A Novel Eco-Friendly Process for the Synthesis and Purification of Ascorbyl-6-Oleates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
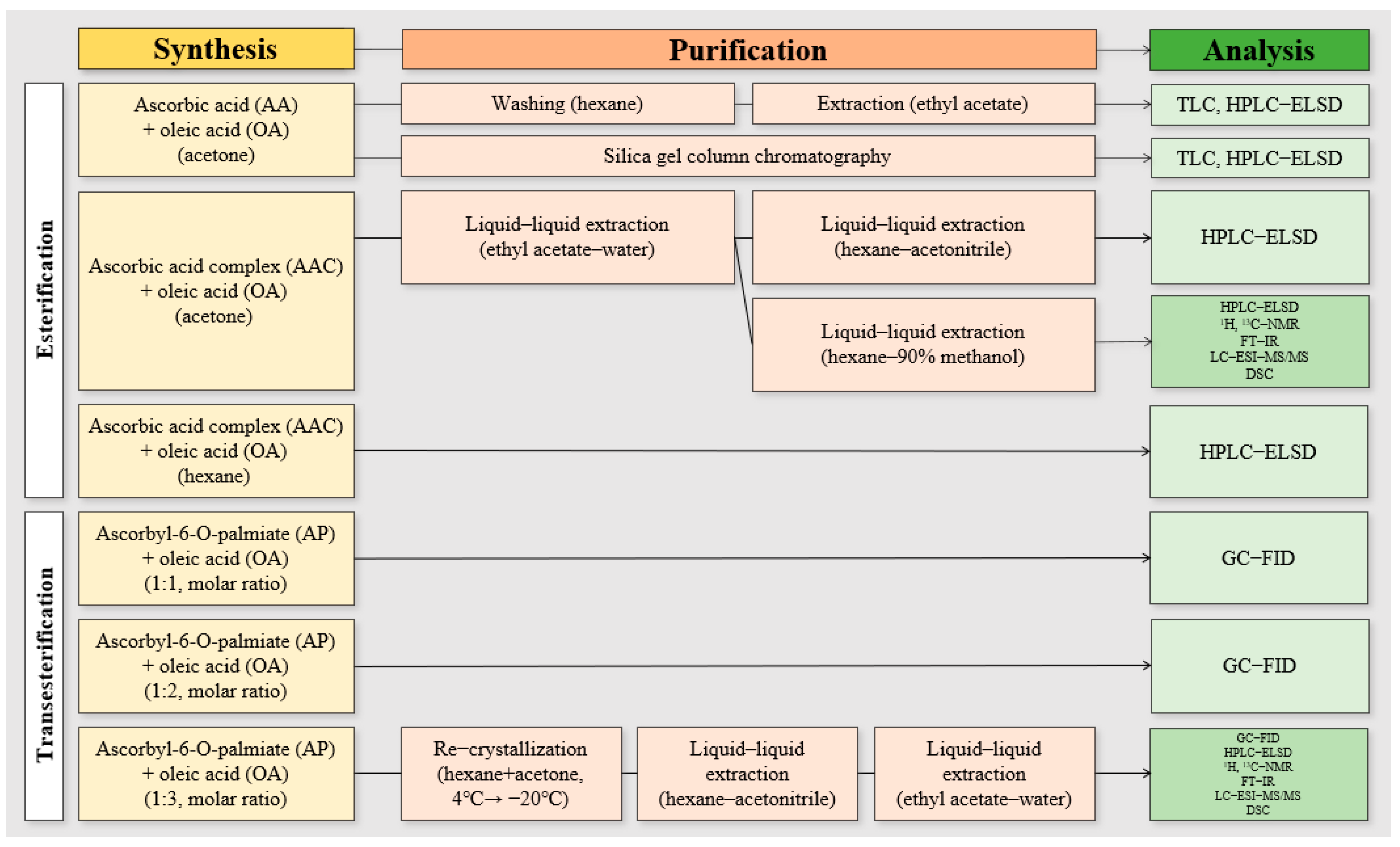
2.2. Synthesis and Purification of AO by Lipase-Catalysed Esterification
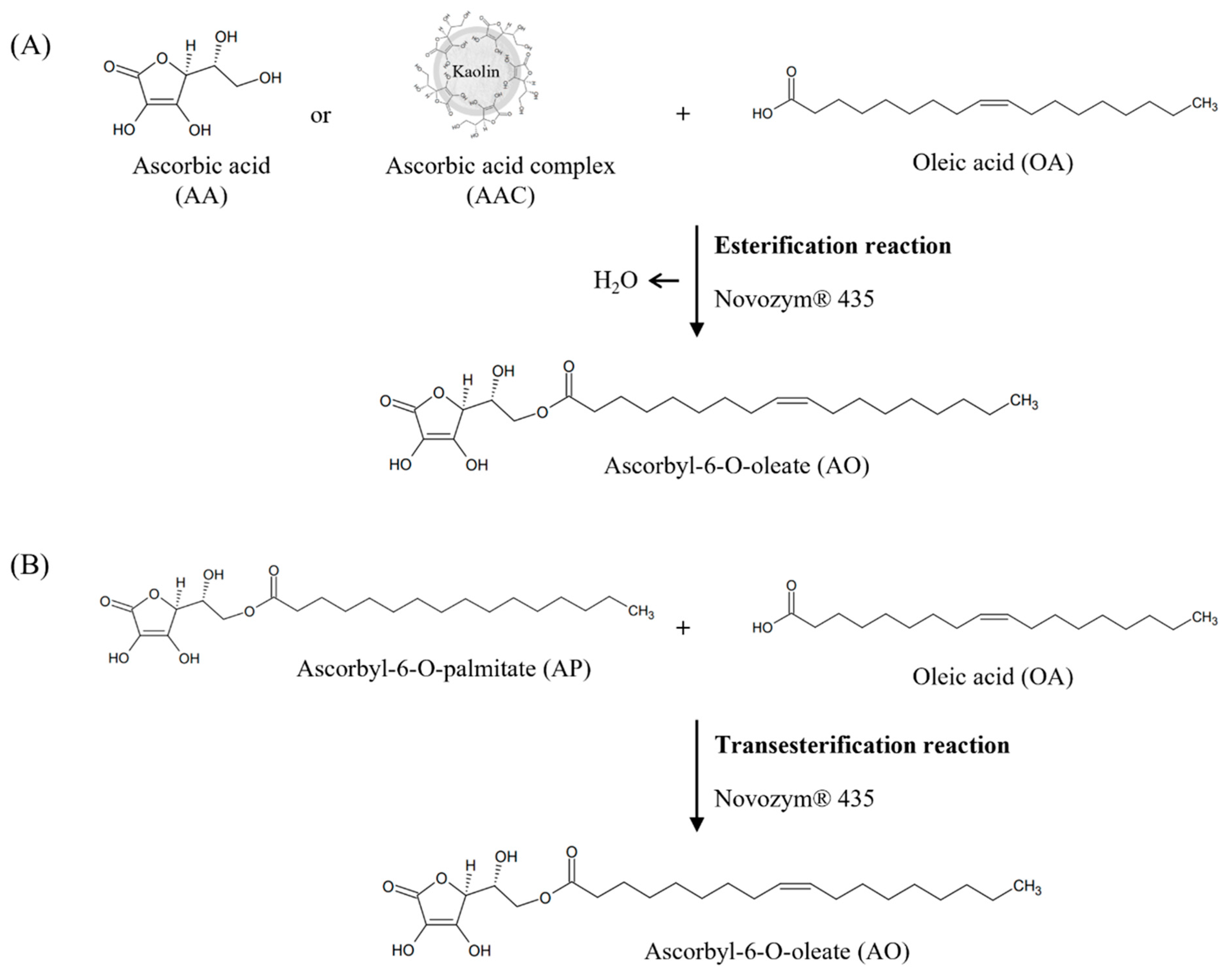
2.2.1. Synthesis of AO Using AA
2.2.2. Synthesis of AO Using AA Complex
2.2.3. Purification of AO Synthesised by Lipase-Catalysed Esterification
2.3. Synthesis and Purification of AO by Lipase-Catalysed Transesterification
2.3.1. Lipase-Catalysed Transesterification Reaction for AO Synthesis
2.3.2. Purification Process of AO Synthesized by Lipase-Catalysed Transesterification
2.4. Analysis
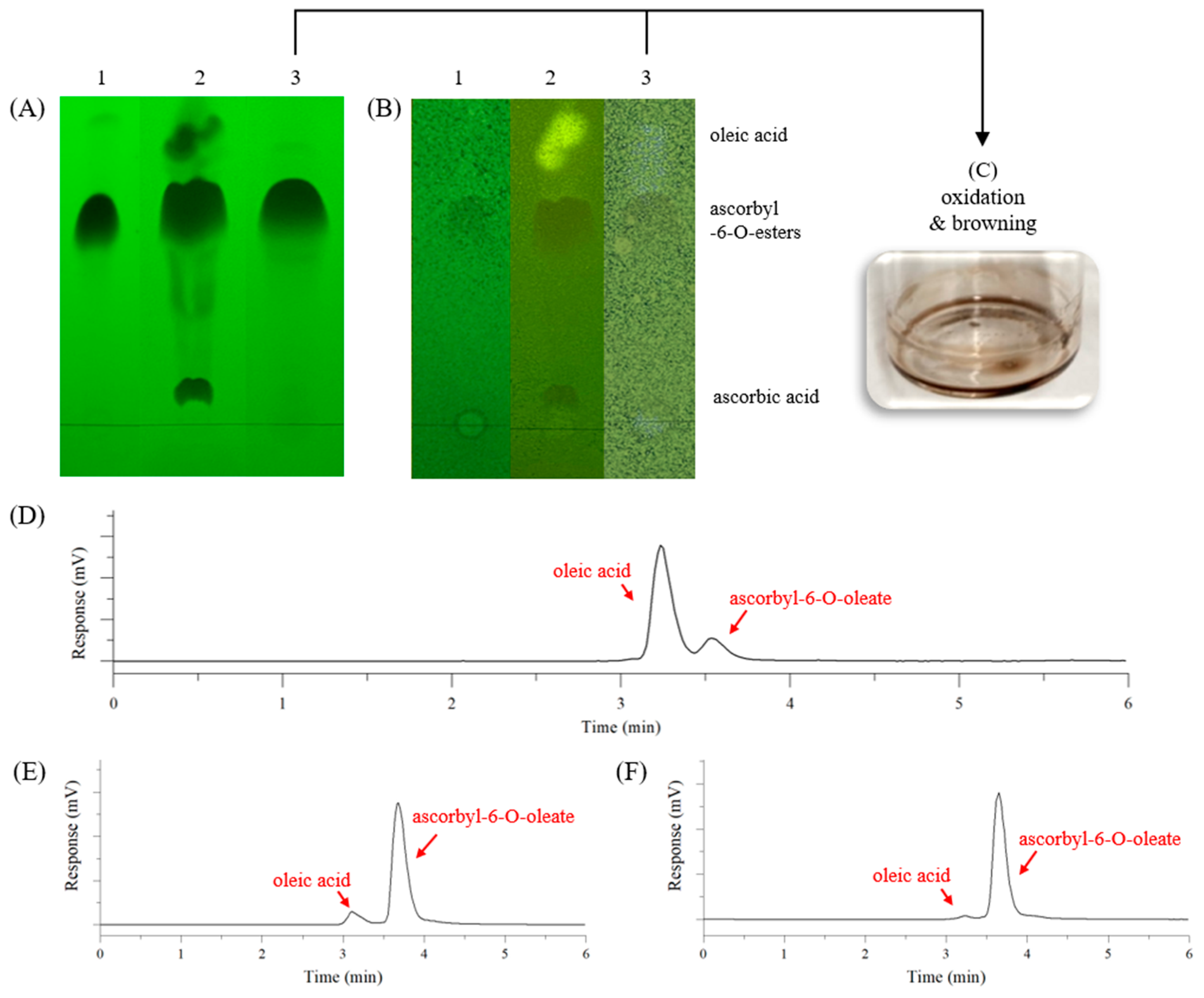
2.4.1. Thin Layer Chromatography (TLC)
2.4.2. High-Performance Liquid Chromatography–Evaporative Light Scattering Detection (HPLC-ELSD)
2.4.3. Gas Chromatography–Flame Ionisation Detection (GC-FID)
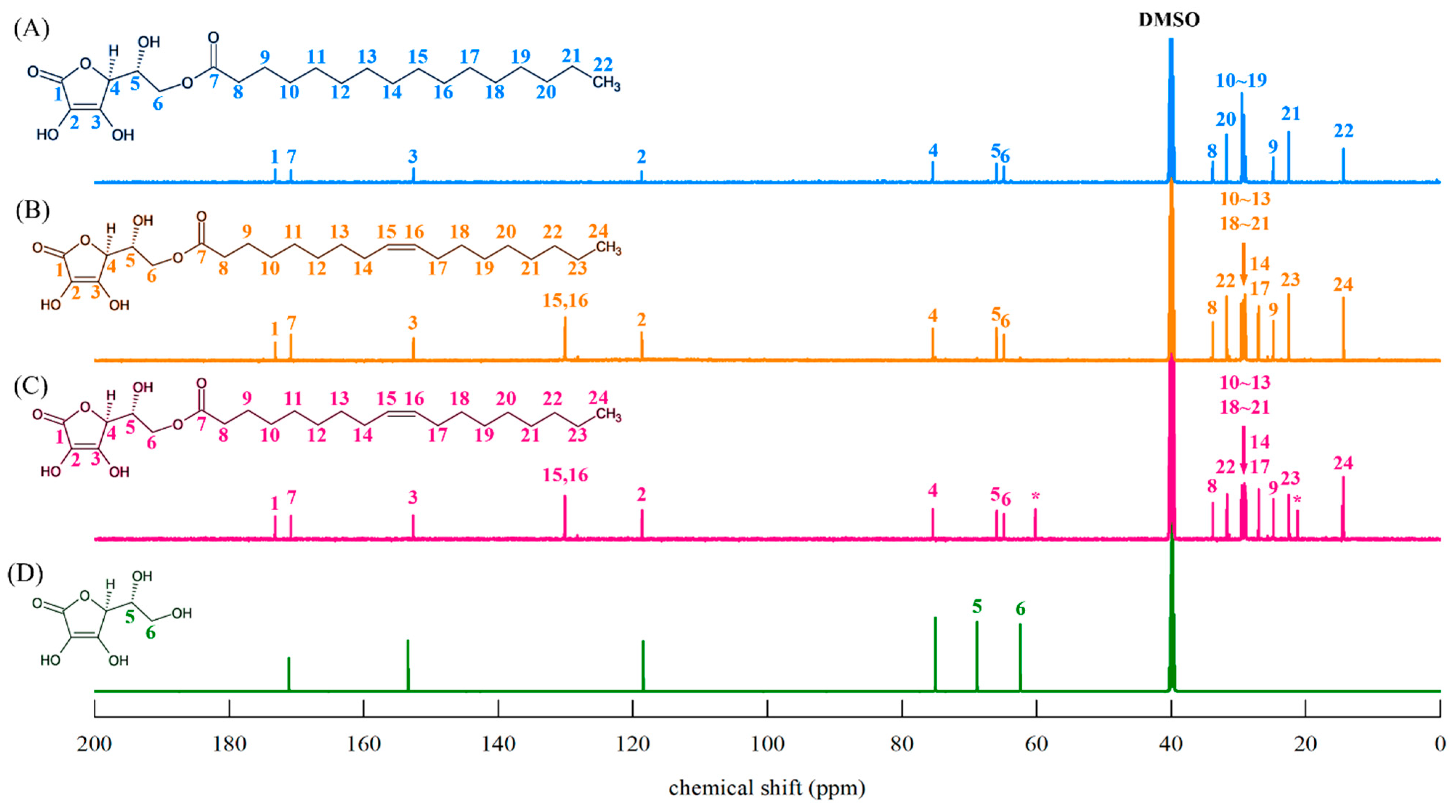
2.4.4. 1H-NMR and 13C-NMR Spectroscopy
2.4.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.6. Liquid Chromatography Electrospray Ionisation Tandem Mass Spectrometry (LC-ESI-MS/MS)
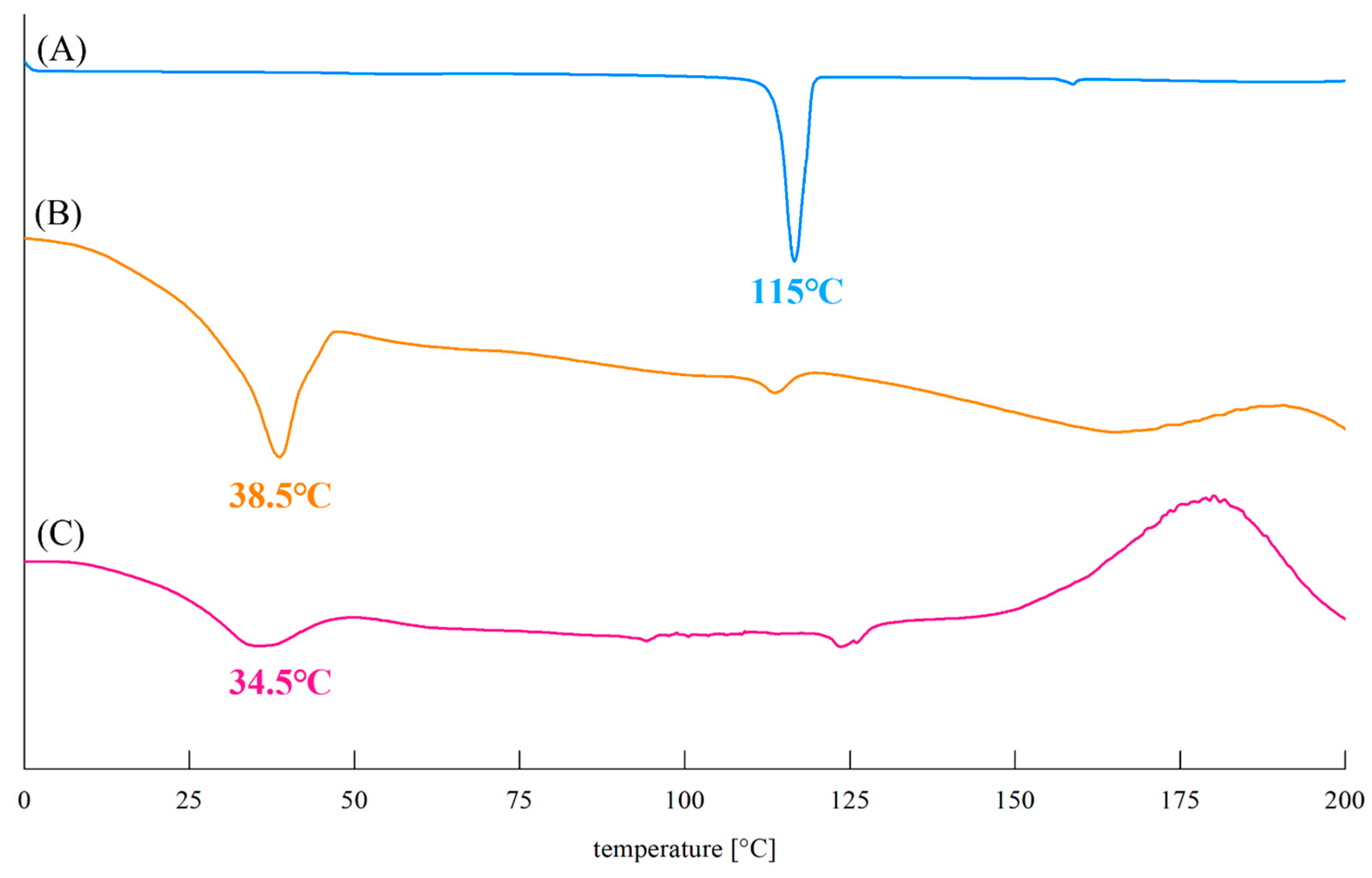
2.4.7. Differential Scanning Calorimetry (DSC)
3. Results
3.1. Synthesis of AO by Lipase-Catalysed Esterification
3.2. Purification of AO Reaction Product Synthesised by Lipase-Catalysed Esterification
3.3. Synthesis of AO by Lipase-Catalysed Transesterification
3.4. Purification of AO by Lipase-Catalysed Transesterification
3.5. AO Analysis
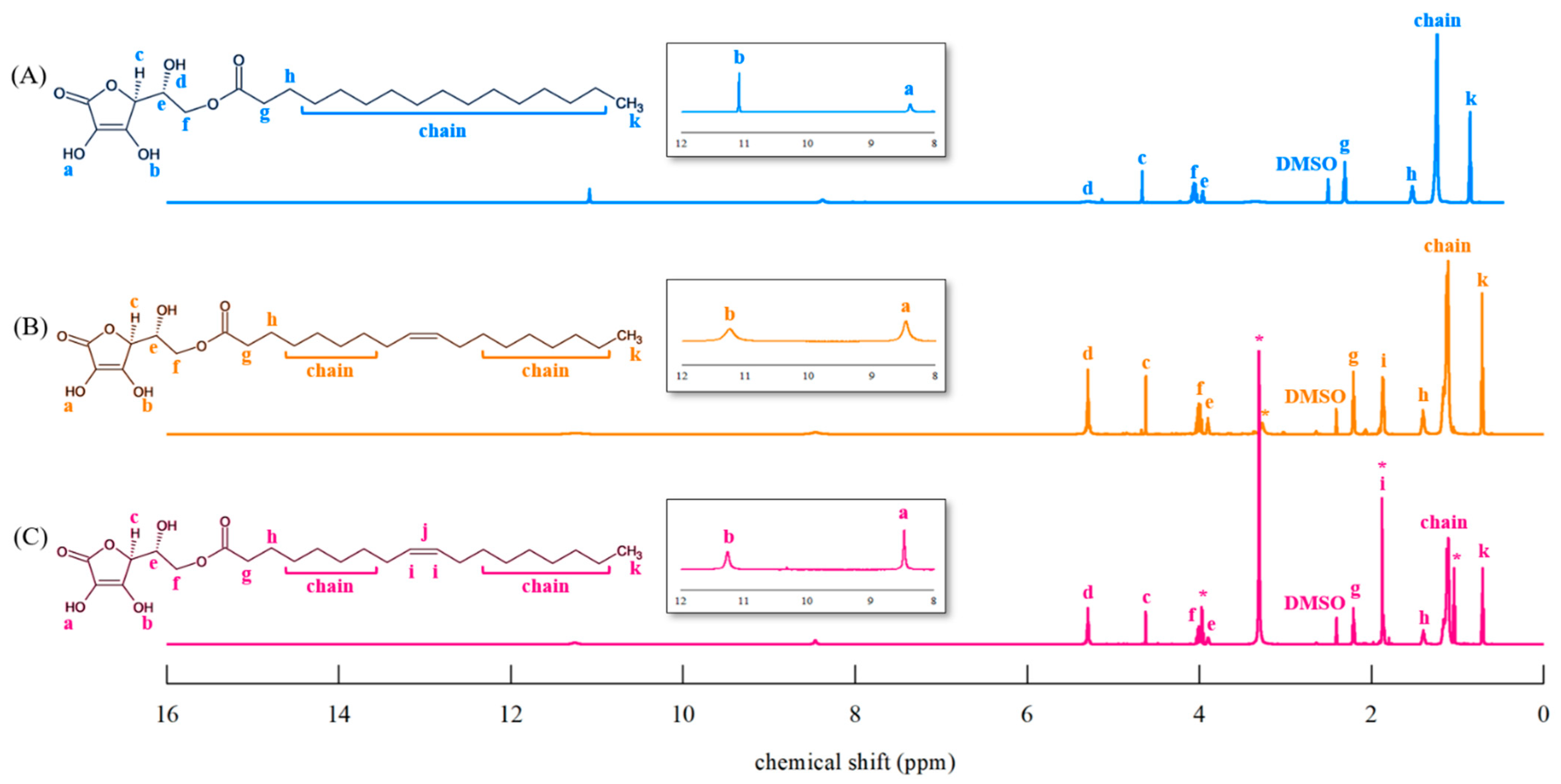
3.5.1. 1H-NMR Spectroscopy
3.5.2. 13C-NMR Spectroscopy
3.5.3. FT-IR Spectroscopy and LC-ESI-MS/MS
3.5.4. DSC
4. Discussion
