Structural Characterization and In Vitro Antioxidant Activity of a Novel Polysaccharide from Summer–Autumn Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Crude Polysaccharides
2.3. Purification of Crude Polysaccharide
2.4. Ultraviolet and FT-IR Analysis
2.5. Molecular Weight Analysis
2.6. Monosaccharide Analysis
2.7. Methylation Analysis
2.8. Nuclear Magnetic Resonance (NMR) Analysis
2.9. Scanning Electronic Microscopy (SEM) Analysis
2.10. Antioxidant Experiments
2.10.1. 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) Scavenging Experiment
2.10.2. •OH Scavenging Experiment
2.10.3. ABTS+ Scavenging Experiment
2.11. Statistical Analysis
3. Results and Discussion
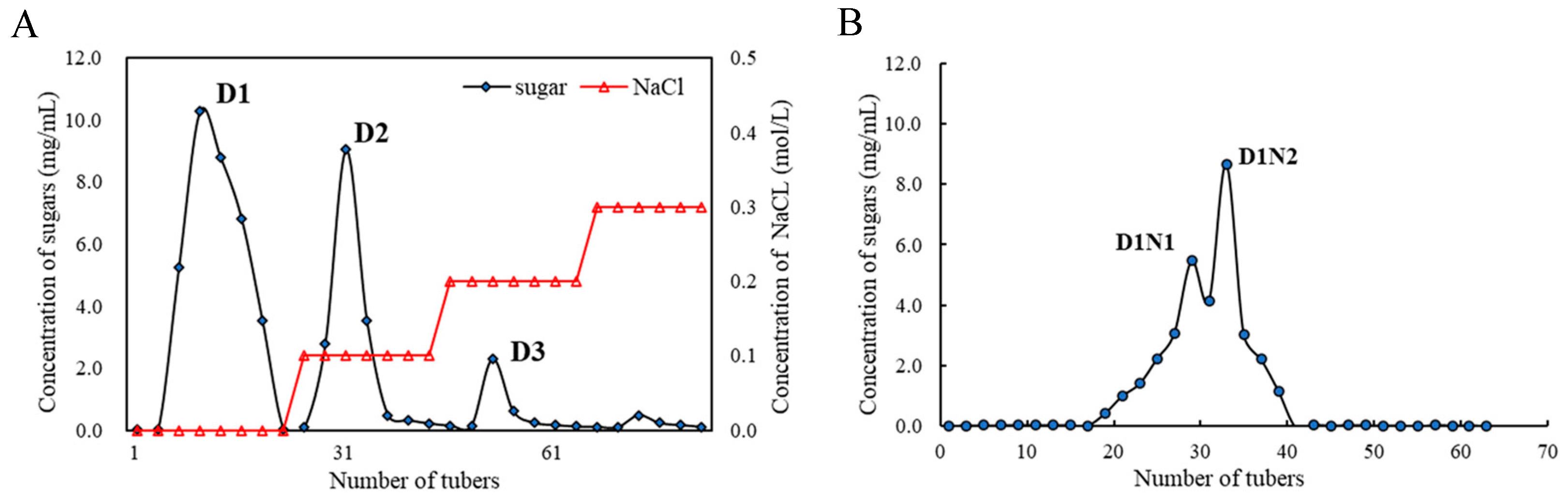
3.1. Extraction and Purification of Polysaccharide from Summer–Autumn Tea
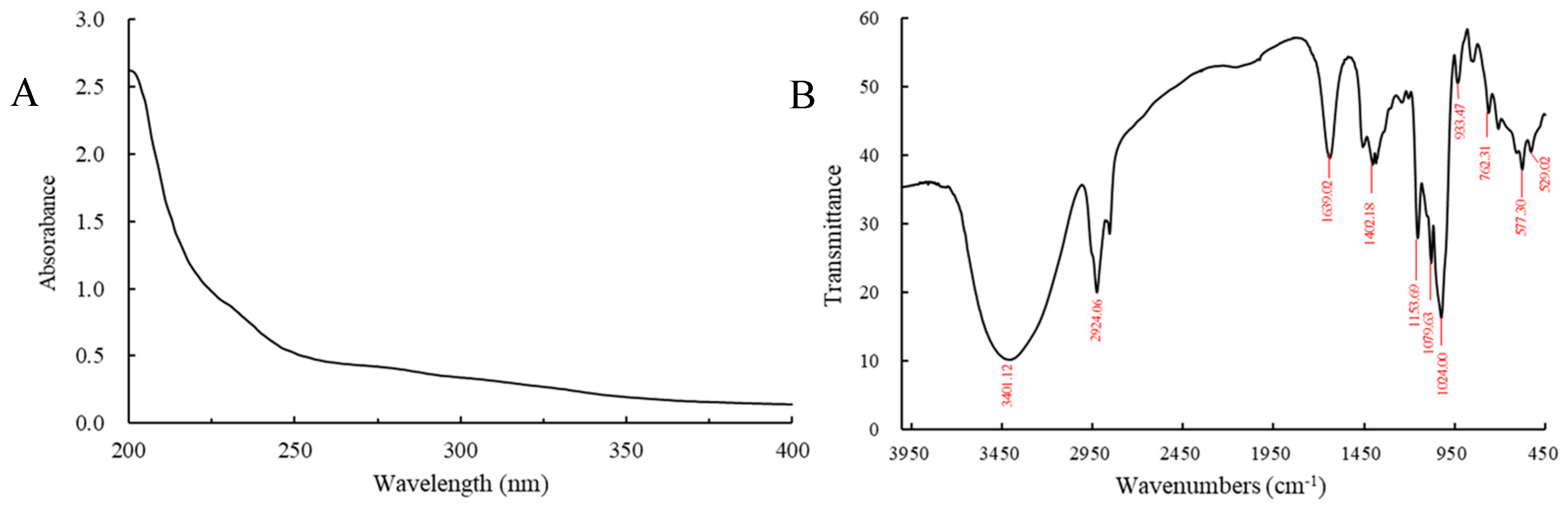
3.2. UV and FT-IR Analysis of D1N1
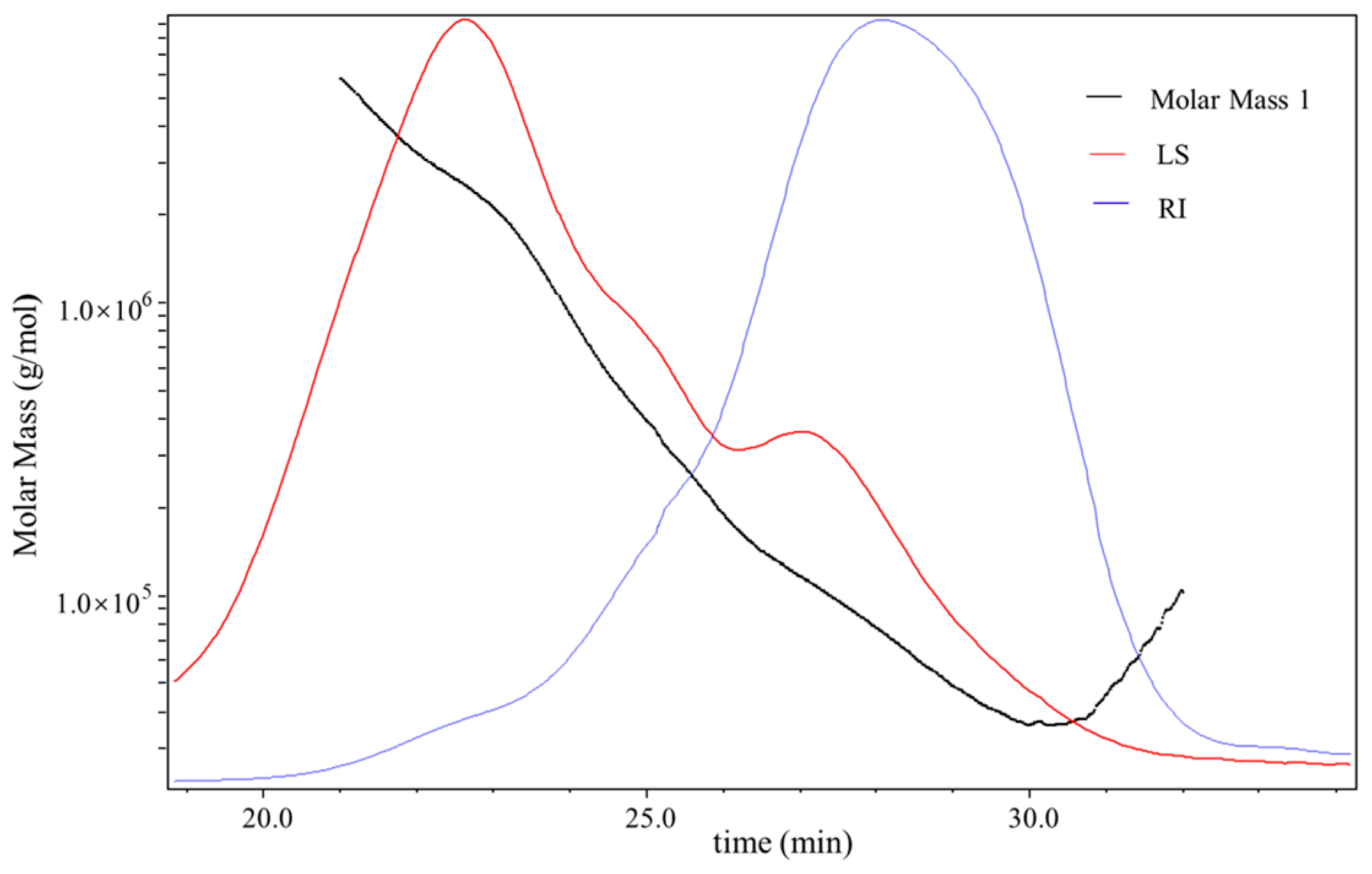
3.3. Molecular Weight (Mw) of D1N1
3.4. Monosaccharide Analysis of D1N1
3.5. Linkage Analysis of D1N1
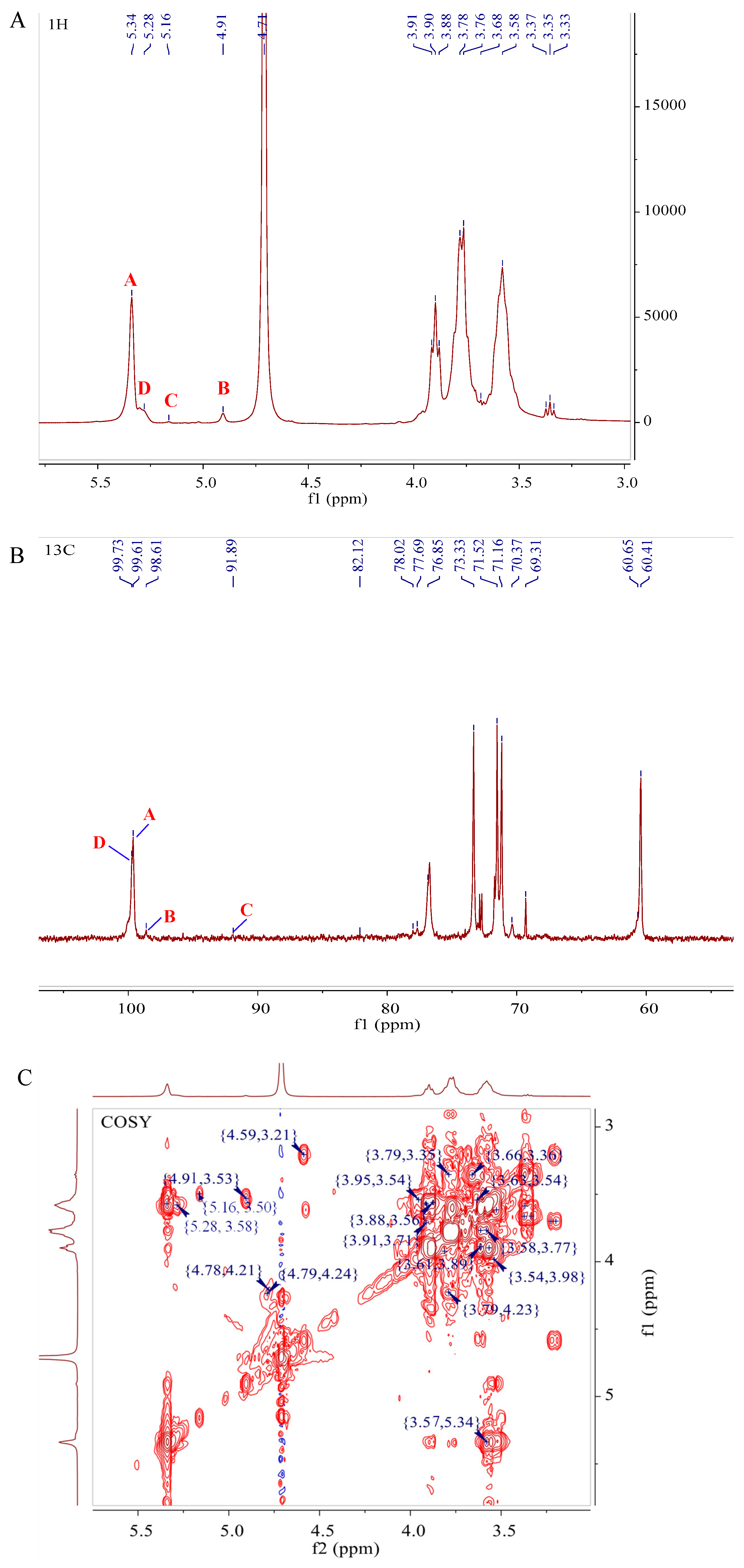
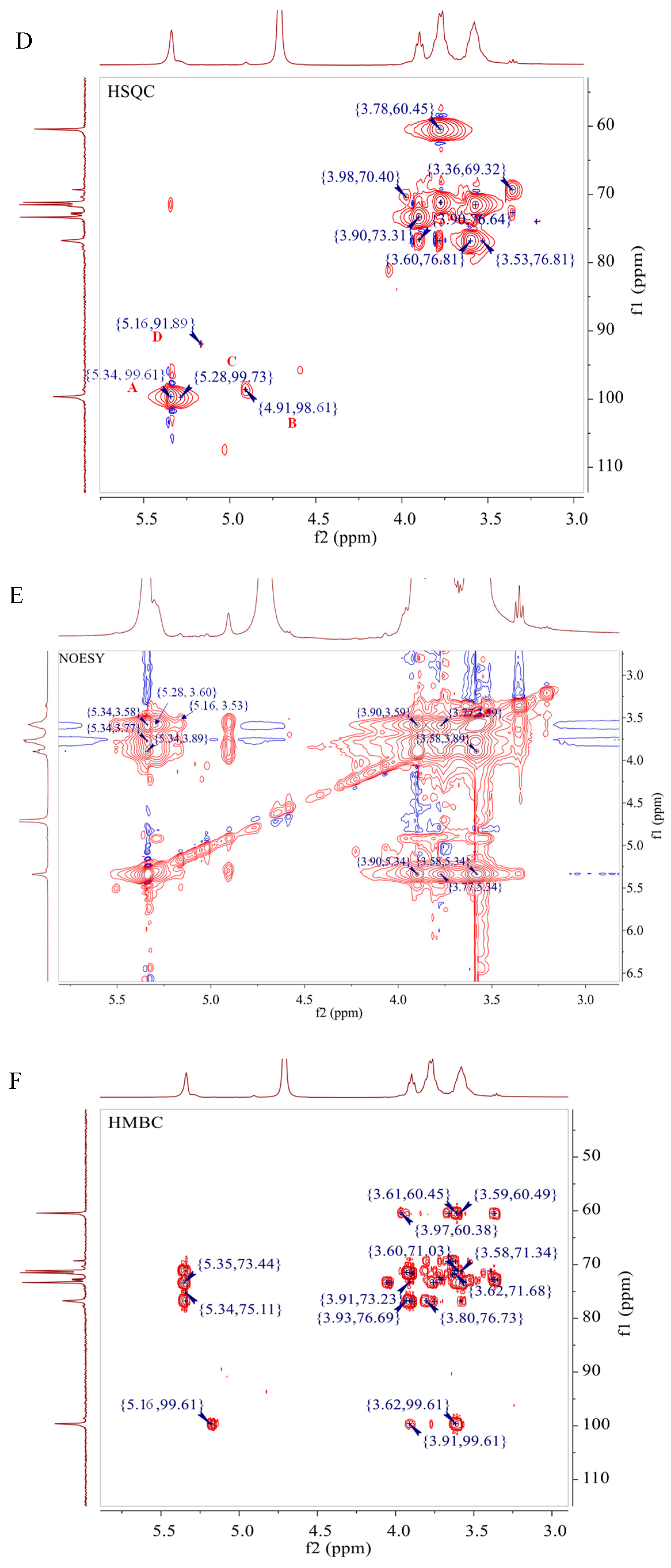
3.6. NMR Analysis of D1N1
3.7. SEM Analysis of D1N1
3.8. Antioxidant Activity of D1N1 In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, H. Polysaccharides from Chinese tea: Recent advance on bioactivity and function. Int. J. Biol. Macromol. 2013, 62, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yuan, Q.; Saeeduddin, M.; Ou, S.; Zeng, X.; Ye, H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym. 2016, 153, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Hu, T.; Wu, P.; Zhan, J.; Wang, W.; Shen, J.; Wang, M.; Ho, C.; Li, S. Structure variety and its potential effects on biological activity of tea polysaccharides. Food Sci. Hum. Wellness 2022, 11, 587–597. