Abstract
Disporopsis aspersa (Hua) Engl. ex K. Krause, locally known as kucai (bitter greens) or yexiahua, is a widely consumed wild vegetable and traditional herbal medicine in western Yunnan. Despite its local significance, its nutrient composition and bioactive properties have not been investigated. This study aims to determine the nutritional content and evaluate the antioxidant and anti-inflammatory activities of the aerial parts extracts of D. aspersa. The levels of protein, amino acids, vitamins, and minerals were measured and compared to those of common vegetables. The results showed that D. aspersa contains 16 amino acids, with a total content of up to 19.13 g/100 g, including 3.0 g/100 g of lysine. In vitro evaluations of its antioxidant and anti-inflammatory activities demonstrated that the ethanolic extract exhibited low cytotoxicity against mouse RAW 264.7 murine macrophages cell line at concentrations of 0–120 μg/mL. The IC50 for nitric oxide (NO) scavenging activity was 72.7 ± 7.43 μg/mL, showing dose dependence. Additionally, the ethanolic extract also exhibited ABTS+· scavenging capacity and total antioxidant capacity. These findings suggest that D. aspersa is rich in carbohydrates, fat, dietary fiber, and amino acids. It also contains various bioactive substances, supporting its traditional practices for both medicinal and dietary purposes by local people. D. aspersa has the potential to be developed into a novel anti-hypertensive food, nutraceutical, or dietary supplement in western Yunnan and neighboring regions, promoting local development.
1. Introduction
Diet plays a crucial role in influencing human health, as the 2022 Global Burden of Cardiovascular Disease project noted, particularly in relation to cardiovascular disease (CVD) [1]. Moreover, there are many overlaps between medicinal and dietary products, especially when it comes to medicinal and edible plants [2,3]. This connection not only emphasizes the importance of a balanced diet in disease prevention, but also highlights the significant market potential of food therapy as a holistic approach to health management. Therefore, wild edible plants (WEPs), which are rich in bioactive compounds, and natural therapies have gained significant interest due to their nutritional and medicinal properties [4,5]. Dietary therapy is becoming increasingly important in preventing and treating diseases and lifestyle disorders in both developing and developed countries [4,6]. Traditional knowledge (TK) of plants is the relationship between a community and the environment around it. Documentation of traditional knowledge is necessary for identifying WEPs to create production systems for sustainable use, commercialization, and conservation [5]. Based on TK, research into the nutrient value of wild vegetable resources and testing the bioactivity of their crude extracts will help us make better use of these resources.
Disporopsis aspersa (Hua) Engler ex K. Krause, a member of the family Asparagaceae, is a wild vegetable commonly consumed in the Yangtze River basin, including Sichuan, Guangxi, Hubei, Hunan, and Yunnan [7,8]. Zhongyao Ziyuan Zhiyao of China record that the rhizome of D. aspersa is used to treat rheumatic pain, rupture, and reducing internal inflammation [9]. Zhongguo Minzuyao Zhiyao listed it as an ethnomedicine of the Tujia people under the name lluang shan qi, with its rhizome being used to treat physical weakness, nourish the spleen and stomach, and alleviate food stagnation [10].
Even though it had preliminarily proved that D. aspersa has antifungal, neuritogenic, and anti-inflammatory activity [11], comprehensive evaluations of this species remain limited. Most previous studies have primarily focused on the anti-cancer effects and its ability to control potato late blight and cucumber downy mildew of its rhizome extracts. Song et al. and Nguyen et al. have verified the anti-cancer activity of the high-isoflavone compounds found in D. aspersa rhizome extracts, reporting its potential anti-cancer properties [12,13]. Zhu et al. conducted studies on the whole plant of D. aspersa, demonstrating that its crude extracts exhibit significant antibacterial activity against Pseudoperonospora cubensis and Phytophthora infestans [8]. Furthermore, other species within the genus Disporopsis also show broad pharmacological activities. Meng et al. screened for active components in Disporopsis fuscopicta that inhibit angiogenesis and confirmed that the isolated compounds effectively hinder the proliferation of human umbilical vein endothelial cells (ECV304) [14]. Tran et al. isolated two new spirostane glycosides from Disporopsis longifolia and evaluated their ability to inhibit nitric oxide (NO) production in lipopolysaccharide (LPS) activated RAW 264.7 cells, finding that both compounds exhibited strong anti-inflammatory activity [15]. This suggests that D. aspersa may possess undiscovered pharmacological potential. Known for its medicinal and edible properties, as well as its versatility and wide distribution, D. aspersa remains an enigmatic wild vegetable in the markets of the western Gaoligong Mountains in Yunnan. Mysteries persist regarding its cytotoxicity, nutritional composition, and the perceptions and management practices of local populations toward this resource.
This study aims to systematically evaluate the ethnobotanical relevance, nutritional composition, and pharmacological bioactivity of the aerial parts of D. aspersa, addressing its high seasonal demand and extensive traditional knowledge. Nutritional analysis will focus on quantifying proteins, lipids, carbohydrates, ash, amino acids, and minerals. Bioactivity assessments will include cytotoxicity, anti-inflammatory properties, and in vitro antioxidant activity of D. aspersa extracts.
2. Materials and Methods
2.1. Ethnobotanical Research
Ethnobotanical surveys were carried out three times in the western section of the Gaoligong Mountains in Yunnan from April 2023 to May 2024, and the entire study was conducted mainly in Longyang District and Tengchong City, Yunnan, China.
Ethnobotany field surveys included market surveys, semi-structured interviews, key informant interviews, and participatory observation [16,17,18]. In total, 18 informants (14 men and 4 women) between the age of 22 and 70 were selected using the snowballing method. The interviews included gathering data on traditional knowledge of Disporopsis aspersa, including the vernacular name, medicinal value, traditional uses, distribution, and growth, along with informants’ basic information (e.g., age, nationality, occupation). Pre-prepared images of the plant and its parts minimized misidentification. Collected information was organized, analyzed, and followed by field investigations to gather voucher specimens and materials.
In the process of investigation, the first recorded specimens, noting collection time, detailed location (e.g., latitude, longitude, altitude), and both local and Latin names, were collected. For the identification of plants, the voucher specimens were studied and compared with reference books (Flora of China, Flora of Yunnan) and online databases (https://www.iplant.cn/ and https://www.worldfloraonline.org/ (accessed on 11 November 2024)).
2.2. Preparation of Disporopsis aspersa
Samples of D. aspersa were selected and collected from markets in Longyang District and Tengchong City, Baoshan, Yunnan Province, in June 2023 and May 2024. Voucher specimens (specimen number: MUC-2024-13) were identified by Professor Long Chunlin and deposited in the herbarium at Minzu University of China, in Beijing.