4.1. Reaction Solvents for AO Synthesis
4.2. Purification of AO from Reactants
4.3. Synthesis of AO Through Transesterification Reaction
4.4. Structural Identification and Analysis of AO
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, M.G.; Kavadia, M.R.; Vadgama, R.N.; Odaneth, A.A.; Lali, A.M. Production of 6-O-l-Ascorbyl Palmitate by Immobilized Candida Antarctica Lipase B. Appl. Biochem. Biotechnol. 2018, 184, 1168–1186. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.A.D.; Catarina, A.S.; Leal, I.C.R.; Sathler, P.C.; De Oliveira, D.; De Oliveira, A.A.S.C.; Cansian, R.L.; Dallago, R.M.; Zeni, J.; Paroul, N. Enzymatic Synthesis of Ascorbyl Oleate and Evaluation of Biological Activities. Food Res. Int. 2022, 161, 111851. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of Free and Encapsulated Natural Antioxidants in Oxidative Stability of Edible Oil: Special Emphasis on Nanoemulsion-Based Encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Karmee, S.K. Biocatalytic Synthesis of Ascorbyl Esters and Their Biotechnological Applications. Appl. Microbiol. Biotechnol. 2009, 81, 1013–1022. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Guo, F.-L.; Wu, L.-M.; Liu, Y.-C.; Liu, Z.-L. Remarkable Enhancement of Antioxidant Activity of Vitamin C in an Artificial Bilayer by Making It Lipo-Soluble. Chem. Phys. Lipids 1996, 83, 39–43. [Google Scholar] [CrossRef]
- Park, K.-Y.; Kim, J. Synthesis and Biological Evaluation of Ascorbyl-Conjugated Peptide Derivatives as Collagen Synthesis Stimulating Agents in Human Skin Fibroblasts. Int. J. Pept. Res. Ther. 2020, 26, 2449–2456. [Google Scholar] [CrossRef]
