Thin-Layer, Intermittent, Near-Infrared Drying of Two-Phase Olive Pomace: Mathematical Modeling and Effect on Recovery of Bioactive Compounds and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Materials
2.3. Drying Assays
2.4. Drying Kinetics Study
2.5. Color Evaluation
2.6. Determination of Bioactive Compounds and Antioxidant Activity
2.6.1. Total Phenolics Content (TPC)
2.6.2. Antioxidant Activity (AA)
2.6.3. Determination of Phenolics and Triterpenic Acids
2.7. Statistical Analysis
3. Results and Discussion
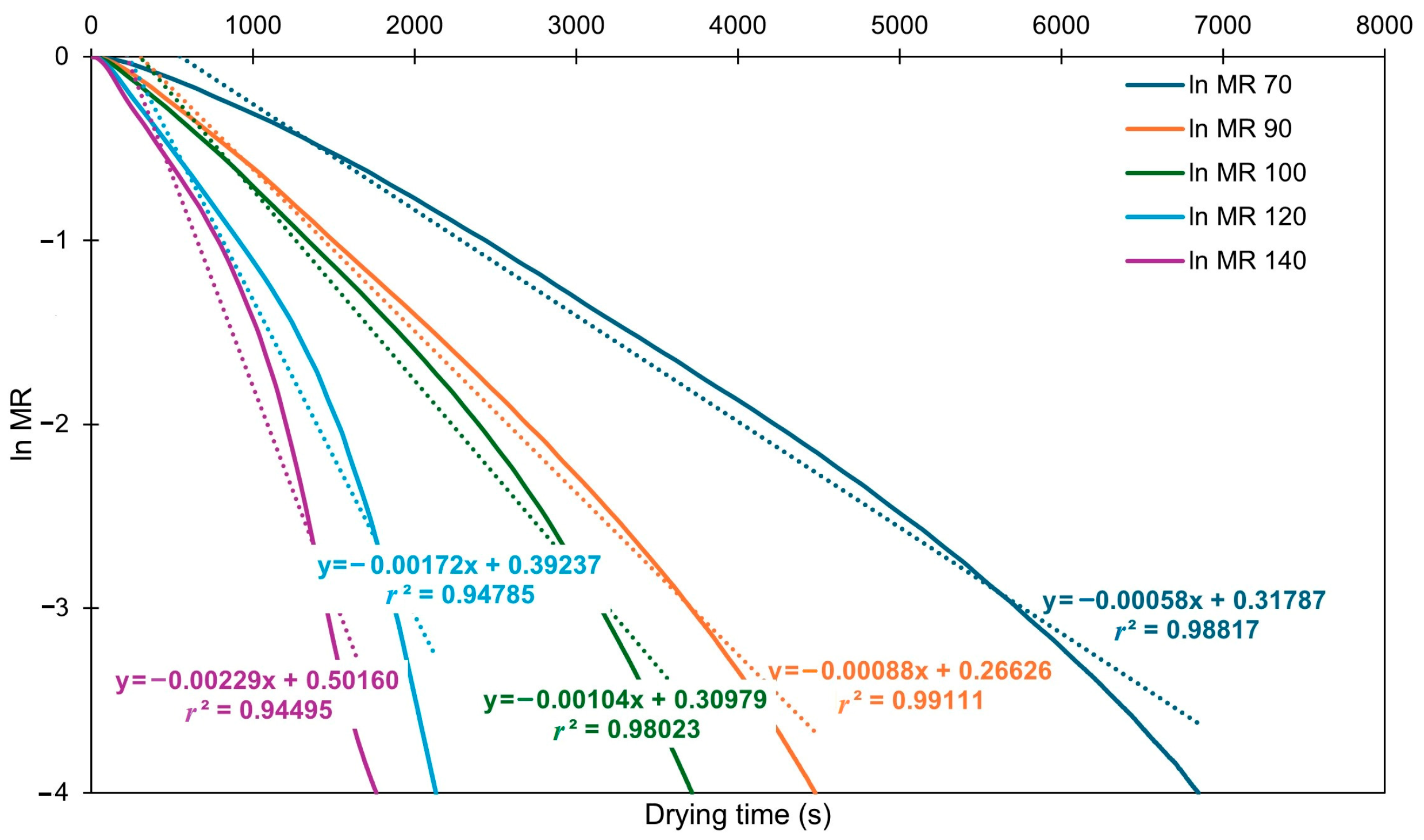
3.1. Drying Kinetics Analysis
3.2. Modeling of Drying Curves
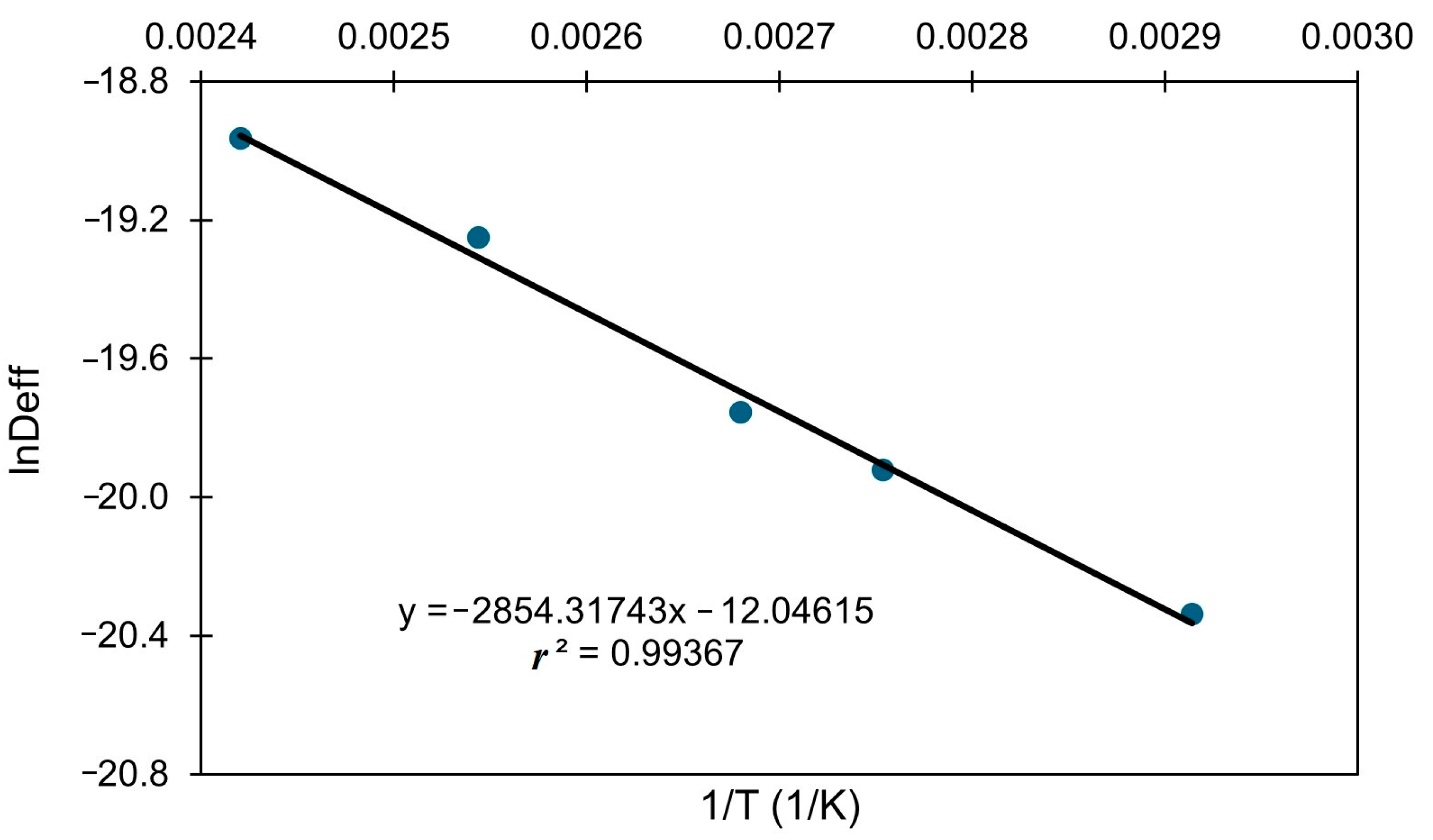
3.3. Effective Diffusivity and Activation Energy
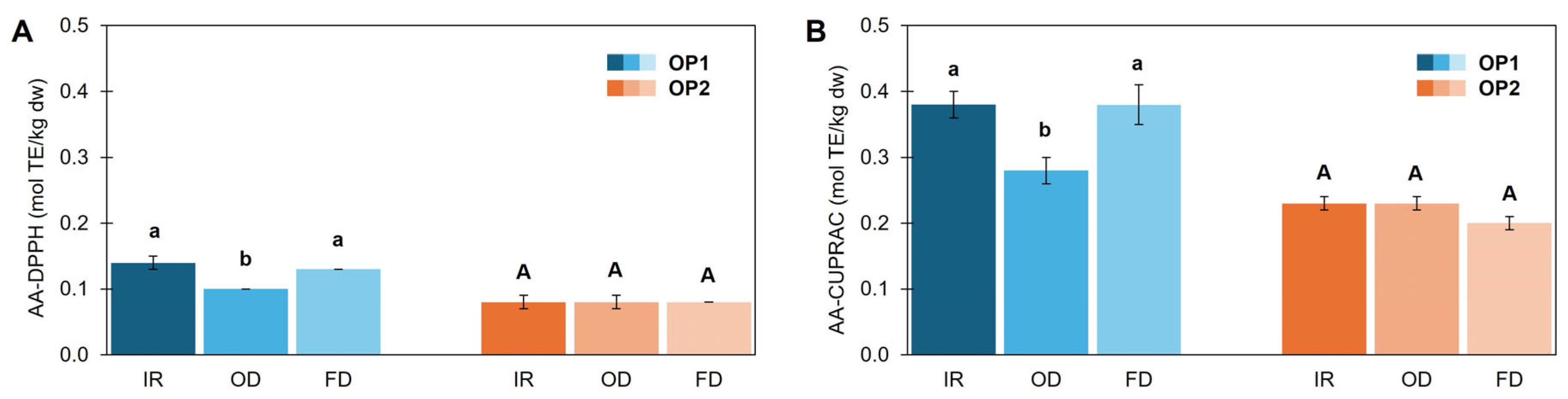
3.4. Temperature Effect on Color and Chemical Composition
3.5. Drying Technique Effect on Color and Chemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | antioxidant activity |
| DR | drying rate |
| FD | freeze-dried |
| H | hydroxytyrosol |
| IR | infrared-dried |
| MA | maslinic acid |
| OA | oleanolic acid |
| OD | oven-dried |
| OP | olive pomace |
| T | tyrosol |
| TPC | total phenolics content |
References
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive By-Products for Functional and Food Applications: Challenging Opportunities to Face Environmental Constraints. IFSET 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Loschi, F.; Zengin, G.; Zancato, M.; Ferrarese, I.; De Zordi, N.; Dall’Acqua, S.; Sut, S. Valorisation of Olive Pomace from Veneto Region: Oven-Dried Material for Food, Nutraceutical, and Cosmetic Application of the OLIVARE Project. Process. Biochem. 2024, 137, 153–163. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are Olive Pomace Powders a Safe Source of Bioactives and Nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional Compounds from Olive Pomace to Obtain High-added Value Foods—A Review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.; Pino, A.; Romeo, F.V.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Pomace and Pâté Olive Cake as Suitable Ingredients for Food and Feed. Microorganisms 2022, 10, 237. [Google Scholar] [CrossRef]
