Enhancing Stability and Antioxidant Activity of Resveratrol-Loaded Emulsions by Ovalbumin–Dextran Conjugates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of OVA–DEX Conjugate
2.2.1. Preparation of OVA–DEX Conjugate
2.2.2. SDS-PAGE
2.2.3. Grafting Degree (GD)
2.2.4. Fourier Transforms Infrared (FT-IR) Spectroscopy
2.2.5. Surface Hydrophobicity (H0)
2.2.6. Heat Stability and Thermal Analysis
2.3. Fabrication and Characterization of Emulsion
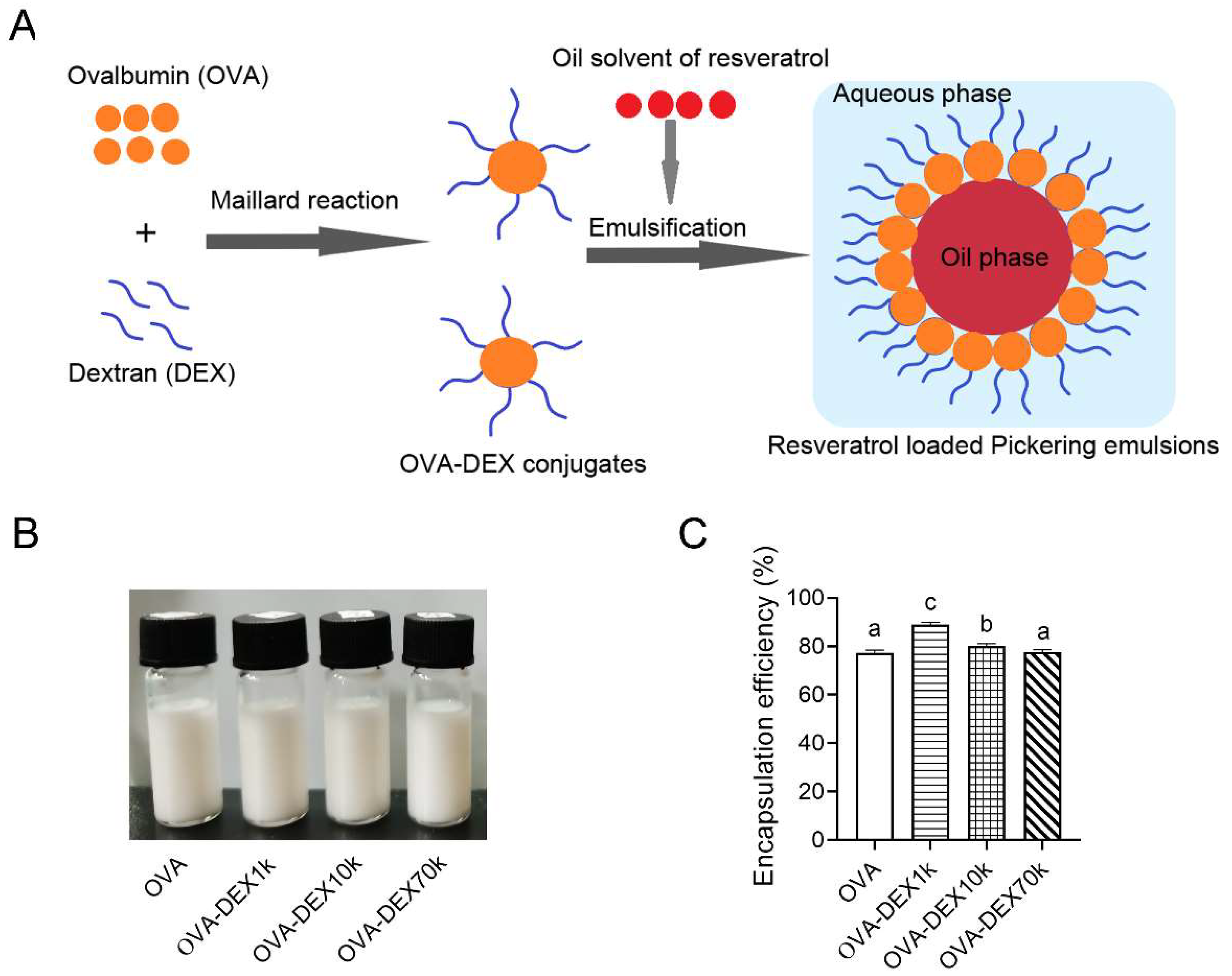
2.3.1. Emulsion Fabrication
2.3.2. Encapsulation Efficiency (EE)
2.3.3. Droplet Size and Polydispersity Index (PDI)
2.3.4. Turbidity
2.3.5. Rheological Properties
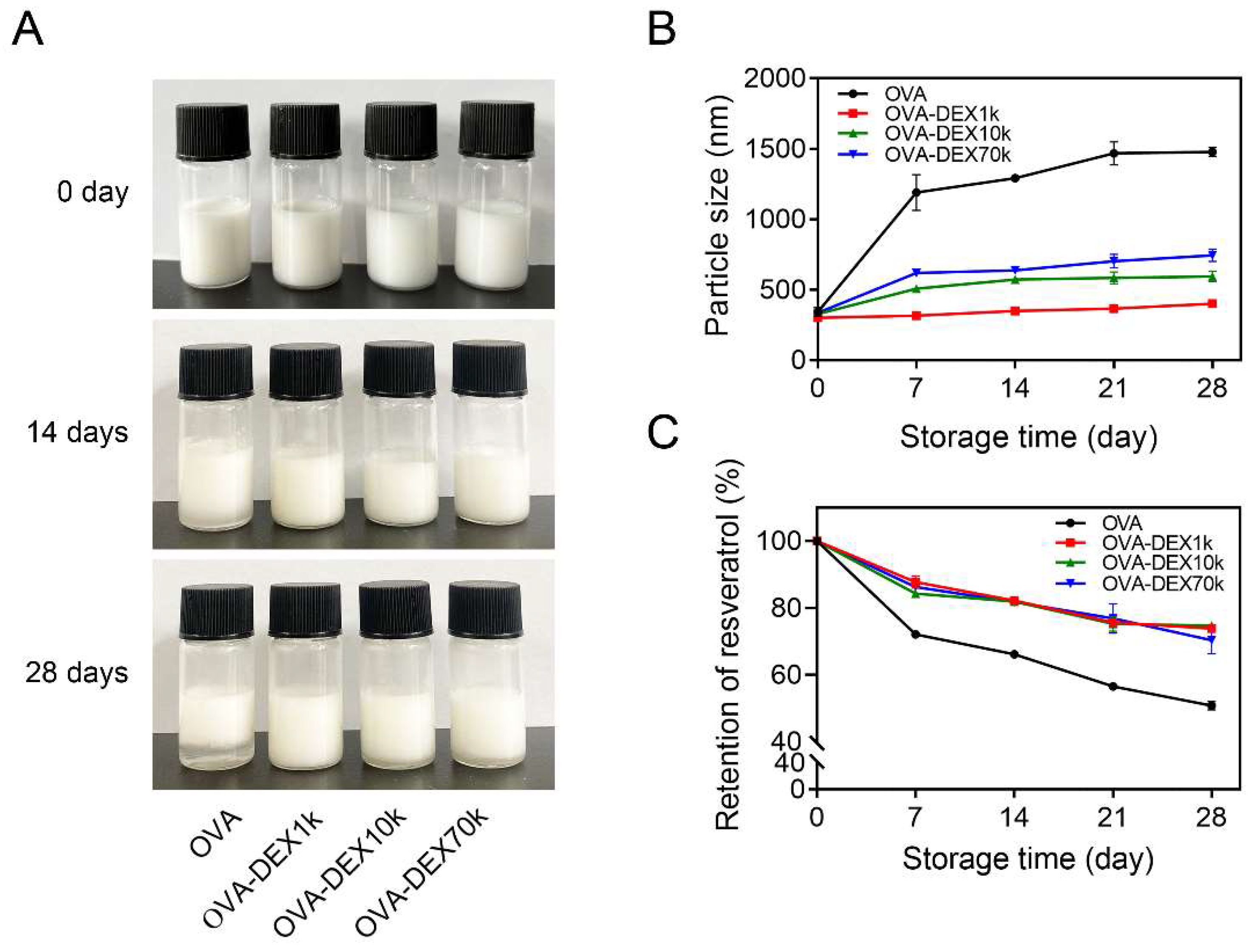
2.4. Storage Stability
2.5. Physicochemical Stability
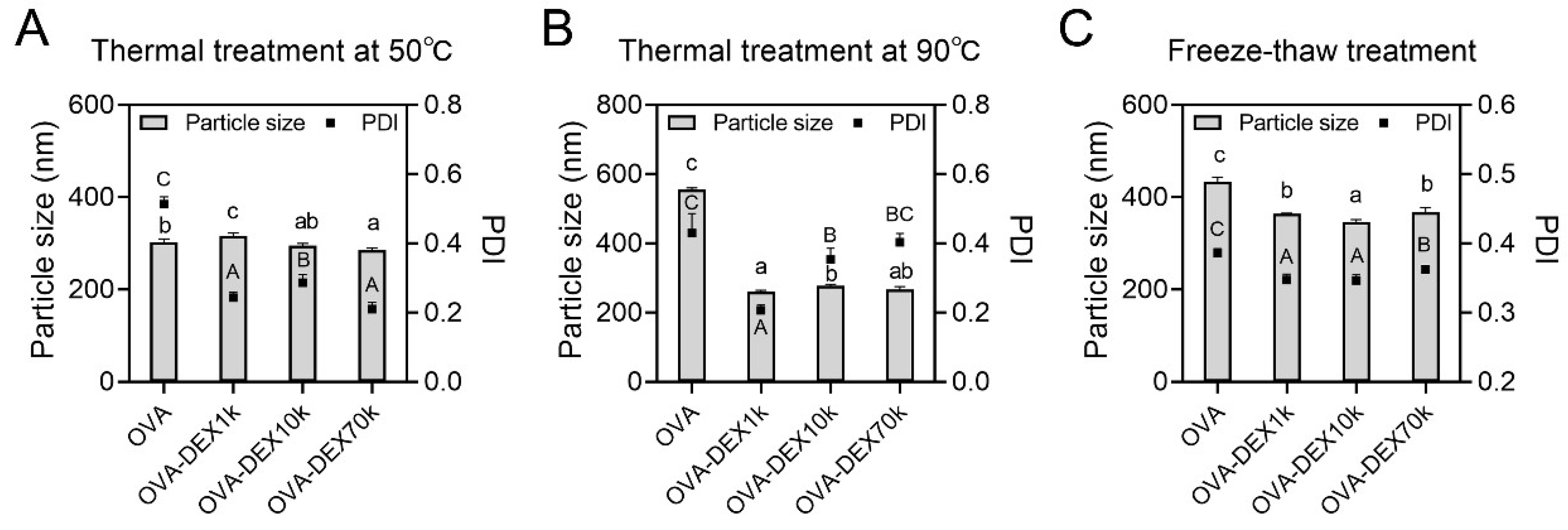
2.5.1. Effect of Thermal Treatment
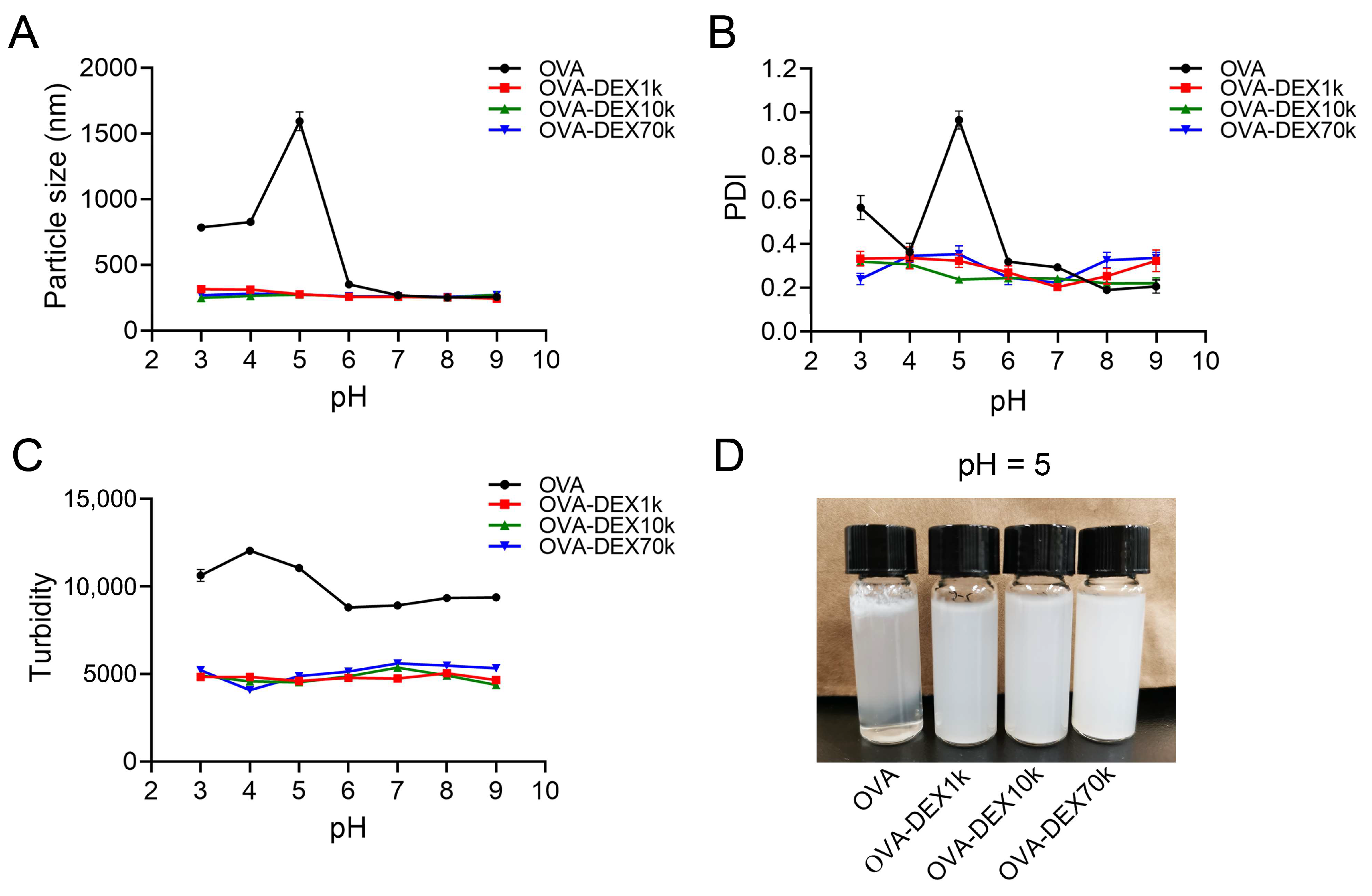
2.5.2. Effect of pH
2.5.3. Effect of Ionic Strength
2.6. Antioxidant Activity Test
2.6.1. ABTS Radical Scavenging
2.6.2. Reducing Power Assay
2.6.3. DPPH Scavenging Capacity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of OVA–DEX Conjugate
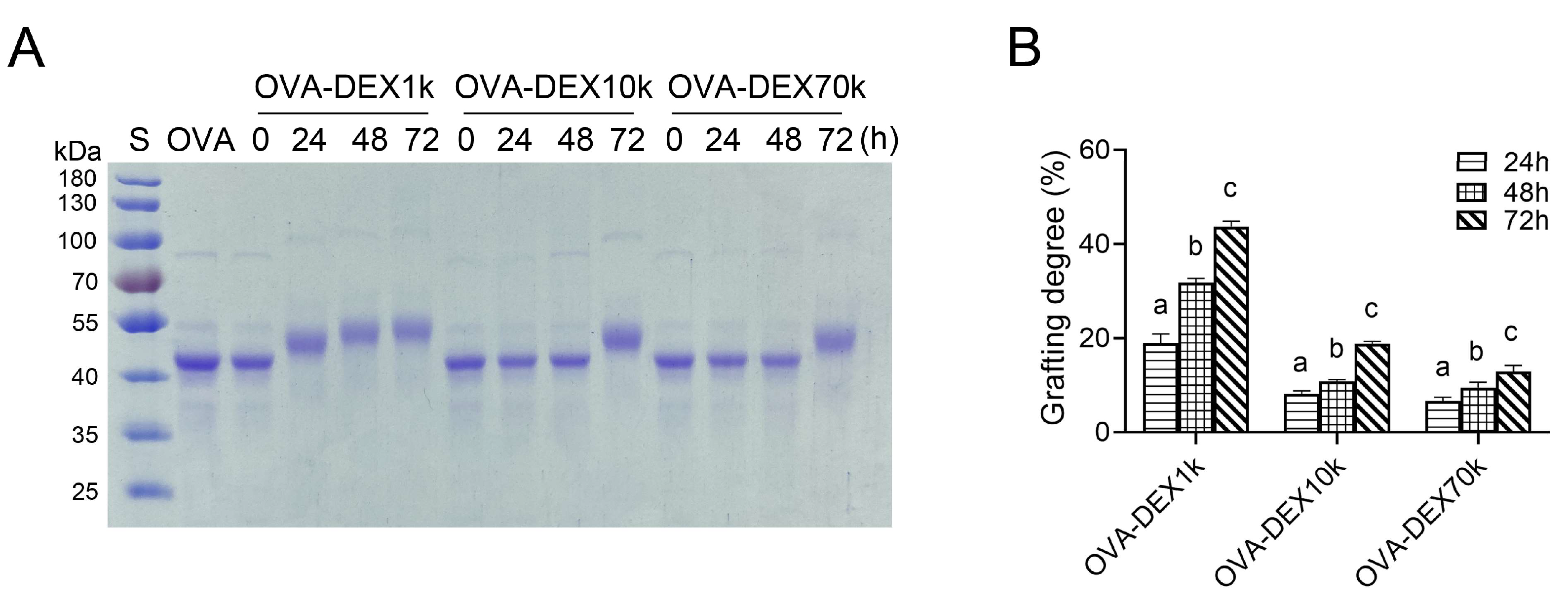
3.1.1. SDS-PAGE and Grafting Degree Analysis
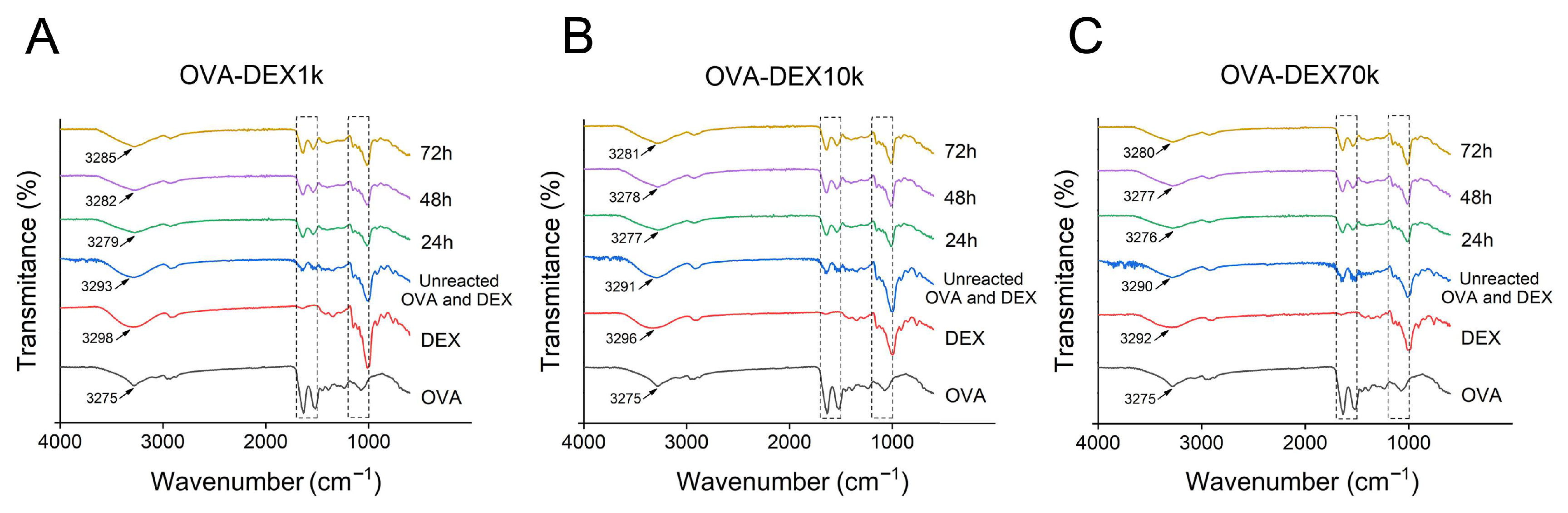
3.1.2. FT-IR Analysis
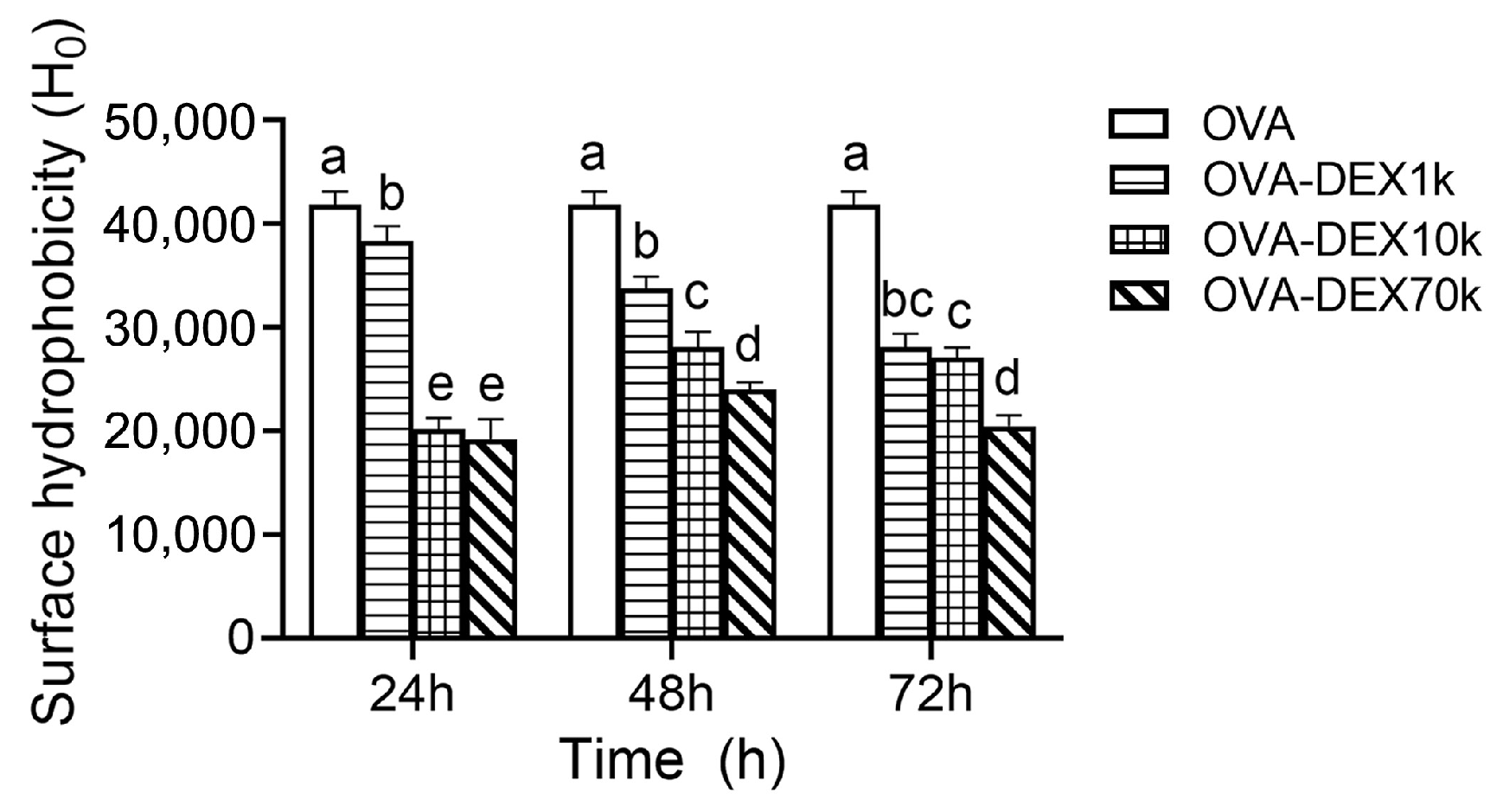
3.1.3. Surface Hydrophobicity
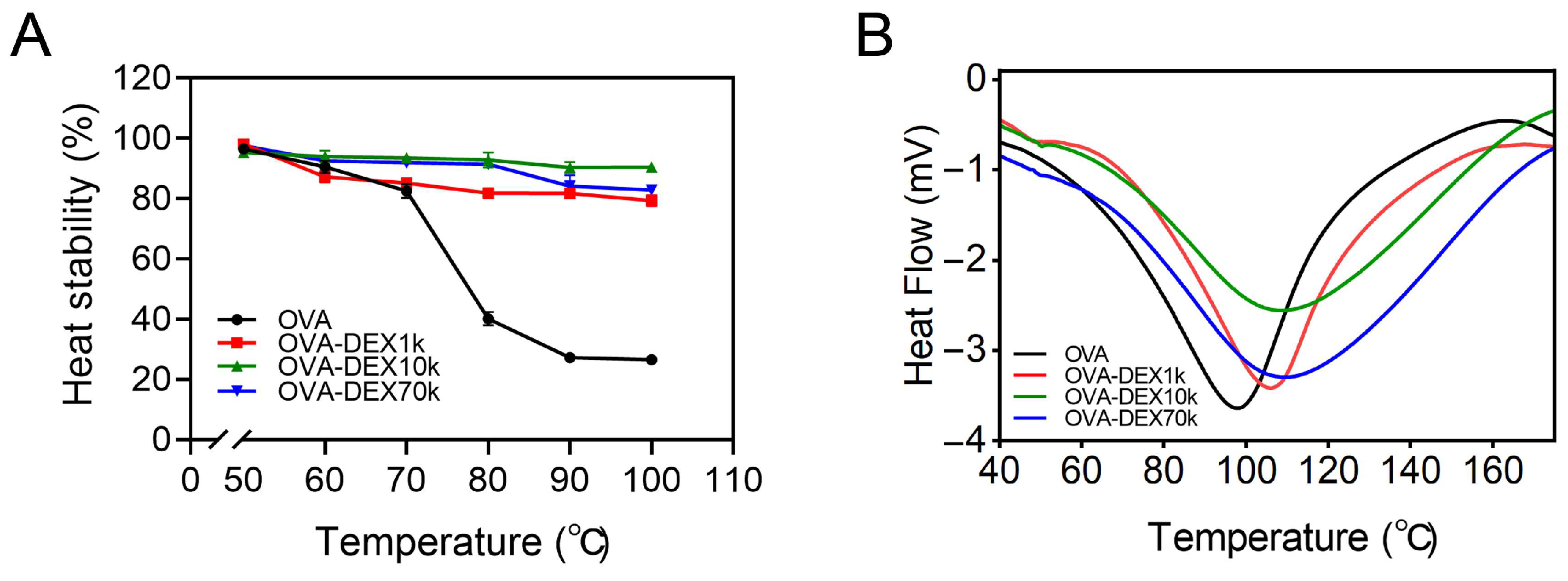
3.1.4. Heat Stability and Thermal Analysis
3.2. Fabrication and Characterization of Emulsion
3.2.1. Encapsulation Efficiency
3.2.2. Storage Stability
3.2.3. Physicochemical Stability
Effect of Thermal Treatment
Effect of pH
Effect of Ionic Strength
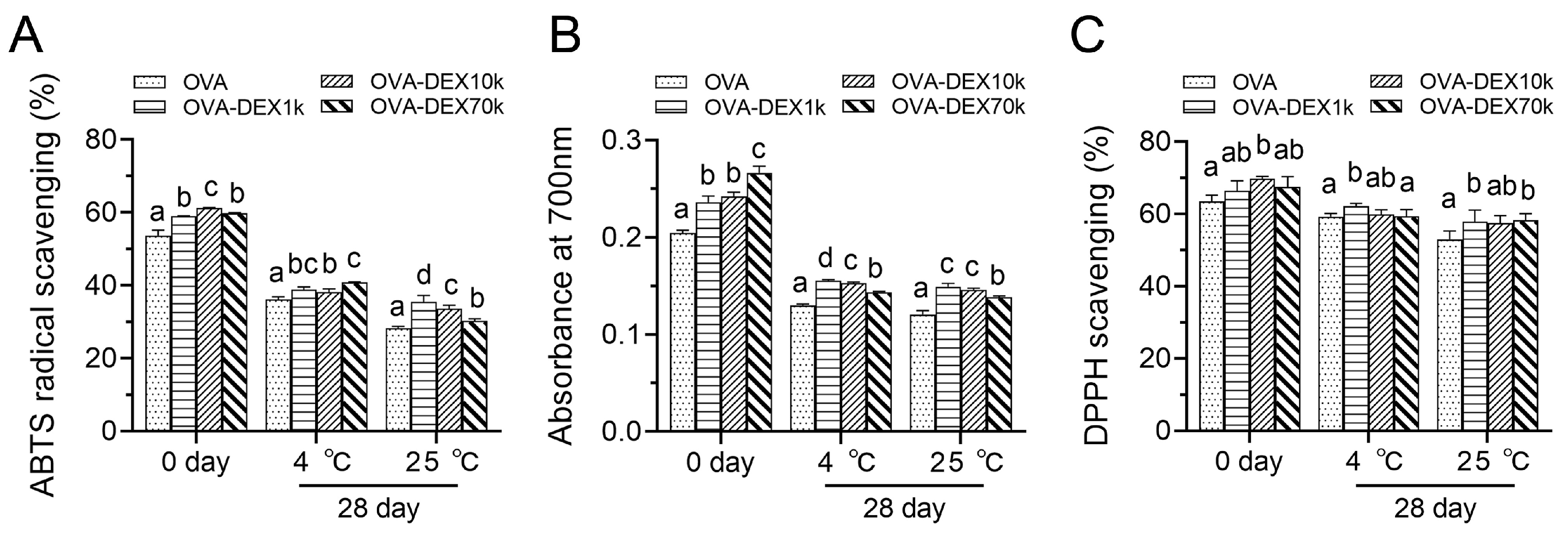
3.2.4. Antioxidant Activity Test
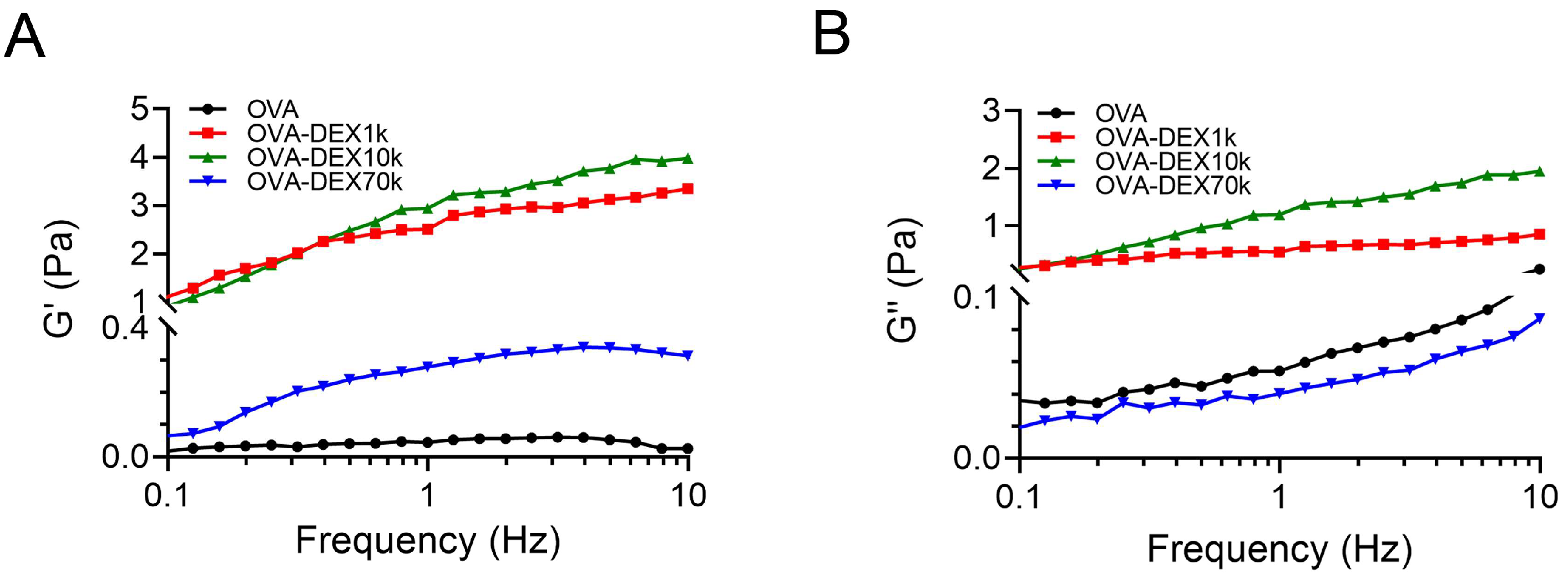