The aerial parts were air-dried, ground, and sieved through a 40-mesh screen. Given its affinity for both water-soluble and lipid-soluble compounds, and combined with traditional knowledge usages of D. aspersa, 95% ethanol was selected as the extraction solvent to obtain an ethanolic extract (EE) [8,15,19]. Approximately 20 g of the ground material were weighed and extracted with 200 mL of 95% ethanol using Soxhlet extraction for 6 h [20]. The mixture was centrifuged at 12,000 r·min−1 for 10 min at 25 °C and the supernatant was collected. The residue underwent two additional extractions and the extracts were combined. The solvent was removed using an R-210 rotary evaporator (Büchi, CHN), and the resulting 2.35 g dried extract was freeze-dried at −80 °C. This process yielded the 95% ethanol extracts (EE), which were stored at −20 °C for future use.
2.3. Proximate Analysis
GB stands for the National Standards of the People’s Republic of China. These standards are formulated or approved by the Standardization Administration of China (SAC) and are designed to unify technical, managerial, and basic requirements across various fields nationwide. They are rigorously researched and validated, widely applied across industries to ensure the quality, safety, and reliability of products, services, and processes.
2.3.1. Moisture Content
Percentages of moisture were determined by an electric constant temperature blower drying oven under 103 °C for 4 h to constant weight per the China national standards method (GB 5009.3-2016) [21,22].
2.3.2. Ash Content
Ash was determined by direct analysis according to the China standards method (GB 5009.4-2016), the sample was fully carbonized to smokeless and weighed [21,23]. The dry sample was then placed in the energy-saving box-type furnace at 550 °C for 4 h, removed, and placed into a desiccator for 30 min after cooling down to 200 °C. The whole process was repeated until to a constant weight.
2.3.3. Fat Content
Total fat content was determined by solvent extraction method according to the China standards method (GB 5009.6-2016) [24,25].
2.3.4. Total Dietary Fiber Content
Dietary content was determined according to the China standards method (GB 5009.88-2014) [26,27]. Take the dried samples and crush them repeatedly until they are completely sieved. After the protein and starch were removed by the enzymolysis of heat-stabilized α-amylase, protease, and glucosidase, the dried sample was precipitated by ethanol, pumped and filtered, the residue was washed with ethanol and acetone, dried and weighed, and the total dietary fiber residue was obtained.
2.3.5. Crude Protein
Protein content was determined by Kjeldahl nitrogen using the China standards method (GB 5009.5-2016) [28]. The percentage of crude protein was estimated as the total nitrogen content multiplied by the conversion factor 6.25.
2.3.6. Carbohydrate and Energy Content
Total carbohydrates were calculated by subtracting the total percentage of other components (saccharides, starch, and dietary fiber) from 100 according to the China standards method (GB/Z 21922-2008) [29,30].
Carbohydrates can be calculated using the following formula:
Energy can be calculated using the following formula:
2.4. Mineral Composition
The mineral elements were determined using an inductively coupled plasma mass spectrometer (ICP-MS) (A1-IE-2855, PONY, Beijing, China). For quantification, an external standard method was used, where the intensity ratio of the target element’s mass spectrometric signal to that of an internal standard element was directly proportional to the concentration of the target element. Potassium, phosphorus, sodium, iron, manganese, magnesium, calcium, copper, and zinc were determined using GB 5009.268-2016 (the second method) [31], while Se was determined using GB 5009.93-2017 (the first method) [32,33].
2.5. Amino Acid Composition
Amino acids were measured according to the method GB 5009.124-2016 by Amino acid Analyzer (A1-IE-2855, PONY) [24,34]. Data were expressed as milligrams of amino acid per 100 g of Disporopsis aspersa.
2.6. Vitamin Composition
Vitamin E was determined using reversed-phase high-performance liquid chromatography (RP-HPLC) (A1-IE-4812, PONY) according to GB 5009.82-2016 [35,36]. Vitamin B1 and vitamin B2 was determined using high-performance liquid chromatography (HPLC), according to GB 5009.84-2016 and GB 5009.85-2016, respectively. Vitamin B6 was determined using microbiological assay according to GB 5009.154-2016 (the second assay) [37,38,39,40]. Vitamin K1 was determined using High-Performance Liquid Chromatography with Fluorescence Detection (HPLC-FLD) according to GB 5009.158-2016 [41].
2.7. Antioxidant Activity
DPPH· scavenging capacity was assessed using a DPPH Antioxidant Assay Kit (Dojindo, Shanghai, China), while ABTS+· scavenging capacity and total antioxidant capacity were measured using ABTS and FRAP Assay Kits (Beyotime, Shanghai, China) [42,43]. The ABTS rapid method evaluates antioxidant capacity by measuring the ability to inhibit ABTS+· formation, whereas the FRAP method assesses the ability of antioxidants to reduce Fe3+-TPTZ under acidic conditions. Both methods utilize Trolox as a standard. Trolox, a water-soluble vitamin E analogue, has antioxidant activity similar to that of vitamin E. DPPH and ABTS+· scavenging capacities are expressed in terms of Trolox equivalents, while total antioxidant capacity is expressed as Fe2+ equivalents.
2.8. Anti-Inflammatory Activity
2.8.1. Cell Culture
RAW 264.7 cell lines are frequently used to evaluate anti-inflammatory activity of plant extracts by measuring inhibition of lipopolysaccharide-induced (LPS-induced) nitric oxide (NO) production [44]. Therefore, this study selected mouse myoblast cell line RAW 264.7 (Cell Resource Center, Beijing, China). RAW 264.7 cells were cultured in DMEM (Gibco, Shanghai, China) high-glucose complete medium containing 10% (v/v) Fetal Bovine Serum (HyClone, Shanghai, China) and 1% penicillin-streptomycin at 37 °C in a 5% CO2 atmosphere.
2.8.2. Cell Viability
To access the potential cytotoxicity and anti-inflammatory activity of the EE, RAW 264.7 cells were pre-induced with LPS for 18 h [45,46], followed by different concentrations of crude extract treatments. The impact of the EE on RAW 264.7 cells viability was detected using the Cell Counting Kit-8 (CCK-8) (Dojindo, China). After the cells adhered, the EE at varying concentrations were applied for 18 h. Then, suck out the supernatant and add 100 µL of 10% CCK-8 solution to cultures, followed by incubation at 37 °C for 1 h. The OD at 450 nm was measured by a Microplate Reader (Epoch TM, San Jose, CA, USA). According to the OD450 values, the cell viability was calculated as follows:
where AS: absorbance of the sample, AC: absorbance of the control, and ASC: absorbance of solvent control.