- Pyrka, I.; Stefanidis, S.; Ordoudi, S.A.; Lalou, S.; Nenadis, N. Oxidative Stability of Virgin Avocado Oil Enriched with Avocado Leaves and Olive-Fruit-Processing By-Products (Leaves, Pomace) via Ultrasound-Assisted Maceration. Foods 2025, 14, 294. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Valorization of Olive Processing By-Products via Drying Technologies: A Case Study on the Recovery of Bioactive Phenolic Compounds from Olive Leaves, Pomace, and Wastewater. Crit. Rev. Food Sci. Nutr. 2023, 63, 9797–9815. [Google Scholar] [CrossRef]
- Doymaz, I.; Gorel, O.; Akgun, N.A. Drying Characteristics of the Solid By-Product of Olive Oil Extraction. Biosyst. Eng. 2004, 88, 213–219. [Google Scholar] [CrossRef]
- Akgun, N.A.; Doymaz, I. Modelling of Olive Cake Thin-Layer Drying Process. J. Food Eng. 2005, 68, 455–461. [Google Scholar] [CrossRef]
- Meziane, S.; Mesbahi, N. Determination of Moisture Diffusivity and Activation Energy in Thin Layer Drying of Olive Pomace. Int. J. Food Eng. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Uribe, E.; Lemus-Mondaca, R.; Vega-Gálvez, A.; López, L.A.; Pereira, K.; López, J.; Ah-Hen, K.; Di Scala, K. Quality Characterization of Waste Olive Cake During Hot Air Drying: Nutritional Aspects and Antioxidant Activity. Food Bioproc. Technol. 2013, 6, 1207–1217. [Google Scholar] [CrossRef]
- Uribe, E.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Zamorano, M.; Quispe-Fuentes, I.; Pasten, A.; Di Scala, K. Influence of Process Temperature on Drying Kinetics, Physicochemical Properties and Antioxidant Capacity of the Olive-Waste Cake. Food Chem. 2014, 147, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Çelen, S.; Aktaş, T.; Karabeyoğlu, S.S.; Akyildiz, A. Drying Behaviour of Prina (Crude Olive Cake) Using Different Type of Dryers. Dry. Technol. 2015, 34, 843–853. [Google Scholar] [CrossRef]
- Koukouch, A.; Idlimam, A.; Asbik, M.; Sarh, B.; Izrar, B.; Bostyn, S.; Bah, A.; Ansari, O.; Zegaoui, O.; Amine, A. Experimental Determination of the Effective Moisture Diffusivity and Activation Energy during Convective Solar Drying of Olive Pomace Waste. J. Renew. Energy 2017, 101, 565–574. [Google Scholar] [CrossRef]
