Improved Functionality, Quality, and Shelf Life of Merguez-Type Camel Sausage Fortified with Spirulina as a Natural Ingredient
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredients and Chemical Reagents
2.2. Experimental Design
2.3. Preparation of Merguez-Type Camel Sausages
2.4. Chemical Analysis of the Spirulina Powder and Camel Sausages
2.5. Total Phenolic Compounds in the Spirulina Powder
2.6. Determination of the pH of Camel Sausages
2.7. Oxidative Stability Analysis (TBARS) of Camel Sausages
2.8. Microbiological Analysis of Camel Sausages
2.9. Instrumental Color Measurement of Camel Sausages
2.10. Determination of Total Volatile Basic Nitrogen (TVB-N) in Camel Sausages
2.11. Cooking Characteristics of Camel Sausages
2.12. Sensory Analysis of Camel Sausages
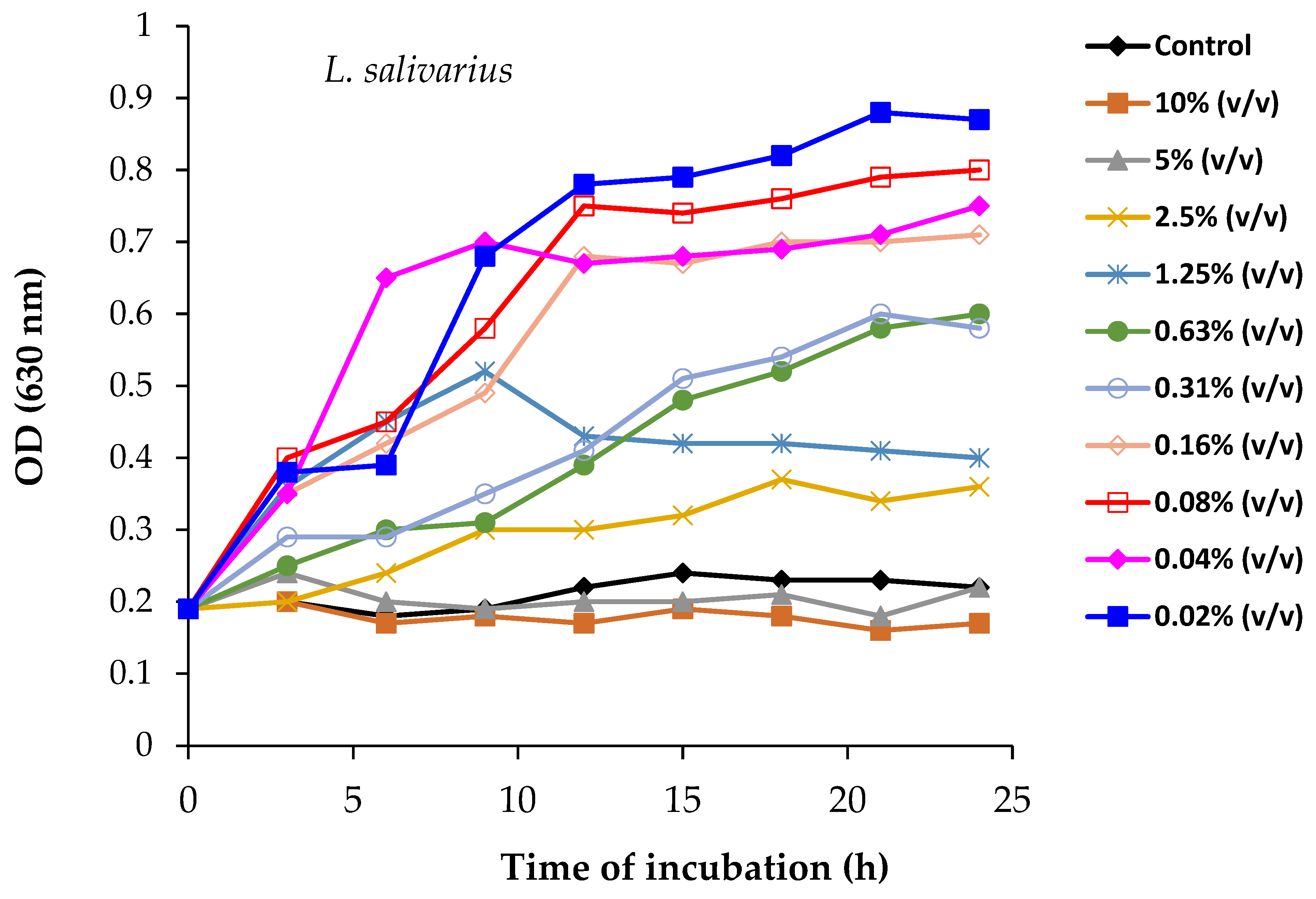
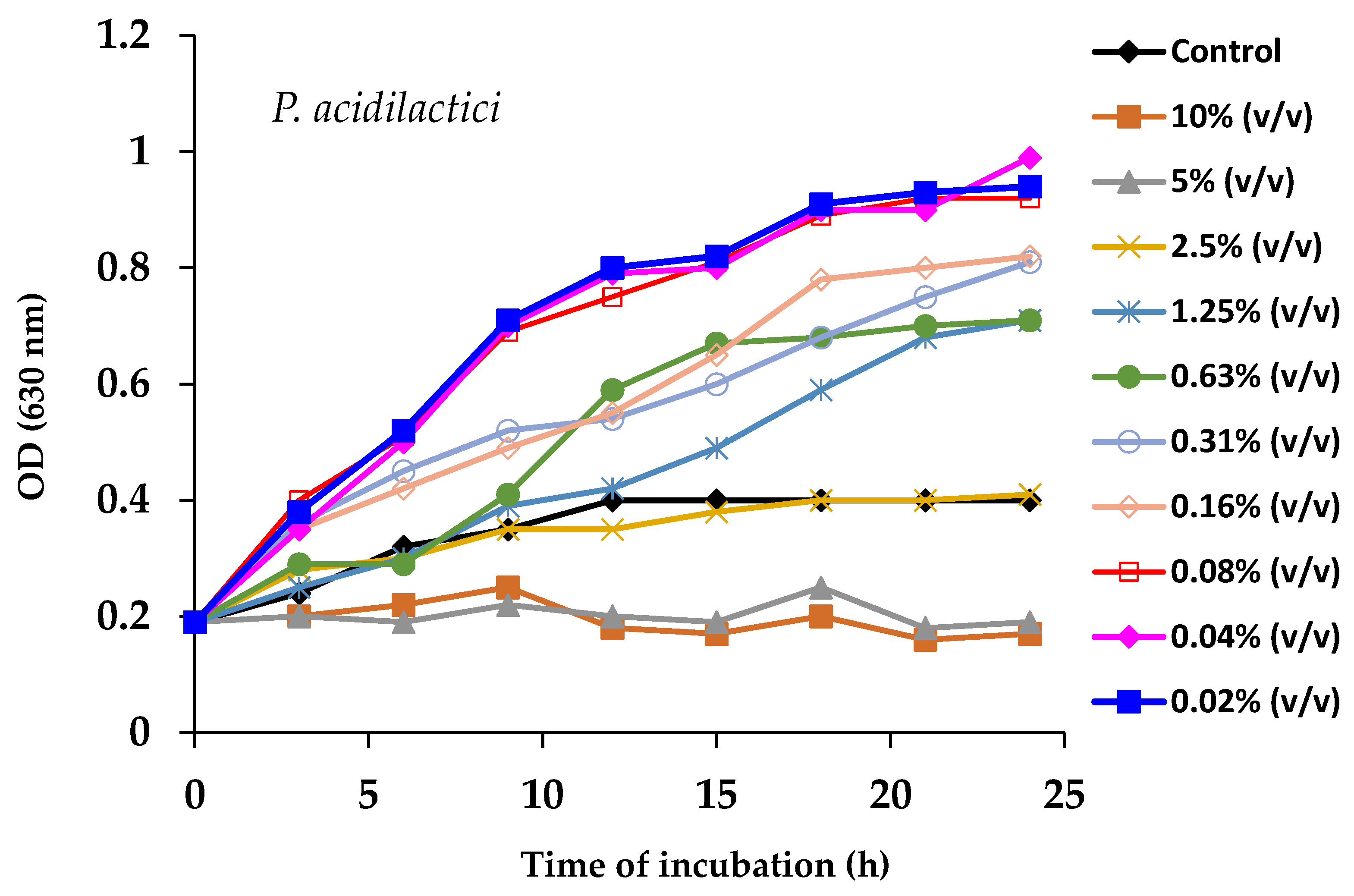
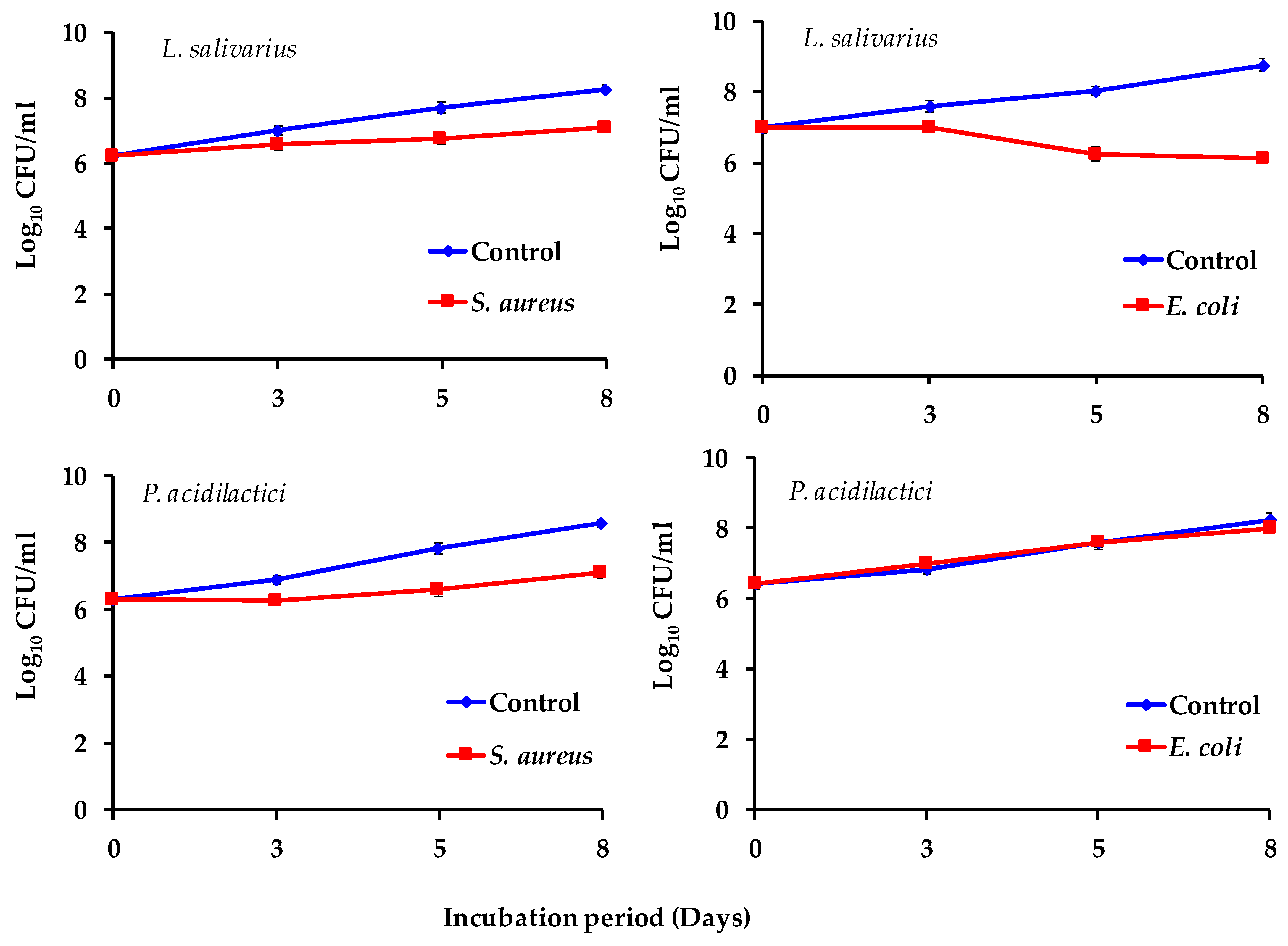
2.13. Prebiotic Effects of Spirulina Powder on Probiotic Cultures and Its Antimicrobial Activity
2.14. Statistical Data Analysis
3. Results and Discussion
3.1. Chemical Composition and Total Phenolic Compounds (TPCs) of Spirulina Powder
3.2. Chemical Composition of Camel Sausages
3.2.1. pH
3.2.2. Lipid Peroxidation (TBARS)
3.2.3. Instrumental Color Parameters
3.2.4. Total Volatile Basic Nitrogen (TVB-N)
3.2.5. Cooking Characteristics and Shrinkage Measurements
3.3. Microbiological Quality Evolution
3.4. Sensorial Quality Evolution
3.5. Prebiotic Effect of Spirulina on the Antagonistic Activity of Probiotic Strains Against Pathogenic Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djenane, D.; Aider, M. The one-humped camel: The animal of future, potential alternative red meat, technological suitability and future perspectives. F1000Research 2024, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Aboudaou, M.; Djenane, F.; García-Gonzalo, D.; Pagán, R. Improvement of the shelf-life status of modified atmosphere packaged camel meat using nisin and Olea europaea Subsp. Laperrinei leaf extract. Foods 2020, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Al-Nabulsi, A.A.; Dhanasekaran, D.K.; Hasan, F.; Rao, S.; Fatima, H.; Ayyash, M.; Holley, R.; Obaid, R.S. Growth behaviour and thermal inactivation of E. coli O157:H7 and Salmonella spp. in ground lean camel meat. Int. J. Food Microbiol. 2020, 316, 108423. [Google Scholar] [CrossRef] [PubMed]
- Elwakil, B.H.; Eskandrani, A.; El-Kady, H.; Shahin, Y.H.; Awaad, A.K. Spirulina platensis as a super prebiotic to enhance the antibacterial effect of Lactobacillus casei. S. Afr. J. Bot. 2024, 173, 417–429. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Effect of cumulative spirulina intake on broiler meat quality, nutritional and health-related attributes. Foods. 2024, 13, 799. [Google Scholar] [CrossRef]
- Fernandes, F.; Martins, R.; Barbosa, M.; Valentão, P. Algae-based supplements claiming weight loss properties: Authenticity control and scientific-based evidence on their effectiveness. Mar. Drugs 2024, 22, 123. [Google Scholar] [CrossRef] [PubMed]
- Atik, D.S.; Gürbüz, B.; Bölük, E.; Palabıyık, I. Development of vegan kefir fortified with Spirulina platensis. Food Biosci. 2021, 42, 101050. [Google Scholar]
- Arslan, R.; Aksay, S. Investigation of sensorial and physicochemical properties of yoghurt colored with phycocyanin of Spirulina platensis. J. Food Process. Preserv. 2022, 46, e15941. [Google Scholar] [CrossRef]
