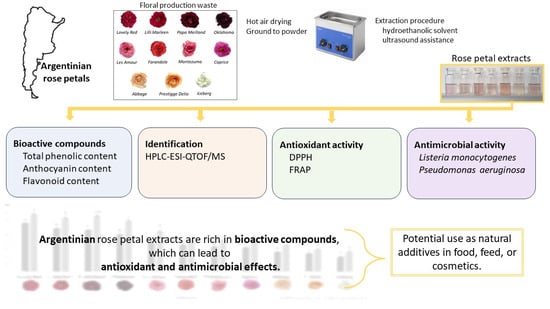
Argentinian Rose Petals as a Source of Antioxidant and Antimicrobial Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction Procedure
2.4. Total Phenolic Content
2.5. Antioxidant Activity
2.6. Anthocyanin Content
2.7. Flavonoid Content
2.8. HPLC-ESI-QTOF/MS Analysis
2.9. Antimicrobial Activity In Vitro
2.9.1. Indicator Strains and Culture Conditions
2.9.2. System Preparation
2.9.3. Antimicrobial Activity
2.10. Data Analysis
3. Results and Discussion
3.1. Bioactive Compounds
3.2. Identification of Phenolic Compounds
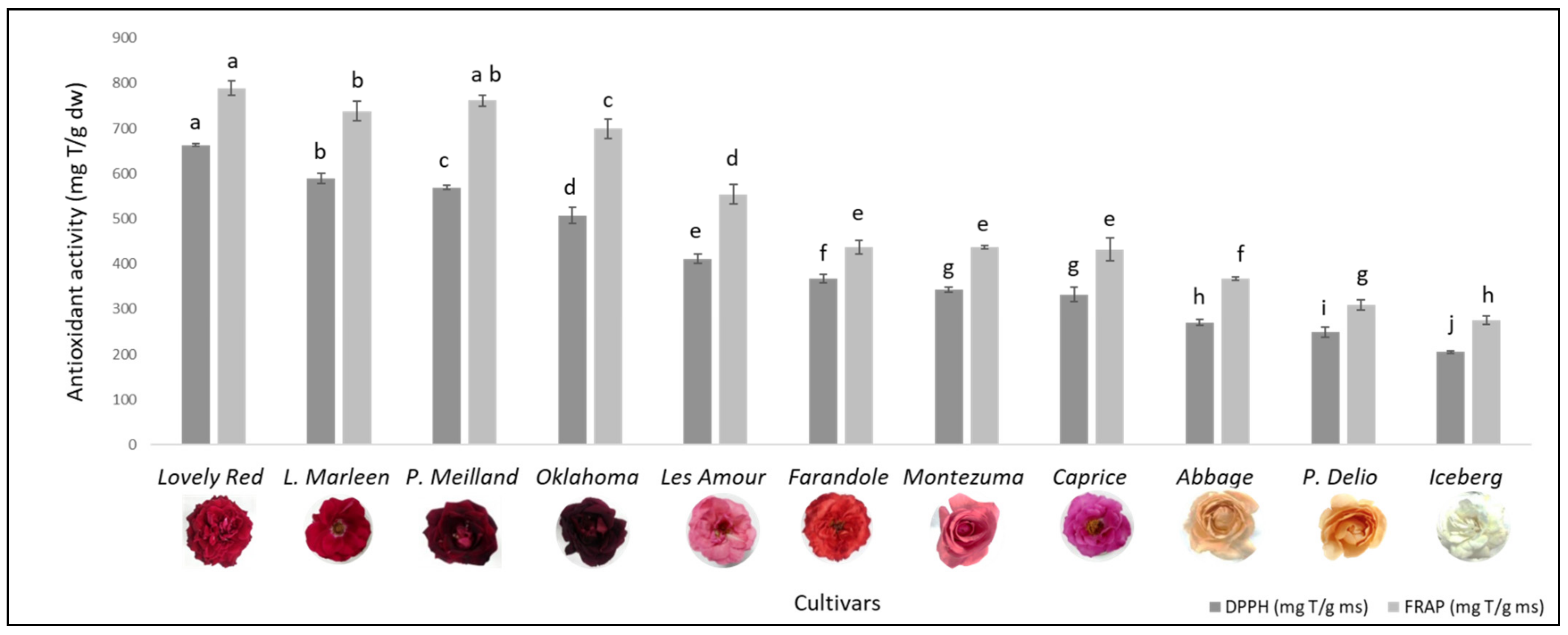
3.3. Antioxidant Activity
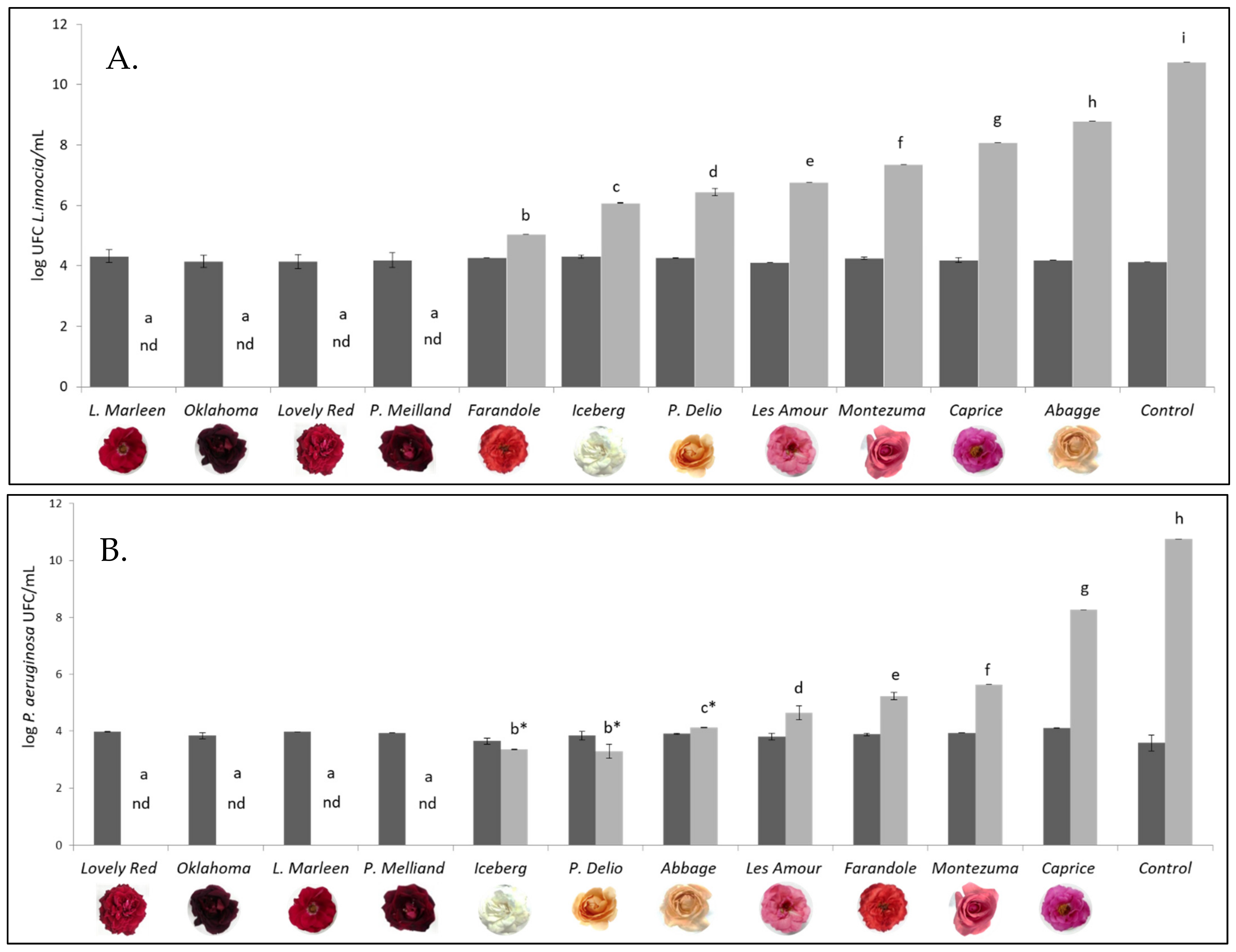
3.4. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| dw | dry weight |
| Trolox | 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| TE | Trolox equivalents |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl |
| FRAP | ferric reducing antioxidant power |
| CE | Cyanidin equivalents |
| GAE | Gallic acid equivalents |
| QE | Quercetin equivalents |
| TSB | Trypticase soy broth |
| CFU | colony forming units |
References
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- dos Santos, A.M.P.; dos Santos, W.N.L.; Silva, E.F.R.; da Silva, E.G.P.; dos Santos, L.O.; da S. Santos, B.R.; da S. Sauthier, M.C.; dos Santos, W.P.C. Evaluation of minerals, toxic elements and bioactive compounds in rose petals (Rosa spp.) using chemometric tools and artificial neural networks. Microchem. J. 2018, 138, 98–108. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Pascal, D.; Hicham, F.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela Raventós, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Halawani, E.M. Antimicrobial activity of Rosa damascena petals extracts and chemical composition by gas chromatography‒mass spectrometry (GC/MS) analysis. Afr. J. Microbiol. Res. 2014, 8, 2359–2367. [Google Scholar] [CrossRef]
- dos Santos, I.Z.; Novaes Reis, S. Edible flowers: Traditional and current use. Ornam. Hort. 2021, 27, 438–445. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Chang, L. Effect of edible rose (Rosa rugosa cv. Plena) flower extract addition on the physicochemical, rheological, functional and sensory properties of set-type yogurt. Food Biosci. 2021, 43, 101249. [Google Scholar] [CrossRef]
