The Degree of Inulin Polymerization Is Important for Short-Term Amelioration of High-Fat Diet (HFD)-Induced Metabolic Dysfunction and Gut Microbiota Dysbiosis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vivo Study Design
2.3. Post In Vivo Study Sample Collection and Analysis
2.4. Serum Proinflammatory and Lipid Analysis Using ELISA
2.5. In Vitro Intestinal Cell Permeability Studies Using SCFAs
2.6. In Vitro Intestinal Cell Permeability Studies Using CFS
2.7. Fecal and Cecal Short-Chain Fatty Acid Quantification
2.8. Statistical Analysis
3. Results and Discussion
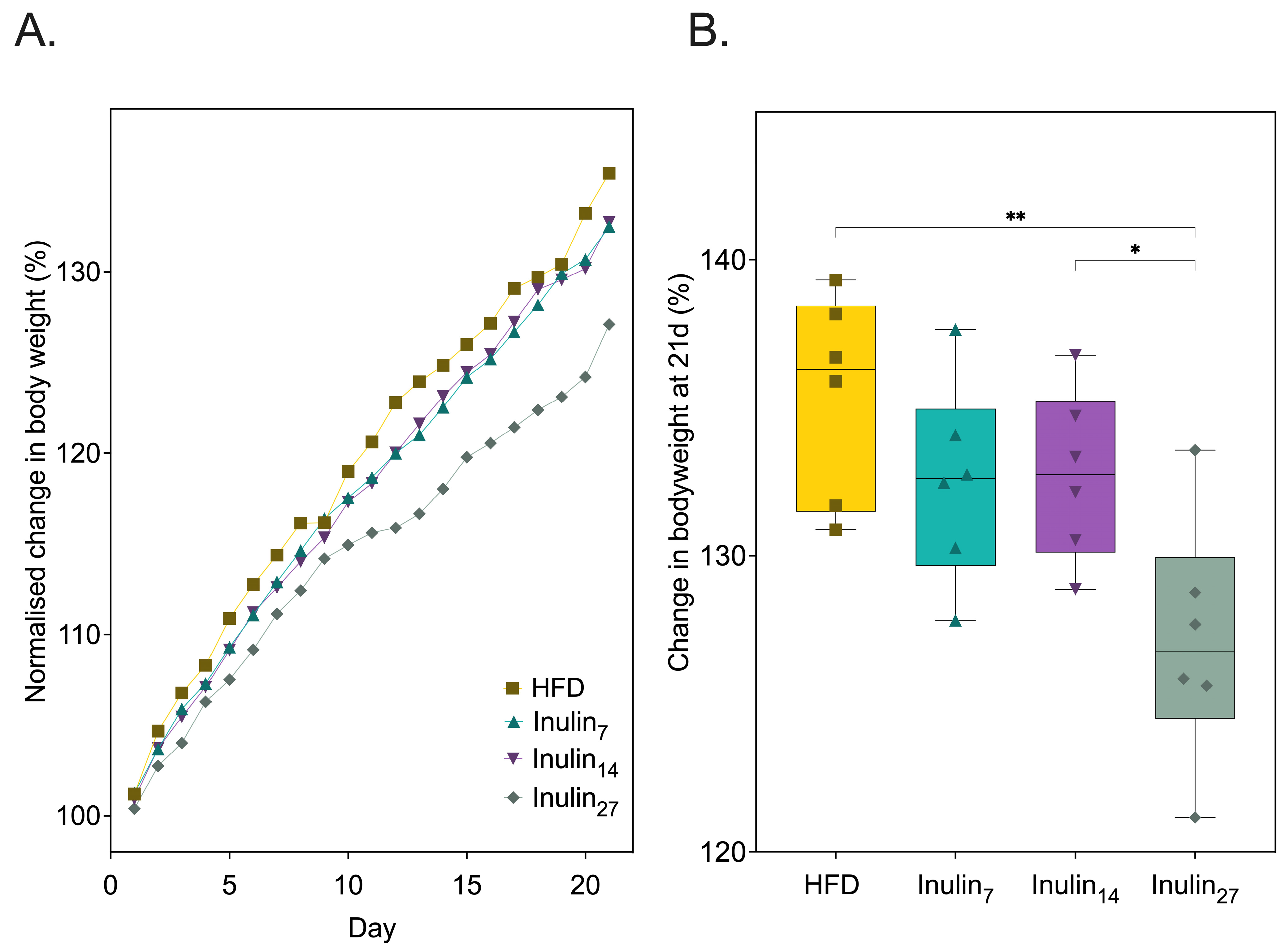
3.1. Impact of Inulin’s DPn on Diet-Indued Metabolic Health of Sprague Dawley Rats
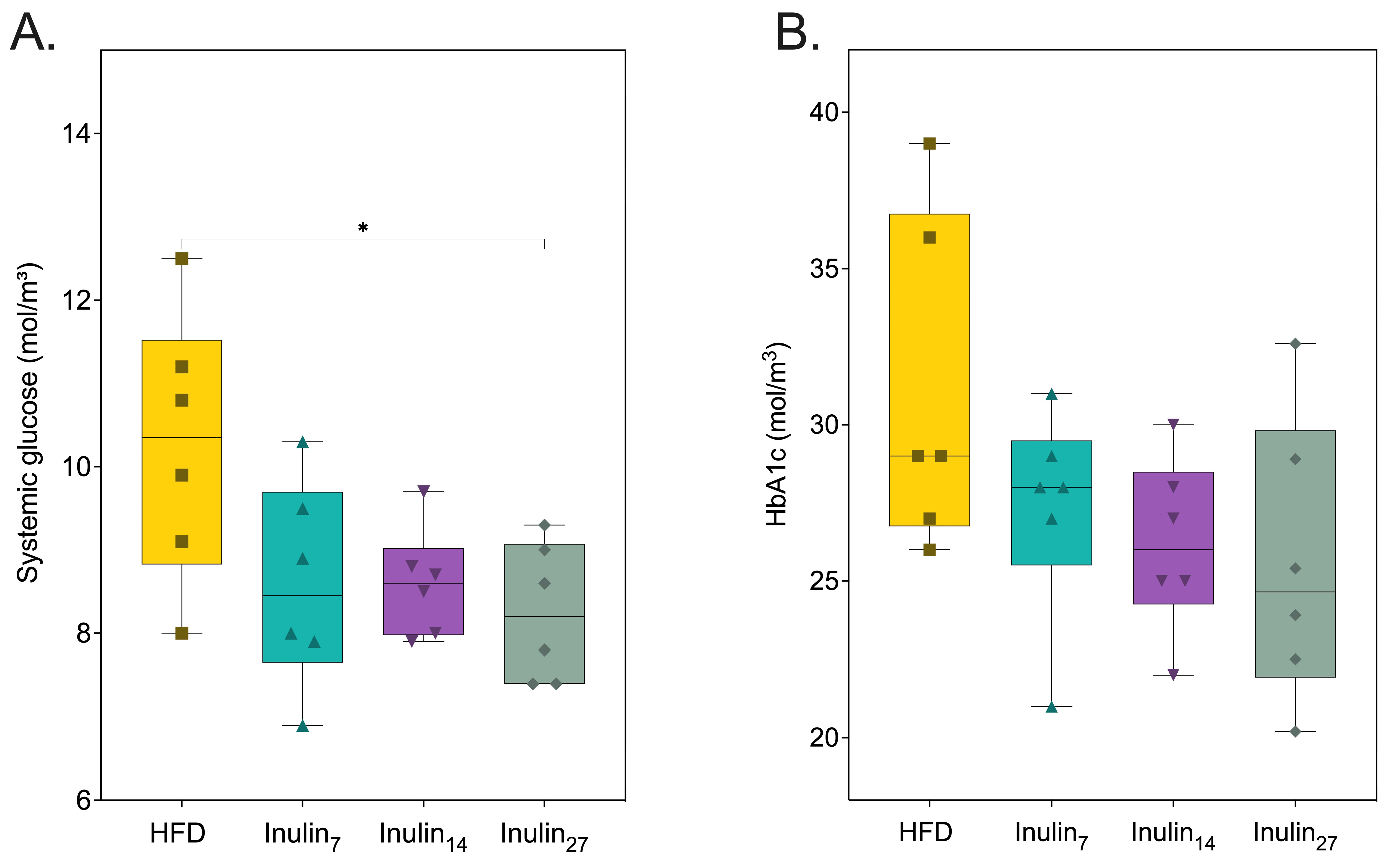
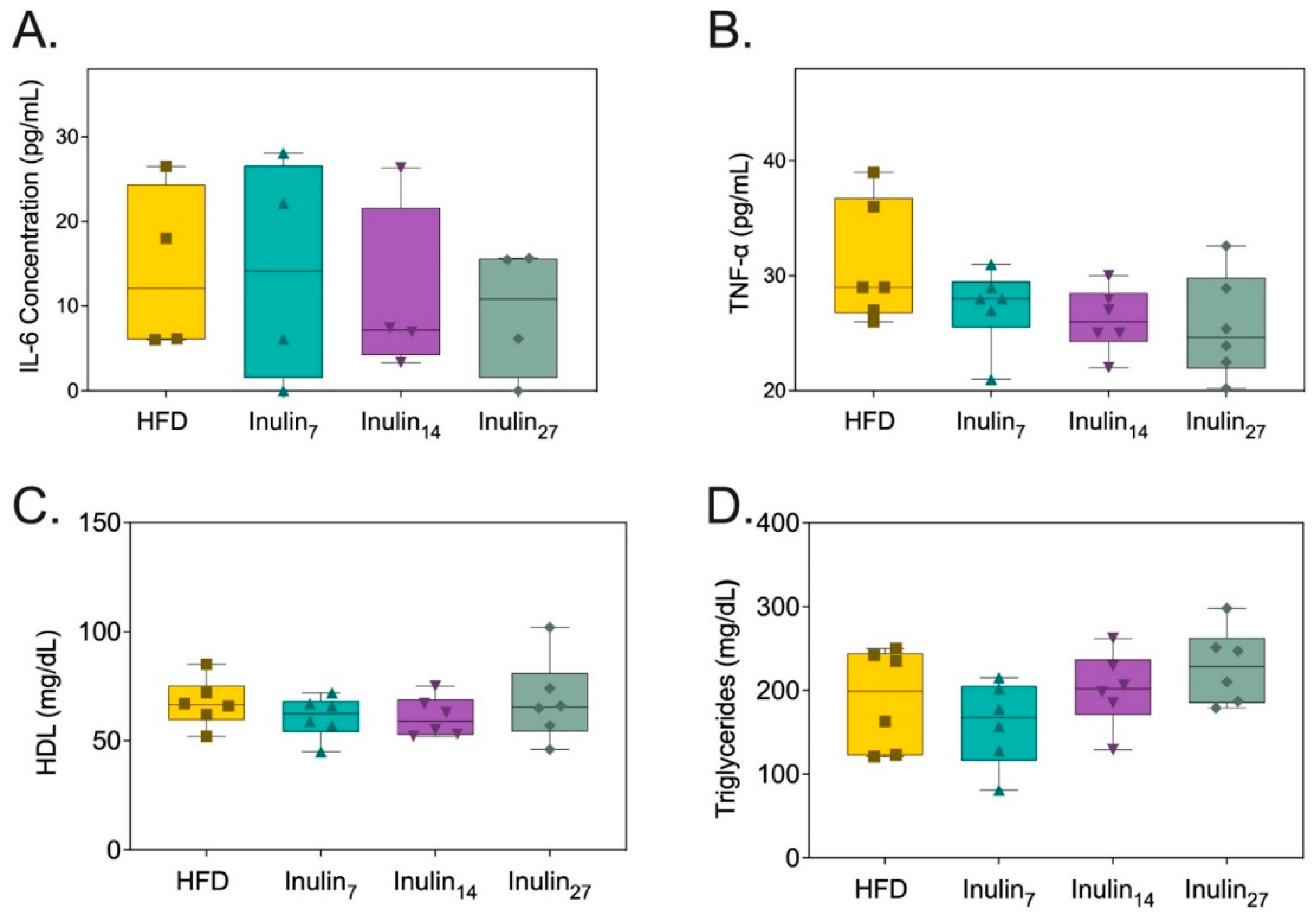
3.2. Metabolic and Inflammatory Biomarker Responses to Inulin Supplementation
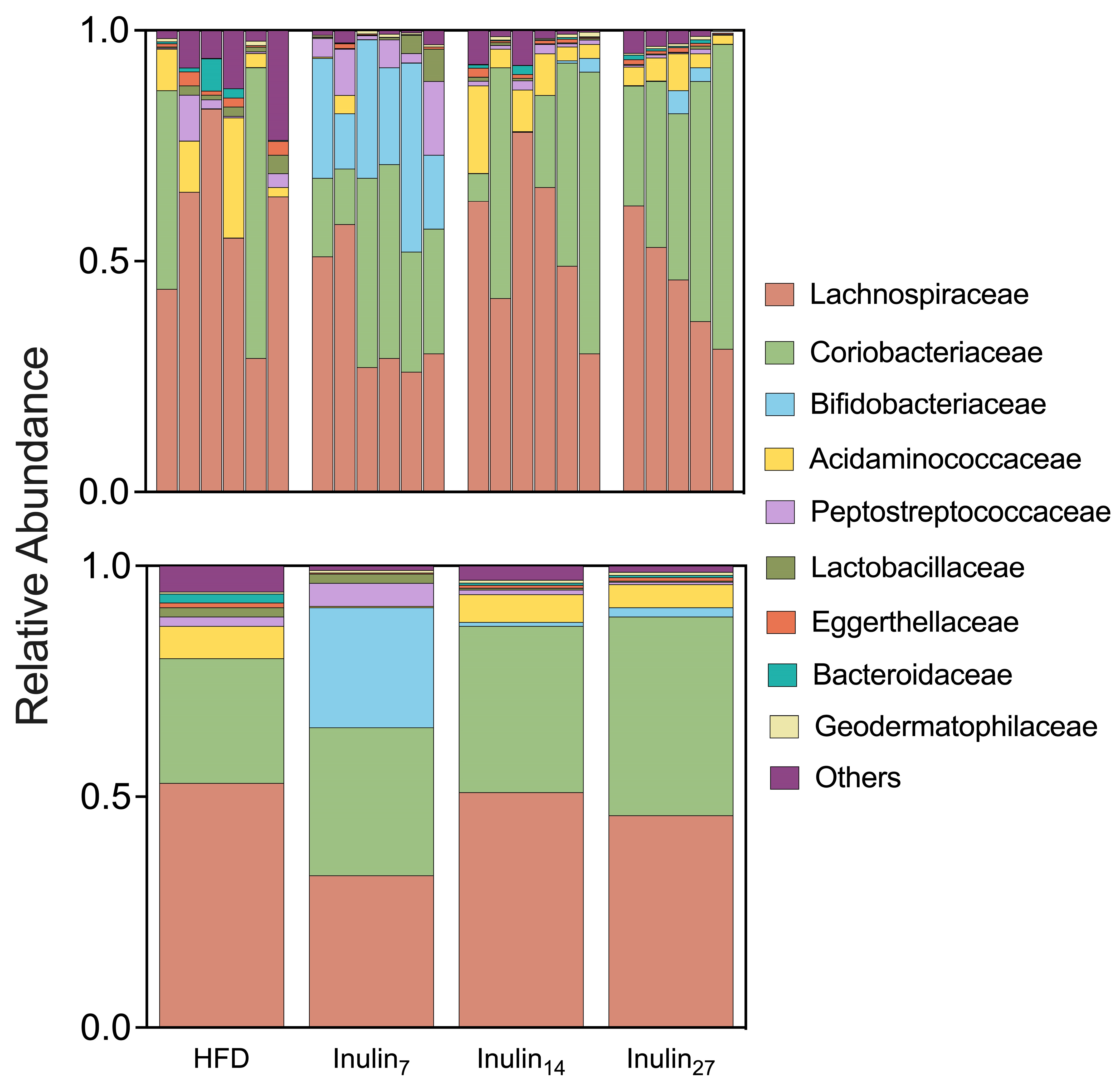
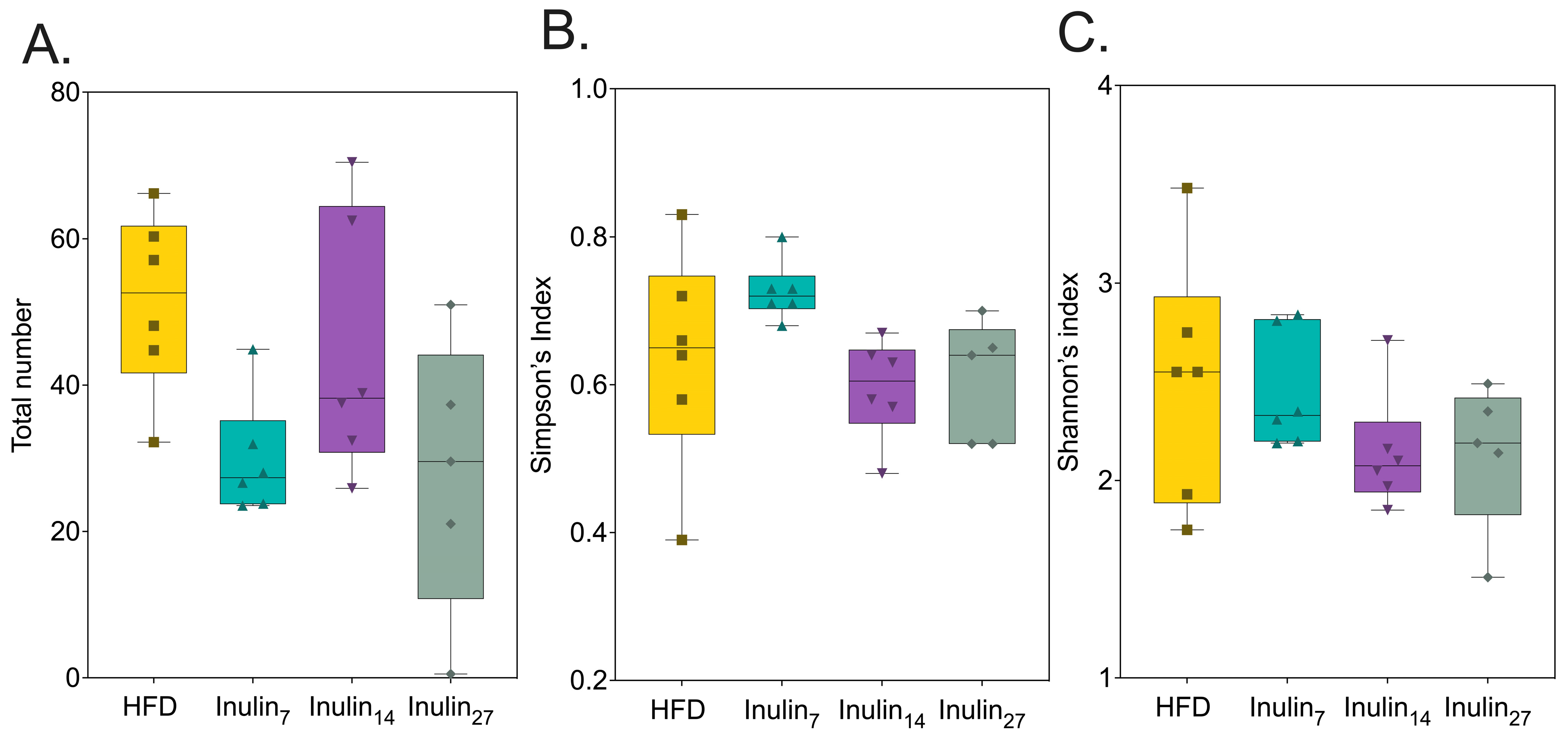
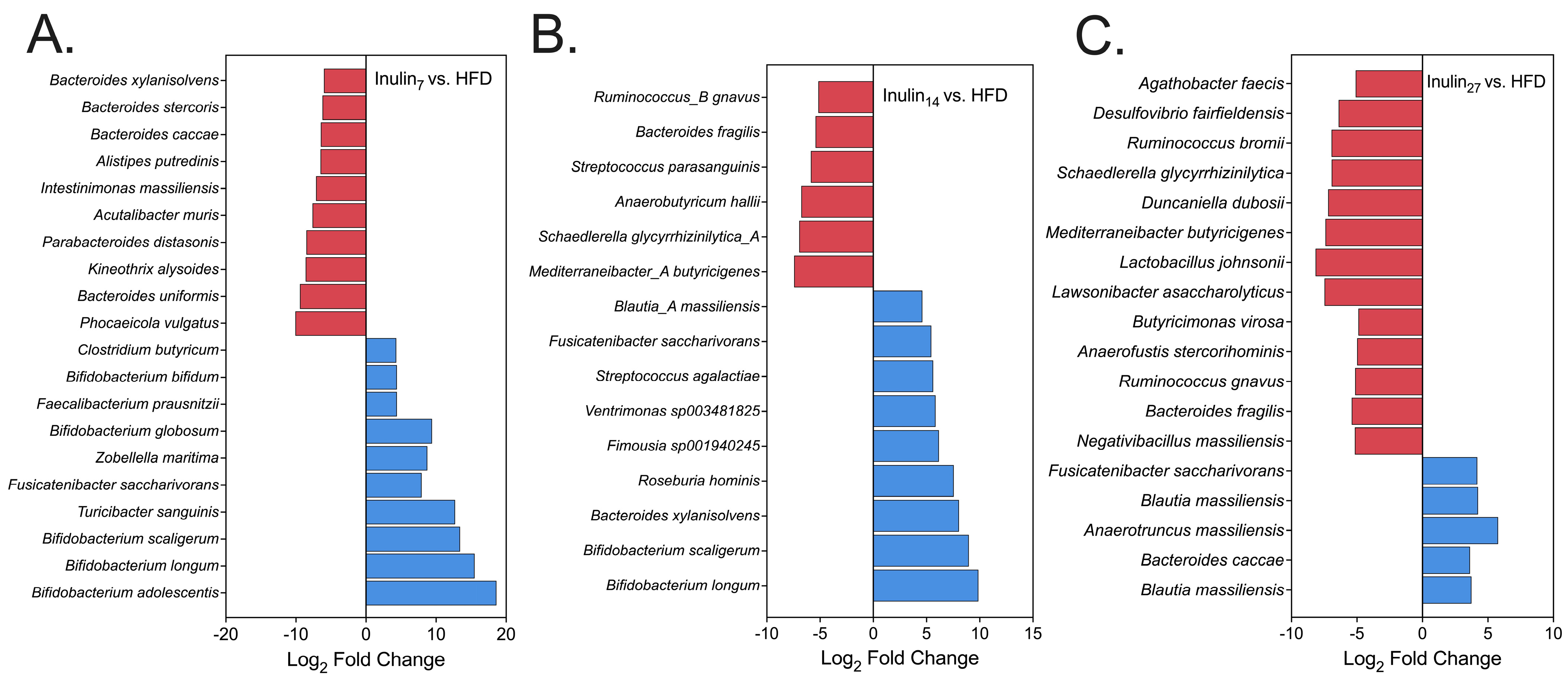
3.3. DPn of Inulin Influencing the Gut Microbiome
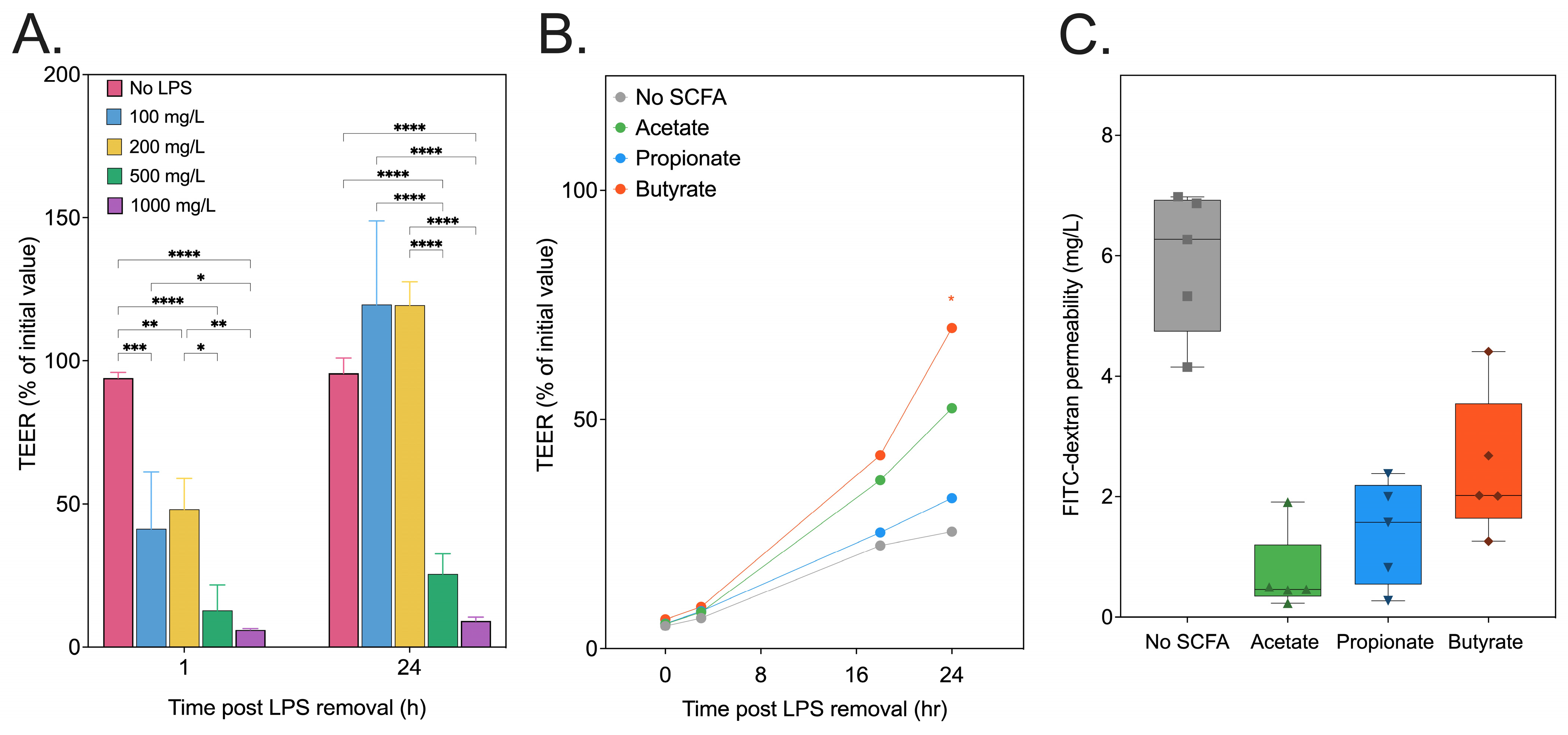
3.4. SCFA Recovery of In Vitro Intestinal Epithelial Permeability
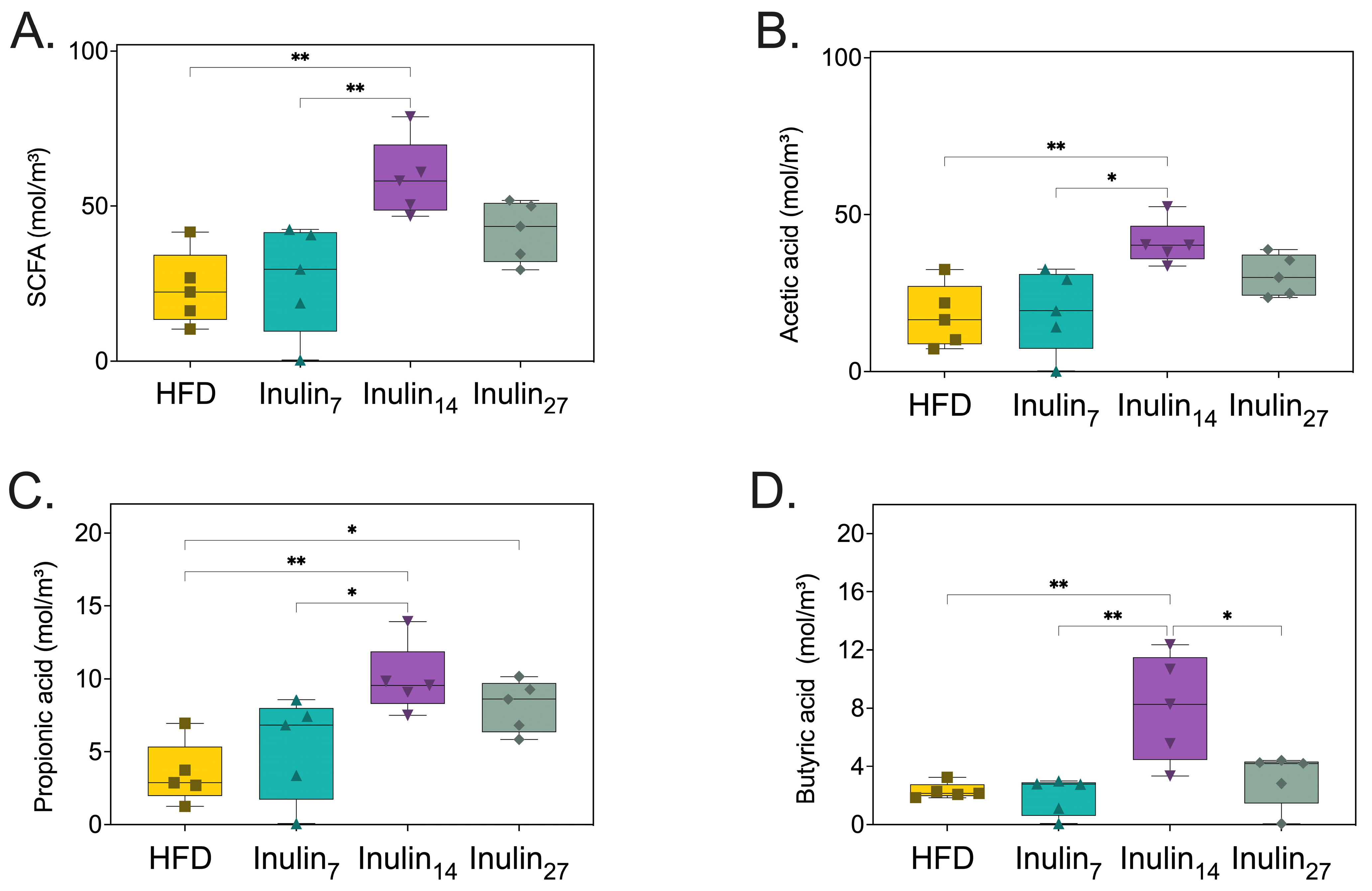
3.5. Short-Chain Fatty Acid Metabolites Following Inulin Administration to HFD-Fed Rats
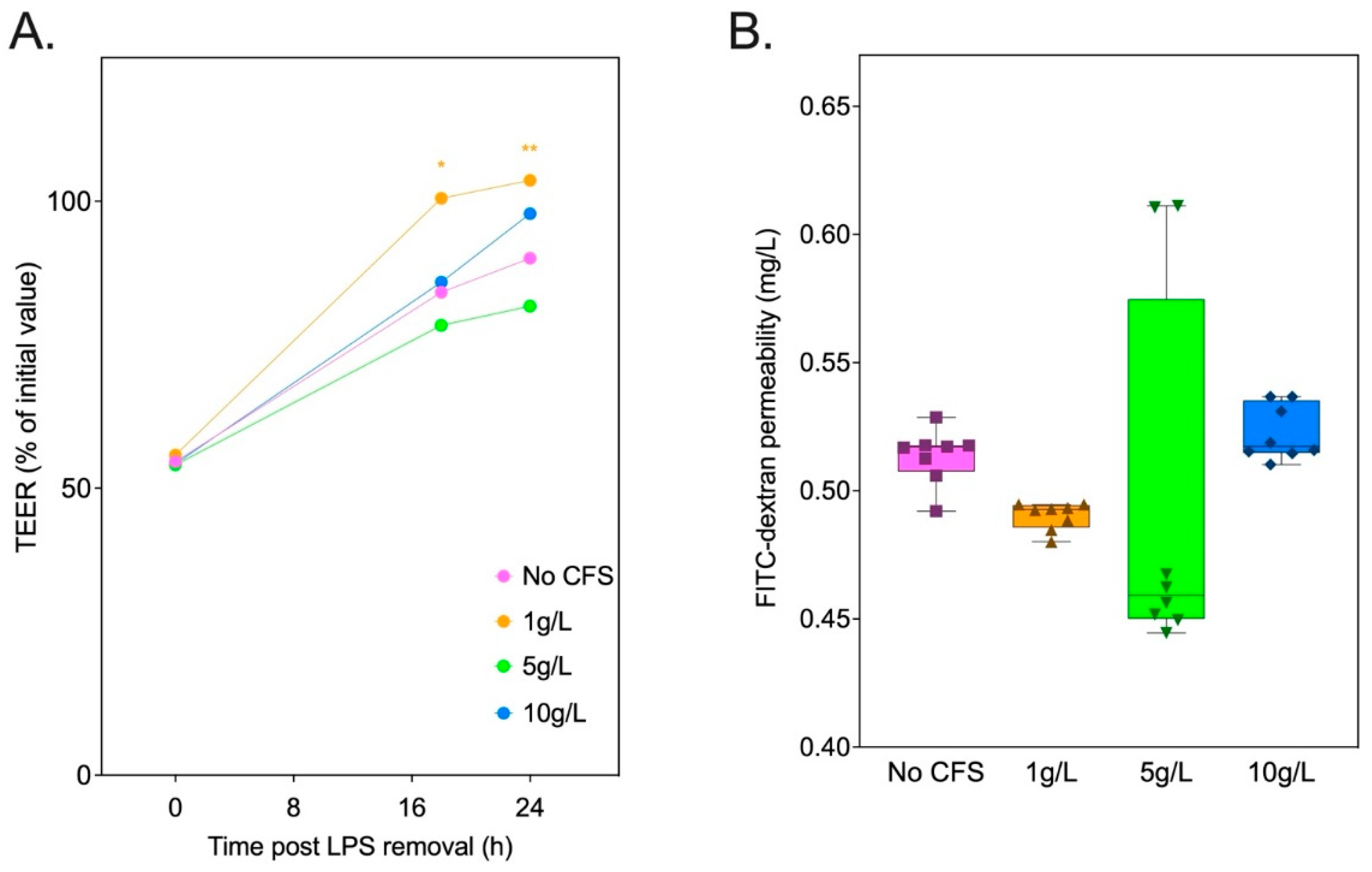
3.6. Inulin-Derived Metabolites Protecting against In Vitro LPS-Induced Intestinal Epithelial Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tremmel, M.; Gerdtham, U.-G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Caveney, E.; Caveney, B.J.; Somaratne, R.; Turner, J.R.; Gourgiotis, L. Pharmaceutical interventions for obesity: A public health perspective. Diabetes Obes. Metab. 2011, 13, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.J.; O’neill, B.V.; Napolitano, A.; Bullmore, E.T. Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci. Ther. 2011, 17, 490–505. [Google Scholar] [CrossRef]
