Nutritional and Functional Properties of Terminalia ferdinandiana Fruits Wild Harvested from Western Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Chemicals, and Reagents
2.2. Total Water-Soluble Carbohydrate Content
2.3. LC-MS/MS Analysis of Sugars
2.4. Analysis of Vitamin C and Ellagic Acid (EA) Content
2.5. Extract Preparation for Phenolic Content and Functional Properties
2.6. (Poly) Phenolic Content
2.6.1. Determination of Total Phenolic Content (TPC)
2.6.2. Determination of Total Flavonoid Content (TFC)
2.7. Evaluation of Antioxidant Activities
2.7.1. FRAP (Ferric Reducing Antioxidant Power of Plasma) Assay
2.7.2. DPPH Radical Scavenging Power
2.8. Determination of Antimicrobial Activity
2.9. In Vitro α-Glucosidase Inhibition Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Total Water-Soluble Carbohydrate and Sugar Contents
3.2. Vitamin C and Ellagic Acid Content
3.3. Total Phenolic and Flavonoid Content
3.4. Antioxidant Activities
3.5. Principal Component Analysis of Correlated Variables and Variance among the Trees
3.6. Antimicrobial Properties
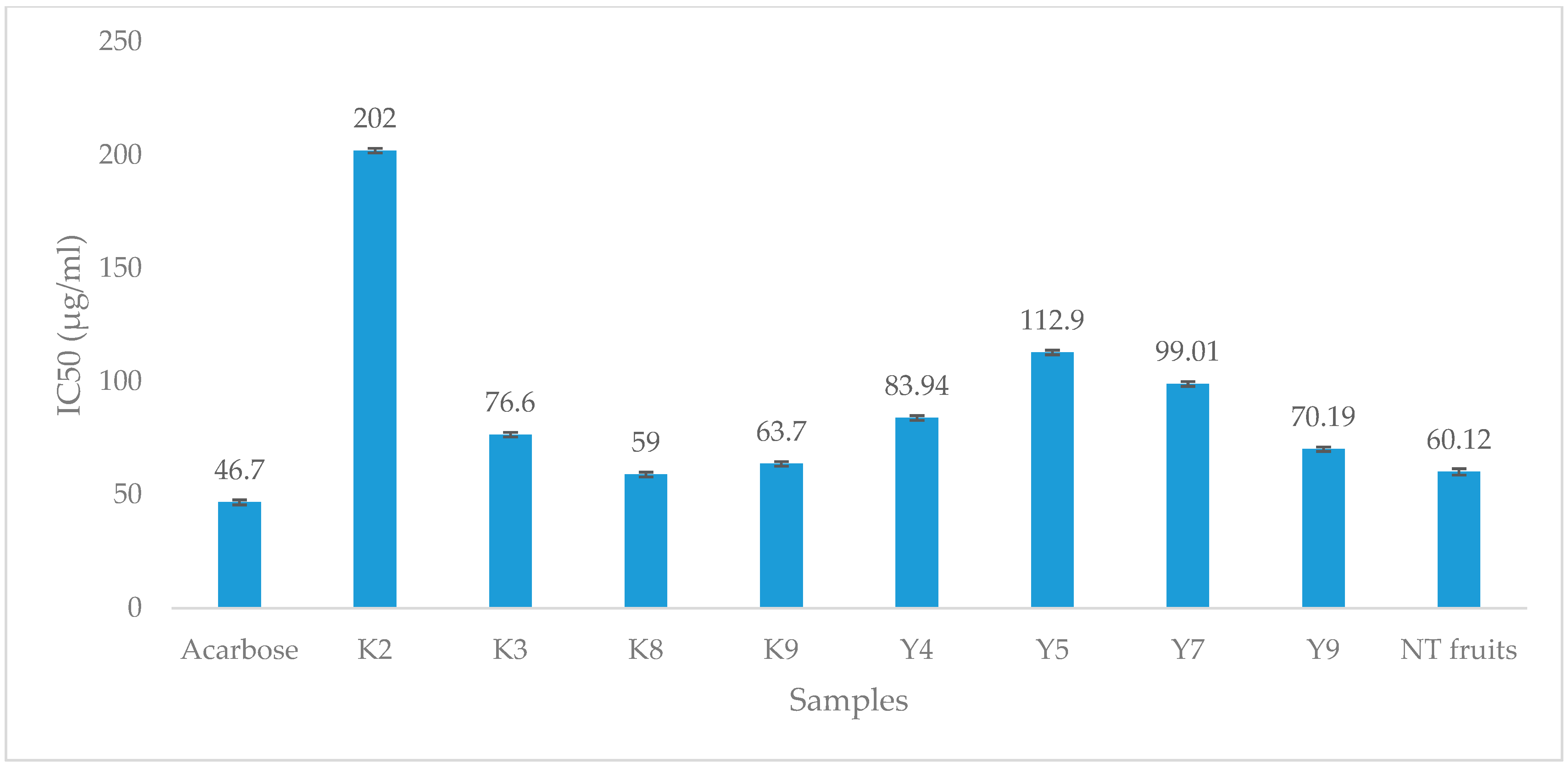
3.7. α-Glucosidase Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargova, V.; Singh, R.B.; Fedacko, J.; Pella, D.; Pella, D.; Wilczynska, A.; Mojto, V. Chapter 20—Effects of Nuts Consumption on Cardiometabolic Diseases and Cancer. In The Role of Functional Food Security in Global Health; Singh, R.B., Watson, R.R., Takahashi, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 347–364. [Google Scholar]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.R.; Blekkenhorst, L.C.; Radavelli-Bagatini, S.; Sim, M.; Woodman, R.J.; Devine, A.; Shaw, J.E.; Hodgson, J.M.; Daly, R.M.; Lewis, J.R. Fruit and Vegetable Knowledge and Intake within an Australian Population: The AusDiab Study. Nutrients 2020, 12, 3628. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Maillot, F.; Dalar, A. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chem. 2014, 151, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Pirie, A.; Parsons, D.; Renggli, J.; Narkowicz, C.; Jacobson, G.A.; Shabala, S. Modulation of flavonoid and tannin production of Carpobrotus rossii by environmental conditions. Environ. Exp. Bot. 2013, 87, 19–31. [Google Scholar]