- Metri-Ojeda, J.; Ramírez-Rodrigues, M.; Rosas-Ordoñez, L.; Baigts-Allende, D. Development and characterization of a low-fat mayonnaise salad dressing based on Arthrospira platensis protein concentrate and sodium alginate. Appl. Sci. 2022, 12, 7456. [Google Scholar] [CrossRef]
- Sengupta, S.; Koley, H.; Dutta, S.; Bhowal, J. Hypocholesterolemic effect of Spirulina platensis (SP) fortified functional soy yogurts on diet-induced hypercholesterolemia. J. Funct. Foods 2018, 48, 54–64. [Google Scholar] [CrossRef]
- Rasouli, F.; Berenji, S.; Shahab Lavasani, A. Optimization of traditional Iranian ice cream formulation enriched with spirulina using response surface methodology. J. Food Technol. Nutr. 2017, 14, 15–28. [Google Scholar]
- Hussein, A.S.; Mostafa, S.; Fouad, S.; Hegazy, N.A.; Zaky, A.A. Production and evaluation of gluten-free pasta and pan bread from Spirulina Algae powder and quinoa flour. Processes 2023, 11, 2899. [Google Scholar] [CrossRef]
- Stejskal, N.; Miranda, J.M.; Martucci, J.F.; Ruseckaite, R.A.; Barros-Velázquez, J.; Aubourg, S.P. Quality enhancement of refrigerated hake muscle by active packaging with a protein concentrate from Spirulina platensis. Food Bioprocess Technol. 2020, 13, 1110–1118. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Kosma, I.; Mataragas, M.; Pappa, E.; Badeka, A.V.; Bosnea, L. Innovative intelligent cheese packaging with whey protein-based edible films containing Spirulina. Sustainability 2023, 15, 13909. [Google Scholar] [CrossRef]
- Lambiase, C.; Braghieri, A.; Barone, C.M.A.; Di Francia, A.; Pacelli, C.; Serrapica, F.; Lorenzo, J.M.; De Rosa, G. Use of cyanobacterium Spirulina (Arthrospira platensis) in buffalo feeding: Effect on Mozzarella cheese quality. Foods 2023, 12, 4095. [Google Scholar] [CrossRef] [PubMed]
- Pestana, J.M.; Puerta, B.; Santos, H.; Madeira, M.S.; Alfaia, C.M.; Lopes, P.A.; Pinto, R.M.A.; Lemos, J.P.C.; Fontes, C.M.G.A.; Lordelo, M.M.; et al. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020, 99, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahyaey, F.; Al-Marzooqi, W.; Shaat, I.; Smith, M.A.; Al-Sabahi, J.; Melak, S.; Bush, R.D. Effect of Spirulina platensis supplementation on carcass characte ristics, fatty acid profile, and meat quality of Omani goats. Animals 2023, 13, 2976. [Google Scholar] [CrossRef]
- Hunduma, D.; Amenu, K.; Desta, H.; Grace, D.; Agga, G.E.; Kerro Dego, O. Prevalence and antimicrobial resistance of Escherichia coli O157:H7 and Salmonella, and the prevalence of Staphylococcus aureus in dairy cattle and camels under pastoral production system. Antibiotics 2024, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Ben Miri, Y.; Ariño, A. Use of Algerian type Ras El-Hanout spices mixture with marination to increase the sensorial quality, shelf life, and safety of whole rabbit carcasses under low-O2 modified atmosphere packaging. Foods 2023, 12, 2931. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 22nd ed.; Current through Revision 1; AOAC International: Gaithersburg, MD, USA, 2023. [Google Scholar]
- Canadian Food Inspection Agency (CFIA). Food Labelling for Industry. Available online: https://inspection.canada.ca/en/food-labels/labelling/industry/nutrition-labelling/nutrition-facts-table (accessed on 27 December 2024).