2.8.3. Determination of NO Production
Cells (4 × 105 cells/well in 2000 μL of medium) were cultured overnight in a six-well plate. After cell adhesion, the varying concentration EEs were applied with or without 1 μg·mL−1 LPS to induce an inflammatory response. Blank controls were served as no LPS and no EE treatment. After 18 h incubation, the culture supernatant from each well was collected to measure the NO levels using an NO detection kit (Sigma-Aldrich, Saint Louis, MO, USA) to assess the level of inflammation in the culture medium.
2.9. Statistical Analysis
The results are presented as mean ± standard deviation (SD), with all experiments performed in triplicate (n = 3) by GraphPad Prism 8.3.0. Data were analyzed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test.
3. Results and Discussion
3.1. Ethnobotanical Knowledge
Disporopsis aspersa (Hua) Engler ex K. Krause (Asparagaceae) is a herbaceous plant with both edible and medicinal properties (Figure 1). Wild D. aspersa in western Yunnan typically grows at altitudes of approximately 3000 m, primarily beneath bamboo forests or along ravines, often coexisting with plants from the genus Allium, Trillium, and Rhododendron. The local harvest season for D. aspersa occurs during its flowering period in April and May, when locals selectively harvest young leaves, tender stems and flower buds, leaving the mature parts untouched. Each area is harvested only once per season. Regarding sustainable harvesting practices, locals report that D. aspersa thrives with regular harvesting and does not face the risk of overharvesting. Specifically, only young shoots taller than 10 cm are harvested, and hardy parts of the plants are left undisturbed. This approach reflects the ecological awareness and plant conservation in local communities.

Figure 1.
The plant of Disporopsis aspersa in the study area. (A) Aboveground part sold on market; (B) mature leaves; (C,D) stir-fried dish of D. aspersa showing flower buds (photographed credit: the authors).
Locals in the western Gaoligong Mountains refer to D. aspersa by several names based on its various morphological features. The name Zhu-jie-cai is derived from its leaf shape, which resembles bamboo leaves. Niu-wei-ba-cai refers to its young shoots, which resemble an ox tail. Zhu-ye-cai is based on its growth near bamboo groves. Ku-cai reflects its characteristic bitter taste when consumed. Its tender leaves can be served in salad, stir-fried, or used to make soups. It is widely consumed by both the Han people and Lisu people in the area.
Ethnobotanical surveys show that D. aspersa is widely recognized by local residents for its dual medicinal and dietary uses (Table 1). The entire plant is consumed as food or used in traditional medicine, with applications including lowering blood pressure, treating coughs, cooling and detoxifying the body, and nourishing the lungs and stomach. The tender leaves are often eaten raw, stir-fried, or cooked in soups, providing a palatable flavor, while the roots are commonly stir-fried, stewed, steamed, or cooked with chicken, imparting a slightly bitter taste. Additionally, D. aspersa is regarded as an excellent topical remedy for cuts and bruises, known locally as Ye-xia-hua (flower under the leaves). In this usage, crushed leaves are applied to wounds and usually changed daily, promoting wound healing and suggesting antibacterial and anti-inflammatory properties. Traditional knowledge suggests that D. aspersa has potential anti-inflammatory and antioxidant properties, and significant nutritional value.

Table 1.
The vernacular names of Disporopsis aspersa in different linguistic groups.
Different linguistic groups have varying usages: the Han people primarily consume it as food, while the Lisu people apply it externally for wounds. Over time, the Lisu people have also begun to consume it as a vegetable, reflecting cultural exchange. This diversity of uses highlights its dual role as both food and medicine. During the course of our research, local residents frequently compared D. aspersa to other medicinal plants such as Panax notoginseng (sanqi), Basella alba, and Polygonatum species, indicating its potential anti-inflammatory effects and medicinal efficacy. In the research regions, traditional knowledge of D. aspersa has been passed down through generations. Additionally, due to its aesthetically pleasing appearance, D. aspersa is often grown as an ornamental plant.
Since the establishment of the Gaoligong Mountains Nature Reserve in the 1980s, to reduce the difficulty of harvesting, the locals transplant D. aspersa into household gardens at lower elevations, around 1500 m. It can be cultivated near large trees or water sources, requiring only natural rainfall for growth, with minimal additional care. Although some individuals believe that wild D. aspersa has a superior aftertaste compared to cultivated varieties, the market prices of both are comparable, indicating the potential for sustainable development. Given its natural distribution in the Yangtze River basin, including Sichuan, Hubei, Hunan, and Yunnan, D. aspersa deserves to be promoted as a seasonal specialty vegetable.
3.2. Nutrient Compositions
3.2.1. Proximate Analysis
The general nutrient composition and dietary fiber content of D. aspersa are summarized in Table 2. Given that Chinese cabbage (Brassica rapa) and spinach (Spinacia oleracea) are among the most commonly consumed leafy vegetables globally, they were used as benchmarks for comparison. Their nutrient composition comes from China Food Composition Tables (Standard Edition) and USDA National Nutrient Database (https://fdc.nal.usda.gov (accessed on 11 November 2024)) [47]. Carbohydrates (49 g/100 g) are the primary component of D. aspersa, followed by total dietary fiber (37.73 g/100 g) and protein (27.13 g/100 g). Although the fat content is the lowest at 5.60 g/100 g, it is still significantly higher than that of Chinese cabbage and spinach. Except for carbohydrates, D. aspersa surpasses both reference vegetables in all other nutritional components.

Table 2.
Proximate composition, mineral, and vitamin content of 100 g Disporopsis aspersa and other two well-known edible greens.
Dietary fiber (DF), derived from the edible parts of plants, is a carbohydrate-like substance that undergoes fermentation and decomposition by the gut microbiota in the colon. Insufficient DF intake is a modifiable risk factor for chronic disease, making increased DF intake an effective prevention strategy [48,49]. Legumes are generally considered to be high in dietary fiber, with common bean seeds containing 16.21 to 24.50 g/100 g of total DF, which is lower than the 37.73 g/100 g found in D. aspersa [50,51]. As a functional nutrient, DF exerts significant physiological effects, such as shortening transit time, delaying gastric emptying, promoting intestinal motility, modulating gut microbiota, and improving metabolic disorders and immune system function. These effects support weight management for individuals with obesity, as well as reducing the incidence of cardiovascular disease (CVD) and colorectal cancer (CRC) [52,53]. Therefore, D. aspersa has the potential to be considered a functional food that meets modern health dietary requirements, offering benefits not only for improving dietary structures in patients but also for disease prevention in the general population.