- Wang, S.Y.; Zheng, W. Effect of Plant Growth Temperature on Antioxidant Capacity in Strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar]
- Attanayake, R.; Eeswaran, R.; Rajapaksha, R.; Weerakkody, P.; Bandaranayake, P.C.G. Biochemical Composition and Expression of Anthocyanin Biosynthetic Genes of a Yellow Peeled and Pinkish Ariled Pomegranate (Punica granatum L.) Cultivar are Differentially Regulated in Response to Agro-Climatic Conditions. J. Agric. Food Chem. 2018, 66, 8761–8771. [Google Scholar]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Res. Int. 2014, 66, 100–106. [Google Scholar]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Bobasa, E.M.; Phan, A.D.T.; Netzel, M.E.; Cozzolino, D.; Sultanbawa, Y. Hydrolysable tannins in Terminalia ferdinandiana Exell fruit powder and comparison of their functional properties from different solvent extracts. Food Chem. 2021, 358, 129833. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Nielsen, S.S. Total Carbohydrate by Phenol-Sulfuric Acid Method. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 137–141. [Google Scholar]
- Hong, H.T.; Phan, A.D.T.; O’Hare, T.J. Temperature and Maturity Stages Affect Anthocyanin Development and Phenolic and Sugar Content of Purple-Pericarp Supersweet Sweetcorn during Storage. J. Agric. Food Chem. 2021, 69, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Terry, L.A. Development of a Rapid Method for the Sequential Extraction and Subsequent Quantification of Fatty Acids and Sugars from Avocado Mesocarp Tissue. J. Agric. Food Chem. 2008, 56, 7439–7445. [Google Scholar] [CrossRef] [PubMed]
- Fountain, K.J.; Hudalla, C.; McCabe, D.; Morrison, D. Waters Application notes UPLC-MS Analysis of Carbohydrates. 2009. Available online: https://www.gimitec.com//file/720003212en.pdf (accessed on 23 September 2021).