- Food Drug Administration (FDA). Nutrition Labeling of Food. 21 CFR 101.9. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-101/subpart-A/section-101.9 (accessed on 10 March 2024).
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; Latimer, G.W.J., Ed.; AOAC International: New York, NY, USA, 2019; pp. 123–158. [Google Scholar]
- Djenane, D.; Aboudaou, M.; Ferhat, M.A.; Ariño, A. Effect of the aromatisation with summer savory (Satureja hortensis L.) essential oil on the oxidative and microbial stabilities of liquid whole eggs during storage. J. Essent. Oil Res. 2019, 31, 444–455. [Google Scholar] [CrossRef]
- Djenane, D.; Sánchez-Escalante, A.; Beltrán, J.A.; Roncalés, P. Extension of the shelf life of beef steaks packaged in a modified atmosphere by treatment with rosemary and displayed under UV-free lighting. Meat Sci. 2003, 64, 417–426. [Google Scholar] [CrossRef]
- Camo, J.; Lorés, A.; Djenane, D.; Beltrán, J.A.; Roncalés, P. Display life of beef packaged with an antioxidant active film as a function of the concentration of oregano extract. Meat Sci. 2011, 88, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.C.C.; Machado, M.; Machado, S.; Costa, A.S.G.; Bessada, S.; Alves, R.C.; Oliveira, M.B.P.P. Algae Incorporation and nutritional improvement: The case of a whole-wheat pasta. Foods 2023, 12, 3039. [Google Scholar] [CrossRef] [PubMed]
- Rose, H.; Bakshi, S.; Kanetkar, P.; Lukose, S.J.; Felix, J.; Yadav, S.P.; Gupta, P.K.; Paswan, V.K. Development and characterization of cultured buttermilk fortified with Spirulina plantensis and its physico-chemical and functional characteristics. Dairy 2023, 4, 271–284. [Google Scholar] [CrossRef]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, functional, textural and sensory evaluation of Spirulina enriched green pasta: A potential dietary and health supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Metekia, W.A.; Ulusoy, B.H.; Habte-Tsion, H.M. Spirulina phenolic compounds: Natural food additives with antimicrobial properties. Int. Food Res. J. 2021, 28, 1109–1118. [Google Scholar] [CrossRef]
- Panaite, T.D.; Cornescu, G.M.; Predescu, N.C.; Cismileanu, A.; Turcu, R.P.; Saracila, M.; Soica, C. Microalgae (Chlorella vulgaris and Spirulina platensis) as a protein alternative and their Effects on productive performances, blood parameters, protein digestibility, and nutritional value of laying hens’ egg. Appl. Sci. 2023, 13, 10451. [Google Scholar] [CrossRef]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Starkute, V.; Jomantaite, I.; Zokaityte, E.; Mockus, E.; Tolpeznikaite, E.; Zokaityte, G.; Petrova, P.; Santini, A.; Rocha, J.M.; et al. Multifunctional nutraceutical composition based on fermented Spirulina, apple cider vinegar, Jerusalem artichoke, and bovine colostrum. Foods 2023, 12, 1690. [Google Scholar] [CrossRef]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michalowska, D. Effect of Spirulina (Arthrospira platensis) supplementation on physical and chemical properties of semolina (Triticum durum) based fresh pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef]
- Al-Fadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of nutraceutical potential of the microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312. [Google Scholar] [CrossRef]
- Ben Mya, O.; Souici, S.; Guenfoud, M. Comparative analysis of nutritional and bioactive components in Algerian and Egyptian spirulina from Tamanrasset and Khatatba Regions. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Chentir, I.; Hamdi, M.; Li, S.; Doumandji, A.; Markou, G.; Nasri, M. Stability, bio-functionality, and bio-activity of crude phycocyanin from a two-phase cultured Saharan Arthrospira sp. strain. Algal Res. 2018, 35, 395–406. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ramamoorthy, D.; Kumar Verma, D.; Kumar, A.; Kumar, N.; Raj Kanak, K.; Marwein, B.M.; Mohan, K. Antioxidant and phytonutrient activities of Spirulina platensis. Energy Nexus 2022, 6, 100070. [Google Scholar] [CrossRef]
- Agustini, T.W.; Suzery, M.; Sutrisnanto, D.; Maaruf, W.F.; Hadiyanto. Comparative Study of Bioactive substances extracted from fresh and dried Spirulina sp. Procedia Environ. Sci. 2015, 23, 282–289. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as functional ingredients in savory food products: Application to wheat crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef]
- Soltanizadeh, N.; Kadivar, M.; Keramat, J.; Bahrami, H.; Poorreza, F. Camel cocktail sausage and its physicochemical and sensory quality. Int. J. Food Sci. Nutr. 2010, 61, 226–243. [Google Scholar] [CrossRef]
- Donadio, G.; Santoro, V.; Dal Piaz, F.; De Tommasi, N. Food matrices affect the peptides produced during the digestion of Arthrospira platensis-based functional aliments. Nutrients 2021, 13, 3919. [Google Scholar] [CrossRef] [PubMed]
- Siladji, C.; Djordjevic, V.; Milijasevic, J.B.; Heinz, V.; Terjung, N.; Sun, W.; Tomasevic, I. Micro- and macroalgae in meat products. Foods 2024, 13, 826. [Google Scholar] [CrossRef]
- Cabrol, M.B.; Glišić, M.; Baltić, M.; Jovanović, D.; Silađi, Č.; Simunović, S.; Tomašević, I.; Raymundo, A. White and honey Chlorella vulgaris: Sustainable ingredients with the potential to improve nutritional value of pork frankfurters without compromising quality. Meat Sci. 2023, 198, 109123. [Google Scholar] [CrossRef] [PubMed]
- Agregan, R.; Franco, D.; Carballo, J.; Tomasevic, I.; Barba, F.J.; Gomez, B.; Muchenje, V.; Lorenzo, J.M. Shelf life study of healthy pork liver pate with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res. Int. 2018, 112, 400–411. [Google Scholar] [CrossRef]
- Shafiei, R.; Mostaghim, T. Improving shelf life of calf fillet in refrigerated storage using edible coating based on chitosan/natamycin containing Spirulina platensis and Chlorella vulgaris microalgae. Food Measure. 2022, 16, 145–161. [Google Scholar] [CrossRef]
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic amines in meat and meat products: A review of the ccience and future perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Ferrocino, I.; Nasi, A.; Ndagijimana, M.; Vernocchi, P.; La Storia, A.; Laghi, L.; Mauriello, G.; Guerzoni, M.E.; Villani, F. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions. Appl. Environ. Microbiol. 2011, 77, 7372–7381. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, M.; He, W.; Li, X.; Qiu, C.; Zhang, J. Unraveling the physicochemical properties and bacterial communities in rabbit meat during chilled storage. Foods 2024, 13, 623. [Google Scholar] [CrossRef] [PubMed]