- Alsuhibani, A.; Alrasheed, M.; Gari, M.; Hincapie, A.L.; Guo, J.J. Descriptive analysis of reported adverse events associated with anti-obesity medications using FDA Adverse Event Reporting System (FAERS) databases 2013–2020. Pharm. Weekbl. 2022, 44, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Folco, E.J. Inflammatory Concepts of Obesity. Int. J. Inflamm. 2011, 2011, 529061. [Google Scholar] [CrossRef] [PubMed]
- Curti, M.L.R.; Jacob, P.; Borges, M.C.; Rogero, M.M.; Ferreira, S.R.G. Studies of gene variants related to inflammation, oxidative stress, dyslipidemia, and obesity: Implications for a nutrigenetic approach. J. Obes. 2011, 2011, 497401. [Google Scholar] [CrossRef] [PubMed]
- Doerner, S.K.; Reis, E.S.; Leung, E.S.; Ko, J.S.; Heaney, J.D.; Berger, N.A.; Lambris, J.D.; Nadeau, J.H. High-Fat Diet-Induced Complement Activation Mediates Intestinal Inflammation and Neoplasia, Independent of Obesity. Mol. Cancer Res. 2016, 14, 953–965. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. The gut microbiome as therapeutic target. Pharmacol. Ther. 2011, 130, 202–212. [Google Scholar] [CrossRef]
- DiBaise, J.K.; Zhang, H.; Crowell, M.D.; Krajmalnik-Brown, R.; Decker, G.A.; Rittmann, B.E. Gut microbiota and its possible relationship with obesity. Mayo Clin. Proc. 2008, 83, 460–469. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Rosario, V.A.D.; Mocellin, M.C.; Kuntz, M.G.; Trindade, E.B. Effects of inulin-type fructans, galacto-oligosaccharides and related synbiotics on inflammatory markers in adult patients with overweight or obesity: A systematic review. Clin. Nutr. 2016, 36, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, P.; Xu, L. Assessing the effects of inulin-type fructan intake on body weight, blood glucose, and lipid profile: A systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2021, 9, 4598–4616. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Law, B.Y.K.; Xu, Y. Effect of Inulin-Type Carbohydrates on Insulin Resistance in Patients with Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 5101423. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Song, W.-S.; Lee, J.; Jo, S.-H.; Lee, J.-S.; Jeon, H.-J.; Kwon, J.-E.; Kim, Y.-R.; Baek, J.-H.; Kim, M.-G.; et al. An Integrative Multiomics Approach to Characterize Prebiotic Inulin Effects on Faecalibacterium prausnitzii. Front. Bioeng. Biotechnol. 2022, 10, 825399. [Google Scholar] [CrossRef] [PubMed]
- Kleessen, B.; Blaut, M. Modulation of gut mucosal biofilms. Br. J. Nutr. 2005, 93 (Suppl. S1), S35–S40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’brien, C.; Jones, N.L.; Sherman, P.M. Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar] [CrossRef]
- Chen, K.; Chen, H.; Faas, M.M.; de Haan, B.J.; Li, J.; Xiao, P.; Zhang, H.; Diana, J.; de Vos, P.; Sun, J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017, 61, 1601006. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2016, 52, 1–8. [Google Scholar] [CrossRef]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Koleva, P.T.; Valcheva, R.S.; Sun, X.; Gänzle, M.G.; Dieleman, L.A. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br. J. Nutr. 2012, 108, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, F.; Zhao, L.; Meng, X.; Li, B. Potential Immunomodulatory Activity of a Selected Strain Bifidobacterium bifidum H3-R2 as Evidenced in vitro and in Immunosuppressed Mice. Front. Microbiol. 2020, 11, 531789. [Google Scholar] [CrossRef]
- Li, L.-L.; Wang, Y.-T.; Zhu, L.-M.; Liu, Z.-Y.; Ye, C.-Q.; Qin, S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci. Rep. 2020, 10, 978. [Google Scholar] [CrossRef]