- Wang, W.; Li, J.; Zhang, H.; Wang, X.; Fan, J.; Zhang, X. Phenolic compounds and bioactivity evaluation of aqueous and methanol extracts of Allium mongolicum Regel. Food Sci. Nutr. 2019, 7, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measurement of ‘‘antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Yin, P.; Yan, L.; Han, J.; Shi, L.; Zhou, X.; Liu, Y.; Ma, C. α-Glucosidase inhibitory activity of polyphenols from the burs of Castanea mollissima Blume. Molecules 2014, 19, 8373–8386. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Akinleye, M.; Omojokun, O.S.; Boligon, A.A.; Athayde, M.L. Starch composition, glycemic indices, phenolic constituents, and antioxidative and antidiabetic properties of some common tropical fruits. J. Ethn. Foods 2015, 2, 64–73. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Kubola, J.; Siriamornpun, S.; Meeso, N. Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem. 2011, 126, 972–981. [Google Scholar] [CrossRef]
- Jovanovic-Malinovska, R.; Kuzmanova, S.; Winkelhausen, E. Oligosaccharide Profile in Fruits and Vegetables as Sources of Prebiotics and Functional Foods. Int. J. Food Prop. 2014, 17, 949–965. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-López, A.; Bello-Perez, L.A. Chapter 9—Carbohydrates. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 175–205. [Google Scholar]
- Zhang, J.; Nie, J.-Y.; Li, J.; Zhang, H.; Li, Y.; Farooq, S.; Bacha, S.A.S.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Abeysuriya, H.I.; Bulugahapitiya, V.P.; Loku Pulukkuttige, J. Total Vitamin C, Ascorbic Acid, Dehydroascorbic Acid, Antioxidant Properties, and Iron Content of Underutilized and Commonly Consumed Fruits in Sri Lanka. Int. J. Food Sci. 2020, 2020, 4783029. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant Activity and Phenolic Content of Oregon Caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Bruni, R.; Righi, D.; Grandini, A.; Tognolini, M.; Pio Prencipe, F.; Poli, F.; Benvenuti, S.; Del Rio, D.; Rossi, D. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J. Ethnopharmacol. 2013, 147, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Akter, S.; Netzel, M.E.; Tinggi, U.; Osborne, S.A.; Fletcher, M.T.; Sultanbawa, Y. Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria. Foods 2019, 8, 281. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants 2020, 9, 305. [Google Scholar] [CrossRef]
- Ruiz-Torralba, A.; Guerra-Hernández, E.J.; García-Villanova, B. Antioxidant capacity, polyphenol content and contribution to dietary intake of 52 fruits sold in Spain. CyTA-J. Food 2018, 16, 1131–1138. [Google Scholar] [CrossRef]