- Yehia, H.M.; Al-Masoud, A.H.; Elkhadragy, M.F.; Korany, S.M.; Nada, H.M.S.; Albaridi, N.A.; Alzahrani, A.A.; AL-Dagal, M.M. Improving the quality and safety of fresh camel meat contaminated with Campylobacter jejuni using citrox, chitosan, and vacuum packaging to extend shelf life. Animals 2021, 11, 1152. [Google Scholar] [CrossRef]
- Widati, A.S.; Rosyidi, D.; Radiati, L.E.; Nursyam, H. The effect of seaweed (Eucheuma cottonii) flour addition on physicochemical and sensory characteristics of an Indonesian-style beef meatball. Int. J. Food Stud. 2021, 10, SI111–SI120. [Google Scholar] [CrossRef]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Chen, C.O.; Xie, S. Polysaccharides in Spirulina platensis improve antioxidant capacity of Chinese-style sausage. J. Food Sci. 2017, 82, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Voloschenko, L.V.; Baidina, I.A.; Shevchenko, N.P.; Trubchaninova, N.C. Functional meat and vegetable pate with spirulina. Earth Environ. Sci. 2021, 845, 012123. [Google Scholar] [CrossRef]
- Djenane, D. Chemical profile, antibacterial and antioxidant activity of Algerian citrus essential oils and their application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef]
- Alavi, N.; Golmakani, M.T. Antioxidant properties of whole-cell Spirulina (Arthrospira platensis) powder expressed in olive oil under accelerated storage conditions. J. Appl. Phycol. 2017, 29, 2971–2978. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Y.; Xue, M.; Dun, Y.; Li, S.; Peng, N.; Liang, Y.; Zhao, S. Purification and identification of antioxidant peptides from enzymatic hydrolysate of Spirulina platensis. J. Microb. Biotechnol. 2016, 26, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Benslima, A.; Ksouda, G.; Montero, V.B.; Hajji, M.; Nasri, M. Safer and healthier reduced nitrites Turkey meat sausages using lyophilized Cystoseira barbata seaweed extract. J. Complement. Integr. Med. 2018, 15, 20170061. [Google Scholar] [CrossRef] [PubMed]
- Moroney, N.C.; O’Grady, M.N.; O’Doherty, J.V.; Kerry, J.P. Effect of a brown seaweed (Laminaria digitata) extract containing laminarin and fucoidan on the quality and shelf-life of fresh and cooked minced pork patties. Meat Sci. 2013, 94, 304–311. [Google Scholar] [CrossRef]
- Munsu, E.; Mohd Zaini, H.; Matanjun, P.; Ab Wahab, N.; Sulaiman, N.S.; Pindi, W. Physicochemical, sensory properties and lipid oxidation of chicken sausages supplemented with three types of seaweed. Appl. Sci. 2021, 11, 11347. [Google Scholar] [CrossRef]
- Martínez, L.; Djenane, D.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of varying oxygen concentrations on the shelf-life of fresh pork sausages packaged in modified atmosphere. Food Chem. 2006, 94, 219–225. [Google Scholar] [CrossRef]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Storage stability of low-fat sodium reduced fresh Merguez sausage prepared with olive oil in konjac gel matrix. Meat Sci. 2013, 94, 438–446. [Google Scholar] [CrossRef]
- Djenane, D.; Roncalés, P. Carbon monoxide in meat and fsh packaging: Advantages and limits. Foods 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Zhang, Y.; Li, Y.; Luo, X.; Chen, X.; Kaur, M.; Mellor, G.; Stark, J.; Hughes, J. Shelf life extension of vacuum packaged chilled beef in the Chinese supply chain. A feasibility study. Meat Sci. 2019, 153, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.B.; Bekhit, A.E.-D.; Waller, M.; Bailes, K.L.; Kerr, M.J.; Hopkins, D.L. The association between total volatile basic nitrogen (TVB-N) concentration and other biomarkers of quality and spoilage for vacuum packaged beef. Meat Sci. 2021, 179, 108551. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.B.; van de Ven, R.; Mao, Y.; Coombs, C.E.O.; Hopkins, D.L. Using instrumental (CIE and reflectance) measures to predict consumers’ acceptance of beef colour. Meat Sci. 2017, 127, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-Y.; Lim, K.-J.; Lee, Y.-H.; Shin, H.-S. Development of a freshness indicator for assessing the quality of packaged pork products during refrigerated storage. Foods 2024, 13, 2097. [Google Scholar] [CrossRef]
- Ziani, K.; Houari Adli, D.E.; Khaled, M.B.; Halla, N.; Mansouri, A.; Kahloula, K.; Djenane, D. Vitamin B12 content in lamb meat: Effect of cooking and freezing temperatures. Emir. J. Food Agric. 2023, 35, 417–427. [Google Scholar] [CrossRef]
- Sánchez-Escalante, A.; Djenane, D.; Torrescano, G.; Beltrán, J.A.; Roncalés, P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001, 58, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Lagerstedt, Å.; Lundström, K.; Lindahl, G. Influence of vacuum or high-oxygen modified atmosphere packaging on quality of beef M. Longissimus dorsi steaks after different ageing times. Meat Sci. 2011, 87, 101–106. [Google Scholar]
- Djenane, D.; Sánchez-Escalante, A.; Beltrán, J.A.; Roncalés, P. Ability of α-tocopherol, taurine and rosemary, in combination with vitamin C, to increase the oxidative stability of beef steaks packaged in modified atmosphere. Food Chem. 2002, 76, 407–415. [Google Scholar] [CrossRef]
- Sánchez-Escalante, A.; Djenane, D.; Torrescano, G.; Beltrán, J.A.; Roncales, P. Antioxidant action of borage, rosemary, oregano, and ascorbic acid in beef patties packaged in modified atmosphere. J. Food Sci. 2003, 68, 339–344. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, E.J.; Cho, Y.S.; Nam, Y.D.; Choi, Y.S.; Kim, D.O.; Seo, D.H.; Nam, T.G. Comparative evaluation of spoilage-related bacterial diversity and metabolite profiles in chilled beef stored under air and vacuum packaging. Food Microbiol. 2019, 77, 166–172. [Google Scholar] [CrossRef]
- ES: 1090/2005; Frozen Poultry and Rabbits. Egyptian Organization for Standardization and Quality Control: Cairo, Egypt, 2005.
- Processing Standards and Ingredient Specifications for Livestock Products No. 2015–94; Korean Ministry of Agriculture and Forestry: Sejong-si, Republic of Korea, 2015.
- GB 2707-2016; Fresh (Frozen) Livestock and Poultry Products. National Standard of the People’s Republic of China, National Health and Family Planning Commission: Beijing, China, 2016.