3.2.2. Mineral Elements and Vitamins
Ten minerals have been selected for detection in D. aspersa. Five of them are essential trace elements for humans, including iron (Fe), zinc (Zn), copper (Cu), chromium (Cr), and magnesium (Mg), as shown in Table 2. Notably, D. aspersa contains particularly high levels of potassium (K) and phosphorus (P), significantly exceeding those found in spinach and Chinese cabbage. The K content in D. aspersa is 4803.33 mg/100 g, which is substantially higher than that of K-rich vegetables such as spinach (919 mg/100 g). Conversely, its sodium (Na) content is relatively low at 5.46 mg/100 g, especially when compared to spinach (242 mg/100 g), classifying D. aspersa as a low-sodium, high-potassium vegetable, making it particularly beneficial for individuals with hypertension. In comparison to Fe-rich wild or cultivated vegetables like spinach, Diplazium esculentum (23.7 mg/100 g) and Maianthemum atropurpureum (20.3 mg/100 g), D. aspersa exhibits comparable levels of Fe (29.67 mg/100 g) and Mg (197.67 mg/100 g) [6]. Furthermore, the zinc content in D. aspersa (5.62 mg/100 g) is slightly higher than that found in well-known zinc-rich vegetables like Brasenia schreberi (1.53–2.88 mg/100 g) and spinach (3.91 mg/100 g) [54].
Recent studies have identified the interaction between excessive Na and K deficiency as key environmental factors in the pathogenesis of hypertension and cardiovascular disease (CVD) [53], with both Na and K playing significant roles in this process. This result nicely explains traditional applications that consuming D. aspersa could lower blood pressure. Increasing K intake through fresh fruits and vegetables can mitigate the detrimental effects of excessive Na, making K supplementation an effective strategy for primary prevention and treatment of hypertension and CVD, which may be more feasible than sodium restriction [55]. Moreover, the effective supplementation of K can rapidly restore energy levels, making D. aspersa a particularly important vegetable resource for local agricultural labor. Thus, D. aspersa serves as a valuable dietary source of K, P, and other nutrients, aiding individuals deficient in these essential elements in maintaining health.
The vitamin E content in D. aspersa is significantly higher than that in spinach and Chinese cabbage. It exceeds that of commonly recognized vitamin E-rich vegetables such as asparagus (Asparagus officinalis, 1.2–1.5 mg/100 g) [56] and beetroot (1.81 mg/100 g). Additionally, the contents of water-soluble vitamins B1, B2, and B6 are relatively high. The high level of vitamin K1 and vitamin B6 in D. aspersa are beneficial for cardiovascular health, as vitamin K1 is essential for blood clotting, playing a key role in the synthesis of several clotting factors. This also indicates that D. aspersa may help prevent cardiovascular diseases. Furthermore, given the local practices of stir-frying, boiling, and using D. aspersa in salads, its consumption could help prevent vitamin and trace element deficiencies, thereby supporting normal physiological functions.
3.2.3. Amino Acids
The 16 amino acids were identified in D. aspersa and the results are listed in Table 3, with a total amino acid content reaching 19.13 g/100 g. Seven of these are essential amino acids (EAAs), totaling 8.11 g/100 g, representing 42.37% of the total and 73.52% of the non-essential amino acids (NAAs). According to the ideal model proposed by the World Health Organization (WHO) and the Food and Agriculture Organization (FAO), high-quality protein should have an EAA to total amino acid (EAA/TAA) ratio of approximately 40%, and an EAA to NAA (EAA/NAA) ratio exceeding 60% [52,57]. Based on these criterion, D. aspersa qualifies as a high-quality protein source, with EAA/TAA and EAA/NAA ratios surpassing Chinese cabbage and spinach.

Table 3.
Amino acids of Disporopsis aspersa compared with other two well-known edible greens.
Additionally, D. aspersa exhibits the highest concentration of lysine (3.00 g/100 g), followed by glutamic acid (2.27 g/100 g), aspartic acid (1.70 g/100 g), histidine (1.60 g/100 g), and leucine (1.41 g/100 g). Lysine, an essential amino acid that cannot be synthesized by the human body, must be acquired through dietary sources. It promotes calcium absorption and utilization, aids in protein synthesis and tissue repair, and supports bone and muscle growth. Furthermore, lysine plays a crucial role in the production of various important substances in the body, including hormones, enzymes, and antibodies, which are vital for maintaining normal physiological functions [58,59]. Glutamic acid, the primary excitatory neurotransmitter in the central nervous system, is essential for brain function, playing a role in learning, memory, and cognition. Additionally, it also contributes to ammonia production in the kidneys, helping maintain the body’s acid-base balance [60].
The findings indicate that D. aspersa is an excellent source of amino acids, providing nearly all the amino acids required by humans. The high content of lysine, aspartic acid, and glutamic acid in D. aspersa contributes to its umami flavor, while histidine and alanine contribute to its sweetness [61,62]. The total content of these five flavor-enhancing amino acids is 9.86 g/100 g, accounting for 51.54% of the total amino acids, giving D. aspersa its characteristic sweet aftertaste.
3.3. Antioxidant Activity
Given its affinity for both water-soluble and lipid-soluble compounds, 95% ethanol was selected as the extraction solvent to obtain an ethanolic extract (EE) [8,19]. The ABTS+· scavenging capacity of the EE of D. aspersa was measured at 55.11 ± 0.31 μmol Trolox·g−1, and its total antioxidant capacity was 57.97 ± 1.04 µmol (Fe2+)·g−1. The trends shown in Table 4 in all three antioxidant capacity evaluation indicators were consistent, indicating a high level of reliability in the antioxidant assessment results.

Table 4.
Antioxidant capacity of the EE of Disporopsis aspersa.
D. aspersa showed potential activity in scavenging hydroxyl radicals at various concentrations, similar to that of essential oil of Disporopsis fuscopicta, which exhibited a scavenging capacity with 0.04 ± 0.008 mmol (Fe2+)·g−1 [63]. According to Li et al. [64], rutin, luteolin, quercetin, and betulinic acid, extracted from the ethyl acetate extract of D. fuscopicta, were all proven to possess strong antioxidant activity. Flavonoids are generally recognized for their great antioxidant capacity [65]. Previous research has isolated a variety of bioactive substances such as rutin, quercetin, and salicylate from D. aspersa, suggesting that flavonoids and phenolic acids may be the main active components responsible for its antioxidant activity [8,13].