- Du, H.; Zhao, A.; Wang, Q.; Yang, X.; Ren, D. Supplementation of inulin with various degree of polymerization ameliorates liver injury and gut microbiota dysbiosis in high fat-fed obese mice. J. Agric. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef]
- Dening, T.J.; Joyce, P.; Kovalainen, M.; Gustafsson, H.; Prestidge, C.A. Spray Dried Smectite Clay Particles as a Novel Treatment against Obesity. Pharm. Res. 2018, 36, 21. [Google Scholar] [CrossRef]
- Joyce, P.; Dening, T.J.; Meola, T.R.; Wignall, A.; Ulmefors, H.; Kovalainen, M.; Prestidge, C.A. Contrasting Anti-obesity Effects of Smectite Clays and Mesoporous Silica in Sprague-Dawley Rats. ACS Appl. Bio Mater. 2020, 3, 7779–7788. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Qiagen. QIAGEN CLC Genomics Workbench; Qiagen: Hilden, Germany, 2023. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Goupry, S.; Antignac, J.; Monteau, F.; Lebizec, B.; Champ, M.; Martin, L.; Dumon, H. Simultaneous measurement of plasma concentrations and 13C-enrichment of short-chain fatty acids, lactic acid and ketone bodies by gas chromatography coupled to mass spectrometry. J. Chromatogr. B 2003, 784, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Paßlack, N.; Al-Samman, M.; Vahjen, W.; Männer, K.; Zentek, J. Chain length of inulin affects its degradation and the microbiota in the gastrointestinal tract of weaned piglets after a short-term dietary application. Livest. Sci. 2012, 149, 128–136. [Google Scholar] [CrossRef]
- Van De Wiele, T.; Boon, N.; Possemiers, S.; Jacobs, H.; Verstraete, W. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J. Appl. Microbiol. 2007, 102, 452–460. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Brooks, L.; Viardot, A.; Tsakmaki, A.; Stolarczyk, E.; Howard, J.K.; Cani, P.D.; Everard, A.; Sleeth, M.L.; Psichas, A.; Anastasovskaj, J.; et al. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol. Metab. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Sakakibara, S.; Yamauchi, T.; Oshima, Y.; Tsukamoto, Y.; Kadowaki, T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem. Biophys. Res. Commun. 2006, 344, 597–604. [Google Scholar] [CrossRef]
- Deng, B. Study the Mechanism of Calcium Signal-and Short Chain Fatty Acids-Regulated Glucose Metabolism. Ph.D. Thesis, Tianjin Medical University, Tianjin, China, 2018. [Google Scholar]
- Jocken, J.W.E.; González Hernández, M.A.; Hoebers, N.T.H.; van der Beek, C.M.; Essers, Y.P.G.; Blaak, E.E.; Canfora, E.E. Short-Chain fatty acids differentially affect intracellular lipolysis in a human white adipocyte model. Front. Endocrinol. 2018, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, H.; Toprak, B.; Colak, A. The independent relationship between hemoglobin A1c and homeostasis model assessment of insulin resistance in non-diabetic subjects. Turk. J. Biochem. 2017, 42, 31–36. [Google Scholar] [CrossRef]
- Miralles-Pérez, B.; Nogués, M.R.; Sánchez-Martos, V.; Fortuño-Mar, À.; Ramos-Romero, S.; Torres, J.L.; Ponomarenko, J.; Amézqueta, S.; Zhang, X.; Romeu, M. Influence of Dietary Inulin on Fecal Microbiota, Cardiometabolic Risk Factors, Eicosanoids, and Oxidative Stress in Rats Fed a High-Fat Diet. Foods 2022, 11, 4072. [Google Scholar] [CrossRef]
- Komatsu, Y.; Aoyama, K.; Yoneda, M.; Ashikawa, S.; Nakano, S.; Kawai, Y.; Cui, X.; Furukawa, N.; Ikeda, K.; Nagata, K. The prebiotic fiber inulin ameliorates cardiac, adipose tissue, and hepatic pathology, but exacerbates hypertriglyceridemia in rats with metabolic syndrome. Am. J. Physiol. Circ. Physiol. 2021, 320, H281–H295. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Dong, S.; Lin, L.; Ma, Q.; Xu, M.; Ni, L.; Fan, Q. Inulin ameliorates metabolic syndrome in high-fat diet-fed mice by regulating gut microbiota and bile acid excretion. Front. Pharmacol. 2023, 14, 1226448. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.-G.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Jobe, M.; Agbla, S.C.; Todorcevic, M.; Darboe, B.; Danso, E.; de Barros, J.-P.P.; Lagrost, L.; Karpe, F.; Prentice, A.M. Possible mediators of metabolic endotoxemia in women with obesity and women with obesity-diabetes in The Gambia. Int. J. Obes. 2022, 46, 1892–1900. [Google Scholar] [CrossRef]
- Lorenz, W.; Buhrmann, C.; Mobasheri, A.; Lueders, C.; Shakibaei, M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: Implications for the development of rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R111–R117. [Google Scholar] [CrossRef] [PubMed]
- Vaure, C.; Liu, Y. A Comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, H.; Torres-Fuentes, C.; van de Wouw, M.; Long-Smith, C.M.; Mitchell, A.; Strain, C.; Berding, K.; Bastiaanssen, T.F.; Rea, K.; Golubeva, A.V.; et al. Bifidobacterium longum counters the effects of obesity: Partial successful translation from rodent to human. EBioMedicine 2021, 63, 103176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Qin, S.; Zhai, S.; Gao, Y.; Li, L. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiol. Lett. 2017, 364, fnx075. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, J.H.A.; Kettunen, H.; Kettunen, A.; Holben, W.E.; Nurminen, P.H.; Rautonen, N.; Mutanen, M. Culture-Independent Microbial Community Analysis Reveals that Inulin in the Diet Primarily Affects Previously Unknown Bacteria in the Mouse Cecum. Appl. Environ. Microbiol. 2002, 68, 4986–4995. [Google Scholar] [CrossRef][Green Version]