- Lei, Y.; Huang, J.; Cheng, Y.; Zhang, Y.; Huang, T.; Huang, M. Changes in bacterial communities and the volatilome of braised chicken with different packaging stored at 4 °C. Food Res. Int. 2022, 155, 111056. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Liu, Q.; Nan, F.; Oliver Chen, C.Y.; Xie, S. Arthrospira (Spirulina) platensis extract improves oxidative stability and product quality of Chinese-style pork sausage. J. Appl. Phycol. 2018, 30, 1667–1677. [Google Scholar] [CrossRef]
- Fu, Q.; Song, S.; Xia, T.; Wang, R. Effects of cherry (Prunus cerasus L.) powder addition on the physicochemical properties and oxidation stability of Jiangsu-type sausage during refrigerated storage. Foods 2022, 11, 3590. [Google Scholar] [CrossRef]
- Meremäe, K.; Rusalepp, L.; Sünter, A.; Raudsepp, P.; Anton, D.; Mäesaar, M.; Elias, T.; Püssa, T.; Roasto, M. Microbial Growth Inhibition Effect, Polyphenolic Profile, and Antioxidative Capacity of Plant Powders in Minced Pork and Beef. Foods 2024, 13, 3117. [Google Scholar] [CrossRef]
- Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Kim, S.-Y.; Kim, C.-J. Effects of sea tangle (Lamina japonica) powder on quality characteristics of breakfast sausages. Food Sci. Anim. Resour. 2010, 30, 55–61. [Google Scholar]
- Mohammed, H.O.; O’Grady, M.N.; O’Sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. Acceptable inclusion levels for selected brown and red Irish seaweed species in pork sausages. Foods 2022, 11, 1522. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Abu-Ghannam, N. Enhancement of the phytochemical and fibre content of beef patties with Himanthalia elongata seaweed. Int. J. Food Sci. Technol. 2013, 48, 2239–2249. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Ben Amira, A.; Gafsi, I.M.; Lahiani, M.; Bejar, M.; Triki, M.; Zouari, A.; Attia, H.; Besbes, S. Toward the enhancement of sensory profile of sausage “Merguez” with chickpea protein concentrate. Meat Sci. 2018, 143, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Pindi, W.; Mah, H.W.; Munsu, E.; Ab Wahab, N. Effects of addition of Kappaphycus alvarezii on physicochemical properties and lipid oxidation of mechanically deboned chicken meat (MDCM) sausages. Br. Food J. 2017, 119, 2229–2239. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Zamuz, S.; Tomašević, I.; Gómez, B.; Rocchetti, G.; Lucini, L.; Remize, F.; Barba, F.J.; Lorenzo, J.M. Influence of different sources of vegetable, whey and microalgae proteins on the physicochemical properties and amino acid profile of fresh pork sausages. LWT Food Sci. Technol. 2019, 110, 316–323. [Google Scholar] [CrossRef]
- Hawashin, M.D.; Al-Juhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Babiker, E.E. Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat Sci. 2016, 122, 32–39. [Google Scholar] [CrossRef]
- Abd El-Hady, E.S.A.; Alfheeaid, H.A.; Abd El-Razik, M.M.; Althwab, S.A.; Barakat, H. Nutritional, physicochemical, and organoleptic properties of camel meat burger incorporating unpollinated Barhi date fruit pulp. Int. J. Food Sci. 2022, 2022, 4581821. [Google Scholar] [CrossRef] [PubMed]
- Al-Juhaimi, F.Y.; Mohamed Ahmed, I.A.; Adiamo, O.Q.; Adisa, A.R.; Ghafoor, K.; Özcan, M.M.; Babiker, E.E. Effect of Argel (Solenostemma argel) leaf powder on the quality attributes of camel patties during cold storage. J. Food Process. Preserv. 2018, 42, e13496. [Google Scholar] [CrossRef]
- ICMSF—International Commission of Microbiological Specifications for Foods. Microorganisms in Foods; Their Significance and Methods of Enumeration; Elliott, R.P., Clark, D.S., Lewis, K.H., Lundbeck, H., Olson, J.C., Jr., Simonsen, B., Eds.; University of Toronto Press: Toronto, ON, Canada, 1986; Volume 1. [Google Scholar]
- Deepitha, R.P.; Xavier, K.A.M.; Layana, P.; Nayak, B.B.; Balange, A.K. Quality improvement of pangasius fillets using aqueous seaweed (Padina Tetrastromatica) extract. LWT Food Sci. Technol. 2021, 137, 110418. [Google Scholar] [CrossRef]
- Alshuniaber, M.A.; Krishnamoorthy, R.; Al-Qhtani, W.H. Antimicrobial activity of polyphenolic compounds from Spirulina against food-borne bacterial pathogens. Saudi J. Biol. Sci. 2021, 28, 459–464. [Google Scholar] [CrossRef]
- Metekia, W.A.; Ulusoy, B.H. Antimicrobial activity of Spirulina platensis extract on total mesophilic and psychrophilic bacteria of fresh tilapia fillet. Sci. Rep. 2023, 13, 13081. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choe, J.; Yong, H.I.; Lee, H.J.; Kim, H.-J.; Jo, C. Combination of sea tangle powder and high-pressure treatment as an alternative to phosphate in emulsion-type sausage. J. Food Process. Preserv. 2018, 42, e13712. [Google Scholar] [CrossRef]
- Ilieva, Y.; Zaharieva, M.M.; Najdenski, H.; Kroumov, A.D. Antimicrobial activity of arthrospira (Former. Spirulina) and dunaliella related to recognized antimicrobial bioactive compounds. Int. J. Mol. Sci. 2024, 25, 5548. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Di Foggia, M.; Quintana, A.M.; Roberti, R. Preliminary study on the activity of phycobiliproteins against Botrytis cinerea. Mar. Drugs 2020, 18, 600. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Bumsted, J.; Ford, E.; Blair, A.; Underwood, K.; Zuelly, S.M.S. Instrumental color measurements have relationships to fat smearing in fresh sausage. Foods 2023, 12, 2813. [Google Scholar] [CrossRef]
- Djenane, D.; Beltrán, J.A.; Camo, J.; Roncalés, P. Influence of vacuum-ageing duration of whole beef on retail shelf life of steaks packaged with oregano (Origanum vulgare L.) active film under high O2. J. Food Sci. Technol. 2016, 53, 4244–4257. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Khosravi-Darani, K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Khaledabad, M.A.; Ghasempour, Z.; Kia, E.M.; Bari, M.R.; Zarrin, R. Probiotic yoghurt functionalised with microalgae and zedo gum: Chemical, microbiological, rheological and sensory characteristics. Int. J. Dairy. Technol. 2019, 73, 67–75. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, C.; Garg, A.P.; Prakash, D. Prebiotic efficiency of blue green algae on probiotics microorganisms. J. Microbiol. Exp. 2017, 4, 00120. [Google Scholar] [CrossRef]
- Akalin, A.; Unal, G.; Dalay, M. Influence of Spirulina platensis biomass on microbiological viability in traditional and probiotic yogurts during refrigerated storage. Ital. J. Food Sci. 2009, 21, 357–364. [Google Scholar]
- De Caire, G.Z.; Parada, J.L.; Zaccaro, M.C.; De Cano, M.M.S. Effect of Spirulina platensis biomass on the growth of lactic acid bacteria in milk. World J. Microbiol. Biotechnol. 2000, 16, 563–565. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Lv, M.; Lei, Q.; Yin, H.; Hu, T.; Wang, S.; Dong, K.; Pan, H.; Liu, Y.; Lin, Q.; Cao, Z. In vitro effects of prebiotics and synbiotics on Apis cerana gut microbiota. Pol. J. Microbiol. 2021, 70, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Rodríguez-Meizoso, I.; Cifuentes, A.; Jaime, L.; García-Blairsy Reina, G.; Señorans, F.J.; Ibáñez, E. Green processes based on the extraction with pressurized fluids to obtain potent antimicrobials from Haematococcus pluvialis microalgae. LWT Food Sci. Technol. 2009, 42, 1213–1218. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F.J. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible seaweeds and Spirulina extracts for food application: In vitro and In situ evaluation of antimicrobial activity towards foodborne pathogenic bacteria. Foods 2020, 9, 1442. [Google Scholar] [CrossRef]
- Piccolo, A. Spirulina, a Livehood and a Business Venture—Report SF/2011/16; Indian Ocean Commission—SmartFish Program; FAO: Roma, Italy, 2012.