- Lu, B.; Li, M.; Yin, R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review (2000–2015). Crit. Rev. Food Sci. Nutr. 2015, 56, S130–S148. [Google Scholar] [CrossRef]
- Hansen, L. Caracterización de los Viveros en el Partido de San Pedro, Buenos Aires, Argentina. Relevancia Tecnológica, Social y Económica 2017. EEA San Pedro, INTA, Ministerio de Agricultura, Ganadería y Pesca. Available online: https://inta.gob.ar/documentos/caracterizacion-de-los-viveros-en-el-partido-de-san-pedro-buenos-aires-argentina-relevancia-tecnologica-social-y-economica (accessed on 16 February 2024).
- Baibuch, S.; Zema, P.; Bonifazi, E.; Cabrera, G.; Mondragón Portocarrero, A.d.C.; Campos, C.; Malec, L. Effect of the Drying Method and Optimization of Extraction on Antioxidant Activity and Phenolic of Rose Petals. Antioxidants 2023, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers: A Rich Source of Phytochemicals with Antioxidant and Hypoglycaemic Activity. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Z.; Fattahi, M. Essential oil, total phenolic, flavonoids, anthocyanins, carotenoids and antioxidant activity of cultivated Damask Rose (Rosa damascena) from Iran: With chemotyping approach concerning morphology and composition. Sci. Hortic. 2021, 288, 110341. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crops Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Ginova, A.; Mihalev, K.; Kondakova, V. Antioxidant Capacity of Petals and Leaves from Different Rose (Rosa damascena Mill.) Plantations in Bulgaria. Int. J. Pure App. Biosci. 2013, 1, 38–43. [Google Scholar]
- Yeon, J.Y.; Kim, W.S. Floral pigment-scent associations in eight cut rose cultivars with various petal colors. Hortic. Environ. Biotechnol. 2020, 61, 633–641. [Google Scholar] [CrossRef]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.-L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.M.; Hardy, W.D.; et al. Isolation and Characterization of Antimicrobial Compounds in Plant Extracts against Multidrug-Resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e61594. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Meenu, M.; Xu, B. A systematic investigation on free phenolic acids and flavonoids profiles of commonly consumed edible flowers in China. J. Pharm. Biomed. Anal. 2019, 172, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, H.; Shokrpour, M.; Fatahi, R.; Naghavi, R.; Mirjalili, M.H. Essential oil, flavonoids and anthocyanins profiling of some Iranian damask rose (Rosa damascena Mill.) genotypes. Ind. Crops Prod. 2023, 205, 117579. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Luo, L.; Pan, H.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q. Determination of Flavonoids and Carotenoids and Their Contributions to Various Colors of Rose Cultivars (Rosa spp.). Front. Plant Sci. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; McCallum, J. Chemistry of Flavonoids. In Fruit and Vegetable Phytochemicals, 1st ed.; de la Rosa, L.A., Alvarez-Parrilla, E., Gonzalez-Aguilar, G.A., Eds.; Wiley: New York, NY, USA, 2009; Chapter 5; pp. 131–153. [Google Scholar] [CrossRef]
- VanderJagt, T.J.; Ghattasa, R.; VanderJagt, D.J.; Crosseyb, M.; Glewa, R.H. Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico. Life Sci. 2002, 70, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Li, H.-B.; Xu, D.-P.; Xu, X.-R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flower. J. Funct. Foods. 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Ahmadi, S.; Javid, H. Novel formulations of ellagic acid for the improvement of antimicrobial, antioxidant, anticancer, antidiabetic, and neuroprotective applications. Nano Micro. Biosyst. 2023, 2, 31–35. [Google Scholar]
- Ferreira, R.Q.; Greco, S.J.; Delarmelina, M.; Weber, K.C. Electrochemical quantification of the structure/antioxidant activity relationship of flavonoids. Electrochim. Acta 2015, 163, 161–166. [Google Scholar] [CrossRef]
- Shaidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Ebrahim, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Ercan, L.; Dogru, M. Antioxidant and Antimicrobial Capacity of Quinic Acid. BEU J. Sci. Technol. 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Olech, M.; Pecio, L.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 2014, 94, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Shohayeb, M.; Abdel-Hameed, E.S.; Bazaid, S.; Maghrabi, I. Antibacterial and antifungal activity of Rosa damascena MILL. essential oil, different extracts of rose petals. Glob. J. Pharmacol. 2014, 8, 1–7. [Google Scholar]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Plumed-Ferrer, C.; Vakevainen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.E.; Eklund, P.; Willfor, S.; Alakomi, H.-L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef]
- Yi, S.; Zhu, J.; Fu, L.; Li, J. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int. J. Food Microbiol. 2010, 144, 111–117. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, Y.; Yi, G.; Li, M.; Liao, L.; Yang, C.; Cho, C.; Zhang, B.; Zhu, J.; Zou, K.; et al. Quinic acid: A potential antibioflm agent against clinical resistant Pseudomonas aeruginosa. Chin. Med. 2021, 16, 72. [Google Scholar] [CrossRef]



| Negative Mode | ||||||
|---|---|---|---|---|---|---|
| N◦ | Rt. (min) | Formula | Compound | [M-H]− (m/z) | MS/MS | Flower Cultivar |
| 1 | 2.5 | C7H12O6 | Quinic acid | 191.0558 | 171, 155, 137, 127 | All cultivars |
| 2 | 12.2 | C41H28O26 | Galloyl-bis-HHDP-hexose | 935.0772 | 451 | Red flowers: Lilli Marleen, Papa Meilland, Oklahoma Pink flowers: Les Amour, Farandole White flowers: Iceberg |
| 3 | 12.5 | C41H26O26 | Unknown ellagitannin | 466.0291 * | 301, 451 | Red flowers: Lilli Marleen, Papa Meilland Pink flowers: Les Amour, Montezuma, Caprice White flowers: Iceberg |
| 4 | 17.8 | C20H18O11 | Quercetin-O-pentoside | 433.0762 | 410, 300 | Red flowers: Papa Meilland Pink flowers: Les Amour, Caprice, Montezuma White flowers: Abagge, Prestigge Delio, Iceberg |
| 5 | 18.1 | C21H20O12 | Quercetin-O-hexoside | 463.0899 | 410, 300 | Pink flowers: Les Amour White flowers: Iceberg |
| 6 | 18.6 | C14H6O8 | Ellagic acid | 300.9995 | 229, 210, 201, 185, 173 | All cultivars |
| 7 | 19.5 | C21H20O11 | Quercetin-O-rhamnoside | 447.0930 | 410, 300, 271, 178, 151 | All cultivars |
| 8 | 19.7 | C28H24O15 | Quercetin-O-galloyl rhamnoside | 599.1042 | 447, 410, 313, 285, 226, 169 | Pink flowers: Les Amour, Montezuma, Caprice White flowers: Prestigge Delio, Iceberg |