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liu, Y.; Chen, X.; Wei, X. Extraction, characterization and antioxidant activities of Se-enriched tea polysaccharides. Int. J. Biol. Macromol. 2015, 77, 76–84. [Google Scholar] [CrossRef]
- Guo, H.; Fu, M.X.; Wu, D.T.; Zhao, Y.X.; Li, H.; Li, H.B.; Gan, R.Y. Structural characteristics of crude polysaccharides from 12 selected Chinese teas, and their antioxidant and anti-diabetic activities. Antioxidants 2021, 10, 1562. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Chen, R.; Li, Q.; Lai, X.; Wen, S.; Cao, J.; Lai, Z.; Li, Z.; Sun, S. Recent insights into the physicochemical properties, bioactivities and their relationship of tea polysaccharides. Food Chem. 2023, 432, 137223. [Google Scholar] [CrossRef]
- Liu, M.; Gong, Z.; Liu, H.; Wang, J.; Wang, D.; Yang, Y.; Zhong, S. Structural characterization and anti-tumor activity in vitro of a water-soluble polysaccharide from dark brick tea. Int. J. Biol. Macromol. 2022, 205, 615–625. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Yin, L.; Fu, S.; Wu, R.; Wei, S.; Yi, J.; Zhang, L.M.; Yang, L. Chain conformation of an acidic polysaccharide from green tea and related mechanism of α-amylase inhibitory activity. Int. J. Biol. Macromol. 2020, 164, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, F.; Wei, X. Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. Carbohydr. Polym. 2012, 88, 146–153. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Lo, T.C.T.; Chang, C.A.; Chiu, K.H.; Tsay, P.K.; Jen, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, B.; Long, F.; Wei, J.; Zhang, Y.; Cui, Y.; Yuan, Y.; Yue, T. Comparison of chemical constituents of Eurotium cristatum-mediated pure and mixed fermentation in summer-autumn tea. LWT-Food Sci. Technol. 2021, 143, 111132. [Google Scholar] [CrossRef]
- Fhatuwani, M.N.; Nokwanda, M.P. Effect of seasonal variations and growth conditions on carbohydrate partitioning in different organs and the quality of bush tea. Hortscience 2018, 53, 999–1005. [Google Scholar] [CrossRef]
- Chen, X.; Fang, Y.; Nishinari, K.; We, H.; Sun, C.; Li, J.; Jiang, Y. Physicochemical characteristics of polysaccharide conjugates prepared from fresh tea leaves and their improving impaired glucose tolerance. Carbohydr. Polym. 2014, 112, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Liu, G.; Xu, H.; Zhang, X. Chemical elucidation of an arabinogalactan from rhizome of Polygonatum sibiricum with antioxidant activities. Int. J. Biol. Macromol. 2021, 190, 730–738. [Google Scholar] [CrossRef]
- Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Wei, Z.J. Physicochemical and antioxidant potential of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 131, 453–460. [Google Scholar] [CrossRef]
- Abuduwaili, A.; Nuerxiati, R.; Mutailifu, P.; Gao, Y.; Lu, C.; Yili, A. Isolation, structural modification, characterization, and bioactivity of polysaccharides from Folium Isatidis. Ind. Crops Prod. 2022, 176, 114319. [Google Scholar] [CrossRef]
- Kuang, M.T.; Xu, J.Y.; Li, J.Y.; Yang, L.; Hou, B.; Zhao, Q.; Hu, J.M. Purification, structural characterization and immunomodulatory activities of a polysaccharide from the fruiting body of Morchella sextelata. Int. J. Biol. Macromol. 2022, 213, 394–403. [Google Scholar] [CrossRef]