- Cruz, J.D.; Vasconcelos, V. Legal Aspects of Microalgae in the European Food Sector. Foods 2024, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- FDA. Agency Response Letter GRAS Notice No. GRN 000127; FDA: Silver Spring, MD, USA, 2003. [Google Scholar]

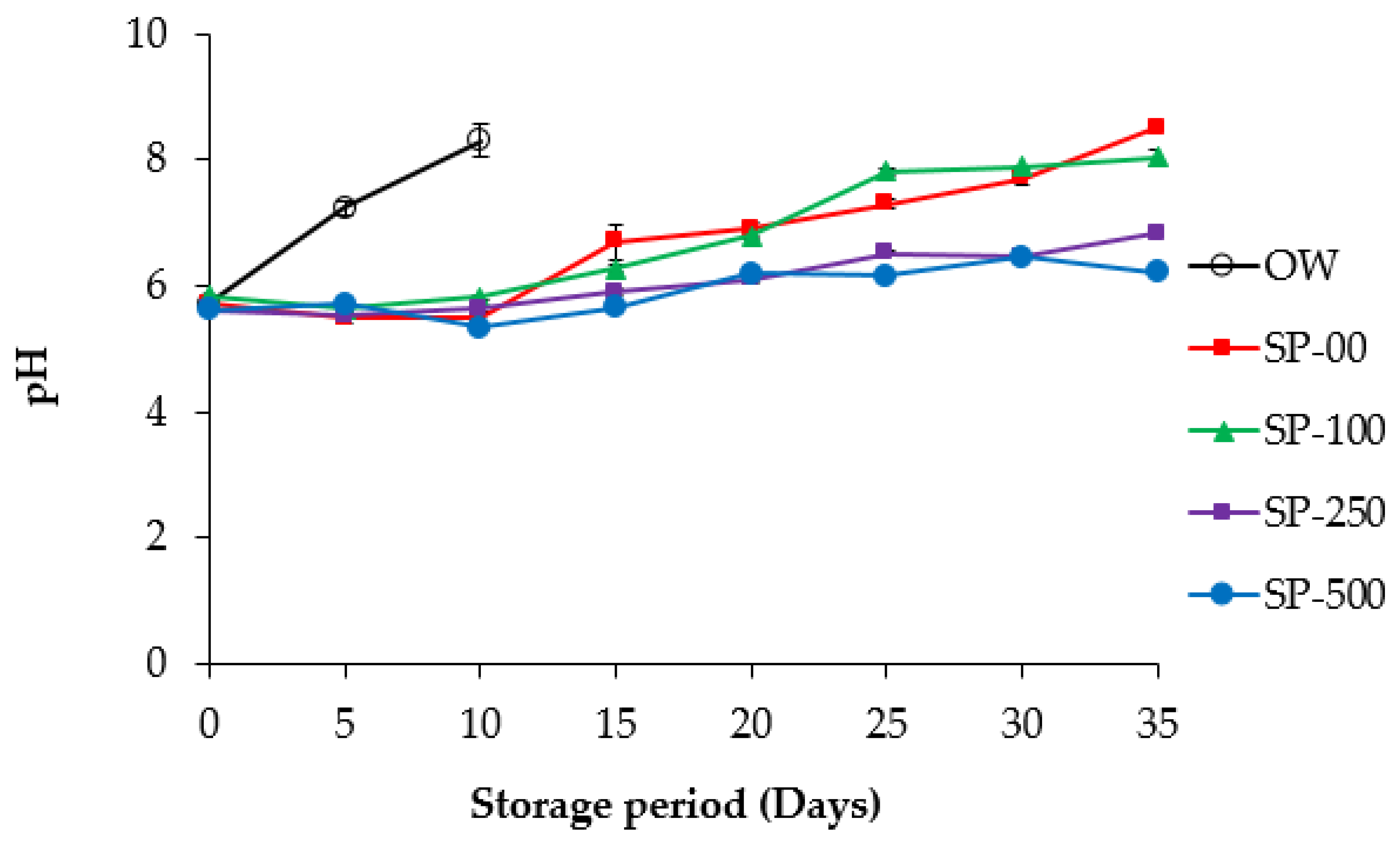
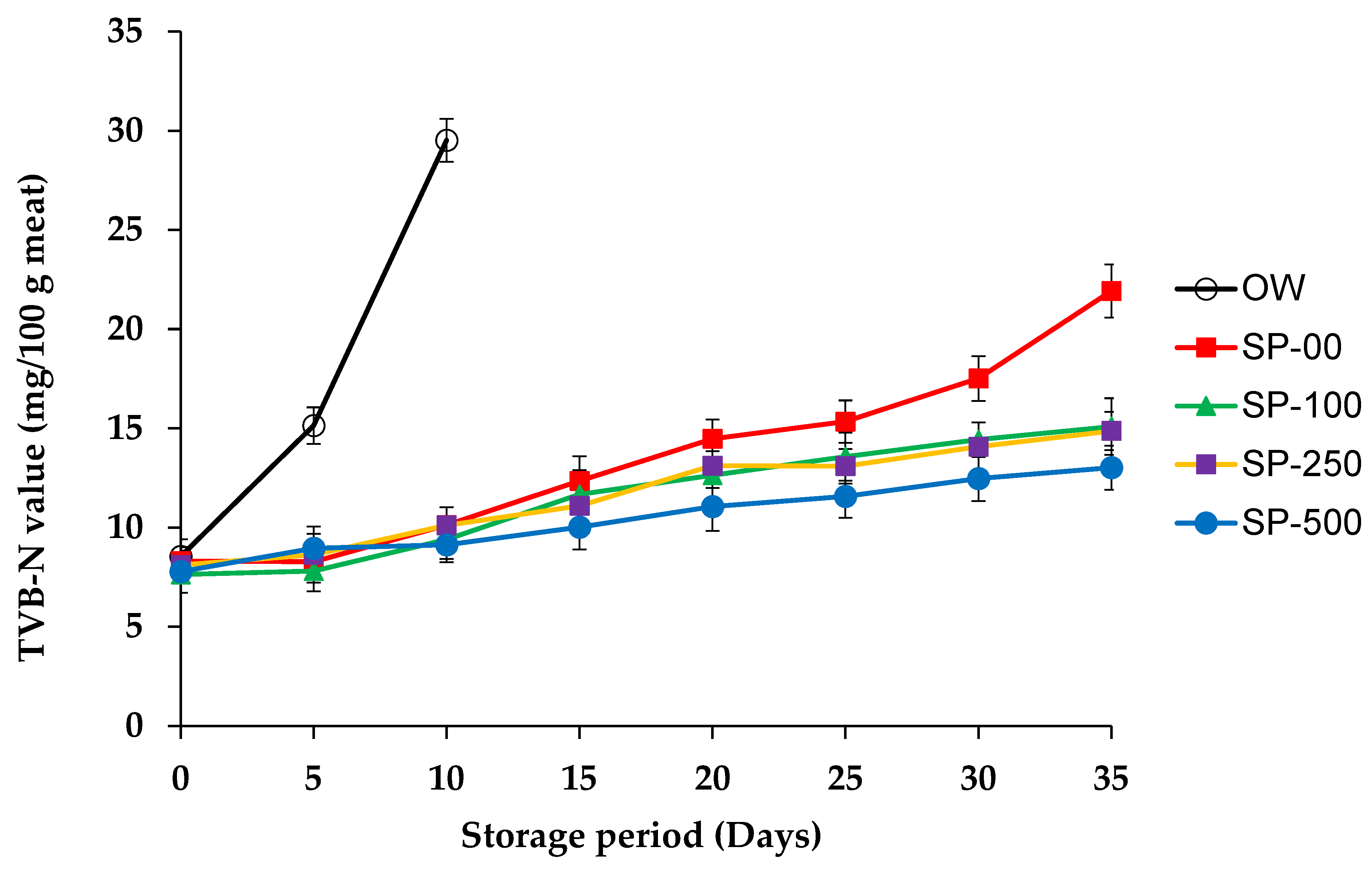


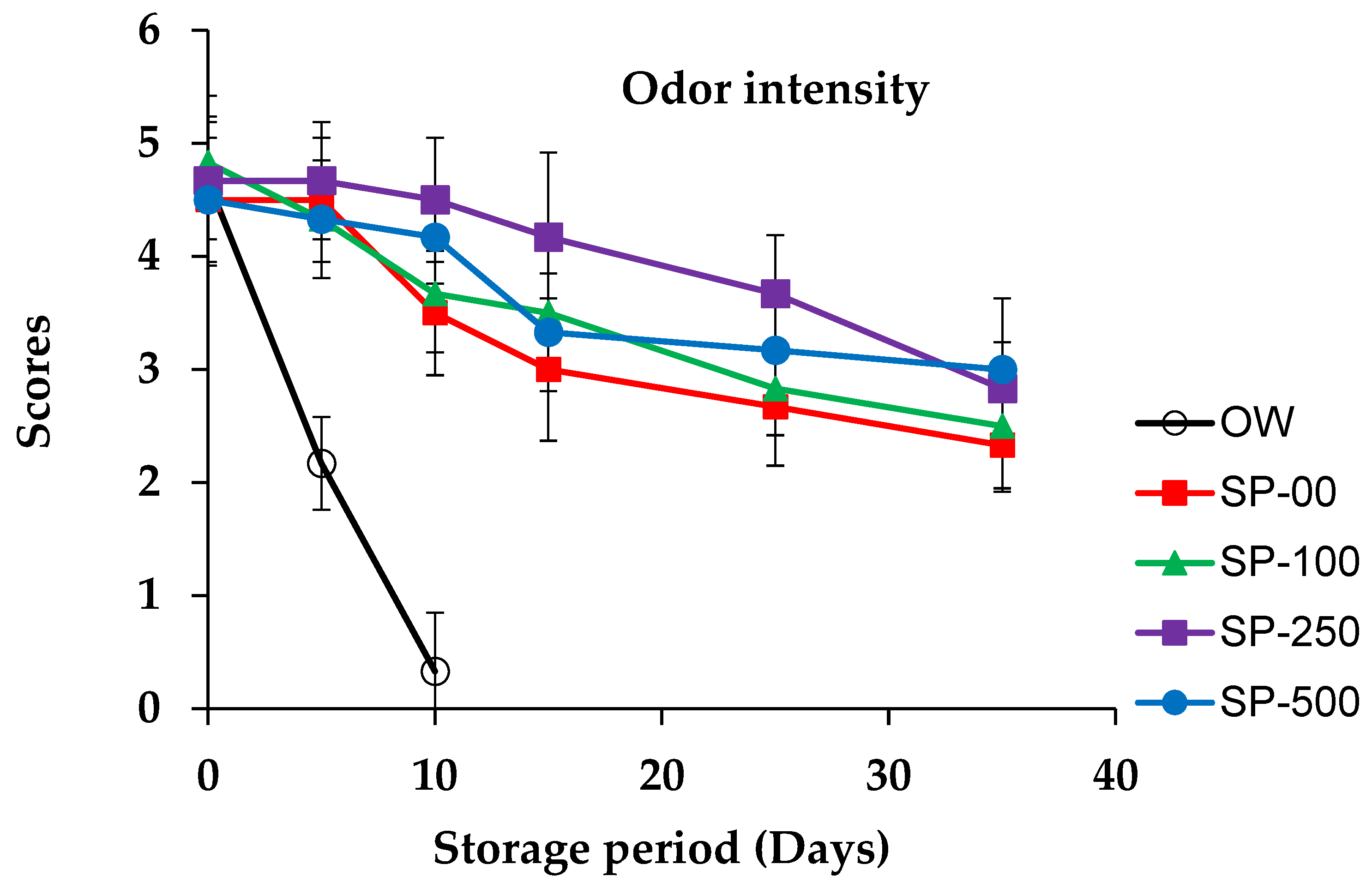
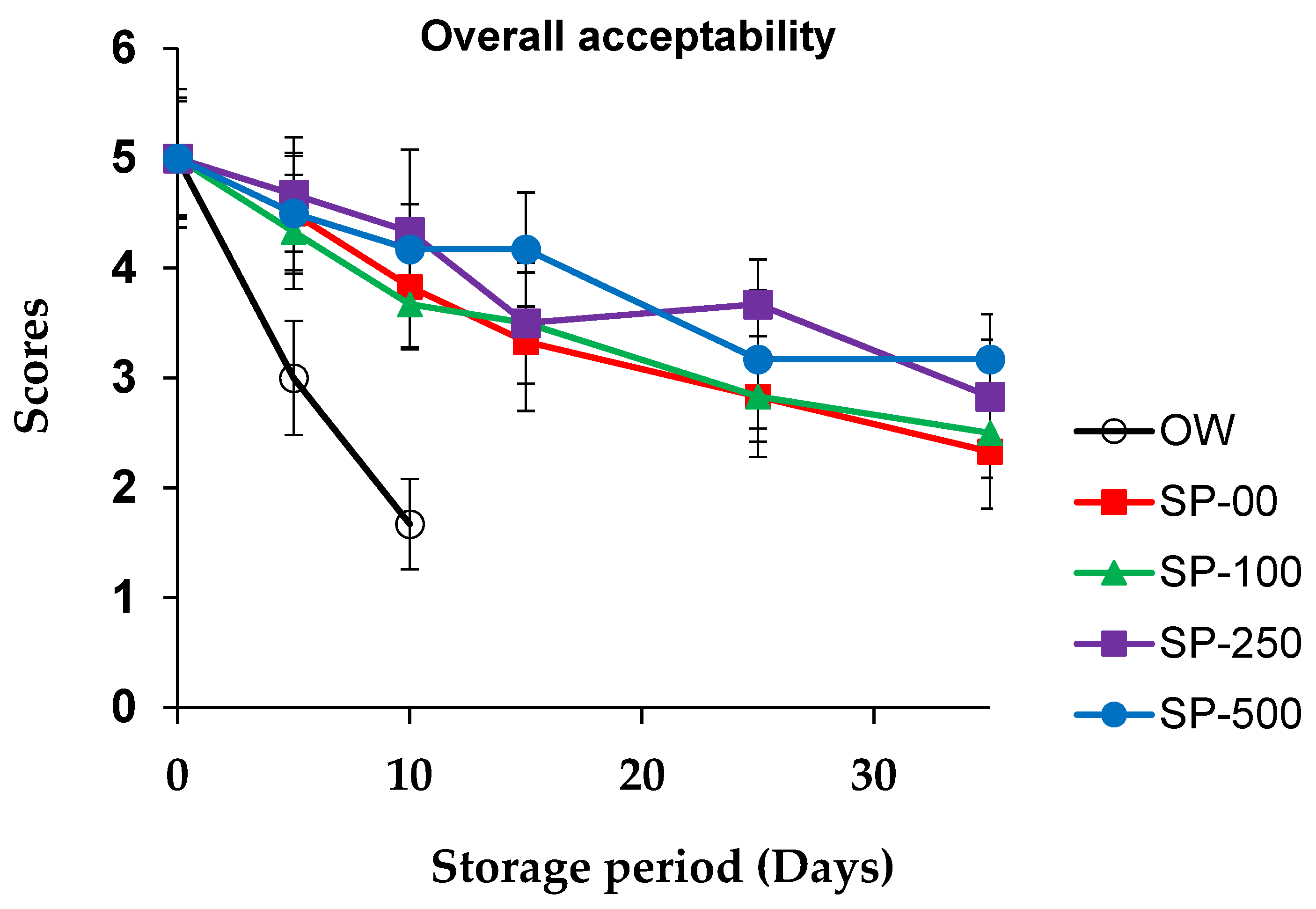
| Ingredients (%) | Control OW 1 and SP-00 | SP-100 | SP-250 | SP-500 |
|---|---|---|---|---|
| Camel meat | 70.00 | 70.00 | 70.00 | 70.00 |
| Camel humped fat | 20.00 | 20.00 | 20.00 | 20.00 |
| Whole liquid eggs | 5.50 | 5.49 | 5.47 | 5.45 |
| Salt | 2.00 | 2.00 | 2.00 | 2.00 |
| Spirulina powder (SP) | 0.00 | 0.01 | 0.025 | 0.05 |
| Merguez spice mixture: | 2.50 | 2.50 | 2.50 | 2.50 |
| -Garlic | 0.80 | 0.80 | 0.80 | 0.80 |
| -Hot pepper | 0.50 | 0.50 | 0.50 | 0.50 |
| -Paprika | 0.30 | 0.30 | 0.30 | 0.30 |
| -Caraway | 0.20 | 0.20 | 0.20 | 0.20 |
| -Black pepper | 0.20 | 0.20 | 0.20 | 0.20 |
| -Coriander | 0.50 | 0.50 | 0.50 | 0.50 |
| Component | Values Per 100 g |
|---|---|
| Moisture | 4.15 ± 0.05 |
| Protein 1 | 66.88 ± 1.16 |
| Fat | 3.13 ± 0.06 |
| Carbohydrates 2 | 20.73 ± 1.38 |
| Ash | 5.11 ± 0.13 |
| Energy (kcal) 3 | 378.62 ± 0.41 |
| Energy (Kj) 4 | 1584.16 ± 1.74 |
| Total phenolic content (TPC) by Folin–Ciocalteu | |
| 8.94 ± 0.16 mg GAE/g | |
| Spirulina Powder Concentrations | |||||
|---|---|---|---|---|---|
| Storage (Days) | OW | SP-00 | SP-100 | SP-250 | SP-500 |
| 0 | 0.54 ± 0.01 aA | 0.51 ± 0.01 aA | 0.58 ± 0.01 aA | 0.60 ± 0.01 aA | 0.52 ± 0.01 aA |
| 5 | 1.89 ± 0.07 bB | 0.78 ± 0.05 aA | 0.80 ± 0.07 aAB | 0.69 ± 0.09 aA | 0.79 ± 0.05 aA |
| 10 | 3.82 ± 0.09 cC | 1.11 ± 0.09 bAB | 1.21 ± 0.08 bC | 0.79 ± 0.07 aA | 0.87 ± 0.09 aAB |
| 15 | nd * (Decomposed) | 1.35 ± 0.10 bB | 1.34 ± 0.09 bC | 1.04 ± 0.08 aAB | 1.01 ± 0.03 aB |
| 20 | nd (Decomposed) | 1.47 ± 0.09 abB | 1.42 ± 0.09 abC | 1.22 ± 0.03 aB | 1.12 ± 0.07 aB |
| 25 | nd (Decomposed) | 2.11 ± 0.05 bC | 2.09 ± 0.10 bD | 1.19 ± 0.06 aB | 1.18 ± 0.08 aBC |
| 30 | nd (Decomposed) | 2.51 ± 0.09 cD | 2.43 ± 0.04 cE | 1.53 ± 0.07 abC | 1.33 ± 0.02 aC |
| 35 | nd (Decomposed) | 2.92 ± 0.09 cE | 2.89 ± 0.07 cF | 1.87 ± 0.06 abE | 1.46 ± 0.09 aC |
| Parameters | Samples | Storage (Days at 1 °C) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | ||
| OW | 51.08 ± 1.28 aBC | 48.42 ± 2.52 aAB | 49.35 ± 2.12 aB | nd * | nd | nd | nd | nd | |
