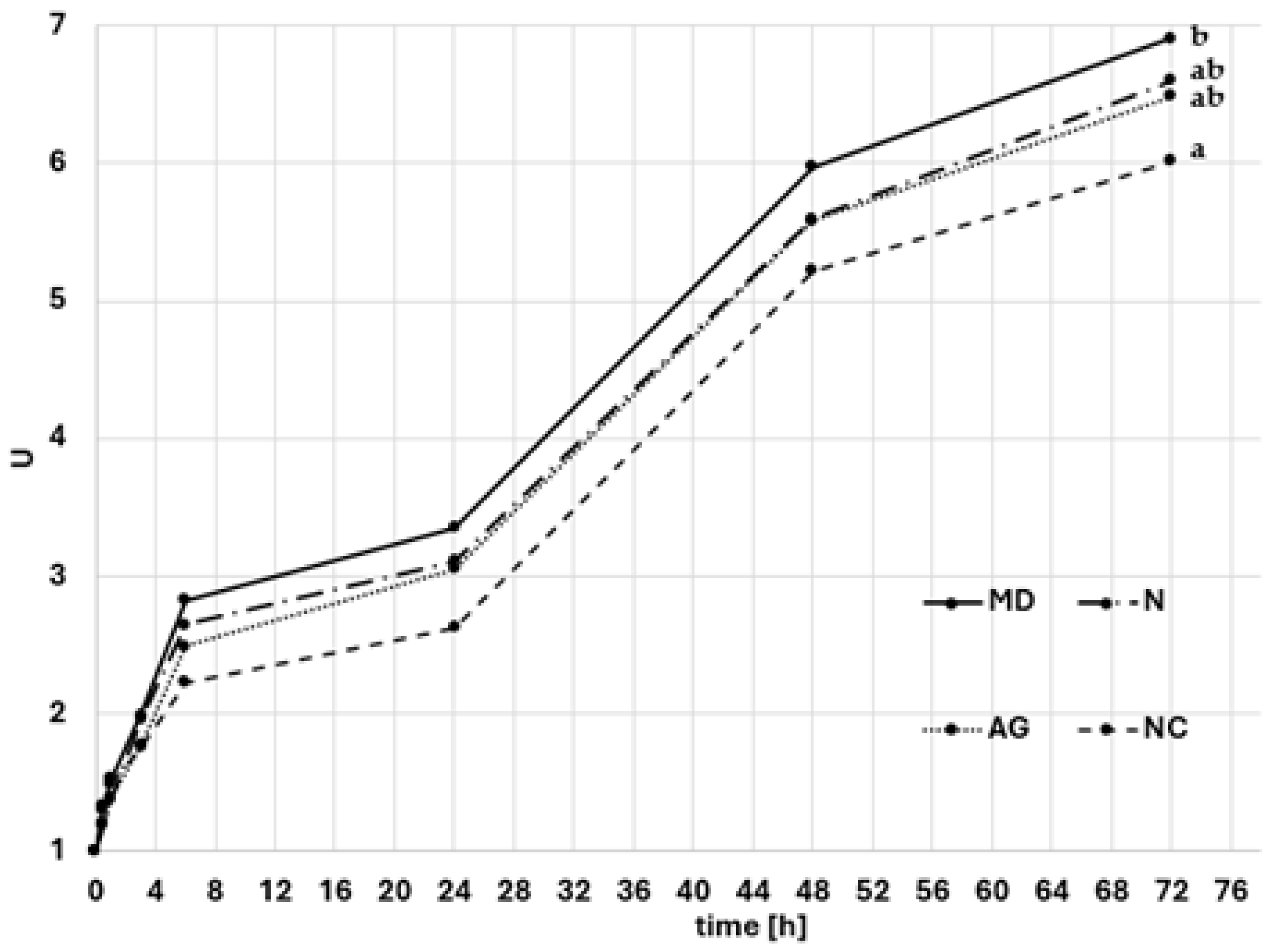
2.3.3. Hygroscopicity
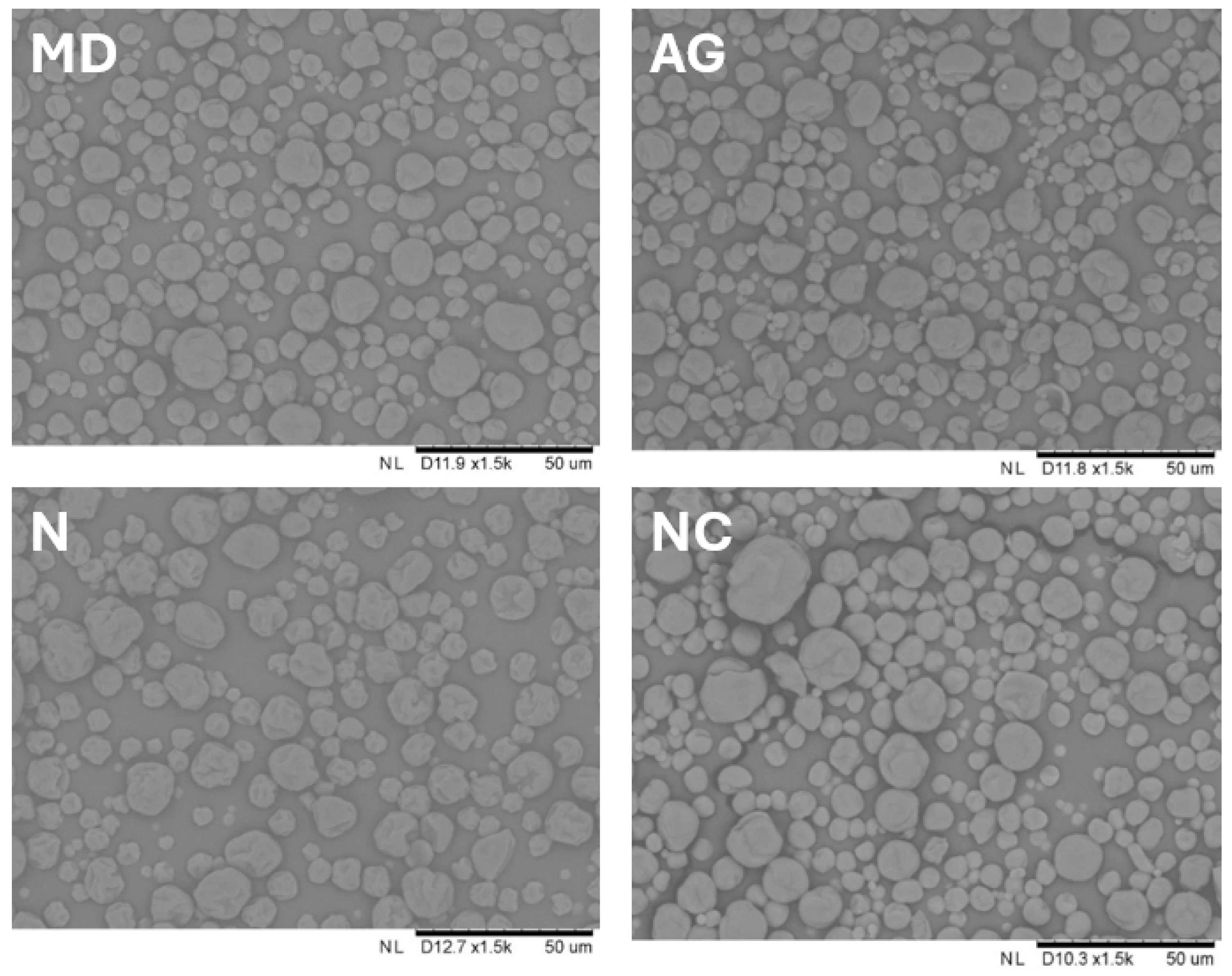
2.3.4. Particle Morphology
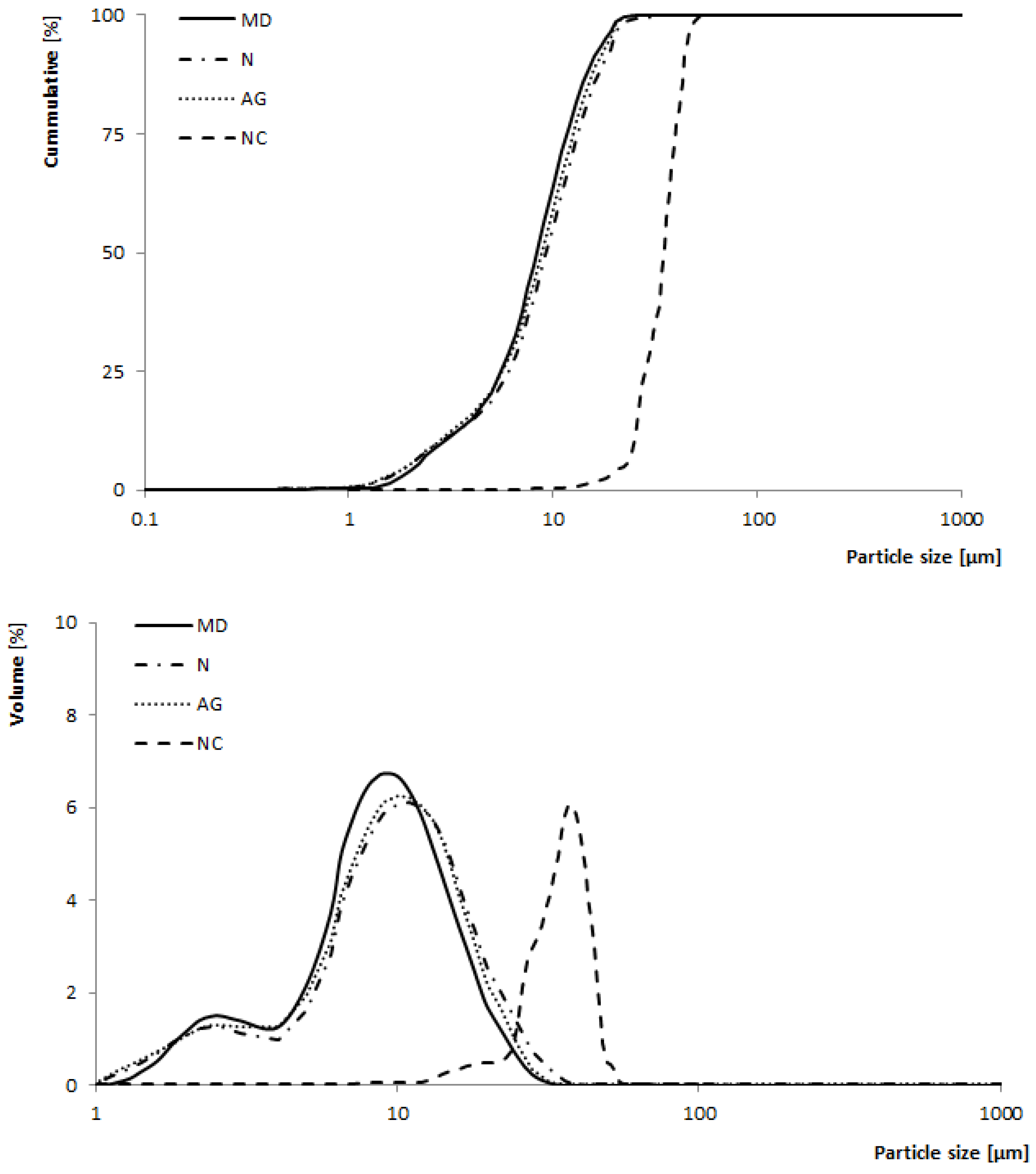
2.3.5. Particle Size Distribution
2.3.6. Loose and Tapped Bulk Densities
2.3.7. Flowability
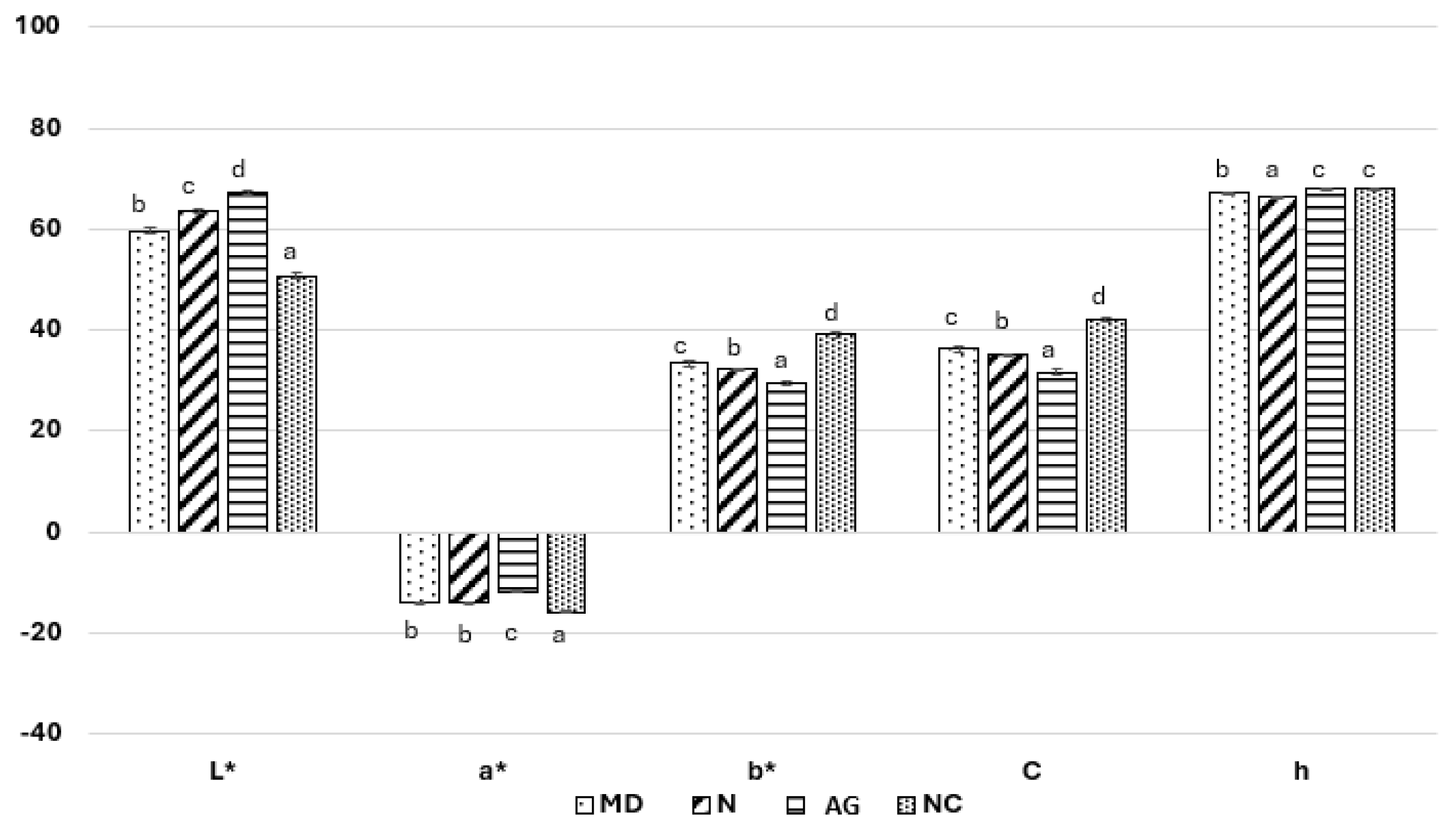
2.3.8. Color
- Chroma (C) expressed as: ;
- Hue (h) expressed as: .
2.4. Chemical Properties of Powders
2.4.1. Total Phenolic Content
2.4.2. Vitamin C
2.4.3. Chlorophyll
2.4.4. Antioxidant Activity
2.4.5. Retention Coefficient
2.5. Statistical Methods
3. Results and Discussion
3.1. Powder Characterization
3.1.1. Dry Matter Content
3.1.2. Water Activity
3.1.3. Hygroscopicity
3.1.4. Particle Size Distribution
3.1.5. Particle Morphology
3.1.6. Loose and Tapped Bulk Densities
3.1.7. Flowability