- Chen, P.; Tong, M.; Zeng, H.; Zheng, B.; Hu, X. Structural characterization and in vitro fermentation by rat intestinal microbiota of a polysaccharide from Porphyra haitanensis. Food Res. Int. 2021, 147, 110546. [Google Scholar] [CrossRef]
- Meng, X.; Che, C.; Zhang, J.; Gong, Z.; Si, M.; Yang, G.; Cao, L.; Liu, J. Structural characterization and immunomodulating activities of polysaccharides from a newly collected wild Morchella sextelata. Int. J. Biol. Macromol. 2019, 129, 608–614. [Google Scholar] [CrossRef]
- Lin, P.; Chen, L.; Huang, X.; Xiao, F.; Fu, L.; Jing, D.; Wang, J.; Zhang, H.; Sun, L.; Wu, Y. Structural characteristics of polysaccharide GP2a in Gardenia jasminoides and Its Immunomodulatory Effect on Macrophages. Int. J. Biol. Macromol. 2022, 23, 11279. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, H.; Hu, Z.; Zhang, Y.; Guo, L.; Shao, M.; Man, C.; Jiang, Y. Research on degradation of polysaccharides during Hericium erinaceus fermentation. LWT-Food Sci. Technol. 2023, 173, 114276. [Google Scholar] [CrossRef]
- Pan, D.; Wang, L.; Chen, C.; Teng, B.; Wang, C.; Xu, Z.; Hu, B.; Zhou, P. Structure characterization of a novel neutral polysaccharide isolated from Ganoderma lucidum fruiting bodies. Food Chem. 2012, 135, 1097–1103. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.E.; Wang, N.; Liu, X.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; et al. Chemical composition and antioxidant activities of polysaccharides from Yingshan cloud mist tea. Oxidative Med. Cell. Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Du, L.; Yuan, M. Extraction, puriffcation and characterization of polysaccharides from Hawk tea. Carbohydr. Polym. 2014, 99, 319–324. [Google Scholar] [CrossRef]