3.4. Anti-Inflammatory Activity
Preliminary experiments to evaluate the effects of the EE of Disporopsis aspersa on cell viability were carried out using a CCK-8 test. RAW 264.7 macrophage cells were treated with the EE at different concentrations (40–120 μg/mL) for 18 h. It is considered that a cell viability rate exceeding 80% indicates that the sample has no cytotoxic effect on the tested cells [66]. As illustrated in Figure 2, the 95% ethanol extracts (EE) exhibited no significant cytotoxicity towards RAW 264.7 cells at concentration below 120 μg/mL. Consequently, further studies on the EE were conducted, keeping the concentration below 120 µg/mL.
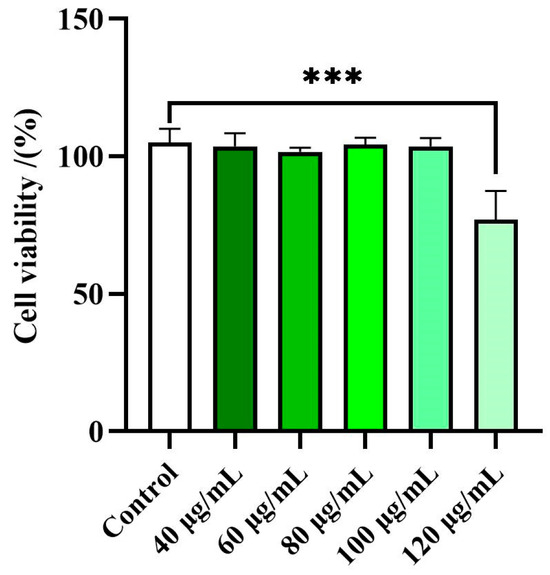
Figure 2.
Effects of the EE of Disporopsis aspersa on RAW 264.7 cell viability. Results are expressed as percentage of cell viability relative to untreated control cells (Control). Each bar shows mean ± SD of three independent experiments performed in triplicate (*** p < 0.01 compared to Control).
The anti-inflammatory effects of the EE were investigated by measuring nitric oxide (NO) production using the Griess reagent. NO is a signaling molecule that plays a crucial role in the progression of inflammation. Therefore, NO inhibitors are considered potential therapeutic targets for therapy anti-inflammatory [67].
As illustrated in Figure 3, the EE exhibited significant NO inhibitory effect (IC50 = 72.7 ± 7.43 μg/mL), demonstrating a certain dose-dependence. Lipopolysaccharide (LPS) significantly induces the production of NO compared to control cells, confirming the successful establishment of the LPS-induced inflammatory model in RAW 264.7 cells. As the concentration of the D. aspersa ethanol extract increased, NO production in RAW264.7 cells gradually decreased. These results indicate that the EE has anti-inflammatory properties, mediated by the inhibition of NO production.
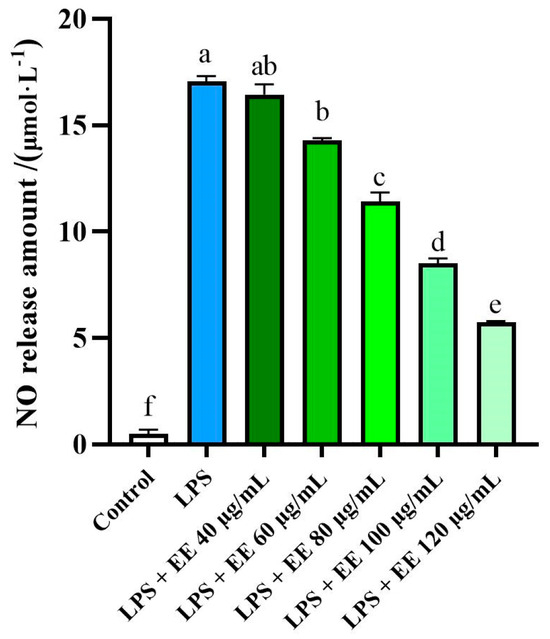
Figure 3.
Effects of the EE on RAW 264.7 cells NO production induced by LPS, expressed as mean ± standard deviation (n = 3). Bars with different letters indicate significant difference (p < 0.05).
Consistent with previous studies, Gang et al. found that 2-isopropyl-5-methyphenyl 2-(naphthalen-1-yl)acetate and (3R,4S)-3,4-dihydroxy-3-methyldihydrofuran-2(3H)-one isolated from D. aspersa exhibit moderate NO inhibition in LPS-induced BV-2 microglial cells [7]. Furthermore, Song et al. revealed that 3-(4′-hydroxy-benzyl)-5, 7-dihydroxy-6-methyl-8-methoxyl-chroman-4-one isolated from D. aspersa significantly inhibit SW620 and MDA-MB-231 cells, with IC50 values of 32.5 μg/mL and 42.7 μg/mL, respectively [12]. Nguyen et al. also showed that several flavonoids display cytotoxicity across six cancer cell lines [13]. Flavonoids, phenolic acids, and steroids are widely recognized for their anti-inflammatory and antioxidant properties, and these compounds are more easily extracted from ethanolic extracts [65,68,69]. The flavonoids isolated from D. aspersa provide a theoretical basis for their potential medicinal applications.
Other Disporopsis species also demonstrate anti-inflammatory potential. Meng et al. identified angiogenesis inhibitors in D. fuscopicta, effective against ECV304 cells [14]. Thu Ha et al. isolated two spirostanol glycosides from D. longifolia that strongly inhibit NO production in LPS-activated RAW 264.7 cells, with IC50 values of 26.6 ± 1.9 μM and 24.5 ± 2.5 μM [15]. According to this study and previous literature, D. aspersa has shown good potential for anti-inflammatory and anti-tumor effects, as well as a certain antioxidant capacity. This provides a scientific basis for the traditional use of healing trauma in ethnobotanical surveys, indicating that this plant may have the potential to support further exploration for natural anti-inflammatory agents.
4. Conclusions
In the western region of the Gaoligong Mountains in Yunnan, the local people have rich traditional knowledge about and practical applications for Disporopsis aspersa. However, the true value of this medicinal food plant remains underestimated due to insufficient evaluation. Understanding the nutritional and medicinal properties of this underutilized vegetable can promote its conservation and broader use in human diets as a valuable source of nutrition and anti-inflammatory agents.
D. aspersa, containing higher levels of calcium, magnesium, potassium, vitamins E, vitamins B2, and vitamins B6, as well as amino acids like Val, Leu and Lys, is a high-energy wild vegetable and an important source of high-quality protein. It also provides substantial dietary fiber and essential minerals, supporting diverse diets and promoting healthier eating patterns while reducing carbon footprints.
