Use of Enriched Mine Water to Grow the Cyanobacterium Arthrospira platensis in Photobioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacteria Culture and Photobioreactors
2.2. Experimental Design
2.3. Chemicals
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakura, A.; Sharmaa, D.; Sainib, R.; Suhagc, R.; Thakur, D. Cultivating blue food proteins: Innovating next-generation ingredients from macro and microalgae. Biocatal. Agric. Biotechnol. 2024, 60, 103278. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Bastiaens, L.; Verspreet, J.; Hayes, M. Applications of microalgae in foods, pharma and feeds and their use as fertilizers and biostimulants: Legislation and Regulatory aspects for consideration. Foods 2023, 12, 3878. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of Microalgae in the functional food and feed industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 201–211. [Google Scholar] [CrossRef]
- Andreotti, V.; Chindris, A.; Brundu, G.; Vallainc, D.; Francavilla, M.; García, J. Bioremediation of aquaculture wastewater from Mugil cephalus (Linnaeus, 1758) with different microalgae species. Chem. Ecol. 2017, 33, 750–761. [Google Scholar] [CrossRef]
- Andreotti, V.; Solimeno, A.; Rossi, S.; Ficara, E.; Marazzi, F.; Mezzanotte, V.; García, J. Bioremediation of aquaculture wastewater with the microalgae Tetraselmis suecica: Semi-continuous experiments, simulation and photo-respirometric tests. Sci. Total Environ. 2020, 738, 139859. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods—Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Market Research Reports. Spirulina Market Analysis & Forecast: 2025–2032. Available online: https://www.coherentmarketinsights.com/market-insight/spirulina-market-972#:~:text=Spirulina%20Market%20Size%20and%20Trends,7.8%25%20from%202025%20to%202032 (accessed on 3 April 2025).