3.2.5. Rheological Properties Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez-Mar, M.I.; Mateos, R.; Garcia-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. STAT3 pathway as a molecular target for resveratrol in breast cancer treatment. Cancer Cell Int. 2021, 21, 468. [Google Scholar] [CrossRef]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1852, 1178–1185. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Li, F.; Li, D.; Huang, Q. Gliadin/Amidated Pectin Core-Shell Nanoparticles for Stabilization of Pickering Emulsion. Int. J. Food Sci. Technol. 2020, 55, 3278–3288. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Lesmes, U.; Mcclements, D.J. Controlling lipid digestibility: Response of lipid droplets coated by β-lactoglobulin-dextran Maillard conjugates to simulated gastrointestinal conditions. Food Hydrocoll. 2012, 26, 221–230. [Google Scholar] [CrossRef]
- Gumus, C.E.; Davidov-Pardo, G.; Mcclements, D.J. Lutein-enriched emulsion-based delivery systems: Impact of Maillard conjugation on physicochemical stability and gastrointestinal fate. Food Hydrocoll. 2016, 60, 38–49. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Xu, G.; Yin, B.; Yao, P. BSA-dextran emulsion for protection and oral delivery of curcumin. Food Hydrocoll. 2016, 61, 11–19. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, F.; Gao, Y.; Panya, A.; Clements, M.; Decker, E.A. Influence of whey protein–beet pectin conjugate on the properties and digestibility of β-carotene emulsion during in vitro digestion. Food Chem. 2014, 156, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Razzak, M.A.; Choi, S.S. Delineating the interaction mechanism of glabridin and ovalbumin by spectroscopic and molecular docking techniques. Food Chem. 2021, 347, 128981. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Cui, B.; Ye, T. Phase behavior of ovalbumin and carboxymethylcellulose composite system. Carbohydr. Polym. Sci. Technol. Asp. Ind. Important Polysacch. 2014, 109, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocoll. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Li, Z.; Kuang, H.; Yang, J.; Hu, J.; Ding, B.; Sun, W.; Luo, Y. Improving emulsion stability based on ovalbumin-carboxymethyl cellulose complexes with thermal treatment near ovalbumin isoelectric point. Sci. Rep. 2020, 10, 3456. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Cui, B.; Li, Y.; Jin, W.; An, Y.; Zhou, B.; Ye, T.; He, L.; Liang, H.; Wang, L. Preparation and characterization of ovalbumin and carboxymethyl cellulose conjugates via glycosylation. Food Hydrocoll. 2014, 37, 86–92. [Google Scholar] [CrossRef]