- Thiele, N.; McGowan, J.; Sloan, K. 2-O-Acyl-3-O-(1-Acyloxyalkyl) Prodrugs of 5,6-Isopropylidene-l-Ascorbic Acid and l-Ascorbic Acid: Antioxidant Activity and Ability to Permeate Silicone Membranes. Pharmaceutics 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Elmore, A.R. Final Report of The Safety Assessment of L-ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as Used in Cosmetics. Int. J. Toxicol. 2005, 24, 51–111. [Google Scholar] [CrossRef]
- Stojanović, M.; Carević, M.; Mihailović, M.; Veličković, D.; Dimitrijević, A.; Milosavić, N.; Bezbradica, D. Influence of Fatty Acid on Lipase-catalyzed Synthesis of Ascorbyl Esters and Their Free Radical Scavenging Capacity. Biotechnol. Appl. Biochem. 2014, 62, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Decker, E.A.; Lee, J. Antioxidant Capacities of α-Tocopherol, Trolox, Ascorbic Acid, and Ascorbyl Palmitate in Riboflavin Photosensitized Oil-in-Water Emulsions. Food Chem. 2012, 133, 68–75. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Misawa, R. Effect of Emulsifier Concentration on the Oxidation of an O/W Emulsion Prepared from Canola Oil. FNS 2018, 9, 683–692. [Google Scholar] [CrossRef][Green Version]
- López-Martínez, A.; Rocha-Uribe, A. Antioxidant Hydrophobicity and Emulsifier Type Influences the Partitioning of Antioxidants in the Interface Improving Oxidative Stability in O/W Emulsions Rich in N-3 Fatty Acids. Eur. J. Lipid Sci. Technol. 2018, 120, 1700277. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Ye, J.; Chen, Y.; Wang, R.; Jin, D.; Liu, Y. Mechanism Study on Nanoparticle Negative Surface Charge Modification by Ascorbyl Palmitate and Its Improvement of Tumor Targeting Ability. Molecules 2022, 27, 4408. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Duarte, D.; Lopez-Cortes, N.; Torres, P.; Comelles, F.; Parra, J.L.; Peña, S.; Ugidos, A.V.; Ballesteros, A.; Plou, F.J. Synthesis and Properties of Ascorbyl Esters Catalyzed by Lipozyme TL IM Using Triglycerides as Acyl Donors. J. Am. Oil Chem. Soc. 2011, 88, 57–64. [Google Scholar] [CrossRef]
- Viklund, F.; Alander, J.; Hult, K. Antioxidative Properties and Enzymatic Synthesis of Ascorbyl FA Esters. J. Am. Oil Chem. Soc. 2003, 80, 795–799. [Google Scholar] [CrossRef]
- Yang, H.; Xie, X.; Seib, P.A. Lipase-Catalyzed Synthesis of C-6 Saturated and Unsaturated Fatty Acid Esters of L-Ascorbic Acid. J. Appl. Glycosci. 2003, 50, 373–378. [Google Scholar] [CrossRef]
- Liu, Y. Biocatalytic Synthesis and Antioxidant Capacities of Ascorbyl Esters by Novozym 435 in Tert-Butanol System Using Different Acyl Donors. Afr. J. Biotechnol. 2011, 10, 17282–17290. [Google Scholar] [CrossRef]
- Holtheuer, J.; Tavernini, L.; Bernal, C.; Romero, O.; Ottone, C.; Wilson, L. Enzymatic Synthesis of Ascorbyl Palmitate in a Rotating Bed Reactor. Molecules 2023, 28, 644. [Google Scholar] [CrossRef] [PubMed]
- Lerin, L.A.; Richetti, A.; Dallago, R.; Treichel, H.; Mazutti, M.A.; Oliveira, J.V.; Antunes, O.A.C.; Oestreicher, E.G.; De Oliveira, D. Enzymatic Synthesis of Ascorbyl Palmitate in Organic Solvents: Process Optimization and Kinetic Evaluation. Food Bioprocess. Technol. 2012, 5, 1068–1076. [Google Scholar] [CrossRef]
- Humeau, C.; Girardin, M.; Coulon, D.; Miclo, A. Synthesis of 6-O-Palmitoyl L-Ascorbic Acid Catalyzed by Candida Antartica Lipase. Biotechnol. Lett. 1995, 17, 1091–1094. [Google Scholar] [CrossRef]
- Humeau, C.; Girardin, M.; Rovel, B.; Miclo, A. Enzymatic Synthesis of Fatty Acid Ascorbyl Esters. J. Mol. Catal. B Enzym. 1998, 5, 19–23. [Google Scholar] [CrossRef]