- Pasten, A.; Uribe, E.; Stucken, K.; Rodríguez, A.; Vega-Gálvez, A. Influence of Drying on the Recoverable High-Value Products from Olive (cv. Arbequina) Waste Cake. Waste Biomass Valor. 2019, 10, 1627–1638. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Avena-Bustillos, R.J.; Olson, D.A.; Crawford, L.M.; Wang, S.C.; McHugh, T.H. Phenolics and Antioxidant Capacity of Pitted Olive Pomace Affected by Three Drying Technologies. J. Food Sci. 2019, 84, 412–420. [Google Scholar] [CrossRef]
- Nsibi, C.; Lajili, M. Experimental Study and Mathematical Modeling Under Various Hot-Air Drying Conditions of Thin Layer Olive Pomaces. Processes 2023, 11, 2513. [Google Scholar] [CrossRef]
- Gögüs, F.; Maskan, M. Drying of Olive Pomace by a Combined Microwave-Fan Assisted Convection Oven. Nahrung/Food 2001, 45, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Celma, A.; Rojas, S.; Lopez-Rodríguez, F. Mathematical Modelling of Thin-Layer Infrared Drying of Wet Olive Husk. Chem. Eng. Process. 2008, 47, 1810–1818. [Google Scholar] [CrossRef]
- Sadi, T.; Meziane, S. Mathematical Modelling, Moisture Diffusion and Specific Energy Consumption of Thin Layer Microwave Drying of Olive Pomace. Int. Food Res. J. 2015, 22, 494–501. [Google Scholar]
- Maamar, M.I.; Badraoui, M.; Mazouzi, M.; Mouakkir, L. Mathematical modeling on vacuum drying of olive pomace. Trends Sci. 2023, 20, 3822. [Google Scholar] [CrossRef]
- Castillo-Luna, A.; Miho, H.; Ledesma-Escobar, C.A.; Priego-Capote, F. Comparison of Drying Techniques for Extraction of Bioactive Compounds from Olive-Tree Materials. Foods 2023, 12, 2684. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Khatib, M.; Bellumori, M.; Civa, V.; Domizio, P.; Innocenti, M.; Balli, D.; Mulinacci, N. Industrial Drying for Agrifood By-Products Re-Use: Cases Studies on Pomegranate Peel (Punica granatum L.) and Stoned Olive Pomace (Pâtè, Olea europaea L.). Food Chem. 2023, 403, 134338. [Google Scholar] [CrossRef] [PubMed]
- Riadh, M.H.; Ahmad, S.A.B.; Marhaban, M.H.; Soh, A.C. Infrared Heating in Food Drying: An Overview. Dry. Technol. 2015, 33, 322–335. [Google Scholar] [CrossRef]
- Sakare, P.; Prasad, N.; Thombare, N.; Singh, R.; Sharma, S.C. Infrared Drying of Food Materials: Recent Advances. Food Eng. Rev. 2020, 12, 381–398. [Google Scholar] [CrossRef]