- Spotti, M.J.; Loyeau, P.A.; Marangon, A.; Noir, H.; Rubiolo, A.C.; Carrara, C.R. Influence of Maillard Reaction Extent on Acid Induced Gels of Whey Proteins and Dextrans. Food Hydrocoll. 2019, 91, 224–231. [Google Scholar] [CrossRef]
- Weng, J.; Qi, J.; Yin, S.; Wang, J.; Yang, X. Fractionation and characterization of soy β-conglycinin-dextran conjugates via macromolecular crowding environment and dry heating. Food Chem. 2016, 196, 1264–1271. [Google Scholar] [CrossRef]
- Diaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Bhavani, A.L.; Nisha, J. Dextran-The polysaccharide with versatile uses. Int. J. Pharma Bio Sci. 2010, 1, 569–573. [Google Scholar]
- Xu, G.; Bao, X.; Yao, P. Protamine and BSA-dextran complex emulsion improves oral bioavailability and anti-tumor efficacy of paclitaxel. Drug Deliv. 2020, 27, 1360–1368. [Google Scholar] [CrossRef]
- Zhang, J.B.; Wu, N.N.; Yang, X.Q.; He, X.T.; Wang, L.J. Improvement of emulsifying properties of Maillard reaction products from β-conglycinin and dextran using controlled enzymatic hydrolysis. Food Hydrocoll. 2012, 28, 301–312. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Kolida, S.; Naeye, T.; Einerhand, A.; Brison, Y.; Remaud-Simeon, M.; Monsan, P.; Gibson, G.R.; Rastall, R.A. In vitro fermentation of linear and alpha-1,2-branched dextrans by the human fecal microbiota. Appl. Environ. Microbiol. 2011, 77, 5307–5315. [Google Scholar] [CrossRef] [PubMed]
- Sarbini, S.R.; Kolida, S.; Naeye, T.; Einerhand, A.W.; Gibson, G.R.; Rastall, R.A. The prebiotic effect of α-1,2 branched, low molecular weight dextran in the batch and continuous faecal fermentation system. J. Funct. Foods 2013, 5, 1938–1946. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Wen, Z.; Zhao, L. Physicochemical stability and invitro bioaccessibility of β-carotene nanoemulsions stabilized with whey protein-dextran conjugates. Food Hydrocoll. 2017, 63, 256–264. [Google Scholar] [CrossRef]