3.1.8. Color
3.2. Chemical Properties
3.2.1. Total Phenolic Content
3.2.2. Vitamin C
3.2.3. Chlorophyll
3.2.4. Antioxidant Activity
4. Conclusions
- The elimination of carriers in young barley leaf juice powders was possible, as a consequence of lowering the drying temperature as an effect of air dehumidification. This method enabled the obtainment of free-flowing carrier-free powder of satisfactory physiochemical properties, including a high retention coefficient (RC) of analyzed bioactive compounds, making it a “clean-label” product.
- In relation to fresh juice, it should be underlined that low-temperature spray-drying caused the highest degradation of a vitamin C in comparison to other bioactive compounds, pointing to the highest thermolability of all tested compounds. Moreover, the protective role of different carriers in the spray-drying of young barley leaf juice needs to be addressed. There was no statistical difference observed between variants with maltodextrin and Nutriose® and the carrier-free powder, considering lower share of raw material in liquid feeds where the carrier was present. Thus, spray-drying without a carrier can be considered as an approach to produce young barley leaf juice powder.
- Dehumidified air-assisted spray-drying enabled powders with a retention of chlorophyll A and B exceeding 80% to be obtained when choosing proper drying conditions—as well as accounting for the carrier type and its presence—making it a method suitable for production of functional powders from very potent young barley grass juice. Similarly to the vitamin C retention coefficient, no statistical difference was noted between variants with maltodextrin and Nutriose® and the carrier-free powder. Therefore, similar conclusions regarding the presence of a carrier can be drawn. However, as the retention coefficients of both chlorophylls are very satisfactory, the elimination of a carrier can be considered in order to obtain a functional product from young barley leaves that can be labeled as “clean”.
- In order to achieve the most satisfying physiochemical properties of the final product when using a carrier, it is recommended to use Arabic gum (AG) as the most suitable carrier for the spray-drying of young barley leaf juice. This carrier showed the lowest hygroscopicity and the most homogenous particle morphology when added to young barley leaf juice.