- Watanabe, Y.; Minemoto, Y.; Adachi, S.; Nakanishi, K.; Shimada, Y.; Matsuno, R. Lipase-catalyzed synthesis of 6-O-eicosapentaenoyl L-ascorbate in acetone and its autoxidation. Biotechnol. Lett. 2000, 22, 637–640. [Google Scholar] [CrossRef]
- Bradoo, S.; Saxena, R.K.; Gupta, R. High Yields of Ascorbyl Palmitate by Thermostable Lipase-mediated Esterification. J. Am. Oil Chem. Soc. 1999, 76, 1291. [Google Scholar] [CrossRef]
- Stojanović, M.; Velićković, D.; Dimitrijević, A.; Milosavić, N.; Knežević-Jugović, Z.; Bezbradica, D. Lipase-Catalyzed Synthesis of Ascorbyl Oleate in Acetone: Optimization of Reaction Conditions and Lipase Reusability. J. Oleo Sci. 2013, 62, 591–603. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Perini, I.; Ambrosi, M.; Tanini, D.; Ninham, B.W.; Capperucci, A.; Nostro, P.L. Ascorbyl-6-O-oleate: A Bioconjugate Antioxidant Lipid. ChemistrySelect 2020, 5, 1938–1944. [Google Scholar] [CrossRef]
- Venturi, V.; Lerin, L.A.; Presini, F.; Giovannini, P.P.; Catani, M.; Buratti, A.; Marchetti, N.; Dilliraj, L.N.; Aprile, S. Enzymatic Synthesis of Ascorbic Acid-Ketone Body Hybrids. Catalysts 2023, 13, 691. [Google Scholar] [CrossRef]
- Tufiño, C.; Bernal, C.; Ottone, C.; Romero, O.; Illanes, A.; Wilson, L. Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate. Molecules 2019, 24, 3227. [Google Scholar] [CrossRef]
- Song, Q.-X.; Wei, D.-Z. Study of Vitamin C Ester Synthesis by Immobilized Lipase from Candida sp. J. Mol. Catal. B Enzym. 2002, 18, 261–266. [Google Scholar] [CrossRef]