| 9 | 20.2 | C20H18O10 | Kaempferol-O-pentoside | 417.0814 | 284, 255, 227 | Red flowers: Oklahoma Pink flowers: Montezuma, Caprice, Farandole, Les Amour |
| 10 | 20.7 | C27H30O15 | Kaempferol-O-hexosyl-deoxyhexoside | 593.1507 | 410, 285 | Pink flowers: Les Amour, Montezuma, Farandole White flowers: Abagge, Prestigge Delio, Iceberg |
| 11 | 21.1 | C21H20O10 | Kaempferol-O-deoxyhexoside | 431.1003 | 410, 284, 255, 227 | Red flowers: Lilli Marleen, Lovely Red, Papa Meilland, Oklahoma Pink flowers: Les Amour, Montezuma, Farandole, White flowers: Abagge, Prestigge Delio, Iceberg |
| Positive mode | ||||||
| N◦ | Rt. (min) | Formula | Compound | [M-H] + (m/z) | MS/MS | Flower cultivar |
| 1 | 8.6 | C27H31O16 | Cyanidin-3,5-O-diglucoside | 611.1609 | 287, 449 | All dark red cultivars |
| 2 | 10.0 | C33H31O14 | Mavidin-3-acetylglucoside-4-vinylphenol | 651.1711 | 489, 471, 379, 327, 309, 277, 185 | All dark red cultivars |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baibuch, S.Y.; Schelegueda, L.I.; Bonifazi, E.; Cabrera, G.; Mondragón Portocarrero, A.C.; Franco, C.M.; Malec, L.S.; Campos, C.A. Argentinian Rose Petals as a Source of Antioxidant and Antimicrobial Compounds. Foods 2024, 13, 977. https://doi.org/10.3390/foods13070977
Baibuch SY, Schelegueda LI, Bonifazi E, Cabrera G, Mondragón Portocarrero AC, Franco CM, Malec LS, Campos CA. Argentinian Rose Petals as a Source of Antioxidant and Antimicrobial Compounds. Foods. 2024; 13(7):977. https://doi.org/10.3390/foods13070977
Chicago/Turabian StyleBaibuch, Sabrina Y., Laura I. Schelegueda, Evelyn Bonifazi, Gabriela Cabrera, Alicia C. Mondragón Portocarrero, Carlos M. Franco, Laura S. Malec, and Carmen A. Campos. 2024. "Argentinian Rose Petals as a Source of Antioxidant and Antimicrobial Compounds" Foods 13, no. 7: 977. https://doi.org/10.3390/foods13070977
APA StyleBaibuch, S. Y., Schelegueda, L. I., Bonifazi, E., Cabrera, G., Mondragón Portocarrero, A. C., Franco, C. M., Malec, L. S., & Campos, C. A. (2024). Argentinian Rose Petals as a Source of Antioxidant and Antimicrobial Compounds. Foods, 13(7), 977. https://doi.org/10.3390/foods13070977