- In the case of the potential application of the produced powders as food colorants, it is recommended to use powders with no additional carrier or with the addition of maltodextrin or Nutriose®, as they were characterized by the greatest share of green color and color chroma.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urbonavičiūtė, A.; Samuolienė, G.; Brazaitytė, A.; Ruzgas, V.; Šabajevienė, G.; Šliogerytė, K.; Sakalauskaitė, J.; Duchovskis, P.; Žukauskas, A. The effect of light quality on the antioxidative properties of green barely leaves. Sodininkystė Ir Darzininkystė 2009, 28, 153–161. [Google Scholar]
- Liu, Y.; Chen, W.; Fan, L. Effects of different drying methods on the storage stability of barley grass powder. J. Sci. Food Agric. 2022, 102, 1076–1084. [Google Scholar] [CrossRef]
- Paulíčková, I.; Ehrenbergerová, J.; Fiedlerová, V.; Gabrovská, D.; Havlová, P.; Holasová, M.; Kopáček, J.; Ouhrabková, J.; Pinkrová, J.; Rysová, J.; et al. Evaluation of barley grass as a potential source of some nutritional substances. Czech J. Food Sci. 2007, 25, 65–72. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szulc, P.; Szczepaniak, O.; Dziedziński, M.; Szymanowska, D.; Szymandera-Buszka, K.; Goryńska-Goldmann, E.; Gazdecki, M.; Telichowska, A.; Ligaj, M. Variability of Hordeum vulgare L. cultivars in yield, antioxidant potential, and cholinesterase inhibitory activity. Sustainability 2020, 12, 1938. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Porównanie składu i właściwości antyoksydacyjnej ekstraktów siewek jęczmienia i pszenicy. Zesz. Probl. Postęp. Nauk. Rol. 2019, 593, 63–72. [Google Scholar]
- Krupa-Małkiewicz, M.; Oszmiański, J.; Lachowicz, S.; Szczepanek, M.; Jaśkiewicz, B.; Pachnowska, K.; Ochmian, I. Effect of nanosilver (nAg) on disinfection, growth, and chemical composition of young barley leaves under in vitro conditions. J. Integr. Agric. 2019, 18, 1871–1881. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, H.; Bai, J.; Zhou, X.; Zhao, Y.; Zhu, Y.; McClements, D.J.; Xiao, X.; Sun, Q. Health-promoting properties of barley: A review of nutrient and nutraceutical composition, functionality, bioprocessing, and health benefits. Crit. Rev. Food Sci. Nutr. 2023, 63, 1155–1169. [Google Scholar] [CrossRef]
- Wangcharoen, W.; Phimphilai, S. Chlorophyll and total phenolic contents, antioxidant activities and consumer acceptance test of processed grass drinks. J. Food Sci. Technol. 2016, 53, 4135–4140. [Google Scholar] [CrossRef] [PubMed]
- Thatiparthi, J.; Dodoala, S.; Koganti, B.; Kvrsg, P. Barley grass juice (Hordeum vulgare L.) inhibits obesity and improves lipid profile in high fat diet-induced rat model. J. Ethnopharmacol. 2019, 238, 111843. [Google Scholar]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Guo, Y.; Baldelli, A.; Singh, A.; Fathordoobady, F.; Kitts, D.; Pratap-Singh, A. Production of high loading insulin nanoparticles suitable for oral delivery by spray drying and freeze drying techniques. Sci. Rep. 2022, 12, 9949. [Google Scholar] [CrossRef] [PubMed]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2012, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Barańska, A.; Michalska-Ciechanowska, A.; Wojdyło, A.; Mykhailyk, V.A.; Korinchevska, T.V.; Samborska, K. Carriers based on dairy by-products and dehumidified-air spray drying as a novel multiple approach towards improved retention of phenolics in powders: Sour cherry juice concentrate case study. J. Sci. Food Agric. 2023, 104, 1497–1510. [Google Scholar] [CrossRef]