- Milia, M.; Corrias, F.; Addis, P.; Chini Zitelli, G.; Cicchi, B.; Torzillo, G.; Andreotti, V.; Angioni, A. Influence of Different Light Sources on the Biochemical Composition of Arthrospira spp. Grown in Model Systems. Foods 2022, 11, 399. [Google Scholar] [CrossRef]
- Sili, C.; Torzillo, G.; Vonshak, A. Arthrospira (Spirulina). In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer Science+Business Media B.V.: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sarker, N.K.; Kaparaju, P. A critical review on the status and progress of microalgae cultivation in outdoor photobioreactors conducted over 35 Years. Energies 2023, 16, 3105. [Google Scholar] [CrossRef]
- Vieira Costa, J.A.; Bastos Freitas, B.C.; Martins Rosa, G.; Moraes, L.; Greque Morais, M.; Mitchell, B.G. Operational and economic aspects of Spirulina-based biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef]
- Nogueira, S.; Souza Junior, J.; Damasceno Maia, H.; Sousa Saboya, J.P.; Lobo Farias, W.R. Use of Spirulina platensis in treatment of fish farming wastewater. Rev. Ciênc. Agron. 2018, 49, 599–606. [Google Scholar] [CrossRef]
- Depraetere, O.; Foubert, I.; Muylaert, K. Decolorisation of piggery wastewater to stimulate the production of Arthrospira platensis. Bioresour. Technol. 2013, 148, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Economou, C.N.; Markou, G.; Nicodemou, A.; Koutinas, M.; Tekerlekopoulou, A.G.; Vayenas, D.V. Cultivation of Arthrospira platensis in brewery wastewater. Water 2022, 14, 1547. [Google Scholar] [CrossRef]
- Spennati, E.; Casazza, A.A.; Dehghani, F.; Perego, P.; Converti, A.; Padula, M.P.; Valtchev, P. Winery waste valorisation as microalgae culture medium: A step forward for food circular economy. Sep. Purif. Technol. 2022, 293, 121088. [Google Scholar] [CrossRef]
- Ljubic, A.; Safafar, H.; Holdt, S.L.; Jacobsen, C. Biomass composition of Arthrospira platensis during cultivation on industrial process water and harvesting. Appl. Phycol. 2018, 30, 943–954. [Google Scholar] [CrossRef]