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel Drying Techniques for the Food Industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A.; Joardder, M.U.H. Intermittent Drying of Food Products: A Critical Review. J. Food Eng. 2014, 121, 48–57. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the Total Phenol Contents and the Color of Fresh and Infrared Dried Olive Leaves. Ind. Crop. Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Pyrka, I.; Mantzouridou, F.T.; Nenadis, N. Optimization of Olive Leaves’ Thin Layer, Intermittent near-Infrared-Drying. IFSET 2023, 84, 103264. [Google Scholar] [CrossRef]
- Derardja, A.E.; Pretzler, M.; Barkat, M.; Rompel, A. Extraction, purification, and characterization of olive (Olea europaea L., cv. Chemlal) polyphenol oxidase. J. Agric. Food Chem. 2024, 72, 3099–3112. [Google Scholar] [CrossRef]
- Pyrka, I.; Koutra, C.; Siderakis, V.; Stathopoulos, P.; Skaltsounis, A.-L.; Nenadis, N. Exploring the Bioactive Content of Liquid Waste and Byproducts Produced by Two-Phase Olive Mills in Laconia (Greece): Is There a Prospect for Added-Value Applications? Foods 2023, 12, 4421. [Google Scholar] [CrossRef] [PubMed]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin Layers. Master’s Thesis, Department of Mechanical Engineering, Purdue University, Purdue, IN, USA, 1949. [Google Scholar]
- Toğrul, İ.T.; Pehlivan, D. Mathematical Modelling of Solar Drying of Apricots in Thin Layers. J. Food Eng. 2002, 55, 209–216. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A New Model for Single-Layer Drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Senadeera, W.; Bhandari, B.R.; Young, G.; Wijesinghe, B. Influence of Shapes of Selected Vegetable Materials on Drying Kinetics during Fluidized Bed Drying. J. Food Eng. 2003, 58, 277–283. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the Estimation of Scavenging Activity of Phenolic Compounds Using Rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) Tests. JAOCS 2002, 79, 1191. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Gomez-Delgado, E.; Medina-Jaramillo, C.; López-Córdoba, A. Analysis of Drying Kinetic of Brewer’s Spent Grains: Effect of the Temperature on the Physical Properties and the Content of Bioactive Compounds. Vitae 2023, 30, 1–21. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajon-Catalan, E.; Micol, V.; Cárcel, J.A.; Garcia-Perez, J.V. Influence of Air Temperature on Drying Kinetics and Antioxidant Potential of Olive Pomace. J. Food Eng. 2013, 119, 516–524. [Google Scholar] [CrossRef]
- Davalos, J.Z.; Valderrama-Negron, A.C.; Barrios, J.R.; Freitas, V.L.; Ribeiro da Silva, M.D. Energetic Structural Properties of Two Phenolic Antioxidants: Tyrosol Hydroxytyrosol. J. Phys. Chem. A 2018, 122, 4130–4137. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Osorio-Revilla, G.I.; Gallardo-Velázquez, T.; Proal-Nájera, J.B. Uso de FTIR-HATR y análisis multivariable para el seguimiento de la degradación de compuestos bioactivos durante el secado de pimiento rojo. Rev. Mex. Ing. Química 2013, 12, 193–204. [Google Scholar]






| Model | Analytical Expression | Temperature | Model Constants | r2 | χ2 | RMSE |
|---|---|---|---|---|---|---|
| Page | MR = exp(−kty) | 70 °C | k = 0.00004, y = 1.30859 | 0.99978 | 0.00002 | 0.02051 |
| 90 °C | k = 0.00012, y = 1.23584 | 0.99964 | 0.00003 | 0.12801 | ||
| 100 °C | k = 0.00014, y = 1.23332 | 0.99928 | 0.00006 | 0.17871 | ||
| 120 °C | k = 0.00016, y = 1.28743 | 0.99670 | 0.00029 | 0.34259 | ||
| 140 °C | k = 0.00017, y = 1.28435 | 0.99623 | 0.00034 | 0.35095 | ||
| Logarithmic | MR = a exp(−kt) + c | 70 °C | a = 1.14427, k = 0.00041, c = −0.04889 | 0.99688 | 0.00027 | 0.01690 |
| 90 °C | a = 1.11630, k = 0.00069, c = −0.03087 | 0.99839 | 0.00013 | 0.15309 | ||
| 100 °C | a = 1.12468, k = 0.00072, c = −0.05769 | 0.99894 | 0.00009 | 0.20357 | ||
| 120 °C | a = 1.15699, k = 0.00104, c = −0.08896 | 0.99729 | 0.00024 | 0.35777 | ||
| 140 °C | a = 1.18179, k = 0.00098, c = −0.12324 | 0.99845 | 0.00014 | 0.36377 | ||
| Midilli | MR = a exp(−ktn) + bt | 70 °C | a = 1.01384, k = 0.00005, n = 1.26512, b = −8.9095 × 10−7 | 0.99992 | 0.00001 | 0.00943 |
| 90 °C | a = 1.02388, k = 0.00018, n = 1.17911, b = −1.3168 × 10−6 | 0.99990 | 0.00001 | 0.00348 | ||
| 100 °C | a = 1.01857, k = 0.00024, n = 1.15943, b = −4.9527 × 10−6 | 0.99988 | 0.00001 | 0.00560 | ||