- Samborska, K.; Edris, A.; Jedlińska, A.; Barańska, A. The production of white mulberry molasses powders with prebiotic carrier by dehumidified air-assisted spray drying. J. Food Process Eng. 2021, 45, e13928. [Google Scholar] [CrossRef]
- Samborska, K.; Budziak-Wieczorek, I.; Matwijczuk, A.; Witrowa-Rajchert, D.; Gagoś, M.; Gładyszewska, B.; Karcz, D.; Rybak, K.; Jaskulski, M.; Barańska, A.; et al. Powdered plant beverages obtained by spray-drying without carrier addition-physicochemical and chemometric studies. Sci. Rep. 2024, 14, 4488. [Google Scholar] [CrossRef]
- Azhar, M.D.; Abd Hashib, S.; Ibrahim, U.K.; Abd Rahman, N. Development of carrier material for food applications in spray drying technology: An overview. Mater. Today Proc. 2021, 47, 1371–1375. [Google Scholar] [CrossRef]
- Lefranc-Millot, C. NUTRIOSE® 06: A useful soluble dietary fibre for added nutritional value. Nutr. Bull. 2008, 33, 234–239. [Google Scholar] [CrossRef]
- Śledź, M.; Nowacka, M.; Wiktor, A.; Witrowa-Rajchert, D. Selected chemical and physico-chemical properties of microwave-convective dried herbs. Food Bioprod. Process. 2013, 91, 421–428. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Guzman, I.; Yousef, G.G.; Brown, A.F. Simultaneous extraction and quantitation of carotenoids, chlorophylls, and tocopherols in Brassica vegetables. J. Agric. Food Chem. 2012, 60, 7238–7244. [Google Scholar] [CrossRef]
- Śledź, M.; Wiktor, A.; Rybak, K.; Nowacka, M.; Witrowa-Rajchert, D. The impact of ultrasound and steam blanching pre-treatments on the drying kinetics, energy consumption and selected properties of parsley leaves. Appl. Acoust. 2016, 103, 148–156. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Peng, H.; Woo, M.W.; Zeng, X.A.; Brennan, M.; Brennan, C.S. Preparation and characterization of whey protein isolate-chlorophyll microcapsules by spray drying: Effect of WPI ratios on the physicochemical and antioxidant properties. J. Food Eng. 2020, 267, 109729. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Koç, G.Ç.; Dirim, S.N. Spray dried spinach juice: Powder properties. J. Food Meas. Charact. 2018, 12, 1654–1668. [Google Scholar] [CrossRef]
- Du, J.; Ge, Z.Z.; Xu, Z.; Zou, B.; Zhang, Y.; Li, C.M. Comparison of the Efficiency of Five Different Drying Carriers on the Spray Drying of Persimmon Pulp Powders. Dry. Technol. 2014, 32, 1157–1166. [Google Scholar] [CrossRef]
- Tonon, R.V.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubinger, M.D. Water sorption and glass transition temperature of spray dried açai (Euterpe oleracea Mart.) juice. J. Food Eng. 2009, 94, 215–221. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef]
- Jedlińska, A.; Barańska, A.; Witrowa-Rajchert, D.; Ostrowska-Ligęza, E.; Samborska, K. Dehumidified Air-Assisted Spray-Drying of Cloudy Beetroot Juice at Low Temperature. Appl. Sci. 2021, 11, 6578. [Google Scholar] [CrossRef]