- Moore, J.; Yu, L. Methods for Antioxidant Capacity Estimation of Wheat and Wheat-Based Food Products. In Wheat Antioxidants; Yu, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 118–172. [Google Scholar]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Sources of Antioxidant Activity in Australian Native Fruits. Identification and Quantification of Anthocyanins. J. Agric. Food Chem. 2006, 54, 9820–9826. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Buren, L.v.; Wagner, E.; Wiseman, S.; Put, F.v.d.; Dacombe, C.; Rice-Evans, C.A. The Antioxidant Activity of Regularly Consumed Fruit and Vegetables Reflects their Phenolic and Vitamin C Composition. Free. Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Potential dietary sources of ellagic acid and other antioxidants among fruits consumed in Brazil: Jabuticaba (Myrciaria jaboticaba (Vell.) Berg). J. Sci. Food Agric. 2012, 92, 1679–1687. [Google Scholar] [CrossRef]
- Cheesman, M.J.; White, A.; Matthews, B.; Cock, I.E. Terminalia ferdinandiana Fruit and Leaf Extracts Inhibit Methicillin-Resistant Staphylococcus aureus Growth. Planta Medica 2019, 85, 1253–1262. [Google Scholar] [CrossRef]
- Savic, I.M.; Jocic, E.; Nikolic, V.D.; Popsavin, M.M.; Rakic, S.J.; Savic-Gajic, I.M. The effect of complexation with cyclodextrins on the antioxidant and antimicrobial activity of ellagic acid. Pharm. Dev. Technol. 2019, 24, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lu, W.; Zhou, X. Phenolic Compounds and In Vitro Antibacterial and Antioxidant Activities of Three Tropic Fruits: Persimmon, Guava, and Sweetsop. Biomed Res. Int. 2016, 2016, 4287461. [Google Scholar] [CrossRef]
- Nakitto, A.M.S.; Muyonga, J.H.; Byaruhanga, Y.B.; Wagner, A.E. Solanum anguivi Lam. Fruits: Their Potential Effects on Type 2 Diabetes Mellitus. Molecules 2021, 26, 2044. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Oyeleye, S.I.; Ejakpovi, I. Erectogenic, Antihypertensive, Antidiabetic, Anti-Oxidative Properties and Phenolic Compositions of Almond Fruit (Terminalia catappa L.) Parts (Hull and Drupe)—In vitro. J. Food Biochem. 2017, 41, e12309. [Google Scholar] [CrossRef]
- Kongstad, K.T.; Özdemir, C.; Barzak, A.; Wubshet, S.G.; Staerk, D. Combined Use of High-Resolution α-Glucosidase Inhibition Profiling and High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry–Solid-Phase Extraction–Nuclear Magnetic Resonance Spectroscopy for Investigation of Antidiabetic Principles in Crude Plant Extracts. J. Agric. Food Chem. 2015, 63, 2257–2263. [Google Scholar] [PubMed]