| SP-00 | 50.35 ± 2.20 aB | 47.54 ± 2.00 aA | 49.35 ± 2.12 aB | 50.15 ± 2.25 aAB | 48.30 ± 1.69 aA | 51.25 ± 0.75 aB | 48.75 ± 1.15 aB | 46.37 ± 0.85 aA | |
| CIE L* | SP-100 | 49.35 ± 2.12 aB | 48.35 ± 1.20 aAB | 52.24 ± 0.57 bC | 51.25 ± 1.15 abB | 49.47 ± 2.09 aA | 51.09 ± 2.07 abB | 51.09 ± 2.24 abC | 48.24 ± 2.35 aAB |
| SP-250 | 47.15 ± 2.25 aA | 49.15 ± 3.05 abB | 48.31 ± 1.10 aA | 49.25 ± 2.08 abA | 49.19 ± 0.79 bA | 50.15 ± 2.10 bB | 50.33 ± 3.13 bC | 49.05 ± 2.35 abB | |
| SP-500 | 46.38 ± 2.35 aA | 46.45 ± 0.85 aA | 47.35 ± 2.10 aA | 49.05 ± 2.07 abA | 47.75 ± 1.15 aA | 47.35 ± 2.02 aA | 46.85 ± 1.10 aA | 47.56 ± 1.17 aA | |
| OW | 18.13 ± 1.17 cAB | 13.42 ± 1.42 bA | 06.18 ± 0.87 aA | nd | nd | nd | nd | nd | |
| SP-00 | 17.58 ± 2.10 eA | 18.24 ± 2.23 eBC | 15.49 ± 1.15 dB | 16.38 ± 1.15 dA | 13.21 ± 2.20 cA | 10.53 ± 0.50 bA | 08.15 ± 1.10 aA | 08.09 ± 1.08 aA | |
| CIE a* | SP-100 | 17.25 ± 1.19 dA | 17.18 ± 1.25 dB | 15.36 ± 0.19 bcB | 15.32 ± 1.20 bcA | 14.01 ± 0.35 bA | 13.73 ± 1.50 bB | 11.35 ± 1.15 aB | 10.12 ± 1.05 aB |
| SP-250 | 16.87 ± 0.87 abA | 16.68 ± 1.25 abB | 15.79 ± 1.18 aB | 16.02 ± 1.37 abA | 16.09 ± 0.20 abB | 15.70 ± 0.58 aC | 14.75 ± 1.12 aC | 14.09 ± 1.05 aC | |
| SP-500 | 17.16 ± 1.21 bA | 17.35 ± 2.20 bB | 17.29 ± 1.10 bC | 16.89 ± 1.20 abAB | 16.56 ± 2.14 abB | 15.63 ± 1.19 aC | 15.15 ± 1.32 aC | 14.65 ± 0.85 aC | |
| OW | 23.29 ± 1.35 aB | 21.75 ± 2.33 aB | 22.85 ± 1.71 aB | nd | nd | nd | nd | nd | |
| SP-00 | 23.19 ± 2.10 cdB | 23.09 ± 2.37 cdB | 23.01 ± 1.40 cdB | 21.57 ± 2.13 cB | 19.28 ± 2.06 bAB | 17.33 ± 2.11 aA | 16.29 ± 0.98 aA | 17.29 ± 1.48 aAB | |
| CIE b* | SP-100 | 21.50 ± 2.35 cAB | 21.10 ± 095 cAB | 19.19 ± 1.65 bcA | 18.31 ± 3.08 bA | 17.11 ± 1.24 abA | 16.84 ± 2.21 aA | 17.17 ± 2.27 abAB | 15.28 ± 1.74 aA |
| SP-250 | 20.47 ± 2.33 cA | 19.49 ± 2.33 cA | 20.08 ± 0.87 cA | 17.29 ± 1.14 abA | 18.42 ± 1.27 abA | 16.29 ± 0.98 aA | 17.11 ± 1.24 abAB | 15.89 ± 2.30 aA | |
| SP-500 | 19.59 ± 2.73 cA | 21.29 ± 2.07 cdAB | 19.28 ± 2.06 cA | 20.53 ± 1.34 cB | 18.79 ± 2.41 bA | 17.19 ± 2.12 abA | 15.89 ± 2.07 aA | 16.19 ± 2.07 aA | |
| Samples | Vacuum Storage (Days) | |||
|---|---|---|---|---|
| 0 | 10 | 20 | 35 | |
| Cooking loss % | ||||
| OW | 32.08 ± 2.18 bD | 25.60 ± 0.58 aB | nd * | nd |
| SP-00 | 31.11 ± 0.99 abD | 32.69 ± 1.48 bD | 30.09 ± 2.01 aBC | 32.48 ± 0.62 bC |
| SP-100 | 29.71 ± 2.19 aC | 31.18 ± 0.95 bC | 28.84 ± 1.50 aB | 31.68 ± 1.66 bC |
| SP-250 | 26.48 ± 0.79 aB | 26.07 ± 1.86 aB | 27.69 ± 2.70 aB | 26.87 ± 0.96 aB |
| SP-500 | 24.41 ± 1.70 bA | 22.55 ± 1.97 aA | 23.88 ± 1.68 abA | 23.56 ± 1.69 abA |