- Chng, G.Y.V.; Chang, L.S.; Pui, L.P. Effects of maltodextrin concentration and inlet temperature on the physicochemical properties of spray-dried kuini powder. Asia Pac. J. Mol. Biol. Biotechnol 2020, 28, 113–131. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of carrier agents on the physicochemical and technofunctional properties and antioxidant capacity of freeze-dried pomegranate juice (Punica granatum) powder. Foods 2020, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.C.; Germer, S.P.M.; Alvim, I.D.; Vissotto, F.Z.; de Aguirre, J.M. Influence of carrier agents on the physicochemical properties of blackberry powder produced by spray drying. Int. J. Food Sci. Technol. 2012, 47, 1237–1245. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, M.; Qian, H.; Mujumdar, A.S.; Wang, Z. Physicochemical and nutraceutical properties of barley grass powder microencapsulated by spray drying. Dry. Technol. 2017, 35, 1358–1367. [Google Scholar] [CrossRef]
- Saker, A.; Cares-Pacheco, M.G.; Marchal, P.; Falk, V. Powders flowability assessment in granular compaction: What about the consistency of Hausner ratio? Powder Technol. 2019, 354, 52–63. [Google Scholar] [CrossRef]
- Leturia, M.; Benali, M.; Lagarde, S.; Ronga, I.; Saleh, K. Characterization of flow properties of cohesive powders: A comparative study of traditional and new testing methods. Powder Technol. 2014, 253, 406–423. [Google Scholar] [CrossRef]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P.; Rattanapon, R. Investigation of phytochemical constituents, phenolic profiles and antioxidant activities of ricegrass juice compared to wheatgrass juice. Funct. Foods Health Dis. 2016, 6, 822–835. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Hadi Peighambardoust, S.; Sarabandi, K.; Jafari, S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Brzezowska, J.; Wojdyło, A.; Gajewicz-Skretna, A.; Ciska, E.; Majerska, J. Chemometric contribution for deeper understanding of thermally-induced changes of polyphenolics and the formation of hydroxymethyl-L-furfural in chokeberry powders. Food Chem. 2021, 342, 128335. [Google Scholar] [CrossRef]
- Chen, X.; Ting, J.L.H.; Peng, Y.; Tangjaidee, P.; Zhu, Y.; Li, Q.; Shan, Y.; Quek, S.Y. Comparing three types of mandarin powders prepared via microfluidic-jet spray drying: Physical properties, phenolic retention and volatile profiling. Foods 2021, 10, 123. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Koç, Ç.G.; Nur Dirim, S. Spray Drying of Spinach Juice: Characterization, Chemical Composition, and Storage. J. Food Sci. 2017, 82, 2873–2884. [Google Scholar]
- Mieszczakowska-Frąc, M.; Celejewska, K.; Płocharski, W. Impact of innovative technologies on the content of vitamin C and its bioavailability from processed fruit and vegetable products. Antioxidants 2021, 10, 54. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R.; Jankowska, B.; Piechowska, P.; Mildner-Szkudlarz, S. Vitamin C and aroma composition of fresh leaves from Kalanchoe pinnata and Kalanchoe daigremontiana. Sci. Rep. 2019, 9, 19786. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The impact of pulsed electric field pretreatment of bell pepper on the selected properties of spray dried juice. Innov. Food Sci. Emerg. Technol. 2021, 65, 102446. [Google Scholar] [CrossRef]