- Yin, L.; Fu, S.; Wu, R.; Wei, S.; Yi, J.; Zhang, L.M.; Yang, L. A neutral polysaccharide from green tea: Structure, effect on α-amylase activity and hydrolysis property. Arch. Biochem. Biophys. 2020, 687, 108369. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, X.; Li, X.; Sun, X.; Kou, Y.; Li, D.; Liu, Y.F.; Zhang, H.; Wu, Y. Pectic polysaccharides from Biluochun Tea: A comparative study in macromolecular characteristics, fine structures and radical scavenging activities in vitro. Int. J. Biol. Macromol. 2022, 195, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Ma, F.; Wang, R.; Zhang, Y.; Bai, J.; Fang, H.; Ma, W.; Liu, W.; Li, Q.; Liu, X. Polysaccharides from Dioscorea opposita Thunb.: Isolation, structural characterization, and anti-inflammatory and anti-tumor effects against hepatocellular carcinoma. Chem. Biol. Technol. Agric. 2023, 10, 43. [Google Scholar] [CrossRef]
- Cui, S. Food Carbohydrates: Chemistry, Physical Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005; pp. 113–164. [Google Scholar]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, M.; Hao, H.; Deng, J.; Li, M.; Sun, Y.; Yang, R.; Wang, H.; Huang, R. Structure characterization of a novel polysaccharide from Chinese wild fruits (Passiflora foetida) and its immune-enhancing activity. Int. J. Biol. Macromol. 2019, 136, 324–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Ran, S.; Wang, K. Purification, structural elucidation and anti-inflammatory activity in vitro of polysaccharides from Smilax china L. Int. J. Biol. Macromol. 