- Lee, D.Y.; Kim, H.W.; Yang, H.; Sung, S.H. Hydrolyzable tannins from the fruits of Terminalia chebula Retz and their alpha-glucosidase inhibitory activities. Phytochemistry 2017, 137, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.N.; Xu, P.Y.; Kawabata, J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007, 105, 628–634. [Google Scholar] [CrossRef]
| Trees/Region | Percent (%) Dry Weight * | Carbohydrate (mg Glu E/g DW) | Sugar Content € | |||||
|---|---|---|---|---|---|---|---|---|
| Glucose (g/100 g DW) | Fructose (g/100 g DW) | Sucrose (g/100 g DW) | Total Sugar (g/100 g DW) | |||||
| Karajarri | Yawuru | Karajarri | Yawuru | |||||
| 1 | 0.1540 | 0.1703 | 584.00 ± 0.84 d | 449.7 ± 15.8 cd | 5.1 ± 0.3 | 6.2 ± 0.4 | 5.3 ± 0.1 | 16.7 ± 0.5 |
| 2 | 0.1470 | 0.1692 | 289.5 ± 11.8 a | 374.7 ± 11.85 b | 7.2 ± 0.4 | 7.3 ± 0.2 | 2.7 ± 0.1 | 17.2 ± 0.2 |
| 3 | 0.1600 | 0.1390 | 296.60 ± 8.80 a | 350.3 ± 4.2 b | 8.0 ± 0.6 | 7.8 ± 0.5 | 4.4 ± 0.3 | 20.2 ± 0.7 |
| 4 | 0.1420 | 0.1576 | 396.6 ± 10.10 c | 253.4 ± 12.15 a | 9.3 ± 0.4 | 9.1 ± 0.6 | 3.8 ± 0.2 | 22.2 ± 0.7 |
| 5 | 0.1502 | 0.1685 | 385.3 ± 9.8 bc | 284.0 ± 7.02 a | 6.2 ± 0.2 | 7.2 ± 0.3 | 0.9 ± 0.01 | 14.3 ± 0.2 |
| 6 | 0.1361 | 0.1870 | 364.8 ± 7.7 bc | 411.2 ± 13.54 bc | 6.7 ± 0.5 | 8.0 ± 0.1 | 3.3 ± 0.2 | 18.0 ± 0.4 |
| 7 | 0.1451 | 0.1672 | 389.3 ± 16.0 c | 387.7 ± 6.50 bc | 5.0 ± 0.3 | 6.0 ± 0.3 | 1.6 ± 0.1 | 12.6 ± 0.3 |
| 8 | 0.1783 | 0.1684 | 377.0 ± 6.9 bc | 403.4 ± 6.0 bc | 6.8 ± 0.1 | 7.5 ± 0.5 | 3.1 ± 0.1 | 17.5 ± 0.3 |
| 9 | 0.1770 | 0.1795 | 334.2 ± 8.4 ab | 366.4 ± 11.40 b | 5.7 ± 0.1 | 7.2 ± 0.3 | 0.2 ± 0.01 | 13.1 ± 0.3 |
| 10 | 0.1684 | 0.1676 | 398.1 ± 2.0 c | 485.60 ± 5.24 d | 6.2 ± 0.3 | 6.8 ± 0.6 | 1.1 ± 0.1 | 14.1 ± 0.5 |
| WA average | 0.16 ± 0.01 | 379.05 ± 18.5 | 6.6 ± 0.3 | 7.3 ± 0.4 | 2.6 ± 0.1 | 16.6 ± 0.4 | ||
| NT | 0.17 α | 253.4 ± 57.0 # | 2.0 ± 0.4 | 3.0 ± 0.4 | 0.2 ± 0.02 | 5.2 ± 0.8 | ||
| Trees | Ellagic Acid | Vitamin C | ||||||
|---|---|---|---|---|---|---|---|---|
| FEA (mg/g DW) | TEA (mg/g DW) | L-AA (mg/g DW) | TVC (mg/g DW) | |||||
| Karajarri | Yawuru | Karajarri | Yawuru | Karajarri | Yawuru | Karajarri | Yawuru | |
| 1 | 20.52 ± 0.50 bcd | 16.60 ± 0.75 bc | 24.26 ± 0.80 a | 30.81 ± 0.45 c | 59.63 ± 1.00 | 47.10 ± 0.30 | 61.44 ± 0.63 | 50.32 ± 1.51 |
| 2 | 24.60 ± 0.32 ef | 31.30 ± 0.22 f | 29.42 ± 0.50 ab | 49.22 ± 1.30 h | 126.43 ± 0.51 | 54.2 ± 0.47 | 131.50 ± 0.20 | 57.12 ± 0.64 |
| 3 | 30.40 ± 0.40 g | 34.40 ± 0.43 g | 42.74 ± 0.31 de | 47.40 ± 0.60 gh | 110.70 ± 1.23 | 66.8 ± 0.22 | 115.00 ± 1.40 | 69.5 ± 0.30 |