- Athanasiadou, V.; Klontza, E.E.; Dimitriou-Christidis, P.; Fountoulakis, M.; Lekkas, D.F. Evaluation of Arthrospira (Spirulina) platensis growth on cheese whey in the context of circular economy. Sustain. Chem. Pharm. 2023, 34, 101173. [Google Scholar] [CrossRef]
- Nicknig, M.A.; Azevedo, A.C.D.; de Oliveira, H.A.; Schneider, I.A.H. Cultivation of microalgae (Scenedesmus sp.) using coal mining wastewater and separation via coagulation/flocculation and dissolved air flotation (DAF). Minerals 2024, 14, 426. [Google Scholar] [CrossRef]
- Levett, A.; Gagen, E.J.; Levett, I.; Erskine, P.D. Integrating microalgae production into mine closure plans. J. Environ. Manag. 2023, 337, 117736. [Google Scholar] [CrossRef]
- Bigi, S.; Tartarello, M.C.; Ruggiero, L.; Graziani, S.; Beaubien, S.E.; Lombardi, S. On-going and future research at the Sulcis site in Sardinia, Italy—characterization and experimentation at a possible future CCS pilot. Energy Procedia 2017, 114, 2742–2747. Available online: http://creativecommons.org/licenses/by-nc-nd/4.0/ (accessed on 3 April 2025). [CrossRef]
- Zarrouk, C. Contribution a L’etude d’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina maxima (Setch. et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- ISO 15705:2002; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD)—Small-Scale Sealed-Tube Method. UNI: Rome, Italy, 2002.
- ISO/TS 15923-2:2017; Water Quality—Determination of Selected Parameters by Discrete Analysis Systems—Part 2: Chromium(VI), Fluoride, Total Alkalinity, Total Hardness, Calcium, Magnesium, Iron, Iron(II), Manganese and Aluminium with Photometric Detection. UNI: Rome, Italy, 2017.
- ISO 15923-1:2013; Water Quality—Determination of Selected Parameters by Discrete Analysis Systems—Part 1: Ammonium, Nitrate, Nitrite, Chloride, Orthophosphate, Sulfate and Silicate with Photometric Detection. UNI: Rome, Italy, 2013.