| Reduction in width % | ||||
| OW | 24.60 ± 1.08 aE | 26.49 ± 0.80 bD | nd | nd |
| SP-00 | 22.51 ± 0.90 bD | 19.60 ± 0.80 aC | 22.75 ± 1.25 bBC | 25.72 ± 0.55 cBC |
| SP-100 | 19.42 ± 0.99 aC | 20.16 ± 2.05 aC | 21.18 ± 1.30 bB | 24.54 ± 0.92 cB |
| SP-250 | 17.57 ± 0.72 abB | 17.53 ± 1.05 abB | 16.66 ± 0.40 aA | 15.65 ± 0.71 aA |
| SP-500 | 14.65 ± 0.83 aA | 13.65 ± 0.53 aA | 15.42 ± 0.98 abA | 15.06 ± 0.63 abA |
| Reduction in length % | ||||
| OW | 17.71 ± 0.34 aC | 17.96 ± 0.31 aD | nd | nd |
| SP-00 | 16.72 ± 0.76 aC | 17.20 ± 0.29 aD | 19.64 ± 0.60 bD | 21.72 ± 1.67 cD |
| SP-100 | 17.17 ± 0.51 bC | 15.60 ± 0.62 aC | 16.77 ± 0.98 abC | 18.48 ± 1.08 cC |
| SP-250 | 14.68 ± 0.61 aB | 13.65 ± 0.41 aB | 14.76 ± 0.51 aB | 15.35 ± 1.01 abB |
| SP-500 | 11.42 ± 0.61 abA | 10.00 ± 0.28 aA | 10.44 ± 1.17 aA | 11.25 ± 0.84 abA |
| Shrinkage % | ||||
| OW | 20.17 ± 1.28 aE | 21.40 ± 1.25 abD | nd | nd |
| SP-00 | 18.96 ± 0.61 aD | 20.40 ± 0.62 bD | 21.81 ± 0.29 bcD | 21.86 ± 0.75 bcB |
| SP-100 | 15.99 ± 0.50 aC | 17.07 ± 0.18 bC | 20.12 ± 0.87 cC | 22.26 ± 0.53 dB |
| SP-250 | 12.82 ± 0.26 aB | 13.36 ± 0.56 aB | 14.92 ± 0.93 abB | 16.85 ± 0.25 cA |
| SP-500 | 10.55 ± 0.55 aA | 11.62 ± 0.78 abA | 13.35 ± 0.55 cA | 16.05 ± 0.40 dA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djenane, D.; Khaled, B.M.; Ben Miri, Y.; Metahri, M.S.; Montañés, L.; Aider, M.; Ariño, A. Improved Functionality, Quality, and Shelf Life of Merguez-Type Camel Sausage Fortified with Spirulina as a Natural Ingredient. Foods 2025, 14, 59. https://doi.org/10.3390/foods14010059
Djenane D, Khaled BM, Ben Miri Y, Metahri MS, Montañés L, Aider M, Ariño A. Improved Functionality, Quality, and Shelf Life of Merguez-Type Camel Sausage Fortified with Spirulina as a Natural Ingredient. Foods. 2025; 14(1):59. https://doi.org/10.3390/foods14010059
Chicago/Turabian StyleDjenane, Djamel, Boumediène Méghit Khaled, Yamina Ben Miri, Mohammed Said Metahri, Luis Montañés, Mohammed Aider, and Agustín Ariño. 2025. "Improved Functionality, Quality, and Shelf Life of Merguez-Type Camel Sausage Fortified with Spirulina as a Natural Ingredient" Foods 14, no. 1: 59. https://doi.org/10.3390/foods14010059
APA StyleDjenane, D., Khaled, B. M., Ben Miri, Y., Metahri, M. S., Montañés, L., Aider, M., & Ariño, A. (2025). Improved Functionality, Quality, and Shelf Life of Merguez-Type Camel Sausage Fortified with Spirulina as a Natural Ingredient. Foods, 14(1), 59. https://doi.org/10.3390/foods14010059