In addition to its nutritional benefits, D. aspersa exhibits potential anti-hypertensive, anti-inflammatory, and antioxidant properties. As a commonly consumed wild edible herb, its medicinal effects influence local dietary choices. Its high vitamin E and dietary fiber content, combined with a low-sodium and high-potassium profile, contribute to its role in regulating blood pressure, benefiting individuals with cardiovascular diseases. Furthermore, D. aspersa also exhibits strong anti-inflammatory and moderate antioxidant activities, supporting its use in wound healing and inflammation control. Based on this study and previous literature, the abundant flavonoids, phenolic acids, and steroids in D. aspersa may contribute to its remarkable anti-inflammatory properties. However, further research is needed to clarify the relationship between these metabolites and their bioactivities.
The genus Disporopsis, to which Disporopsis aspersa belongs, is primarily distributed in Southeast Asia and has a wide geographical range. This study scientifically validates the traditional knowledge of D. aspersa, offering insights for research on Disporopsis species in other regions and providing clues for the development and utilization of edible plants in the Asparagaceae family by other countries. This would not only restore cultural and food heritage but also create sustainable production and consumption alternatives.
Author Contributions
Conceptualization, C.L.; methodology, Q.C., M.W. and J.Z.; formal analysis, Q.C.; investigation, Q.C., M.W., X.H., Q.Z. and C.X.; resources, Q.C. and C.X.; data curation, Q.C.; writing—original draft preparation, Q.C.; writing—review and editing, Q.C., M.W., X.H., J.Z., Q.Z., C.X. and C.L.; visualization, Q.C. and J.Z.; supervision, C.L.; project administration, C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Baoshan Administration of Gaoligongshan National Nature Reserve (202305AF150121 & GBP-2022-01), and the National Natural Science Foundation of China (32370407, 31761143001 & 31870316).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We are very grateful to the staff of the PONY Testing Company (Beijing) for testing nutritional ingredients. Special thanks to all of informants of western Yunnan, including the collectors, retailers, and restaurateurs for their unreserved information and patience. We also show our thanks and respect for our College of Life and Environmental Sciences, Minzu University of China.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reddy, K.R.; Aggarwal, M.; Freeman, A.M. Food Is Medicine: The Time Is Now. Am. J. Med. 2024, 137, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lu, X.; Lin, F.; Naeem, A.; Long, C. Ethnobotanical Study on Wild Edible Plants Used by Dulong People in Northwestern Yunnan, China. J. Ethnobiol. Ethnomed. 2022, 18, 3. [Google Scholar] [CrossRef]
- Sun, J.; Xiong, Y.; Li, Y.; Yang, Q.; Chen, Y.; Jiang, M.; Li, Y.; Li, H.; Bi, Z.; Huang, X.; et al. Medicinal Dietary Plants of the Yi in Mile, Yunnan, China. J. Ethnobiol. Ethnomed. 2020, 16, 48. [Google Scholar] [CrossRef]
- Bi, J.; Fang, H.; Zhang, J.; Lu, L.; Gu, X.; Zheng, Y. A Review on the Application, Phytochemistry and Pharmacology of Polygonatum odoratum, an Edible Medicinal Plant. J. Future Foods 2023, 3, 240–251. [Google Scholar] [CrossRef]
- Kaur, S.; Roy, A. A Review on the Nutritional Aspects of Wild Edible Plants. Curr. Tradit. Med. 2021, 7, 542–553. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Ji, Y.; Li, P.; Cao, W.; Wu, S.; Kennelly, E.; Long, C. Nutraceutical Study on Maianthemum Atropurpureum, a Wild Medicinal Food Plant in Northwest Yunnan, China. Front. Pharmacol. 2021, 12, 710487. [Google Scholar] [CrossRef]
- Gang, F.; Zhu, F.; Yang, C.; Li, X.; Yang, H.; Sun, M.; Wu, W.; Zhang, J. Antifungal, Anti-Inflamatory and Neuritogenic Activity of Newly-Isolated Compounds from Disporopsis Aspersa. Nat. Prod. Res. 2020, 34, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yuan, C.; Gang, F.; Yang, C.; Wu, W.; Zhang, J. Bioassay-Guided Isolation of Antifungal Compounds from Disporopsis aspersa (Hua) Engl. ex Diels against Pseudoperonospora cubensis and Phytophthora infestans. Chem. Biodivers. 2018, 15, e1800090. [Google Scholar] [CrossRef]
- China National Medicinal Materials Corporation. Zhongyao Ziyuanzhi Yao of China; Zeng, M., Zeng, J., Eds.; Science Press: Beijing, China, 1994; p. 2069. ISBN 7-5067-3251-3. [Google Scholar]
- Jia, M.; Li, X. Zhongguo Minzuyao Zhiyao; China Medical Science Press: Beijing, China, 2005; p. 2069. ISBN 7-03-003138-5. [Google Scholar]
- Sarapan, A.; Hodkinson, T.R.; Suwanphakdee, C. Assessment of Morphological, Anatomical and Palynological Variation in the Medicinal Plant Disporopsis longifolia Craib (Asparagaceae) for Botanical Quality Control. Plants 2023, 12, 259. [Google Scholar] [CrossRef]
- Song, H.; Chen, S. Separation and purification of anticancer active ingredients from Disporopsis aspersa by high-speed counter-current chromatography. Coal Chem. Ind. 2009, 32, 45–46. [Google Scholar]
- Nguyen, V.H.; Chen, C.X.; Van, H.T.; Le, N.T.; Chu, V.H.; Truong, H.A.V.; Nguyen, Q.H.; Dam, S.M. Chemical Profiles and Antibacterial Activity of Essential Oils from Rhizomes of Aspidistra phanluongii. Chem. Nat. Compd. 2022, 58, 562–564. [Google Scholar] [CrossRef]
- Meng, M.; Yang, Z.; He, L. Screening the Efective Component of Rhizoma tupistrae Chinensis with Inhibiting Angiogenesis. Chin. J. Inf. Tradit. Chin. Med. 2008, 1, 46–47. [Google Scholar]
- Thu Ha, T.T.; Vui, D.K.; Hoang, N.H.; Tai, B.H.; Van Kiem, P. Dispolongiosides A and B, Two New Fucose Containing Spirostanol Glycosides from the Rhizomes of Disporopsis longifolia Craib., and Their Nitric Oxide Production Inhibitory Activities. Nat. Prod. Commun. 2021, 16, 1934578X211055013. [Google Scholar] [CrossRef]
- Xie, J.; Liu, F.; Jia, X.; Zhao, Y.; Liu, X.; Luo, M.; He, Y.; Liu, S.; Wu, F. Ethnobotanical Study of the Wild Edible and Healthy Functional Plant Resources of the Gelao People in Northern Guizhou, China. J. Ethnobiol. Ethnomed. 2022, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Panmei, R.; Gajurel, P.R.; Singh, B. Ethnobotany of Medicinal Plants Used by the Zeliangrong Ethnic Group of Manipur, Northeast India. J. Ethnopharmacol. 2019, 235, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, S.; Wu, Z.; Lin, C.; Zhang, Q.; Xu, C.; Li, J.; Long, C. Study on Medicinal Food Plants in the Gaoligongshan Biosphere Reserve, the Richest Biocultural Diversity Center in China. J. Ethnobiol. Ethnomed. 2024, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Nie, F. Chemical Constituents from Disporopsis pernyi. Chin. Tradit. Pat. Med. 2010, 11, 1936–1938. [Google Scholar]
- Ngamlai, E.V.; Lalthanpuii, P.B.; Lalbiaknii, P.C.; Ralte, V.; Lalnunmawia, F. Antioxidant Property and Free Radical Scavenging Activity of Hedyotis scandens (Roxb). Rubiaceae. Curr. Trends Biotechnol. Pharm. 2022, 16, 46–55. [Google Scholar] [CrossRef]
- Shi, S.; Li, W.; Li, Q.; Yang, F.; Liu, L.; Cui, L. Analysis on the Moisture, Total Ash and Lead, Cadmium, Arsernic, Mercury of Lycii fructus from Different Habitats. J. Henan Univ. (Med. Sci.) 2020, 39, 13–16. [Google Scholar]
- GB 5009.3-2016; Moisture: National Food Safety Standard—Determination of Moisture in Foods. National Health Commission of PRC: Beijing, China, 2016.