- Pasquini, V.; Biancacci, C.; Milia, M.; Moccia, D.; Solari, P.; Angioni, A.; Addis, P. Effects of different culture media on growth, composition, quality and palatability of the green algae Ulva sp. cultivated in cylindrical photobioreactors. Algal Res. 2024, 84, 103749. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Auckland, New Zealand, 2008. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Vonshak, A. Laboratory techniques for the culturing of microalgae. In Handbook for Microalgal Mass Culture; Richmond, A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 117–145. [Google Scholar]
- Ragaza, J.A.; Hossain, M.S.; Meiler, K.A.; Velasquez, S.F.; Kumar, V. A review on Spirulina: Alternative media for cultivation and nutritive value as an aquafeed. Rev. Aquac. 2020, 12, 2371–2395. [Google Scholar] [CrossRef]
- AlFadhly, N.K.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies affecting the growth and cultivation of genus Spirulina: An investigative review on current trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Lowrey, J.; Brooks, M.S.; McGinn, P.J. Heterotrophic and mixotrophic cultivation of microalgae for biodiesel production in agricultural wastewaters and associated challenges—A critical review. J. Appl. Phycol. 2014, 27, 1485–1498. [Google Scholar] [CrossRef]
- Nasir, N.M.; Bakar, N.S.; Lananan, F.; Abdul Hamid, S.H.; Lam, S.S.; Jusoh, A. Treatment of African catfish, Clarias gariepinus wastewater utilizing phytoremediation of microalgae, Chlorella sp. with Aspergillus niger bio-harvesting. Bioresour. Technol. 2015, 190, 492–498. [Google Scholar] [CrossRef]
- Lananan, F.; Abdul Hamid, S.H.; Din, W.N.S.; Ali, N.A.; Khatoon, H.; Jusoh, A.; Endut, A. Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing effective microorganism (EM-1) and microalgae (Chlorella sp.). Int. Biodeterior. Biodegrad. 2014, 95, 127–134. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, S.; Cheng, C.; Guo, W.; Nagarajan, D.; Ren, N.; Lee, D.; Chang, J. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Yusni, E.; Hartono, E.S. Growth of Spirulina sp. microalgae in the media of fish waste treatment from Cemara market, Medan, North Sumatera. Earth Environ. Sci. 2021, 782, 042002. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A. Heavy metal analysis in commercial Spirulina products for human consumption. Saudi J. Biol. Sci. 2013, 20, 383–388. [Google Scholar] [CrossRef]
- Yuan, D.; Yaoa, M.; Wanga, L.; Lia, Y.; Gonga, Y.; Hu, Q. Effect of recycling the culture medium on biodiversity and population dynamics of bio-contaminants in Spirulina platensis mass culture systems. Algal Res. 2019, 44, 101718. [Google Scholar] [CrossRef]
- Mufidatun, A.; Koerniawan, M.D.; Siregar, U.J.; Suwanti, L.T.; Budiman, A.; Suyono, E.A. The Effect of pH on Contamination Reduction and Metabolite Contents in Mass Cultures of Spirulina (Arthrospira platensis Gomont). Int. J. Adv. Sci. Eng. Inf. Technol. 2023, 13, 84–90. [Google Scholar] [CrossRef]
- Pereira, M.I.B.; Aguiar, E.M.; Chagas, B.M.E.; Borba, L.H.F.; Sassi, R.; Medeiros, G.F.; Silva, E.P.E.; Rangel, A.H.N. Mixotrophic cultivation of Spirulina platensis in dairy wastewater: Effects on the production of biomass, biochemical composition and antioxidant capacity. PLoS ONE 2019, 14, e0224294. [Google Scholar] [CrossRef]
- Tarazona Delgado, R.; Guarieiro, M.D.S.; Antunes, P.W.; Cassini, S.T.; Terreros, H.M.; Fernandes, V.D.O. Effect of nitrogen limitation on growth, biochemical composition, and cell ultrastructure of the microalga Picocystis salinarum. J. Appl. Phycol. 2021, 33, 2083–2092. [Google Scholar] [CrossRef]
- Bellamy-Carter, J.; Sound, J.K.; Leney, A.C. Probing heavy metal binding to phycobiliproteins. FEBS J. 2022, 15, 4646–4656. [Google Scholar] [CrossRef] [PubMed]
- Jeeji Bai, N.; Seshadri, C.V. On coiling and uncoiling of trichomes in the genus Spirulina. Algol. Stud. 1980, 26, 32–47. [Google Scholar]