| 4 | 22.70 ± 0.07 cde | 30.01 ± 0.60 f | 33.41 ± 0.54 bc | 43.30 ± 0.81 fg | 94.83 ± 0.43 | 118.63 ± 2.75 | 98.00 ± 1.00 | 122.33 ± 3.25 |
| 5 | 15.00 ± 0.40 a | 24.75 ± 0.25 d | 23.30 ± 1.4 a | 36.10 ± 0.60 de | 24.51 ± 0.01 | 87.65 ± 0.68 | 25.20 ± 0.16 | 90.82 ± 1.10 |
| 6 | 23.10 ± 0.50 de | 31.70 ± 0.34 f | 41.00 ± 0.75 de | 42.70 ± 2.30 fg | 111.51 ± 0.42 | 40.95 ± 0.10 | 114.20 ± 0.74 | 42.47 ± 0.27 |
| 7 | 20.00 ± 0.24 bc | 12.28 ± 0.60 a | 33.32 ± 0.80 bc | 19.00 ± 0.40 a | 55.60 ± 0.44 | 61.22 ± 0.31 | 57.77 ± 0.74 | 63.70 ± 0.25 |
| 8 | 18.64 ± 0.33 b | 27.24 ± 0.42 e | 40.53 ± 3.61 cd | 40.00 ± 0.12 ef | 95.00 ± 0.80 | 57.05 ± 0.30 | 97.40 ± 0.60 | 58.27 ± 0.40 |
| 9 | 26.64 ± 0.30 f | 14.80 ± 0.43 b | 44.62 ± 1.00 e | 31.63 ± 0.80 cd | 77.30 ± 1.22 | 53.56 ± 0.22 | 81.00 ± 1.20 | 54.03 ± 0.13 |
| 10 | 19.53 ± 1.82 bcd | 17.60 ± 0.10 c | 28.52 ± 0.40 ab | 26.00 ± 0.35 b | 75.40 ± 1.43 | 53.20 ± 2.80 | 80.71 ± 3.60 | 54.00 ± 3.0 |
| Average | 22.2 ± 0.8 | 24.1 ± 2.5 | 34.0 ± 1.4 | 36.6 ± 3.1 | 83.1 ± 5.4 | 64.0 ± 7.3 | 86.2 ± 5.6 | 66.1 ± 7.5 |
| Trees | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | FRAP (µmole Fe2+ E/g DW) | DPPH (µmole TE/g DW) | ||||
|---|---|---|---|---|---|---|---|---|
| Karajarri | Yawuru | Karajarri | Yawuru | Karajarri | Yawuru | Karajarri | Yawuru | |
| 1 | 112.70 ± 2.70 bc | 110.72 ± 2.70 ab | 4.00 ± 0.06 cd | 5.30 ± 0.04 d | 1422.72 ± 44.90 b | 1235.75 ± 23.70 a | 1204.50 ± 7.23 a | 1157.00 ± 8.44 ab |
| 2 | 184.71 ± 4.80 e | 133.22 ± 2.00 cd | 4.10 ± 0.05 de | 5.62 ± 0.08 e | 2315.55 ± 55.12 d | 1565.20 ± 32.33 bc | 1274.13 ± 5.41 ab | 1288.23 ± 16.00 bc |
| 3 | 190.50 ± 3.20 e | 124.00 ± 2.00 bc | 3.53 ± 0.05 ab | 3.13 ± 0.05 a | 2266.12 ± 53.00 d | 1530.23 ± 24.00 b | 1381.00 ± 14.40 bc | 1271.27 ± 47.41 bc |
| 4 | 165.60 ± 3.00 d | 195.40 ± 3.30 e | 3.74 ± 0.05 bc | 5.04 ± 0.08 d | 1979.26 ± 43.00 c | 2350.20 ± 60.25 d | 1313.30 ± 19.22 abc | 1390.50 ± 18.10 c |
| 5 | 77.74 ± 2.00 a | 147.50 ± 4.00 d | 4.20 ± 0.05 de | 5.10 ± 0.05 d | 1011.20 ± 24.10 a | 1711.92 ± 53.33 c | 1229.93 ± 30.13 a | 1314.40 ± 29.60 bc |
| 6 | 160.70 ± 3.43 d | 106.55 ± 3.34 a | 3.80 ± 0.07 bc | 5.72 ± 0.06 e | 1943.82 ± 42.60 c | 1288.05 ± 33.00 a | 1431.87 ± 30.23 c | 1300.30 ± 32.82 bc |
| 7 | 104.65 ± 3.45 b | 111.70 ± 3.70 ab | 4.6 ± 0.06 f | 6.54 ± 0.09 f | 1350.10 ± 45.00 b | 1257.11 ± 54.01 a | 1303.56 ± 36.20 abc | 1005.47 ± 28.00 a |
| 8 | 120.54 ± 3.20 bc | 99.52 ± 7.70 a | 4.34 ± 0.05 ef | 3.90 ± 0.04 b | 1542.46 ± 31.70 b | 1250.50 ± 37.00 a | 1314.80 ± 44.00 abc | 1269.40 ± 46.54 bc |