- GB 5009.4-2016; Ash: National Food Safety Standard—Determination of Ash in Foods. National Health Commission of PRC: Beijing, China, 2016.
- Wang, Y.; Lliang, M.; Xie, T.; Yang, B.; Peng, J.; He, R.; Zhu, H.; Huang, Y. Analysis of Nutritional Components and Acute Toxicity of Bauhinia variegata. J. Guangxi Acad. Sci. 2022, 38, 69–75. [Google Scholar]
- GB 5009.6-2016; Fat: National Food Safety Standard—Determination of Fat in Foods (Method 2). National Health Commission of PRC: Beijing, China, 2016.
- Wang, Y.; Huang, L.; Chen, J.; Sun, M.; Jiang, X.; Mi, X. Determination of Dietary Fiber in Foods by Dietary Fiber Analyzer. J. Food Saf. Food Qual. 2018, 9, 523–527. [Google Scholar]
- GB 5009.88-2014; Dietary Fiber: National Food Safety Standard—Determination of Dietary Fibers in Food. National Health and Family Planning Commission of PRC: Beijing, China, 2014.
- GB 5009.5-2016; Protein: National Food Safety Standard—Determination of Protein in Foods (Method 1). National Health Commission of PRC: Beijing, China, 2016.
- Tan, L.; Li, J.; Li, Y.; Wang, H.; Gao, X.; Zhao, J.; Ma, J.; Ji, T.; Wang, H. Analysis of Nutritional Compositions and Evaluation of Quality in Potentilla anserina L. from Qinghai Different Producing Areas. J. Food Sci. Biotechnol. 2022, 41, 95–111. [Google Scholar]
- GB/Z 21922-2008; Energy: Fundamental Terminology and Definition of Nutritional Component in Foods. Ministry of Health of PRC; Standardization Administration of PRC: Beijing, China, 2008.
- GB 5009.268-2016; Trace Elements: National Food Safety Standard—Determination of Multi-elements in Foods (Method 2). National Health Commission of PRC: Beijing, China, 2016.
- Zhang, P.; Liang, A.; Wang, D.; Yang, C.; Wu, X.; Yin, W. Validation of determination method of Sodiumand lead in food of GB 5009.268-2016. Mod. Prev. Med. 2017, 44, 4256–4262. [Google Scholar]
- GB 5009.93-2017; Selenium: National Food Safety Standard—Determination of Selenium in Foods (Method 1). National Health Commission of PRC: Beijing, China, 2017.
- GB 5009.124-2016; Amino Acids: National food Safety Standard—Determination of Amino Acids in Foods. National Health Commission of PRC: Beijing, China, 2016.
- Zhang, L.; Qi, L.; Zhou, M. Determination of Vitamins A, D and E in Health Foods Using Column-Switching Liquid Chromatography. Food Sci. 2022, 43, 225–230. [Google Scholar]
- GB 5009.82-2016; Vitamin A, D and E: National Food Safety Standard—Determination of Vitamin A, D and E in Foods. National Health and Family Planning Commission of PRC: Beijing, China, 2016.
- Zhao, X.; Zhao, X.; Zhang, S.; Wang, Y.; Niu, Z.; Lu, Y.; Peng, X.; Ma, X.; Tan, J. Analysis of Vitamin B1 and B2 Content of 9 Edible Wild Mushrooms in Yunnan Province. Adv. Anal. Chem. 2023, 13, 361–367. [Google Scholar] [CrossRef]
- GB 5009.84-2016; Vitamin B1: National Food Safety Standard—Determination of Vitamin B1 in Foods. National Health and Family Planning Commission of PRC: Beijing, China, 2016.
- GB 5009.85-2016; Vitamin B2: National Food Safety Standard—Determination of Vitamin B2 in Foods (Method 1). National Health and Family Planning Commission of PRC; China Food and Drug Administration: Beijing, China, 2016.
- GB 5009.154-2016; Vitamin B6: National Food Safety Standard—Determination of Vitamin B6 in Foods. National Health and Family Planning Commission of PRC: Beijing, China, 2016.
- GB 5009.158-2016; Vitamin K1: National Food Safety Standard—Determination of Vitamin K1 in Foods. National Health and Family Planning Commission of PRC: Beijing, China, 2016.