2019, 139, 233–243. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, C.; Wang, X.; Rui, X.; Zhang, Q.; Chen, X.; Dong, M.; Li, W. Structural characterization and immunomodulatory activity of intracellular polysaccharide from the mycelium of Paecilomyces cicadae TJJ1213. Food Res. Int. 2021, 147, 110515. [Google Scholar] [CrossRef]
- Rong, L.; Li, G.; Zhang, Y.; Xiao, Y.; Qiao, Y.; Yang, M.; Wei, L.; Bi, H.; Gao, T. Structure and immunomodulatory activity of a water-soluble α-glucan from Hirsutella sinensis mycelia. Int. J. Biol. Macromol. 2021, 189, 857–868. [Google Scholar] [CrossRef]
- Li, J.; Deng, Q.; Yu, X.; Wang, W. Structural studies of a new fraction obtained by gradient ethanol precipitation from Acacia seyal gum. Food Hydrocoll. 2020, 107, 105932. [Google Scholar] [CrossRef]
- Wei, L.; Huang, L.; Du, L.; Sun, Q.; Chen, C.; Tang, J.; Teng, J.; Wei, B. Structural characterization and in vitro antioxidant, hypoglycemic and hypolipemic activities of a natural polysaccharide from Liupao Tea. Foods 2023, 12, 2226. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Zhang, K.; Sun, F.; Zhou, W.; Wu, Z.; Li, X. Purification, characterization, and emulsification stability of high-and low-molecular-weight fractions of polysaccharide conjugates extracted from green tea. Food Hydrocoll. 2022, 129, 107667. [Google Scholar] [CrossRef]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the anti-oxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Huangfu, L.T.; Dong, L.L.; Liu, S.L. Functional groups and antioxidant activities of polysaccharides from five categories of tea. Ind. Crops Prod. 2014, 58, 31–35. [Google Scholar] [CrossRef]