- Joshi, M.P.; Uday, A.; Rajamani, S. Elucidating N-Acyl Amino Acids as a Model Protoamphiphilic System. Commun. Chem. 2022, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Viklund, F. Surfactants Based on Natural Products: Enzymatic Synthesis & Functional Characterization. Doctoral Dissertation, Royal Institute of Technology, Stockholm, Sweden, 2003. [Google Scholar]
- Noroozi-Pesyan, N.; Dabbagh, A.H. Alumina and Silica Oxides as Catalysts for the Oxidation of Benzoins to Benzils under Solvent-Free Conditions. Molecules 2005, 10, 1364–1368. [Google Scholar] [CrossRef]
- Shephard, A.B.; Nichols, S.C.; Braithwaite, A. Moisture Induced Solid Phase Degradation of L-Ascorbic Acid. Talanta 1999, 48, 585–593. [Google Scholar] [CrossRef]
- Hoerr, C.W.; Harwood, H.J. The Solubilities of Oleic and Linoeic Acids in Common Organic Solvents. J. Phys. Chem. 1952, 56, 1068–1073. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process 2016, 4, 7. [Google Scholar] [CrossRef]
- Li, C.-P.; Du, M. Role of Solvents in Coordination Supramolecular Systems. Chem. Commun. 2011, 47, 5958. [Google Scholar] [CrossRef]
- Kleiman, M.; Ryu, K.A.; Esser-Kahn, A.P. Determination of Factors Influencing the Wet Etching of Polydimethylsiloxane Using Tetra-n-butylammonium Fluoride. Macro Chem. Phys. 2016, 217, 284–291. [Google Scholar] [CrossRef]
- Kim, H.; Koo, H.; Oh, W.Y.; Myoung, S.; Ahn, S.; Kim, M.-J.; Lee, J. Changes of Molecular Mobility of Ascorbyl Palmitate and α-Tocopherol by Phospholipid and Their Effects on Antioxidant Properties in Bulk Oil. Food Chem. 2023, 403, 134458. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Studies on the Enzymatic Synthesis of Lipophilic Derivatives of Natural Antioxidants. J. Am. Oil Chem. Soc. 1999, 76, 1505. [Google Scholar] [CrossRef]