- Torzillo, G.; Vonshak, A. Environmental Stress Physiology with Reference to Mass Cultures. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 1st ed.; Richmond, A., Hu, Q., Eds.; Wiley: Chichester, UK, 2013; pp. 154–196. [Google Scholar] [CrossRef]
- Magwell, P.F.R.; Minyaka, E.; Fotsop, O.W.; Leng, M.S.; Lehman, L.G. Influence of sulphate nutrition on growth performance and antioxidant enzyme activities of Spirulina platensis. J. Agric. Sci. 2021, 13, 115. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Grettenberger, C.L.; Abou-Shanab, R.; Hamilton, T.L. Limiting factors in the operation of photosystems I and II in cyanobacteria. Microb. Biotechnol. 2024, 17, e14519. [Google Scholar] [CrossRef]
- Woldie, A.A.; Chowdhary, A.K.; Sekine, M.; Kishi, M.; Zegeye, M.B.; Kurosawa, N.; Toda, T. Growth characteristics and molecular identification of indigenous Limnospira strains from Ethiopian soda lakes as a protein source. Biocatal. Agric. Biotechnol. 2024, 60, 103336. [Google Scholar] [CrossRef]
- Etisa, D.; Mebrat, Y. Recent changes in the phytoplankton community of Soda Lake Chitu, Ethiopia, in response to some environmental factors. Fish. Aquat. Sci. 2024, 27, 23–34. [Google Scholar] [CrossRef]
- Ho, S.H.; Liao, J.F.; Chen, C.Y.; Chang, J.S. Combining light strategies with recycled medium to enhance the economic feasibility of phycocyanin production with Spirulina platensis. Bioresour. Technol. 2018, 247, 669–675. [Google Scholar] [CrossRef]
- Beshi, M.; Assaye, H.; Tilahun, G.; Kishi, M.; Tanaka, K.; Addisu, S.; Toda, T. Optimization of Growth Conditions for Arthrospira fusiformis in Laboratory and Outdoor Cultivation Settings. SSRN 2024. [Google Scholar] [CrossRef]
- Habib, M.A.B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. FAO Fisheries and Aquaculture Circular. No. 1034 2008, FAO, Rome. Available online: http://www.fao.org/3/i0424e/i0424e00.pdf (accessed on 3 April 2025).