- Xu, G.Y.; Liao, A.M.; Huang, J.H.; Zhang, J.G.; Thakur, K.; Wei, Z.J. Evaluation of structural, functional, and anti-oxidant potential of differentially extracted polysaccharides from potatoes peels. Int. J. Biol. Macromol. 2019, 129, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.L.; Yu, X.B. Isolation, purification, characterization, and antioxidant activity of low-molecular-weight polysaccharides from Sparassis latifolia. Int. J. Biol. Macromol. 2019, 137, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Huo, J.; Zhao, T.; Ren, J.; Wei, X. Effect of different drying methods on chemical composition and bioactivity of tea polysaccharides. Int. J. Biol. Macromol. 2013, 62, 714–719. [Google Scholar] [CrossRef] [PubMed]
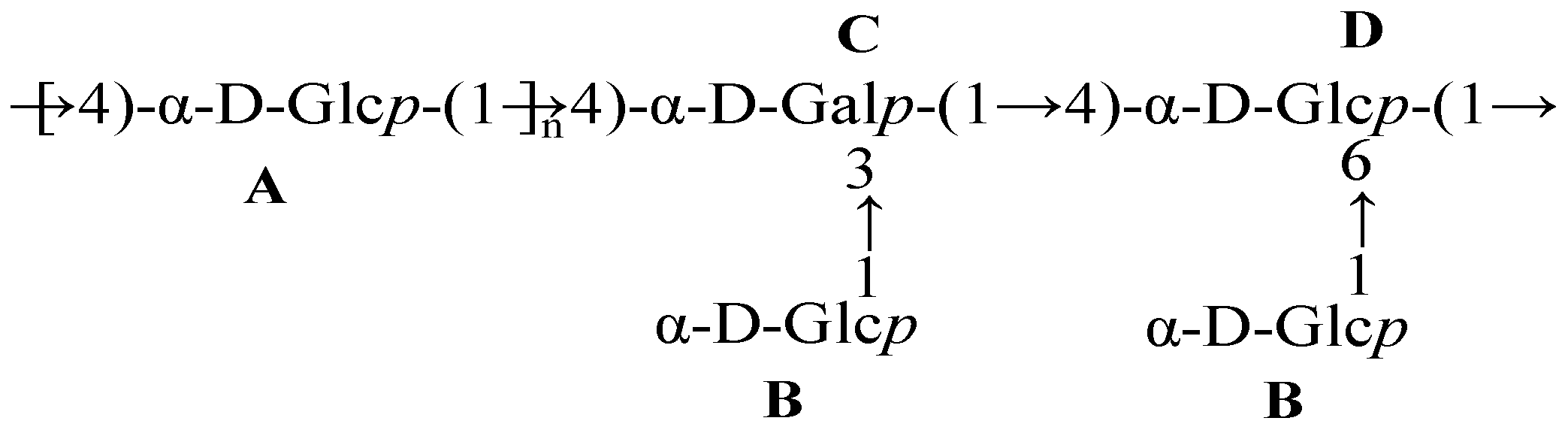


| Sample | DIN1 | |||
|---|---|---|---|---|
| Monosaccharide composition | Rhamnose | Arabinose | Galactose | Glucose |
| Molar ratio (%) | 0.44 | 2.43 | 1.83 | 95.30 |
| Sample | Type of Linkage | Methylated Sugars | Retention Time | Mass Fragments | Relative Molar Ratio (%) |
|---|---|---|---|---|---|
| D1N1 | t-Glc(p) | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl glucitol | 8.994 | 55.1, 71.1, 87.1, 102.1, 129.1, 145.1, 162.1, 205.1, 239.3 | 11.83 |
| 4-Glc(p) | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 14.244 | 71, 87, 102, 118, 129, 142, 162, 233 | 82.36 | |
| 3,4-Gal(p) | 1,3,4,5-tetra-O-acetyl-2,6-di-O-methyl galactitol | 16.308 | 59, 87, 99, 118, 129, 143, 185 | 2.31 | |