- Park, K.-M.; Lee, D.E.; Sung, H.; Lee, J.; Chang, P.-S. Lipase-Catalysed Synthesis of Erythorbyl Laurate in Acetonitrile. Food Chem. 2011, 129, 59–63. [Google Scholar] [CrossRef]
- Premaratne, W.A.P.J.; Priyadarshana, W.M.G.I.; Gunawardena, S.H.P.; De Alwis, A.A.P. Synthesis of Nanosilica from Paddy Husk Ash and Their Surface Functionalization. J. Sci. Univ. Kelaniya 2013, 8, 33–48. [Google Scholar] [CrossRef]
- He, W.S.; Zhao, L.; Sui, J.; Li, X.; Huang, S.; Ding, H.; Zhu, H.; Chen, Z.Y. Enzymatic Synthesis of a Novel Antioxidant Octacosanol Lipoate and Its Antioxidant Potency in Sunflower Oil. J. Agric. Food Chem. 2024, 72, 21781–21793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Deng, C.; Zhong, H.; Gu, T.; Goh, K.-L.; Han, Z.; Zheng, M.; Zhou, Y. Green and Efficient Synthesis of Highly Liposoluble and Antioxidant L-Ascorbyl Esters by Immobilized Lipases. J. Clean. Prod. 2022, 379, 134772. [Google Scholar] [CrossRef]
- Ogawa, S.; Hirase, R.; Ohishi, T.; Hara, S.; Takahashi, I. Thermal Behavior of Anhydrous Ascorbic Acid 6-Palmitate with Trace of Decomposition. ChemistrySelect 2020, 5, 10022–10028. [Google Scholar] [CrossRef]


| Ion Source Type | ESI, Negative Ion Mode | ||
|---|---|---|---|
| Gas temp | 300 °C | ||
| Gas flow rate | 9 L/min | ||
| Nebulizer gas | 45 psi | ||
| Sheath gas temp | 320 °C | ||
| Sheath gas flow | 11 L/min | ||
| Scan Type | Product ion (MS2 Scan range: m/z 100~500) | ||
| Compound | Fragment (V) | CE (V) | |
| Ascorbyl-6-O-palmitate | 220 | 20 | |
| Ascorbyl-6-O-oleate | 170 | 20 | |
| Reaction Time (h) | Synthesis Yields (%) of Ascorbyl-6-O-Oleate | ||
|---|---|---|---|
| AA-OA (1) | AAC-OA (2) | ||
| Acetone | Acetone | Hexane | |
| 24 | 18.3 ± 0.1 b (3) | 13.8 ± 0.5 c | - (4) |