| 120 °C | a = 1.01421, k = 0.00031, n = 1.18674, b = −0.00001 | 0.99839 | 0.00014 | 0.01349 | ||
| 140 °C | a = 1.01980, k = 0.00039, n = 1.14839, b = −0.00002 | 0.99887 | 0.00010 | 0.01238 |
| Sample | L* | a* | b* | Color Visualization * |
|---|---|---|---|---|
| OP1-70 | 32.28 ± 0.13 a | 10.50 ± 0.17 a | 21.54 ± 0.55 a | |
| OP1-90 | 30.09 ± 0.02 b | 12.11 ± 0.05 b | 21.54 ± 0.14 a | |
| OP1-100 | 32.70 ± 0.17 c | 13.64 ± 0.14 c | 24.98 ± 0.30 b | |
| OP1-120 | 30.53 ± 0.18 d | 16.21 ± 0.06 d | 25.77 ± 0.37 c | |
| OP1-140 | 18.80 ± 0.27 e | 11.82 ± 0.10 e | 13.13 ± 0.48 d |
| Sample | L* | a* | b* | Color Visualization * |
|---|---|---|---|---|
| OP1-IR | 18.80 ± 0.27 a | 11.82 ± 0.10 a | 13.13 ± 0.48 a | |
| OP1-OD | 19.55 ± 0.29 a | 12.19 ± 0.37 b | 17.01 ± 0.11 b | |
| OP1-FD | 47.63 ± 0.39 b | 5.58 ± 0.16 c | 26.69 ± 0.55 c | |
| OP2-IR | 13.27 ± 0.16 A | 5.69 ± 0.63 A | 9.09 ± 1.45 A | |
| OP2-OD | 16.35 ± 0.22 B | 10.51 ± 0.81 B | 12.81 ± 0.57 A | |
| OP2-FD | 41.08 ± 0.19 C | 8.64 ± 0.28 B | 23.16 ± 0.55 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrka, I.; Nenadis, N. Thin-Layer, Intermittent, Near-Infrared Drying of Two-Phase Olive Pomace: Mathematical Modeling and Effect on Recovery of Bioactive Compounds and Antioxidant Activity. Foods 2025, 14, 2042. https://doi.org/10.3390/foods14122042
Pyrka I, Nenadis N. Thin-Layer, Intermittent, Near-Infrared Drying of Two-Phase Olive Pomace: Mathematical Modeling and Effect on Recovery of Bioactive Compounds and Antioxidant Activity. Foods. 2025; 14(12):2042. https://doi.org/10.3390/foods14122042
Chicago/Turabian StylePyrka, Ioanna, and Nikolaos Nenadis. 2025. "Thin-Layer, Intermittent, Near-Infrared Drying of Two-Phase Olive Pomace: Mathematical Modeling and Effect on Recovery of Bioactive Compounds and Antioxidant Activity" Foods 14, no. 12: 2042. https://doi.org/10.3390/foods14122042
APA StylePyrka, I., & Nenadis, N. (2025). Thin-Layer, Intermittent, Near-Infrared Drying of Two-Phase Olive Pomace: Mathematical Modeling and Effect on Recovery of Bioactive Compounds and Antioxidant Activity. Foods, 14(12), 2042. https://doi.org/10.3390/foods14122042