| 4,6-Glc(p) | 1,4,5,6-tetra-O-acetyl-2,3-tri-O-methyl glucitol | 18.414 | 59, 74, 85, 102, 118, 127, 142, 162, 201, 261 | 3.50 |
| Code | Glycosyl Residues | Chemical Shifts (ppm) | |||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| A | →4)-α-d-Glcp-(1→ | 5.34 | 3.57 | 3.98 | 3.60 | 3.77 | 3.62, 3.78 |
| 99.61 | 71.53 | 70.4 | 76.81 | 71.18 | 60.45 | ||
| B | α-d-Glcp-(1→ | 4.91 | 3.53 | 3.90 | 3.36 | 3.57 | 3.62, 3.92 |
| 98.61 | 71.40 | 73.31 | 69.31 | 71.53 | 60.79 | ||
| C | →3,4)-α-d-Galp-(1→ | 5.16 | 3.50 | 3.76 | 3.90 | 3.49 | 3.65, 3.90 |
| 91.89 | 70.00 | 76.78 | 76.64 | 70.30 | 61.30 | ||
| D | →4,6)-α- d -Glcp-(1→ | 5.28 | 3.58 | 3.87 | 3.53 | 3.90 | 3.87 |
| 99.73 | 72.52 | 71.33 | 76.81 | 71.82 | 69.80 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Cao, Z.; Tian, J.; Lv, W.; Wang, H. Structural Characterization and In Vitro Antioxidant Activity of a Novel Polysaccharide from Summer–Autumn Tea. Foods 2024, 13, 821. https://doi.org/10.3390/foods13060821
Cao M, Cao Z, Tian J, Lv W, Wang H. Structural Characterization and In Vitro Antioxidant Activity of a Novel Polysaccharide from Summer–Autumn Tea. Foods. 2024; 13(6):821. https://doi.org/10.3390/foods13060821
Chicago/Turabian StyleCao, Miao, Zheng Cao, Juanjuan Tian, Wenping Lv, and Hongxin Wang. 2024. "Structural Characterization and In Vitro Antioxidant Activity of a Novel Polysaccharide from Summer–Autumn Tea" Foods 13, no. 6: 821. https://doi.org/10.3390/foods13060821
APA StyleCao, M., Cao, Z., Tian, J., Lv, W., & Wang, H. (2024). Structural Characterization and In Vitro Antioxidant Activity of a Novel Polysaccharide from Summer–Autumn Tea. Foods, 13(6), 821. https://doi.org/10.3390/foods13060821