- Pattananandecha, T.; Sirilun, S.; Duangjitcharoen, Y.; Sivamaruthi, B.S.; Suwannalert, P.; Peerajan, S.; Chaiyasut, C. Hydrolysed inulin alleviates the azoxymethane-induced preneoplastic aberrant crypt foci by altering selected intestinal microbiota in Sprague–Dawley rats. Pharm. Biol. 2016, 54, 1596–1605. [Google Scholar] [CrossRef]
- Stewart, M.L.; Timm, D.A.; Slavin, J.L. Fructooligosaccharides exhibit more rapid fermentation than long-chain inulin in an in vitro fermentation system. Nutr. Res. 2008, 28, 329–334. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Gómez del Pugar, E.M.; López-Almela, I.; Moya-Pérez, Á.; Codoñer-Franch, P.; Sanz, Y. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems 2020, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. Npj Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hänninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.-H.; Shin, J.-H.; Lee, D.; Lee, S.-N.; Seo, J.-G.; Shin, J.-H.; Nam, Y.-D.; Kim, H.; Sun, X. Pharmaceutical efficacy of novel human-origin Faecalibacterium prausnitzii strains on high-fat-diet-induced obesity and associated metabolic disorders in mice. Front. Endocrinol. 2023, 14, 1220044. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Mariadason, J.M.; Barkla, D.H.; Gibson, P.R.; Bluemel, S.; Williams, B.; Knight, R.; Schnabl, B. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am. J. Physiol. Liver Physiol. 1997, 272, G705–G712. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; Van Loo, J.A.E.; Gibson, G.R. The Bifidogenic Nature of Chicory Inulin and Its Hydrolysis Products. J. Nutr. 1998, 128, 11–19. [Google Scholar] [CrossRef]
- Van der Meulen, R.; Makras, L.; Verbrugghe, K.; Adriany, T.; De Vuyst, L. In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. indicates different degradation mechanisms. Appl. Environ. Microbiol. 2006, 72, 1006–1012. [Google Scholar] [CrossRef]
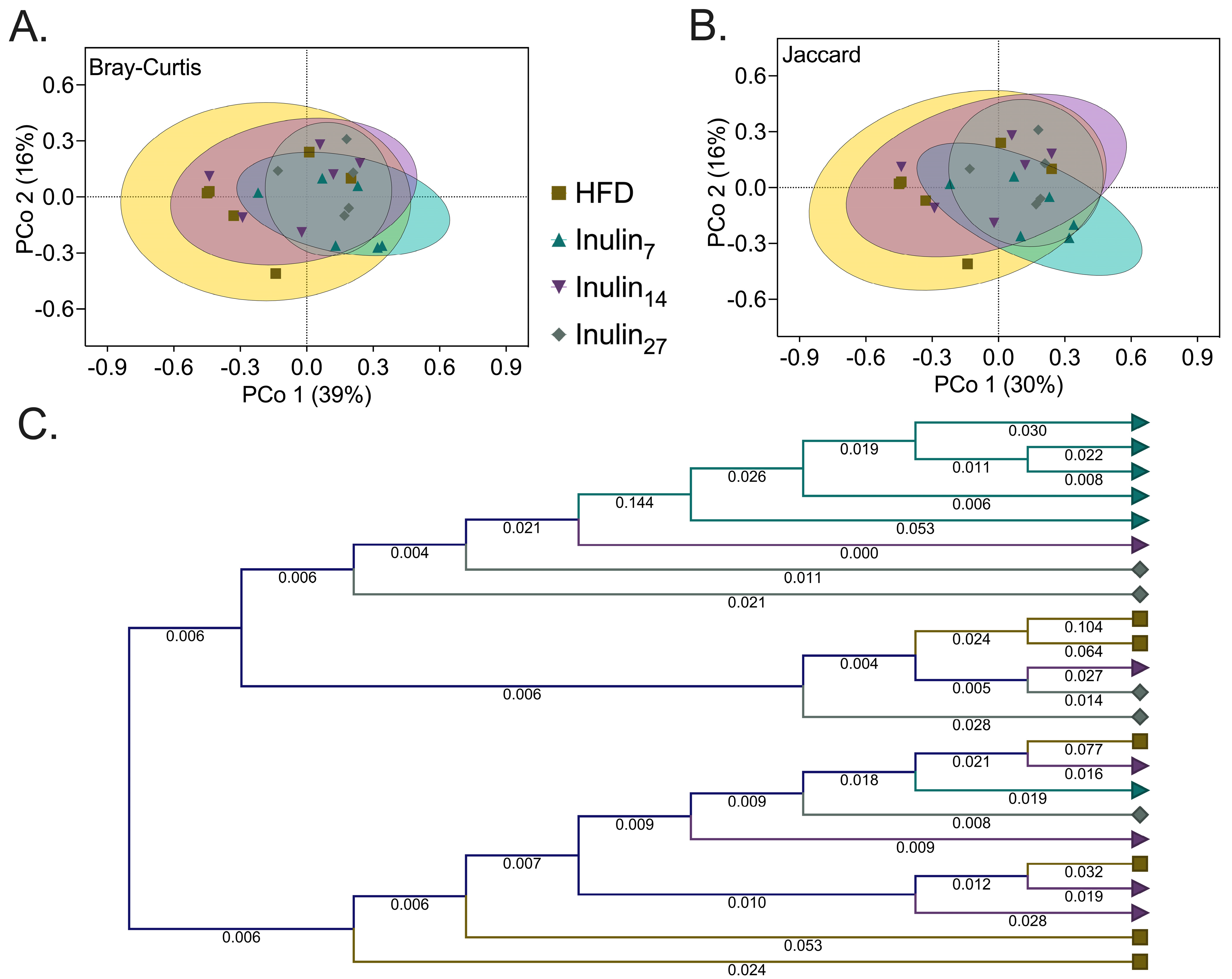