- Rawel, H.M.; Rohn, S.; Kruse, H.P. Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid. Food Chem. 2002, 78, 443–455. [Google Scholar] [CrossRef]
- Sun, J.; Mu, Y.; Obadi, M.; Dong, S.; Xu, B. Effects of single-mode microwave heating and dextran conjugation on the structure and functionality of ovalbumin–dextran conjugates. Food Res. Int. 2020, 137, 109468. [Google Scholar] [CrossRef]
- Olson, B.J.; Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Pharmacol. 2007, 38, A.3A.1–A.3A.29. [Google Scholar] [CrossRef]
- Yi, J.; Liu, Y.; Zhang, Y.; Gao, L. Fabrication of Resveratrol-Loaded Whey Protein-Dextran Colloidal Complex for the Stabilization and Delivery of β-Carotene Emulsions. J. Agric. Food Chem. 2018, 66, 9481–9489. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef]
- Zhou, Y.; Teng, F.; Tian, T.; Sami, R.; Wu, C.; Zhu, Y.; Zheng, L.; Jiang, L.; Wang, Z.; Li, Y. The impact of soy protein isolate-dextran conjugation on capsicum oleoresin (Capsicum annuum L.) nanoemulsions—ScienceDirect. Food Hydrocoll. 2020, 108, 105818. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Lu, Z.; Wu, F.; Fu, G.; Zheng, B.; Tian, Y. Structural characteristics and emulsifying properties of lotus seed protein isolate-dextran glycoconjugates induced by a dynamic high pressure microfluidization Maillard reaction. LWT-Food Sci. Technol. 2022, 160, 113309. [Google Scholar] [CrossRef]
- Aoki, T.; Hiidome, Y.; Kitahata, K.; Sugimoto, Y.; Ibrahim, H.R.; Kato, Y. Improvement of heat stability and emulsifying activity of ovalbumin by conjugation with glucuronic acid through the Maillard reaction. Food Res. Int. 1999, 32, 129–133. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Q.; Diao, C.; Wang, H. Enhanced physicochemical stability of lutein-enriched emulsions by polyphenol-protein-polysaccharide conjugates and fat-soluble antioxidant. Food Hydrocoll. 