| 48 | 19.2 ± 0.1 ab | 19.3 ± 0.5 ab | - |
| 72 | 19.7 ± 0.1 a | 19.7 ± 0.1 a | - |
| Reaction Time (h) | Conversion Rates (%) of Ascorbyl-6-O-Oleate | ||
|---|---|---|---|
| Ascorbyl-6-O-Palmitate: Oleic Acid | |||
| 1:1 | 1:2 | 1:3 | |
| 24 | 38.6 ± 0.1 f | 57.2 ± 0.1 d | 69.8 ± 0.1 b |
| 48 | 49.0 ± 0.1 e | 64.9 ± 0.1 c | 73.2 ± 0.1 a |
| 72 | 50.1 ± 0.1 e | 64.8 ± 0.1 c | 73.8 ± 0.1 a |
| Area % | |||||
|---|---|---|---|---|---|
| HPLC | GC | ||||
| Ascorbyl-6-O-Esters | Fatty Acid | Ascorbyl-6-O-Oleate | Ascorbyl-6-O-Palmitate | ||
| Hexane layers after recrystallisation | 4 °C | NA | NA | 90.5 ± 0.3 | 9.5 ± 0.3 |
| −20 °C | NA | NA | 91.0 ± 0.6 | 9.0 ± 0.6 | |
| Final purified product | 94.3 ± 0.1 | 5.7 ± 0.1 | 91.1 ± 0.2 | 8.9 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.-E.; Kim, S.-Y.; So, H.; Prayitno, V.; Lee, K.-T.; Shin, J.-A. A Novel Eco-Friendly Process for the Synthesis and Purification of Ascorbyl-6-Oleates. Foods 2025, 14, 70. https://doi.org/10.3390/foods14010070
Ji H-E, Kim S-Y, So H, Prayitno V, Lee K-T, Shin J-A. A Novel Eco-Friendly Process for the Synthesis and Purification of Ascorbyl-6-Oleates. Foods. 2025; 14(1):70. https://doi.org/10.3390/foods14010070
Chicago/Turabian StyleJi, Ha-Eun, Se-Young Kim, Heejin So, Vivian Prayitno, Ki-Teak Lee, and Jung-Ah Shin. 2025. "A Novel Eco-Friendly Process for the Synthesis and Purification of Ascorbyl-6-Oleates" Foods 14, no. 1: 70. https://doi.org/10.3390/foods14010070
APA StyleJi, H.-E., Kim, S.-Y., So, H., Prayitno, V., Lee, K.-T., & Shin, J.-A. (2025). A Novel Eco-Friendly Process for the Synthesis and Purification of Ascorbyl-6-Oleates. Foods, 14(1), 70. https://doi.org/10.3390/foods14010070