| 9 | 154.20 ± 4.00 d | 113.10 ± 2.00 ab | 3.60 ± 0.05 b | 3.32 ± 0.06 a | 1803.21 ± 34.12 c | 1353.20 ± 32.00 a | 1410.00 ± 39.03 c | 1212.81 ± 24.66 bc |
| 10 | 126.60 ± 5.00 c | 103.10 ± 2.30 a | 3.30 ± 0.08 a | 4.44 ± 0.03 c | 1488.20 ± 33.00 b | 1255.32 ± 15.05 a | 1223.11 ± 28.30 a | 1208.35 ± 33.44 bc |
| Average | 140.0 ± 6.6 | 124.5 ± 9.2 | 3.9 ± 0.07 | 4.8 ± 0.3 | 1714.4 ± 74.4 | 1476.7 ± 110.6 | 1311.2 ± 17.2 | 1242.8 ± 33.2 |
| Trees/Std | Zone of Inhibition Measured in mm | MIC (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| MRSA2 | SA | PA | AB | S. put | SA | MRSA2 | S. put | |
| 4Y | 13.8 ± 0.3 c | 14.4 ± 0.6 c | 6.1 ± 0.7 a | 6.0 ± 0.9 a | 10.0 ± 0.2 b | 4 | 4 | 4 |
| 5Y | 12.2 ± 0.8 abc | 12.0 ± 0.1 b | 7.0 ± 0.5 a | 8.0 ± 0.5 a | 10.0 ± 0.5 b | 4 | 4 | 4 |
| 7Y | 10.2 ± 0.9 a | 10.0 ± 0.7 a | 6.0 ± 0.1 a | 6.6 ± 0.4 a | 7.8 ± 0.2 a | |||
| 9Y | 13.0 ± 1.0 bc | 10.5 ± 0.8 ab | 6.5 ± 0.5 a | 7.0 ± 1.0 a | 10.0 ± 0.3 b | 4 | 4 | 4 |
| 2K | 12.7 ± 0.6 AB | 13.3 ± 0.5 C | 8.0 ± 0.1 B | 8.3 ± 0.6 B | 9.5 ± 0.6 BC | 4 | 4 | 4 |
| 3K | 14.1 ± 0.5 B | 14.7 ± 0.3 CD | 6.0 ± 0.1 A | 8.2 ± 0.7 B | 13.4 ± 0.9 D | 4 | 4 | 4 |
| 8K | 11.1 ± 0.4 A | 8.4 ± 0.3 A | 6.0 ± 0.7 A | 5.1 ± 0.6 A | 6.8 ± 0.3 A | |||
| 9K | 13.6 ± 0.5 B | 15.1 ± 0.8 D | 6.0 ± 0.1 A | 8.5 ± 0.3 B | 11.0 ± 0.8 C | 4 | 4 | 4 |
| Oxy (0.06 mg/mL) | 22.1 ± 1.0 | 28.8 ± 0.4 | - | 8.7 ± 0.3 | 32.6 ± 0.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobasa, E.M.; Phan, A.D.T.; Netzel, M.E.; Akter, S.; Cozzolino, D.; Sultanbawa, Y. Nutritional and Functional Properties of Terminalia ferdinandiana Fruits Wild Harvested from Western Australia. Foods 2024, 13, 2888. https://doi.org/10.3390/foods13182888
Bobasa EM, Phan ADT, Netzel ME, Akter S, Cozzolino D, Sultanbawa Y. Nutritional and Functional Properties of Terminalia ferdinandiana Fruits Wild Harvested from Western Australia. Foods. 2024; 13(18):2888. https://doi.org/10.3390/foods13182888
Chicago/Turabian StyleBobasa, Eshetu M., Anh Dao Thi Phan, Michael E. Netzel, Saleha Akter, Daniel Cozzolino, and Yasmina Sultanbawa. 2024. "Nutritional and Functional Properties of Terminalia ferdinandiana Fruits Wild Harvested from Western Australia" Foods 13, no. 18: 2888. https://doi.org/10.3390/foods13182888
APA StyleBobasa, E. M., Phan, A. D. T., Netzel, M. E., Akter, S., Cozzolino, D., & Sultanbawa, Y. (2024). Nutritional and Functional Properties of Terminalia ferdinandiana Fruits Wild Harvested from Western Australia. Foods, 13(18), 2888. https://doi.org/10.3390/foods13182888