2019, 101, 105447. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, Y.; Liu, J.; Hedi, W.; Zhang, T. Molecular structural modification of egg white protein by pH-shifting for improving emulsifying capacity and stability. Food Hydrocoll. 2021, 121, 107071. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Huang, Q.; Geng, F. Microwave pretreatment enhanced the properties of ovalbumin-inulin-oil emulsion gels and improved the storage stability of pomegranate seed oil. Food Hydrocoll. 2020, 113, 106548. [Google Scholar] [CrossRef]
- Hayashi, Y.; Li, C.P.; Enomoto, H.; Ibrahim, H.R.; Aoki, T. Improvement of Functional Properties of Ovotransferrin by Phosphorylation through Dry-heating in the Presence of Pyrophosphate. Asian Australas. J. Anim. Sci. 2008, 21, 596–602. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, W.; Han, M.; Bu, Y.; Li, X.; Li, J. Preparation, characterization, and gel characteristics of nanoemulsions stabilized with dextran-conjugated clam Meretrix meretrix linnaeus protein isolate. Food Chem. 2022, 375, 131664. [Google Scholar] [CrossRef]
- Razi, S.M.; Motamedzadegan, A.; Shahidi, A.; Rashidinejad, A. The effect of basil seed gum (BSG) on the rheological and physicochemical properties of heat-induced egg albumin gels—ScienceDirect. Food Hydrocoll. 2018, 82, 268–277. [Google Scholar] [CrossRef]
- Kevij, H.T.; Salami, M.; Mohammadian, M.; Khodadadi, M. Fabrication and investigation of physicochemical, food simulant release, and antioxidant properties of whey protein isolate-based films activated by loading with curcumin through the pH-driven method. Food Hydrocoll. 2020, 108, 106026. [Google Scholar] [CrossRef]
- Feng, J.; Cai, H.; Wang, H.; Li, C.; Liu, S. Improved oxidative stability of fish oil emulsion by grafted ovalbumin-catechin conjugates. Food Chem. 2018, 241, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, R.; Zhang, Y.; Guan, X. Characteristics of two cedarwood essential oil emulsions and their antioxidant and antibacterial activities. Food Chem. 2021, 346, 128970. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sun, C.; Wang, D.; Yuan, F.; Gao, Y. Glycosylation improves the functional characteristics of chlorogenic acid-lactoferrin conjugate. RSC Adv. 2015, 5, 78215–78228. [Google Scholar] [CrossRef]
- Kim, S.; Cavaco-Paulo, A. Laccase-catalysed protein-flavonoid conjugates for flax fibre modification. Appl. Microbiol. Biotechnol. 2012, 93, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Perisic, N.; Afseth, N.K.; Ofstad, R.; Hassani, S.; Kohler, A. Characterising protein, salt and water interactions with combined vibrational spectroscopic techniques. Food Chem. 2013, 138, 679–686. [Google Scholar] [CrossRef]
- Razzak, M.A.; Lee, J.E.; Choi, S.S. Structural insights into the binding behavior of isoflavonoid glabridin with human serum albumin. Food Hydrocoll. 2019, 91, 290–300. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Carrara, C.R.; Santiago, L.G. Impact of environment conditions on physicochemical characteristics of ovalbumin heat-induced nanoparticles and on their ability to bind PUFAs. Food Hydrocoll. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Noji, M.; So, M.; Yamaguchi, K.; Hojo, H.; Onda, M.; Akazawa-Ogawa, Y.; Hagihara, Y.; Goto, Y. Heat-Induced Aggregation of Hen Ovalbumin Suggests a Key Factor Responsible for Serpin Polymerization. Biochemistry 2018, 57, 5415–5426. [Google Scholar] [CrossRef]
- Azakami, H.; Mukai, A.; Kato, A. Role of amyloid type cross beta-structure in the formation of soluble aggregate and gel in heat-induced ovalbumin. J. Agric. Food Chem. 2005, 53, 1254–1257. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Z.; Cheng, Y.; Xu, H.; Velickovic, T.C.; He, K.; Sun, F.; He, Z.; Liu, Z.; Wu, X. Changes in Allergenicity of Ovalbumin In Vitro and In Vivo on Conjugation with Quercetin. J. Agric. Food Chem. 2020, 68, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Angardi, V.; Ettehadi, A.; Yücel, Z. Critical Review of Emulsion Stability and Characterization Techniques in Oil Processing. J. Energy Resour. Technol. Trans. ASME 2021, 144, 040801. [Google Scholar] [CrossRef]