| Group 1 | Group 2 | Pseudo-f Statistic | p-Value | ||
|---|---|---|---|---|---|
| Bray–Curtis | Jaccard | Bray–Curtis | Jaccard | Bray–Curtis | Jaccard |
| HFD | Inulin7 | 3.53714 | 2.53989 | 0.01732 | 0.01732 |
| HFD | Inulin14 | 0.89221 | 0.83340 | 0.44589 | 0.53030 |
| Inulin7 | Inulin14 | 3.43465 | 2.63353 | 0.03030 | 0.02381 |
| HFD | Inulin27 | 2.20498 | 1.85110 | 0.07576 | 0.07576 |
| Inulin7 | Inulin27 | 3.21460 | 2.84335 | 0.03247 | 0.01299 |
| Inulin14 | Inulin27 | 1.16436 | 0.94797 | 0.33333 | 0.44805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariaee, A.; Wardill, H.R.; Wignall, A.; Prestidge, C.A.; Joyce, P. The Degree of Inulin Polymerization Is Important for Short-Term Amelioration of High-Fat Diet (HFD)-Induced Metabolic Dysfunction and Gut Microbiota Dysbiosis in Rats. Foods 2024, 13, 1039. https://doi.org/10.3390/foods13071039
Ariaee A, Wardill HR, Wignall A, Prestidge CA, Joyce P. The Degree of Inulin Polymerization Is Important for Short-Term Amelioration of High-Fat Diet (HFD)-Induced Metabolic Dysfunction and Gut Microbiota Dysbiosis in Rats. Foods. 2024; 13(7):1039. https://doi.org/10.3390/foods13071039
Chicago/Turabian StyleAriaee, Amin, Hannah R. Wardill, Anthony Wignall, Clive A. Prestidge, and Paul Joyce. 2024. "The Degree of Inulin Polymerization Is Important for Short-Term Amelioration of High-Fat Diet (HFD)-Induced Metabolic Dysfunction and Gut Microbiota Dysbiosis in Rats" Foods 13, no. 7: 1039. https://doi.org/10.3390/foods13071039
APA StyleAriaee, A., Wardill, H. R., Wignall, A., Prestidge, C. A., & Joyce, P. (2024). The Degree of Inulin Polymerization Is Important for Short-Term Amelioration of High-Fat Diet (HFD)-Induced Metabolic Dysfunction and Gut Microbiota Dysbiosis in Rats. Foods, 13(7), 1039. https://doi.org/10.3390/foods13071039