- Looi, Y.F.; Ong, S.P.; Julkifle, A.; Alias, M.S. Effects of pretreatment and spray drying on the physicochemical properties and probiotics viability of Moringa (Moringa oleifera Lam) leaf juice powder. J. Food Process Preserv. 2019, 43, e13915. [Google Scholar] [CrossRef]
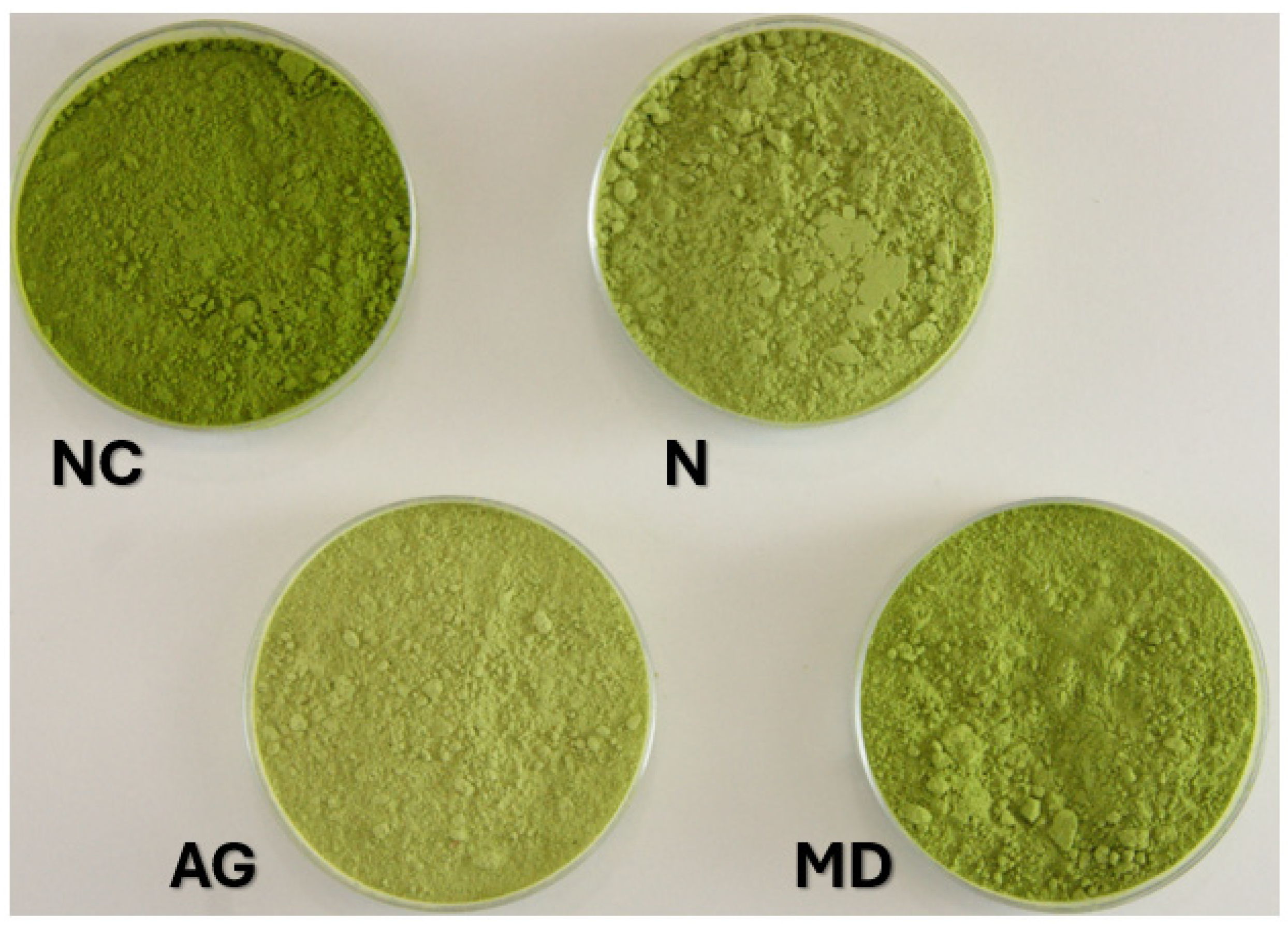
| NC | MD | N | AG | |
|---|---|---|---|---|
| DM [%] | 94.86 ± 0.04 a | 96.51 ± 0.26 b | 96.29 ± 0.04 ab | 95.98 ± 0.01 ab |
| aw [-] | 0.27 ± 0.02 b | 0.18 ± 0.01 ab | 0.15 ± 0.01 a | 0.20 ± 0.01 ab |
| D50 [µm] | 11.61 ± 0.65 b | 8.77 ± 0.57 a | 9.41 ± 0.41 a | 8.89 ± 0.30 a |
| ρL [kg/m3] | 486.38 ± 14.54 a | 601.37 ± 9.08 c | 549.42 ± 9.97 b | 565.07 ± 17.10 b |
| ρT [kg/m3] | 628.89 ± 11.75 a | 715.57 ± 13.94 c | 670.70 ± 10.06 b | 658.58 ± 4.18 b |
| HR [-] | 1.24 ± 0.02 b | 1.19 ± 0.01 a | 1.20 ± 0.03 a | 1.20 ± 0.01 a |
| Juice | NC | MD | N | AG | |
|---|---|---|---|---|---|
| TPC [mg GAE/100 g solids] | 2719.19 ± 33.98 e | 1370.35 ± 19.76 d | 695.35 ± 34.96 b | 524.05 ± 15.81 a | 990.68 ± 47.11 c |
| RC [%] | - | 50.40 ± 0.73 b | 102.29 ± 5.14 c | 25.70 ± 0.78 a | 48.58 ± 2.31 b |
| Vit. C [mg/100 g solids] | 124.24 ± 12.63 b | 28.55 ± 1.50 a | 25.67 ± 1.94 a | 23.99 ± 2.14 a | 26.74 ± 1.86 a |
| RC [%] | - | 22.98 ± 1.21 a | 27.55 ± 2.08 ab | 25.75 ± 2.30 ab | 28.70 ± 2.00 b |
| Chlorophyll A [mg/g solids] | 7.01 ± 0.31 e | 5.67 ± 0.46 d | 4.80 ± 0.27 c | 3.80 ± 0.28 b | 2.87 ± 0.26 a |
| RC [%] | - | 80.84 ± 6.56 bc | 91.22 ± 5.07 c | 72.24 ± 5.32 b | 54.62 ± 4.97 a |
| Chlorophyll B [mg/g solids] | 2.83 ± 0.14 c | 2.47 ± 0.15 c | 1.52 ± 0.12 b | 1.42 ± 0.26 ab | 1.00 ± 0.11 a |
| RC [%] | - | 87.05 ± 5.21 b | 71.72 ± 5.44 b | 67.04 ± 12.41 b | 46.95 ± 4.96 a |
| EC50 [mg solids/mL] | 0.31 ± 0.06 a | 0.50 ± 0.09 b | 0.87 ± 0.06 c | 1.15 ± 0.08 d | 0.52 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barańska-Dołomisiewicz, A.; Żubernik, J.; Samborska, K.; Jedlińska, A.; Witrowa-Rajchert, D. Effects of Spray-Drying Conditions on the Functional and Physicochemical Properties of Young Barley Grass Juice Powders. Foods 2025, 14, 1663. https://doi.org/10.3390/foods14101663
Barańska-Dołomisiewicz A, Żubernik J, Samborska K, Jedlińska A, Witrowa-Rajchert D. Effects of Spray-Drying Conditions on the Functional and Physicochemical Properties of Young Barley Grass Juice Powders. Foods. 2025; 14(10):1663. https://doi.org/10.3390/foods14101663
Chicago/Turabian StyleBarańska-Dołomisiewicz, Alicja, Joanna Żubernik, Katarzyna Samborska, Aleksandra Jedlińska, and Dorota Witrowa-Rajchert. 2025. "Effects of Spray-Drying Conditions on the Functional and Physicochemical Properties of Young Barley Grass Juice Powders" Foods 14, no. 10: 1663. https://doi.org/10.3390/foods14101663
APA StyleBarańska-Dołomisiewicz, A., Żubernik, J., Samborska, K., Jedlińska, A., & Witrowa-Rajchert, D. (2025). Effects of Spray-Drying Conditions on the Functional and Physicochemical Properties of Young Barley Grass Juice Powders. Foods, 14(10), 1663. https://doi.org/10.3390/foods14101663