- Hou, Z.; Gao, Y.; Yuan, F.; Liu, Y.; Li, C.; Xu, D. Investigation into the Physicochemical Stability and Rheological Properties of β-Carotene Emulsion Stabilized by Soybean Soluble Polysaccharides and Chitosan. J. Agric. Food Chem. 2010, 58, 8604–8611. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; Mcclements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-R.; Yang, Q.; Du, Y.-N.; Chen, H.-Q. Chitosan can improve the storage stability of ovalbumin fibrils at pH higher than isoelectric point. Food Hydrocoll. 2023, 136, 108286. [Google Scholar] [CrossRef]
- Geng, F.; Xie, Y.; Wang, J.; Li, S.; Jin, Y.; Ma, M. Large-scale purification of ovalbumin using polyethylene glycol precipitation and isoelectric precipitation. Poult. Sci. 2019, 98, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Diftis, N.; Kiosseoglou, V. Improvement of emulsifying properties of soybean protein isolate by conjugation with carboxymethyl cellulose. Food Chem. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Diftis, N.; Kiosseoglou, V. Stability against heat-induced aggregation of emulsions prepared with a dry-heated soy protein isolate-dextran mixture. Food Hydrocoll. 2006, 20, 787–792. [Google Scholar] [CrossRef]
- Ray, M.; Rousseau, D. Stabilization of oil-in-water emulsions using mixtures of denatured soy whey proteins and soluble soybean polysaccharides. Food Res. Int. 2013, 52, 298–307. [Google Scholar] [CrossRef]
- Jiménez-Castaño, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Xu, T.; Gu, Z.; Cheng, L.; Li, C.; Li, Z.; Hong, Y. Stability, oxidizability, and topical delivery of resveratrol encapsulated in octenyl succinic anhydride starch/chitosan complex-stabilized high internal phase Pickering emulsions. Carbohydr. Polym. 2023, 305, 120566. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.; George, S.; Saharan, V.K. Curcumin Encapsulation in Multilayer Oil-in-Water Emulsion: Synthesis Using Ultrasonication and Studies on Stability and Antioxidant and Release Activities. Langmuir 2019, 35, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fan, L.; Liu, Y.; Li, J. Fabrication of chitosan-protocatechuic acid conjugates to inhibit lipid oxidation and improve the stability of β-carotene in Pickering emulsions: Effect of molecular weight of chitosan. Int. J. Biol. Macromol. 2022, 217, 1012–1026. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, D.; Munari, M. Progress of the Maillard reaction and antioxidant action of Maillard reaction products in preheated model systems during storage. J. Agric. Food Chem. 2000, 48, 3555–3559. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Liu, C.; Liu, C. Application of LF-NMR to the characterization of camellia oil-loaded pickering emulsion fabricated by soy protein isolate. Food Hydrocoll. 2021, 112, 106329. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Meng, L.; Lv, X.; Wang, L.; Zhao, P.; Wang, J.; Zhang, X.; Chen, J.; Wu, Z. Enhancing Stability and Antioxidant Activity of Resveratrol-Loaded Emulsions by Ovalbumin–Dextran Conjugates. Foods 2024, 13, 1246. https://doi.org/10.3390/foods13081246
Zhang W, Meng L, Lv X, Wang L, Zhao P, Wang J, Zhang X, Chen J, Wu Z. Enhancing Stability and Antioxidant Activity of Resveratrol-Loaded Emulsions by Ovalbumin–Dextran Conjugates. Foods. 2024; 13(8):1246. https://doi.org/10.3390/foods13081246
Chicago/Turabian StyleZhang, Wen, Lingli Meng, Xinyi Lv, Limin Wang, Pei Zhao, Jinrong Wang, Xinping Zhang, Jinyu Chen, and Zijian Wu. 2024. "Enhancing Stability and Antioxidant Activity of Resveratrol-Loaded Emulsions by Ovalbumin–Dextran Conjugates" Foods 13, no. 8: 1246. https://doi.org/10.3390/foods13081246
APA StyleZhang, W., Meng, L., Lv, X., Wang, L., Zhao, P., Wang, J., Zhang, X., Chen, J., & Wu, Z. (2024). Enhancing Stability and Antioxidant Activity of Resveratrol-Loaded Emulsions by Ovalbumin–Dextran Conjugates. Foods, 13(8), 1246. https://doi.org/10.3390/foods13081246