- Pelizer, L.H.; Danesi, E.D.G.; de O Rangel, C.; Sassano, C.E.; Carvalho, J.C.M.; Sato, S.; Moraes, I.O. Influence of inoculum age and concentration in Spirulina platensis cultivation. J. Food Engin. 2003, 56, 371–375. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
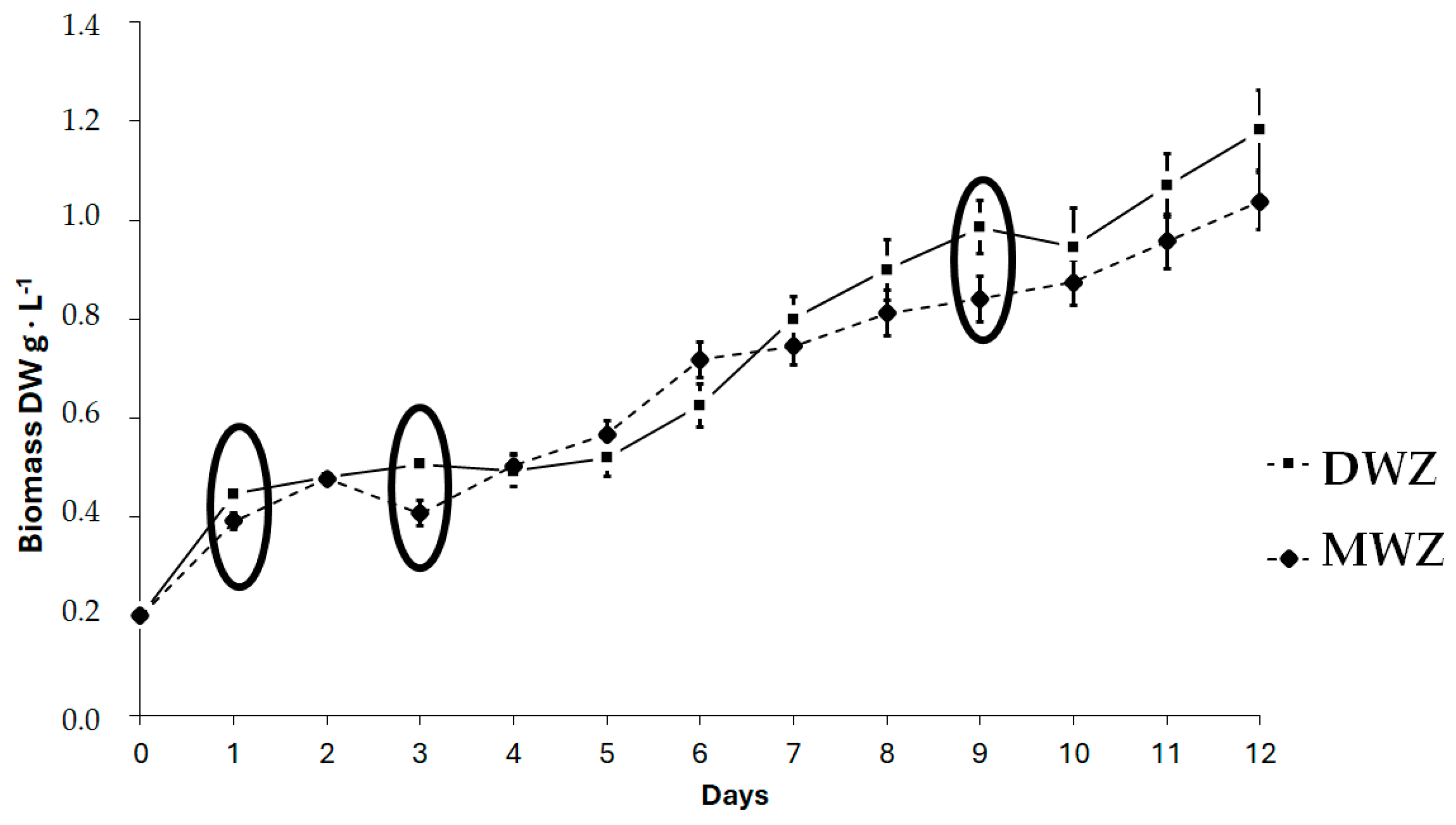
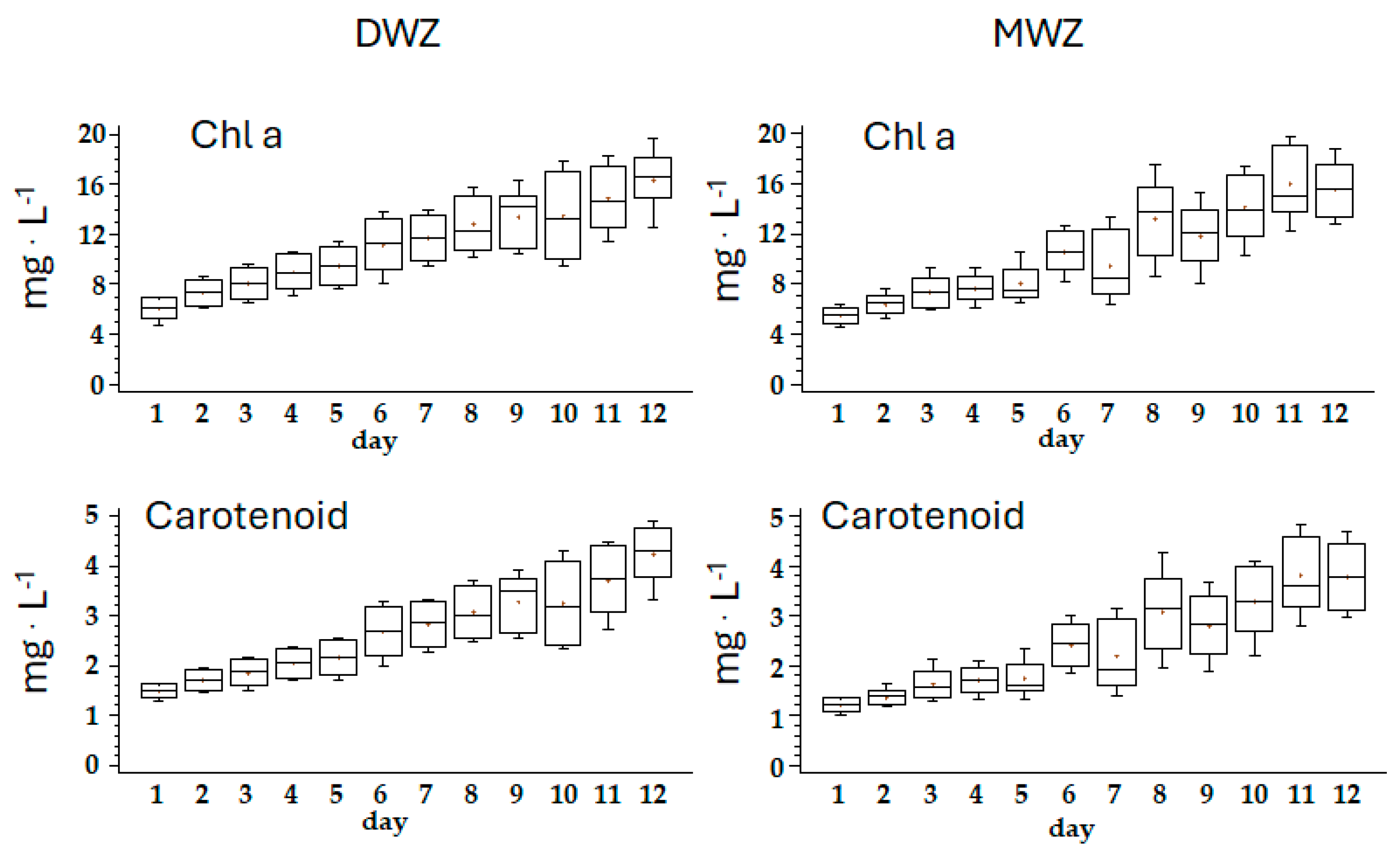
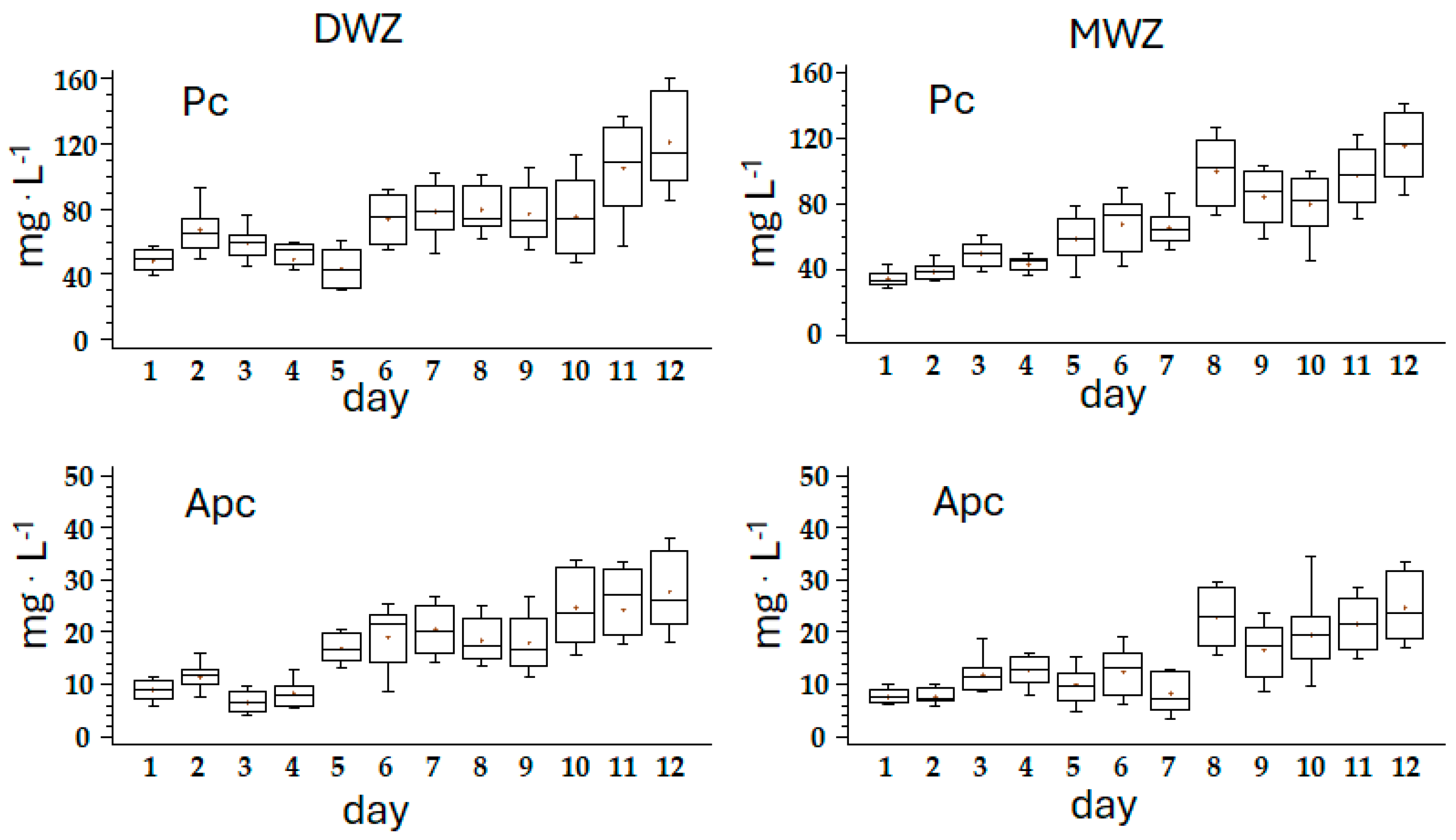
| Ingredients | g·L−1 |
|---|---|
| NaHCO3 | 16.80 |
| K2HPO4 | 0.50 |
| NaNO3 | 2.50 |
| K2SO4 | 1.00 |
| NaCl | 1.00 |
| MgSO4·7H2O | 0.20 |
| EDTA-Na2·2H2O | 0.08 |
| CaCl2·2H2O | 0.04 |
| FeSO4·2H2O | 0.01 |
| H3BO3 | 2.860 |
| MnCl2·4H2O | 1.810 |
| ZnSO4·7H2O | 0.222 |
| Na2MoO4·H2O | 0.007 |
| CuSO4·5H2O | 0.079 |
| Physicochemical Properties | Values (conc ± RSD%) |
|---|---|
| COD (mg·O2−1) | 5.80 ± 0.10 |
| NO3− (mg·L−1) | 2.07 ± 0.03 |
| NO2− (mg·L−1) | 0.02 ± 0.01 |
| NH4− (mg·L−1) | 3.34 ± 0.49 |
| SO42− (mg·L−1) | 547.2 ± 1.31 |
| Hardness °F | 19.4 ± 0.5 |
| Cl− (mg·L−1) | 606.6 ± 1.53 |
| PO43− (mg·L−1) | 4.72 ± 0.07 |
| Fe (mg·L−1) | <0.001 |
| Ba (mg·L−1) | 0.33 ± 0.01 |
| B (mg·L−1) | 0.31 ± 0.03 |
| Al (mg·L−1) | 0.014 ± 0.003 |
| As (mg·L−1) | 0.035 ± 0.005 |
| Zn (mg·L−1) | 0.004 ± 0.001 |
| F− (mg·L−1) | 5.85 ± 0.02 |
| pH | 8.3 |
| Dry Weight | Chl a | Carot | Pc | Apc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | t | P (MC) | t | P (MC) | t | P (MC) | t | P (MC) | t | P (MC) | |
| DWZ vs. MWZ | 1 | 2.460 | * | 1.854 | ns | 4.638 | *** | 6.278 | *** | 1.651 | ns |
| 2 | 0.124 | ns | 2.571 | * | 4.341 | *** | 6.354 | *** | 4.729 | *** | |
| 3 | 2.986 | ** | 1.410 | ns | 1.964 | * | 2.644 | * | 4.770 | *** | |
| 4 | 0.239 | ns | 2.561 | * | 3.014 | * | 1.143 | ns | 3.988 | *** | |
| 5 | 1.027 | ns | 2.351 | * | 2.819 | ** | 2.947 | ** | 5.553 | *** | |
| 6 | 1.619 | ns | 0.646 | ns | 1.238 | ns | 0.946 | ns | 3.134 | ** | |
| 7 | 0.919 | ns | 2.311 | * | 2.663 | * | 2.294 | * | 6.723 | *** | |
| 8 | 1.140 | ns | 0.401 | ns | 0.042 | ns | 2.757 | * | 2.054 | ns | |
| 9 | 2.038 | * | 1.617 | ns | 1.941 | ns | 1.074 | ns | 0.656 | ns | |