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.-L.; Dong, X.; Zhou, P.; Li, P.; Liu, E.-H. Chemical and Biological Comparison of the Fruit Extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Luo, M.; Wang, W.; Zhao, C.; Gu, C.; Zu, Y.; Fu, Y.; Yao, X.; Duan, M. Variation of Active Constituents and Antioxidant Activity in Pyrola [P. incarnata Fisch.] from Different Sites in Northeast China. Food Chem. 2013, 141, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Yang, C.S.; Chen, J.J. Main Bioactive Components and Their Biological Activities from Natural and Processed Rhizomes of Polygonum sibiricum. Antioxidants 2022, 11, 1383. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Pieraccini, G.; Romoli, R.; Biondi, N.; Niccolai, A.; Rodolfi, L.; Tredici, M.R.; Luceri, C. A Comparative Study of Metabolites Profiles, Anti-Inflammatory and Antioxidant Activity of Methanolic Extracts from Three Arthrospira Strains in RAW 264.7 Macrophages. Algal Res. 2023, 73, 103171. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, M.-B.; Jeong, S.; Lee, H.; Baek, S.H.; Uddin, S.; Lee, S.W.; Lee, S.G. Examination of the Antioxidant and Anti-Inflammatory Effects of Extracts from the Bark of Bangladesh Medicinal Plants. Food Eng. Progress. 2024, 28, 10–19. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z. China Food Composition Tables · Standard Edition; Peking University Medical Press: Beijing, China, 2018; p. 362. ISBN 978-7-5659-1699-1. [Google Scholar]
- Veluvali, A.; Snyder, M. Dietary Fiber Deficiency in Individuals with Metabolic Syndrome: A Review. Curr. Opin. Clin. Nutr. 2023, 26, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, S. A Review of Healthy Role of Dietary Fiber in Modulating Chronic Diseases. Food Res. Int. 2024, 191, 114682. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-Chemical and Functional Properties of Legume Protein, Starch, and Dietary Fiber—A Review. Legume Sci. 2022, 4, e117. [Google Scholar] [CrossRef]
- Li, S.; Hong, F.; Wang, Y.; Liu, T.; Zhao, J.; Gou, D. Research Progress on Extraction, Funetional Properties and Application of Water Insoluble Dietary Fiber from Legumes. Food Res. Dev. 2024, 45, 202–209. [Google Scholar]
- Yang, Z.; Yang, M.; Deehan, E.C.; Cai, C.; Madsen, K.L.; Wine, E.; Li, G.; Li, J.; Liu, J.; Zhang, Z. Dietary Fiber for the Prevention of Childhood Obesity: A Focus on the Involvement of the Gut Microbiota. Gut Microbes 2024, 16, 2387796. [Google Scholar] [CrossRef]
- Szczepańska, E.; Białek-Dratwa, A.; Janota, B.; Kowalski, O. Dietary Therapy in Prevention of Cardiovascular Disease (CVD)—Tradition or Modernity? A Review of the Latest Approaches to Nutrition in CVD. Nutrients 2022, 14, 2649. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Huang, L.; Yang, K.; Wang, J.; Xiong, H.; Wu, Z.; Qin, R. Research Progress on Resources and Nutritional Components of Brasenia schreberi. Bot. Res. 2019, 8, 7–14. [Google Scholar]
- Fang, J.; Chen, Y.; Gong, R.; Wang, L. Dietary Sodium and Potassium Intake and Hypertension. Progress. Physiol. Sci. 2023, 54, 235–239. [Google Scholar] [CrossRef]
- Olas, B. A Review of the Pro-Health Activity of Asparagus officinalis L. and Its Components. Foods 2024, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, B.; Luo, Z.; Yuan, Y.; Zhao, Z.; Liu, M. Composition Analysis and Nutritional Value Evaluation of Amino Acids in the Fruit of 161 Jujube cultivars. Plants 2023, 12, 1744. [Google Scholar] [CrossRef]
- Zhu, J.Y.; van de Leemput, J.; Han, Z. The Roles of Histone Lysine Methyltransferases in Heart Development and Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, X. Protein Lysine Four-Carbon Acylations in Health and Disease. J. Cell. Physiol. 2024, 239, e30981. [Google Scholar] [CrossRef] [PubMed]
- Voss, C.M.; Arildsen, L.; Nissen, J.D.; Waagepetersen, H.S.; Schousboe, A.; Maechler, P.; Ott, P.; Vilstrup, H.; Walls, A.B. Glutamate Dehydrogenase Is Important for Ammonia Fixation and Amino Acid Homeostasis in Brain During Hyperammonemia. Front. Neurosci. 2021, 15, 646291. [Google Scholar] [CrossRef]
- Kurihara, K. Glutamate: From Discovery as a Food Flavor to Role as a Basic Taste (Umami). Am. J. Clin. Nutr. 2009, 90, 719S–722S. [Google Scholar] [CrossRef] [PubMed]
- Yuniarti, T.; Prayudi, A.; Setiarto, R.H.B.; Martosuyono, P.; Supenti, L.; Suhrawardan, H.; Maulani, A. Formulation and Organoleptic Characteristics of Flavor Enhancer from Shrimp Head Protein Hydrolysate. Food Res. 2024, 8, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhang, L.; Yang, D.; Zhao, C. Contribution of Phenolics and Essential Oils to the Antioxidant and Antimicrobial Properties of Disporopsis pernyi (Hua) Diels. J. Med. Food 2014, 17, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Feng, T.; Huang, Y.; Lin, Q. Component and Content Analysis of Phenolics in Disporopsis pernyi (Hua) Diels. Food Res. Dev. 2019, 40, 183–187. [Google Scholar]
- Khan, H.; Saeed, M.; Muhammad, N.; Perviz, S. Phytochemical Analysis, Antibacterial, and Antifungal Assessment of Aerial Parts of Polygonatum verticillatum. Toxicol. Ind. Health 2016, 32, 841–847. [Google Scholar] [CrossRef]
- Li, W.; Gao, X.; Huang, X.; Li, H.; Mao, W.; Liu, L.; Long, C. Bioactivities of Ethanol Extracts from Rosa macrophylla and Their Material Basis. J. Plant Resour. Environ. 2024, 33, 97–106. [Google Scholar]
- Phan, U.T.T.; Nguyen, H.D.; Nguyen, T.K.O.; Tran, T.H.; Le, T.H.; Tran, T.T.P. Anti-Inflammatory Effect of Piper longum L. Fruit Methanolic Extract on Lipopolysaccharide-Treated RAW 264.7 Murine Macrophages. Heliyon 2024, 10, e26174. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, M.; Cai, F.; Liu, L.; Cheng, Z.; Zhao, J.; Zhang, Q.; Long, C. A Comprehensive Review of Medicinal Toxicodendron (Anacardiaceae): Botany, Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2024, 318, 116829. [Google Scholar] [CrossRef] [PubMed]
- Gamba, M.; Raguindin, P.F.; Asllanaj, E.; Merlo, F.; Glisic, M.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Bioactive Compounds and Nutritional Composition of Swiss Chard (Beta vulgaris L. Var. Cicla and Flavescens): A Systematic Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).