| 10 | 0.786 | ns | 0.482 | ns | 0.150 | ns | 0.538 | ns | 1.831 | ns | |
| 11 | 1.347 | ns | 1.062 | ns | 0.317 | ns | 0.763 | ns | 0.709 | ns | |
| 12 | 1.462 | ns | 0.887 | ns | 1.776 | ns | 0.508 | ns | 0.993 | ns |
| Lipids | Carbohydrates | Proteins | NDF | Ashes | |
|---|---|---|---|---|---|
| DWZ | 9.22 ± 1.01 | 31.72 ± 1.57 | 46.03 ± 2.14 | 2.69 ± 0.37 | 9.00 ± 0.32 |
| MWZ | 6.25 ± 0.88 | 25.66 ± 2.12 | 52.64 ± 2.51 | 2.34 ± 0.11 | 8.18 ± 1.41 |
| Source | DF | MS | Pseudo-F | P (MC) | |
|---|---|---|---|---|---|
| Lipids | Trial (Tr) | 1 | 5.36 | 19.6 | ** |
| Res | 6 | 0.27 | |||
| Carbohydrates | Trial (Tr) | 1 | 5.45 | 21.2 | ** |
| Res | 6 | 0.26 | |||
| Proteins | Trial (Tr) | 1 | 5.09 | 16.02 | ** |
| Res | 6 | 0.32 | |||
| NDF | Trial (Tr) | 1 | 2.48 | 3.29 | ns |
| Res | 6 | 0.75 | |||
| Ashes | Trial (Tr) | 1 | 1.24 | 1.3 | ns |
| Res | 6 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milia, M.; Andreotti, V.; Giglioli, A.; Pasquini, V.; Addis, P.; Angioni, A. Use of Enriched Mine Water to Grow the Cyanobacterium Arthrospira platensis in Photobioreactors. Foods 2025, 14, 1665. https://doi.org/10.3390/foods14101665
Milia M, Andreotti V, Giglioli A, Pasquini V, Addis P, Angioni A. Use of Enriched Mine Water to Grow the Cyanobacterium Arthrospira platensis in Photobioreactors. Foods. 2025; 14(10):1665. https://doi.org/10.3390/foods14101665
Chicago/Turabian StyleMilia, Massimo, Valeria Andreotti, Angelica Giglioli, Viviana Pasquini, Pierantonio Addis, and Alberto Angioni. 2025. "Use of Enriched Mine Water to Grow the Cyanobacterium Arthrospira platensis in Photobioreactors" Foods 14, no. 10: 1665. https://doi.org/10.3390/foods14101665
APA StyleMilia, M., Andreotti, V., Giglioli, A., Pasquini, V., Addis, P., & Angioni, A. (2025). Use of Enriched Mine Water to Grow the Cyanobacterium Arthrospira platensis in Photobioreactors. Foods, 14(10), 1665. https://doi.org/10.3390/foods14101665








